Abstract
Mitochondrial thymidine kinase 2 (TK2) is a key enzyme in the salvage of pyrimidine deoxynucleosides needed for mitochondrial DNA synthesis. TK2 phosphorylates thymidine (dThd), deoxycytidine (dCyd), and many other antiviral pyrimidine nucleoside analogs. Zidovudine (AZT) is the first nucleoside analog approved for anti-HIV therapy, and it is still used in combination with other drugs. One of the side effects of long-term treatment with nucleoside analogs is mitochondrial DNA depletion, which has been ascribed to competition by AZT for the endogenous dThd phosphorylation carried out by TK2. Here we studied the kinetics of AZT and 3′-fluorothymidine phosphorylation by recombinant human TK2 and the effects of these and other pyrimidine nucleoside analogs on the phosphorylation of dThd and dCyd. Thymidine analogs strongly inhibited dThd phosphorylation but not dCyd phosphorylation, which instead was stimulated ∼30%. We found that recombinant human TK2 contained the feedback inhibitor dTTP in a 1:1 molar ratio and that incubation with dThd and AZT could completely remove the enzyme-bound dTTP, but dCyd was less efficient in this regard. The release of feedback inhibitor by dThd and dThd analogs most likely accounts for the observed kinetics. Similar effects were also observed with native rat liver mitochondrial TK2, strongly indicating a physiologic role for this process, which most likely is an important factor in the mitochondrial toxicity observed with antiviral nucleoside analogs.
INTRODUCTION
Clinical use of anti-HIV nucleoside analogues is often associated with diverse side effects such as myopathy, cardiomyopathy, peripheral neuropathy, and lipodystrophy (4–6, 16). Mitochondrial toxicity accompanying mitochondrial DNA (mtDNA) depletion was first observed in muscle tissues of patients receiving zidovudine (AZT) therapy (1). Depletion of mtDNA has also been reported in patients treated with didanosine (ddI), stavudine (d4T), and zalcitabine (ddC). Except for muscles, mtDNA depletion has also been found in heart, liver, and peripheral blood cells (12, 17, 24). Fluorothymidine (FLT) was initially developed as an antiviral drug but was discontinued due to severe bone marrow and liver toxicity in clinical trials (8). Fosalvudine tidoxil, a new FLT derivative in clinical trial for HIV treatment, induced liver mtDNA depletion in an experimental animal model (23). Labeled FLT is often used as a tracer for positron emission tomography (3).
Mitochondrial DNA depletion syndrome is a mitochondrial disorder characterized by a quantitative reduction of mtDNA in a tissue-specific manner, usually in liver or muscle (13). Deficiency in thymidine kinase 2 (TK2) activity or alteration of TK2 specificities, due to point mutations, insertion, and deletion of the TK2 gene, is associated with tissue-specific mitochondrial DNA depletion and severe myopathy (19). The underlining mechanism of this mtDNA disorder has been suggested to be an imbalance in the mitochondrial deoxynucleotide pools (18, 27). Organello studies with isolated rat mitochondria as well as heart perfusion studies have shown that the TK2-catalyzed reaction is the rate-limiting step in the mitochondrial dTTP synthesis (10, 11, 20). Furthermore, treatment with AZT inhibited mitochondrial TK2 activity, which resulted in a significantly decreased mitochondrial dTTP level but not dCTP level (14). In light of these results, we decided to reevaluate the role of TK2 in mitochondrial toxicity caused by antiviral nucleoside analogues. We studied the effects of AZT and FLT, as well as those of several other pyrimidine nucleoside analogues used in anti-HIV therapy, on the phosphorylation of thymidine (dThd) and deoxycytidine (dCyd) with human TK2. Native TK2 purified from rat liver mitochondria was also investigated. Striking differences in the effects of these analogs on the phosphorylation of dCyd and dThd catalyzed by TK2 were found, and this most likely accounts for the dTTP and dCTP pool imbalance observed in the presence of pyrimidine nucleoside analogs (14). We found that recombinant human TK2 contains dTTP bound in an equimolar amount, that dTTP could be released by incubation with dThd and dThd analogs, and that the enzyme-bound dTTP most likely accounts for the kinetic effects observed with dThd analogs.
MATERIALS AND METHODS
Materials.
Nucleosides 2′-deoxycytidine (dCyd), 2′-deoxythymidine (dThd), 2′,3′-dideoxycytidine (ddC), 3′-azido-2′,3′-dideoxythymidine (AZT), 3′-fluoro-2′,3′-deoxythymidine (FLT), β-l-2′,3′-dideoxy-3′-thiacytidine (3TC), and 2′,3′-didehydro-2′,3′-dideoxythymidine (d4T) were purchased from Sigma. 1-(2′-Deoxy-2′-fluoro-1-β-d-arabinosyl)-5-iodouracil (FIAU) and 1-(2′-deoxy-2′-fluoro-1-β-d-arabinosyl)-5-methyluracil (FMAU) were kindly provided by J. Fox, Memorial Sloan Kettering Cancer Institute, New York, NY. Radioactive dThd ([methyl-3H]dThd; 20 Ci/mmol) and [γ-32P]ATP (3,000 Ci/mmol) were from PerkinElmer, and radioactive dCyd ([5-3H]dCyd, 27 Ci/mmol) was from Moravek Biochemicals Inc.
Enzyme assays.
Recombinant TK2 was expressed and purified as previously described (26). TK2 activity was determined using either [3H]dThd, [3H]dCyd, or [γ-32P]ATP as the labeled substrate. The standard reaction mixture (50 μl) contained 50 mM Tris HCl, pH 7.6, 2 mM ATP, 5 mM MgCl2, 0.5 mg/ml bovine serum albumin, 5 mM dithiothreitol, variable concentrations of 3H-labeled deoxynucleoside, and a fixed concentration of each inhibitor in order to determine the Ki values of the inhibitor. The concentration of TK2 used was 0.2 nM. Due to the fact that the phosphorylation of dThd by TK2 exhibits negative cooperativity (15), the concentration range for dThd was chosen to be between 1 and 10 μM in this study, which will yield linear double-reciprocal (Lineweaver-Burk) plots, and the Km value for dThd determined here corresponded to Km1 (15). The dCyd concentration was varied from 1 to 160 μM. The data were analyzed by the Sigma Plot enzyme kinetic module, version 1.1 (SPSS Inc.), using the Michaelis-Menten equation.
Isolation of intact mitochondria and purification of rat liver mitochondrial TK2.
Mitochondria were prepared by a differential centrifugation method (7) using fresh tissue from adult rats (female; weight, 300 to 350 g; Sprague-Dawley), and the whole procedure was done at 4°C. The pellet of mitochondria was resuspended in the mitochondria preparation buffer (20 mM HEPES/KOH, pH 7.4, 250 mM sucrose, 2 mM EGTA), and the suspension was stored at −70°C. Mitochondrial proteins were extracted by addition of 0.5% NP-40 and sonication in an ice-water bath. Mitochondrial TK2 was purified essentially as described previously (12). To determine the molecular forms of TK2 in mitochondria, purified TK2 was resolved on an SDS–12% polyacrylamide gel, the bands were excised and digested with trypsin, and the resulting peptides were analyzed by mass spectrometry.
Determination of enzyme-bound feedback inhibitor content by HPLC.
Recombinant human TK2 (20 μM) was incubated at 37°C for 5 min in the presence of nucleosides and/or nucleotides (200 μM), and then the samples were dialyzed against buffer containing 50 mM Tris HCl, pH 7.6, 1 mM MgCl2, and 100 mM NaCl at 4°C with two buffer changes. Protein concentrations of the dialyzed samples were determined before treatment with 10% perchloric acid (PCA) to precipitate the proteins, and after centrifugation the supernatant was neutralized with KOH. The salt precipitates were removed by centrifugation, and the supernatant was mixed with high-pressure liquid chromatography (HPLC) buffer (1:4) before injection. HPLC analysis was performed using a reverse-phase column (Source 5RPC; GE Healthcare) with isocratic elution in buffer containing 50 mM ammonium phosphate, pH 6.5, 5 mM tetrabutylammonium hydrogen sulfate, and 30% methanol at a flow rate of 1 ml/min. UV absorbance was monitored at 260 nm and 280 nm, and the absorbance ratio of 280 nm/260 nm (dTTP, 0.73; dCTP, 0.96; dATP, 0.15; and dGTP, 0.67) was used to identify the deoxynucleotide peaks and was compared with the ratios for authentic standards. Retention times for standards are 3.25 min for dCTP, 4.01 min for dTTP, 5.1 min for dGTP, 6.43 min for dATP, and 5.36 min for ATP.
RESULTS
Phosphorylation of AZT and FLT by human recombinant TK2.
Both AZT and FLT are known to be poor substrates for TK2 (26). Here the phosphorylation of AZT and FLT was studied using the phosphoryl transfer assay with [γ-32P]ATP as the labeled substrate. The Km values for AZT and FLT were 4.5 μM and 6.5 μM, respectively, and the Vmax values were 28.9 and 23.5 nmol/min/mg, respectively, which gave efficiencies (Vmax/Km) 2 and 3% of the efficiency with dThd (Fig. 1A). These results are similar to those of earlier studies using tritium-labeled nucleoside analogs (12).
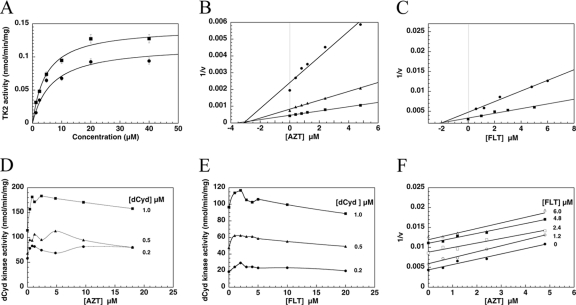
Fig. 1.
Characterization of recombinant human TK2 with AZT and FLT. (A) Substrate saturation curve of AZT (■) and FLT (●). (B) Dixon plots of 1/v versus AZT concentration. The concentrations of dThd were 0.2 μM (●), 0.5 μM (▴), and 1.0 μM (■). (C) Dixon plots of 1/v versus FLT concentration. The concentrations of dThd were 0.2 μM (●) and 0.6 μM (■). (D) Stimulation of dCyd phosphorylation by AZT. dCyd concentrations were 0.2, 0.5, and 1.0 μM. (E) Stimulation of dCyd phosphorylation by FLT. dCyd concentrations were 0.2, 0.5, and 1.0 μM. (F) Plots of 1/v versus AZT concentration (at fixed concentrations of FLT of 0, 1.2, 2.4, 4.8, and 6.0 μM). The concentrations of dThd and ATP were 0.6 μM and 2 mM, respectively. All measurements were repeated at least 3 times, and data are plotted as means ± standard errors of the means (error bars).
Inhibition of dThd phosphorylation and stimulation of dCyd phosphorylation by AZT, FLT, and other nucleoside analogs.
In tissues or cells, the concentrations of dThd and dCyd are submicromolar (21), and therefore, in an attempt to mimic physiological conditions, the kinetic assays were performed with either [3H]dThd or [3H]dCyd as substrates at 0.2 to 2.0 μM with purified recombinant human TK2 and with AZT and FLT as inhibitors. The TK2 activity with dThd was inhibited by AZT, and the mode of inhibition was competitive, with a Ki value of 3.0 μM (Fig. 1B). FLT inhibited dThd phosphorylation competitively, with a Ki value of 1.4 μM (Fig. 1C).
The phosphorylation of dCyd was, however, not inhibited by AZT over the same concentration range (0.4 to 19.2 μM); rather, a stimulation of ∼30% was observed (Fig. 1D). FLT also stimulated dCyd phosphorylation over a concentration range of 1 to 20 μM (Fig. 1E), and slight inhibition was observed at concentrations higher than 100 μM (data not shown). We also tested other thymidine analogs, such as d4T and arabinosyl-thymidine (araT), which stimulated dCyd phosphorylation over the same concentration range (data not shown).
The inhibitory effect of other nucleoside analogues was studied with dThd (1 to 10 μM) or dCyd (1 to 160 μM) as substrate and different fixed concentrations of nucleoside analogs. Inhibition by d4T was similar to that by AZT, with Ki values of d4T of 24 μM toward dThd and >500 μM toward dCyd (Table 1). The monophosphate product of AZT, i.e., AZTMP, did not inhibit either dThd or dCyd phosphorylation at a 500 μM concentration (data not shown). The anti-HIV nucleoside 3TC, which is a poor substrate with TK2, inhibited dCyd and dThd phosphorylation only at very high concentrations (Table 1). Therefore, at therapeutic concentrations, 3TC would not inhibit TK2 activity. At low concentrations, ddC did not inhibit either dThd or dCyd phosphorylation by TK2, and at very high concentrations, ddC inhibited dCyd phosphorylation, with a Ki value of 496 μM. FIAU, a nucleoside analogue used in hepatitis B treatment, inhibited dThd and dCyd phosphorylation by TK2, with Ki values of 43 and 141 μM, respectively. FMAU strongly inhibited dThd phosphorylation, with a Ki value of 8.8 μM, but the inhibition toward dCyd was much less pronounced, with a Ki value of 286 μM.
Table 1.
Pyrimidine nucleoside analogues as inhibitors of recombinant human TK2a
| Substrate | Inhibitor | Km (μM) | Ki (μM) |
|---|---|---|---|
| dThd | 1.9 ± 0.2 | ||
| AZT | 3.0 ± 0.3 | ||
| FLT | 1.4 ± 0.2 | ||
| d4T | 24 ± 2.0 | ||
| FIAU | 42.8 ± 5.2 | ||
| FMAU | 8.8 ± 1.0 | ||
| 3TC | 465 ± 12 | ||
| ddC | >1,000 | ||
| dCyd | 11 ± 1.0 | ||
| AZT | >200 | ||
| FLT | >200 | ||
| d4T | >500 | ||
| FIAU | 141 ± 6.0 | ||
| FMAU | 286 ± 12 | ||
| 3TC | 397 ± 9.0 | ||
| ddC | 496 ± 5.0 |
Data were from at least three independent measurements performed with variable dThd/dCyd concentrations at three fixed inhibitor concentrations. The Ki values were calculated from plots of slopes of Lineweaver-Burk plots versus inhibitor concentration and are given as means ± standard errors of the means.
The inhibition of human TK2 phosphorylation of dThd by AZT and FLT are mutually exclusive.
With dThd as substrate, the inhibitory effects of AZT and FLT were studied in a multi-inhibitor assay in which the [3H]dThd concentration was kept constant (0.6 μM), the FLT concentration varied (0 to 6.0 μM) at different fixed AZT concentrations (0 to 4.8 μM), and the formation of [3H]dTMP was measured with the DE-81 filter method. Dixon plots of 1/v (where v is velocity) versus FLT concentration at different fixed concentrations of AZT gave parallel lines (Fig. 1F). Similarly, 1/v versus the AZT concentration at different fixed FLT concentrations also gave parallel lines (data not shown), indicating that inhibition by both the inhibitors FLT and AZT is mutually exclusive and that they most likely inhibit via the same mechanism.
When the assays were performed with [γ-32P]ATP as labeled substrate, all the products, i.e., dTMP, AZTMP, and FLT monophosphate (FLTMP), were quantified in the same reaction. We could then observe a concentration-dependent decrease of dTMP formation and concentration-dependent increases of AZTMP and FLTMP formation. Furthermore, the maximal inhibition of dTMP production was ∼50% in the presence of both AZT and FLT (data not shown).
AZT and FLT inhibited dThd phosphorylation but stimulated dCyd phosphorylation with native rat liver mitochondrial TK2.
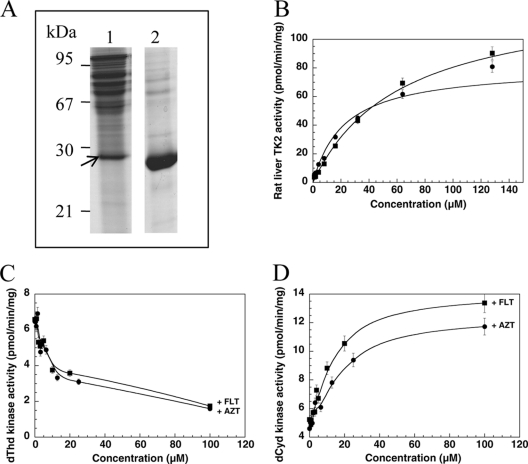
In order to clarify if the stimulatory effect observed with AZT and FLT on dCyd phosphorylation was a special property of recombinant TK2, rat liver mitochondrial TK2 was purified by anion-exchange chromatography and affinity chromatography (Fig. 2A) (15). Mass spectrometry analysis of peptides generated by in-gel trypsin digestion was performed (see Table S1 in the supplemental material) and showed that the band with an apparent molecular mass of 28 kDa corresponded to rat TK2. The amino acid sequence identity between rat and human TK2 was 82%, and the apparent molecular mass of native rat mitochondrial TK2 is 28 kDa, similar to that of the recombinant human TK2, which corresponded to the mature mitochondrial form of TK2 with a cleavage site, as suggested for mouse TK2 (25). Thus, the identity of the purified native TK2 protein was confirmed.
Fig. 2.
Characterization of rat liver mitochondrial TK2. (A) SDS-PAGE analysis of purified native rat liver mitochondrial TK2 (lane 1, the arrow indicates the TK2 band) and recombinant human TK2 (lane 2); (B) substrate saturation curves (■, dThd; ●, dCyd); (C) inhibition of dThd phosphorylation by AZT (■) and FLT (●); (D) stimulation of dCyd phosphorylation by AZT and FLT. All measurements were repeated at least 3 times, and data are plotted as mean ± standard errors of the means (error bars).
Substrate kinetic studies were performed with this purified native TK2, and the apparent Km values for dThd and dCyd were 19 and 41 μM, respectively, and the Vmax values for dThd and dCyd were 70 and 89 pmol/min/mg, respectively (Fig. 2B). As in the case of recombinant human TK2, AZT and FLT inhibited dThd phosphorylation with 50% inhibitory concentrations of ∼10 μM, while stimulation of dCyd phosphorylation was observed (Fig. 2C and D). Thus, rat liver mitochondrial TK2 showed properties similar to those of the recombinant human TK2. However, the stimulatory effect of AZT and FLT on dCyd phosphorylation was more profound, with native rat TK2 having an ∼3-fold increase in dCyd kinase activity in the presence of AZT or FLT.
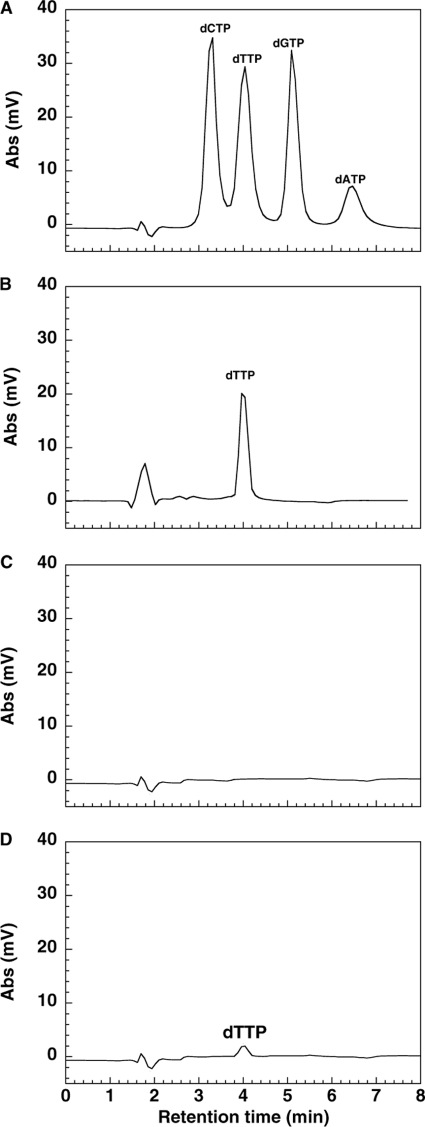
Efficiency in removal of enzyme-bound feedback inhibitor dTTP.
Feedback inhibitors dTTP, dCTP, and dATP have been shown to bind to a recombinant human TK2 preparation (2). Therefore, we suspected that the presence of enzyme-bound feedback inhibitors might affect the kinetic behavior of TK2. The content of dTTP in the TK2 preparations was determined as described in Materials and Methods and found to be approximately equimolar to TK2, and there were no other deoxynucleoside triphosphates detected (Fig. 3B). The enzyme-bound dTTP could be removed by incubation with either nucleoside or nucleoside in combination with ATP, as described by Barroso et al. (2). We incubated TK2 at 37°C for 5 min with a 10-fold excess of nucleoside, and after extensive dialysis at 4°C, we analyzed the content of enzyme-bound dTTP by HPLC. There was no detectable dTTP present in enzyme incubated with dThd or AZT (Fig. 3C); however, there was ∼10% of enzyme-bound dTTP remaining in TK2 incubated with dCyd (Fig. 3D). The most efficient way to remove enzyme-bound dTTP was to combine nucleoside with ATP (data not shown).
Fig. 3.
HPLC analysis of enzyme-bound feedback inhibitor. (A) HPLC standards; (B) recombinant human TK2; (C) recombinant human TK2 (20 μM) incubated with 200 μM dThd or AZT; (D) recombinant human TK2 incubated with dCyd. After dialysis, the protein was precipitated with 10% PCA and removed by centrifugation, and the supernatant was analyzed by HPLC after neutralization. Abs, absorbance.
Inhibition of dTTP-free human TK2 by AZT and FLT.
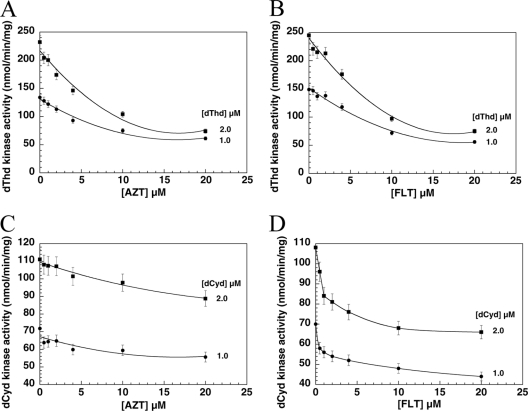
Using dTTP-free enzyme, we repeated the kinetic studies with dThd and dCyd. With 1 and 2 μM dThd and dCyd as the labeled substrate and variable concentrations of AZT or FLT (0 to 20 μM), the phosphorylation of both dThd and dCyd was inhibited by AZT and FLT (Fig. 4). The Ki values were 12 μM for AZT and 21 μM for FLT with dThd as substrate and 120 μM for AZT and 16 μM for FLT with dCyd as substrate (Fig. 4). The dTTP-free enzyme was less stable (half-life = 3 days) than dTTP containing TK2 (half-life = 21 days) when they were stored on ice.
Fig. 4.
Effects of AZT and FLT on phosphorylation of dThd and dCyd by dTTP-free recombinant human TK2. Data were from 3 independent measurements and are plotted as means ± standard errors of the means (error bars).
DISCUSSION
Nucleoside analogs used in anti-HIV therapy are prodrugs and have to be activated to their triphosphate forms by cellular enzymes to exert their therapeutic effect. In this study we have investigated the effect of pyrimidine nucleoside analogs on the phosphorylation of dThd and dCyd by recombinant human TK2. A striking finding was that these analogs showed opposite effects, e.g., inhibition of dThd phosphorylation but stimulation of dCyd phosphorylation at physiological relevant dThd and dCyd concentrations. AZT and FLT are poor substrates of human TK2, having overall efficiencies of ∼2 to 3% of the efficiency with dThd. Both AZT and FLT acted as strong inhibitors of dThd phosphorylation with low Ki values. Multi-inhibitor assays showed that inhibition by AZT and FLT is mutually exclusive, which indicates that these inhibitors compete with dThd for the same binding site. However, AZT and FLT stimulated dCyd phosphorylation by human TK2 by ∼30%. With native TK2 purified from rat liver mitochondria, the stimulation by AZT and FLT was ∼3-fold; thus, it was considerably higher than that with human TK2. The fact that other dThd analogs, i.e., araT and d4T, also inhibited dThd phosphorylation, with no or little inhibitory effect on dCyd phosphorylation, suggests that this family of compounds would elicit similar side effects with regard to mitochondrial pyrimidine nucleoside metabolism.
In order to understand the effects of these pyrimidine nucleoside analogs, other possible regulators of TK2 activity such as feedback inhibitors were investigated. Both dTTP and dCTP are the end products of dThd and dCyd phosphorylation, and they have been shown to inhibit TK2 activity with Ki values of 0.8 to 2.5 μM (27). Earlier studies showed that dTTP and, to some extent, dCTP and dATP bound to a recombinant human TK2 preparation, and the presence of these feedback inhibitors affected the initial reaction rate (2). In the present study, we found an equimolar occupancy of recombinant TK2 with dTTP, but no dCTP or other nucleotides were detected. Our kinetic studies strongly indicate that native rat liver mitochondrial TK2 also contained enzyme-bound dTTP.
The catalytic rate for AZT or FLT phosphorylation is very low compared to that for dThd or dCyd phosphorylation, but these analogs are efficient in removing dTTP from the enzyme compared with the efficiency of dCyd. Thus, the stimulatory effect of AZT and FLT on dCyd phosphorylation can be explained by the possibility that these dThd analogs more efficiently release enzyme-bound dTTP, which will increase the number of active TK2 molecules that can react with dCyd. The underlying kinetic mechanism relies on the fact that binding of nucleoside substrates is not usually the rate-limiting step, and phosphorylation is dependent on a conformational change of the enzyme substrate complex to allow phosphorylation to occur, as has been shown in the case of the homologous enzyme deoxycytidine kinase (22). The results presented here indicate that binding of dThd and dThd analogs releases the dTTP inhibitor but that the conformational changes rarely lead to phosphorylation in the case of the dThd analogs. dCyd can thus benefit from the presence of dThd analogs, and only at very high concentrations of the latter would competitive inhibition be seen. Further enzyme kinetic analysis as well as structure determinations of TK2 may provide a detailed mechanism of this phenomenon.
The kinetic effects on dThd and dCyd phosphorylation by dThd analogs most likely lead to an imbalance of dTMP and dCMP production in mitochondria. Normally, the ratio of dThd and dCyd phosphorylation with TK2 is approximately 1:0.7, calculated from Vmax/Km values. In general, mitochondrial dThd and dCyd concentrations are <1 μM, and the highest AZT concentration in patients is ∼3 μM (11). Under these conditions, TK2 activity with dThd will be reduced by ∼50% and that with dCyd will be increased by ∼30%, and thus, the ratio will be changed to 1:1.95. This might eventually lead to dTTP and dCTP pool imbalances and mtDNA mutations or depletion.
Studies with isolated rat mitochondria have shown that AZT competitively inhibited dThd phosphorylation (10, 11, 20). Treatment of perfused rat heart with AZT resulted in an ∼50% decrease of the dTTP pool, while the dCTP pool was not affected (14), in accordance with the findings of our study with purified enzyme. These results demonstrate that inhibition of TK2 activity has a strong impact on the dTTP pool at the tissue level. However, TK2 overexpression in mouse heart caused increased mtDNA copy number and mtDNA-coded polypeptide abundance and caused cardiomyopathy after AZT treatment (9). Clearly, a disturbance in TK2 activity has a deteriorating effect on mtDNA replication and mitochondrial homeostasis.
The work presented here provided new insights concerning the effects of pyrimidine nucleoside analogs on TK2 activity which may change our view on the mechanism of mtDNA depletion observed in patients with TK2 mutations and/or AIDS patients treated with pyrimidine nucleoside analogs.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the Swedish Research Council and partly by a grant from the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 28 March 2011.
REFERENCES
- 1. Arnaudo E., et al. 1991. Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet 337:508–510 [DOI] [PubMed] [Google Scholar]
- 2. Barroso J. F., Elholm M., Flatmark T. 2003. Tight binding of deoxyribonucleotide triphosphates to human thymidine kinase 2 expressed in Escherichia coli. Purification and partial characterization of its dimeric and tetrameric forms. Biochemistry 42:15158–15169 [DOI] [PubMed] [Google Scholar]
- 3. Barwick T., Bencherif B., Mountz J. M., Avril N. 2009. Molecular PET and PET/CT imaging of tumour cell proliferation using F-18 fluoro-l-thymidine: a comprehensive evaluation. Nucl. Med. Commun. 30:908–917 [DOI] [PubMed] [Google Scholar]
- 4. Brinkman K., Smeitink J., Romijn J., Reiss P. 1999. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet 354:1112–1115 [DOI] [PubMed] [Google Scholar]
- 5. Brinkman K., ter Hofstede H., Burger D., Meitink J., Koopmans P. 1998. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS 12:1735–1744 [DOI] [PubMed] [Google Scholar]
- 6. Chariot P., et al. 1999. Zidovudine-induced mitochondrial disorder with massive liver steatosis, myopathy, lactic acidosis, and mitochondrial DNA depletion. J. Hepatol. 30:150–160 [DOI] [PubMed] [Google Scholar]
- 7. Fischer J. C., et al. 1986. Estimation of NADH oxidation in human skeletal muscle mitochondria. Clin. Chim. Acta 155:263–273 [DOI] [PubMed] [Google Scholar]
- 8. Flexner C. C., et al. 1994. Relationship between plasma concentrations of 3′-deoxy-3′-fluorothymidine (alovudine) and antiretroviral activity in two concentration-controlled trials. J. Infect. Dis. 170:1394–1403 [DOI] [PubMed] [Google Scholar]
- 9. Hosseini S. H., et al. 2007. Targeted transgenic overexpression of mitochondrial thymidine kinase (TK2) alters mitochondrial DNA (mtDNA) and mitochondrial polypeptide abundance: transgenic TK2, mtDNA, and antiretrovirals. Am. J. Pathol. 170:865–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lynx M. D., Bentley A. T., McKee E. E. 2006. 3′-Azido-3′-deoxythymidine (AZT) inhibits thymidine phosphorylation in isolated rat liver mitochondria: a possible mechanism of AZT hepatotoxicity. Biochem. Pharmacol. 71:1342–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lynx M. D., McKee E. E. 2006. 3′-Azido-3′-deoxythymidine (AZT) is a competitive inhibitor of thymidine phosphorylation in isolated rat heart and liver mitochondria. Biochem. Pharmacol. 72:239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montaner J. S., et al. 2004. Nucleoside-related mitochondrial toxicity among HIV-infected patients receiving antiretroviral therapy: insights from the evaluation of venous lactic acid and peripheral blood mitochondrial DNA. Clin. Infect. Dis. 38(Suppl. 2):S73–S79 [DOI] [PubMed] [Google Scholar]
- 13. Moraes C., et al. 1991. mtDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am. J. Hum. Genet. 48:492–501 [PMC free article] [PubMed] [Google Scholar]
- 14. Morris G. W., Iams T. A., Slepchenko K. G., McKee E. E. 2009. Origin of pyrimidine deoxynucleotide pools in perfused rat heart: implications for 3′-azido-3′-deoxythymidine-dependent cardiotoxicity. Biochem. J. 422:513–520 [DOI] [PubMed] [Google Scholar]
- 15. Munch-Petersen B., Cloos L., Tyrsted G., Eriksson S. 1991. Diverging substrate specificity of pure human thymidine kinases 1 and 2 against antiviral dideoxynucleosides. J. Biol. Chem. 266:9032–9038 [PubMed] [Google Scholar]
- 16. Nolan D., et al. 2003. Mitochondrial DNA depletion and morphologic changes in adipocytes associated with nucleoside reverse transcriptase inhibitor therapy. AIDS 17:1329–1338 [DOI] [PubMed] [Google Scholar]
- 17. Reiss P., et al. 2004. Greater and more rapid depletion of mitochondrial DNA in blood of patients treated with dual (zidovudine + didanosine or zidovudine + zalcitabine) vs. single (zidovudine) nucleoside reverse transcriptase inhibitors. HIV Med. 5:11–14 [DOI] [PubMed] [Google Scholar]
- 18. Saada A., Shaag A., Elpeleg O. 2003. mtDNA depletion myopathy: elucidation of the tissue specificity in the mitochondrial thymidine kinase (TK2) deficiency. Mol. Genet. Metab. 79:1–5 [DOI] [PubMed] [Google Scholar]
- 19. Saada A., et al. 2001. Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy. Nat. Genet. 29:342–344 [DOI] [PubMed] [Google Scholar]
- 20. Susan-Resiga D., Bentley A. T., Lynx M. D., Laclair D. D., McKee E. E. 2007. Zidovudine inhibits thymidine phosphorylation in the isolated perfused rat heart. Antimicrob. Agents Chemother. 51:1142–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Traut T. 1994. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 140:1–22 [DOI] [PubMed] [Google Scholar]
- 22. Turk B., Awad R., Usova E., Bjork I., Eriksson S. 1999. A pre-steady-state kinetic analysis of substrate binding to human recombinant deoxycytidine kinase: a model for nucleoside kinase action. Biochemistry 38:8555–8561 [DOI] [PubMed] [Google Scholar]
- 23. Venhoff A. C., Lebrecht D., Reuss F. U., Heckl-Östreicher B., Wehr R., Walker U. A., Venhoff N. 2009. Mitochondrial DNA depletion in rat liver induced by fosalvudine tidoxil, a novel nucleoside reverse transcriptase inhibitor prodrug. Antimicrob. Agents Chemother. 53:2748–2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walker U. A., et al. 2004. Depletion of mitochondrial DNA in liver under antiretroviral therapy with didanosine, stavudine, or zalcitabine. Hepatology 9:311–317 [DOI] [PubMed] [Google Scholar]
- 25. Wang L., Eriksson S. 2000. Cloning and characterization of full length mouse thymidine kinase 2: the N-terminal sequence directs import of the precursor protein into mitochondria. Biochem. J. 351:469–476 [PMC free article] [PubMed] [Google Scholar]
- 26. Wang L., et al. 1999. Human thymidine kinase 2: molecular cloning and characterisation of the enzyme activity with antiviral and cytostatic nucleoside substrates. FEBS Lett. 443:170–174 [DOI] [PubMed] [Google Scholar]
- 27. Wang L., Saada A., Eriksson S. 2003. Kinetic properties of mutant human thymidine kinase 2 suggest a mechanism for mitochondrial DNA depletion myopathy. J. Biol. Chem. 278:6963–6968 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.