Abstract
Each year, infections with the protozoan parasite Plasmodium falciparum kill 1 million people, mostly children in Africa. Intermittent preventive treatment (IPT) with sulfadoxine-pyrimethamine (SP) reduces the incidence of malaria and aims to prevent mortality in infants, children, and pregnant women. There is contradictory evidence as to whether this strategy may generate additional protection against reinfection beyond the limited duration of the intervention. Previous work established that ablation of either liver-stage maturation or subsequent life cycle conversion by causal prophylactic drugs elicits protective immune responses against reinfections when drugs are no longer present. Here we show in the rodent malaria model that pyrimethamine, a component of SP, inhibits liver-stage development in vitro and in vivo, confirming the causal prophylactic activity of pyrimethamine. Repeated exposure to high doses of Plasmodium berghei sporozoites during pyrimethamine prophylaxis induced complete protection in C57BL/6 mice against challenge with high doses of sporozoites delivered intravenously 35 to 199 days later. Immunizations by infectious mosquito bites induced limited, inoculation-dependent protection against subsequent challenge by infected mosquito bites but provided partial protection against experimental cerebral malaria. Short-term pyrimethamine prophylaxis during intravenous transmission of sporozoites from a pyrimethamine-resistant strain delayed, but did not prevent, blood-stage infection. Our data provide a rationale for the notion of sustained protective efficacy of IPT based on the capacity of arrested, drug-sensitive liver-stage and/or suppressed blood-stage parasites to mount lasting protection.
INTRODUCTION
Malaria, caused by Plasmodium falciparum, is one of the most pernicious parasitic diseases, mostly affecting children and pregnant women in sub-Saharan Africa (2, 20). For many decades chloroquine, arguably the safest, most affordable antimalarial drug, which is available globally, was the first-line treatment throughout Africa (10). After the global spread of chloroquine-resistant P. falciparum, national malaria control programs started to change the first-line treatment to a coformulation of sulfadoxine and pyrimethamine (SP) in the early to mid-1990s (10). Coinciding with this deployment of an efficacious replacement drug, substantial declines in malaria morbidity and mortality were reported from several study sites (1, 13, 26, 29, 36). Unfortunately, parasite resistance to pyrimethamine has spread rapidly (11, 34, 40), leading to the introduction of artemisinin-based combination therapy (ACT) in all countries where malaria is endemic (http://www.who.int/malaria/am_drug_policies_by_region_afro/en/index.html). However, there is still a consensus on the benefits of SP in intermittent preventive treatment (IPT) programs for infants, pregnant women, and, to a lesser extent, children (18, 41).
The aim of IPT is to reduce the frequency of malaria episodes without preventing every blood-stage infection. IPT offers protection by clearance of preexisting, mostly asymptomatic infections, followed by a short period of posttreatment chemoprophylaxis (41), and permits the natural acquisition of immunity against the pathogenic blood-stage parasites (38). Thereby, IPT avoids the rebound effect that was initially seen in extended continuous chemoprophylaxis, where individuals experienced higher attack rates after intervention (3, 17, 23). An intriguing finding of a follow-up study on one of the first trials of IPT in infants (IPTi) was that the protective efficacy of SP appeared to extend into the second year, when the drug was no longer administered (35).
Experimental studies in the rodent malaria model demonstrated the potential of inoculation with live sporozoites during prophylactic antimalarial administration for the induction of lasting protection against reinfection (6, 7, 16, 32). The drugs tested so far in studies of immunoprophylaxis act at different points in the Plasmodium preerythrocytic cycle: primaquine kills intrahepatic parasites (32); antibiotics, such as azithromycin, lead to delayed death inside the infected hepatocyte, resulting in the emergence of noninfectious liver-stage merozoites (16); and chloroquine cover results in the suppression of emerging blood-stage infections (6, 7). These experimental findings were validated by a recent proof-of-concept study with human volunteers. In that study, exposure to 12 to 15 infected mosquitoes during chloroquine prophylaxis elicited sterile protection against challenge by infected-mosquito bites (33).
The inhibitory effect of the antifolate drug pyrimethamine on hepatic stages of the malaria parasite was first described more than 4 decades ago as a result of direct and indirect observations (8, 24). In this study, we wanted, first, to test whether causal prophylaxis with pyrimethamine during the inoculation of mice with sporozoites can generate immune system-mediated protection against challenge with sporozoites when the drug is no longer present. Second, we studied the prophylactic efficacy of pyrimethamine against a pyrimethamine-resistant Plasmodium strain and the effect of pyrimethamine resistance on potential secondary, immune system-mediated protection against reinfection with sporozoites (38). We hypothesized that the results may also help explain some of the controversial results of IPT trials.
MATERIALS AND METHODS
All animal research was conducted in accordance with European Union and German regulations and was approved by the state authorities (Landesamt für Gesundheit und Soziales, Berlin, Germany).
Inoculation of mice with sporozoites during pyrimethamine prophylaxis.
Groups of C57BL/6 mice (Charles River Laboratories) were inoculated either by intravenous (i.v.) injection of freshly dissected Plasmodium berghei ANKA (clone 507, constitutively expressing green fluorescent protein [GFP]) sporozoites or by exposure of anesthetized mice to the bites of infected Anopheles stephensi mosquitoes. Infected mosquitoes used for infected-mosquito bite (termed “by-bite” below) experiments were anesthetized on ice, presorted under an epifluorescence microscope, and put into individual cups 1 day before the inoculation or challenge (see the next section) was performed. Pyrimethamine (Sigma) was either administered orally with the drinking water for 42 h at a concentration of 70 μg/ml or injected intraperitoneally on three consecutive days at the doses indicated. The irradiation dose for the experiments with irradiated sporozoites was 12,000 cGy. The intraperitoneal treatment with chloroquine diphosphate salt (Sigma) dissolved in sterile phosphate-buffered saline (PBS) consisted of 1.6 mg/mouse for 7 days until mice were parasitemia free as assessed by Giemsa-stained blood smears. To assess the chemoprophylactic efficacy of pyrimethamine, animals were monitored for asexual blood-stage parasites over a period of at least 14 days by microscopic examination of Giemsa-stained blood smears.
Challenge with Plasmodium sporozoites.
Mice that had been subjected to the inoculation/treatment protocol and age-matched control mice were challenged by intravenous injection of freshly dissected sporozoites or by exposure to bites by infected mosquitoes. Control mice were not age matched at the intermediate (day 64) and latest (day 199) challenge time points or in the rechallenge experiments. In these experiments, 6- to 8-week-old control mice were used. Mice were checked daily for the appearance of blood-stage parasites by microscopy of blood smears, starting at day 3 after sporozoite injection, and for symptoms of cerebral malaria (i.e., ataxia, paraplegia, deviation of the head, and coma).
In vitro liver-stage development.
For determination of the in vitro activity of pyrimethamine on preerythrocytic forms, 100,000 hepatoma (HuH7) cells were seeded in 24-well plates 1 day before the addition of 40,000 ANKA wild-type (WT) or sera1(−) sporozoites (31). After the addition of the sporozoites, the plates were centrifuged at 2,000 rpm for 5 min and were incubated for 2 h at 37°C. After the infected cells were washed three times with sterile Hanks balanced salt solution (HBSS) medium, they were treated with trypsin and were transferred to chamber slides (Lab-Tek; Nunc) for incubation with serially diluted pyrimethamine (concentration range, 1 μM to 100 μM) in complete Dulbecco's modified Eagle medium (DMEM) containing 10% heat-inactivated fetal calf serum and 1% penicillin-streptomycin. Cells were fixed with cold methanol 48 h after infection and were stained using an antibody against P. berghei heat shock protein 70 (PbHSP70) (39) and Hoechst 33342 to determine the sizes and numbers of liver-stage parasites. Inhibition of parasite growth was determined by comparing the sizes of preerythrocytic forms to the host cell nucleus size and by counting the number of parasites per slide.
Quantification of parasite loads in the liver.
To determine inhibition of liver-stage development either by direct drug action (causal prophylaxis) or by immune responses against liver-stage parasites generated by the inoculation/treatment protocol (immunization), parasite loads in the livers of infected C57BL/6 mice were determined by quantitative real-time PCR 42 h after intravenous injection of 10,000 or 25,000 sporozoites as described previously (9, 16).
RESULTS
Pyrimethamine blocks intrahepatocytic replication of Plasmodium berghei liver-stage parasites.
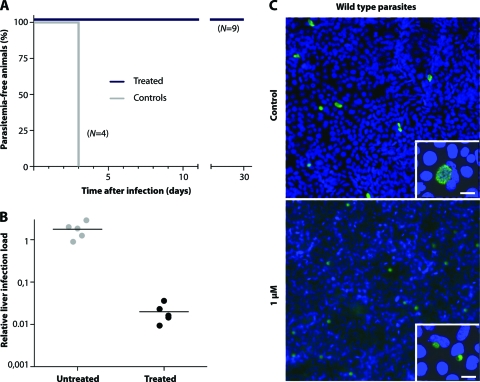
We first established that oral pyrimethamine treatment of C57BL/6 mice within the first 42 h after intravenous application of 10,000 Plasmodium berghei ANKA sporozoites prevented asexual blood-stage infection even after prolonged monitoring (0/9 mice had asexual blood-stage infection), whereas untreated controls showed parasitemia at day 3 after sporozoite inoculation (4/4 mice) (Fig. 1A). In order to differentiate between a suppressive and a causal-prophylactic effect on P. berghei infection, we administered 25,000 sporozoites to C57BL/6 mice (n = 5) under pyrimethamine cover for 42 h. Control animals (n = 5) received dimethyl sulfoxide (DMSO) in water. In mice that received oral pyrimethamine treatment, the hepatic liver burden was reduced by 99% from that in the untreated controls, as determined by quantitative PCR (Fig. 1B) (P, <0.01 by an unpaired t test). We therefore confirm that pyrimethamine acts as a true causal-prophylactic drug, by inhibiting liver-stage maturation in vivo.
Fig. 1.
Pyrimethamine treatment of preerythrocytic malaria parasites prevents blood-stage infection and inhibits the growth of susceptible and pyrimethamine-resistant parasites in vitro and in vivo. (A) Kaplan-Meier curve shows the time to patent blood-stage infection after oral treatment with pyrimethamine for 42 h. (B) Quantification by real-time PCR of parasite loads in infected livers at 42 h after sporozoite inoculation under oral treatment with pyrimethamine. WT parasites were used for infection of C57BL/6 mice. Relative expression levels of the Pb18S gene were normalized to the levels of the mouse GAPDH gene. (C) In vitro development of treated (1 μM pyrimethamine) and untreated (control) pyrimethamine-susceptible exoerythrocytic forms (green). Insets show the same images at a higher magnification. Bars, 10 μm.
To further characterize the action of pyrimethamine on P. berghei liver-stage development, we used an established in vitro assay. We infected cultured hepatoma cells (HuH7) with 10,000 P. berghei sporozoites and measured liver-stage development by an immunofluorescence assay (IFA) using an anti-PbHSP70 antibody (39). Forty-eight hours after infection, liver-stage parasites in cultures that were exposed to pyrimethamine at a concentration of 1, 3.33, 10, or 100 μM were viable but significantly smaller than the controls (Fig. 1C; see also Fig. 3C), suggesting intrahepatocytic persistence of metabolically active but growth-arrested parasites.
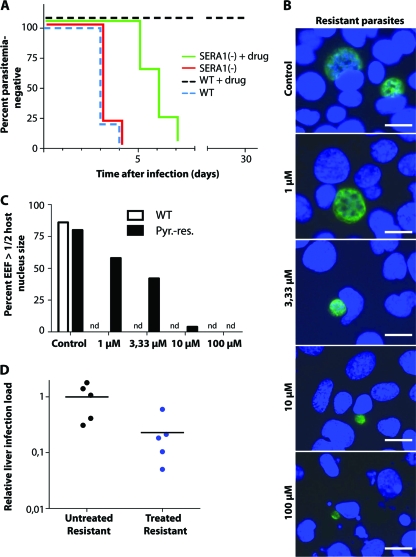
Fig. 3.
Mice infected with pyrimethamine-resistant sporozoites show a delay in the onset of blood-stage infection after oral treatment with pyrimethamine. (A) Kaplan-Meier curve showing the time to patency of pyrimethamine-resistant (Pyr.-res.) and pyrimethamine-susceptible sporozoites. Animals were treated orally with pyrimethamine for 42 h. Each experimental group consisted of five C57BL/6 mice. (B) In vitro development of pyrimethamine-resistant exoerythrocytic forms (EEF) with different drug concentrations. Bars, 10 μm. (C) The effect of pyrimethamine on the growth of pyrimethamine-resistant and -susceptible parasites is illustrated by comparison of the EEF size to the size of the host cell nucleus. Fifty EEF were counted for each drug concentration. nd, none determined. (D) Quantification by real-time PCR of parasite loads in infected livers at 42 h after inoculation of pyrimethamine-resistant parasites with or without oral pyrimethamine prophylaxis. Relative expression levels of the Pb18S gene were normalized to those of the mouse GAPDH gene.
Protection against sporozoite challenge in mice after repeated exposure to live sporozoites during pyrimethamine prophylaxis.
We next wanted to test whether sporozoite inoculation during parallel administration of pyrimethamine can induce immune responses protective against reinfection with sporozoites (Table 1). First, animals received three consecutive sporozoite doses during oral administration of pyrimethamine. For this protocol, 10,000 sporozoites were injected intravenously into age-matched naïve C57BL/6 mice. Three groups of animals were then challenged intravenously with 10,000 sporozoites in the absence of pyrimethamine at three different time points after the last inoculation/treatment step (Table 1): (i) an early time point, i.e., day 35 (n = 9), (ii) an intermediate time point, 2 months (n = 4), and (iii) a late time point in a long-term protection experiment, 199 days (n = 4). Regardless of the time to challenge, none of the challenged animals developed parasitemia after 5 months of follow-up. All control animals developed blood-stage infections and symptoms of cerebral malaria on days 3 to 4 and 7 to 8 after sporozoite challenge, respectively. We conclude that intravenous inoculation of high doses of live, drug-sensitive sporozoites during pyrimethamine administration induced sterilizing protection against homologous challenge with equally high doses of sporozoites.
Table 1.
Protection against reinfection by exposure to sporozoites under pyrimethamine causal prophylaxisa
| Drug | Initial challenge |
Rechallenge |
||||
|---|---|---|---|---|---|---|
| Time to challenge (days) | No. of animals protected/infected (%) | Prepatencyb (days) | Time to rechallenge (days) | No. of animals protected/infected (%) | Prepatency (days) | |
| Pyrimethamine | 35 | 9/9 (100)c | N/A | 130 | 5/5 (100) | N/A |
| 64 | 4/4 (100) | N/A | 158 | 1/4 (25) | (8) | |
| 199 | 4/4 (100) | N/A | ||||
| Noned | 0/5 (0) | 3 | ||||
Pyrimethamine was administered orally with the drinking water for 42 h at a concentration of 70 μg/ml to C57BL/6 mice. Animals were immunized three times by intravenous injection of 10,000 sporozoites each time. Boosts were given at 2- to 4-week intervals under oral pyrimethamine. Animals were challenged by intravenous injection of 10,000 sporozoites or by bites from 5 to 10 infectious mosquitoes. Rechallenge was carried out by intravenous injection of 10,000 sporozoites. Animals were monitored for parasitemia for at least 14 days after challenge.
Defined as the time to detection of the first blood-stage parasite. N/A, not applicable.
In another experiment, 3 protected animals were challenged with 7,500 infected red blood cells; they developed parasitemia on the same day as the controls (day 5), indicating liver-stage-specific protection of the sporozoite immunization protocol.
Data for control animals are displayed from combined experiments. For all challenges, age-matched naïve control animals (at least 2) were infected in parallel.
Protection is long-lasting and liver-stage specific.
We next rechallenged protected mice 5 months after the last challenge by intravenous injection of 10,000 P. berghei ANKA sporozoites (Table 1). All control animals became positive at day 3 (n = 5), and 4 out of 5 developed symptoms of cerebral malaria 7 days after challenge. In the test group, all previously exposed mice remained sterilely protected against rechallenge with the homologous P. berghei ANKA strain (n = 9). We conclude that pyrimethamine-mediated ablation of liver-stage development has the potential to induce lasting protection, an effect similar to that of an equivalent protocol with primaquine (32).
To test whether sterile protection elicited by the sporozoite/pyrimethamine regimen is liver-stage specific, we infected 3 protected animals with 7,500 P. berghei ANKA-infected red blood cells (Table 1). The period to patency was 5 days in both control and immunized mice, indicating that the protective immune mechanisms were targeting the liver phase of the parasite.
Incomplete and dose-dependent protection by sporozoite inoculation via mosquito bite during pyrimethamine prophylaxis.
To assess whether inoculation of mice with sporozoites via the natural transmission route (that is, via infected-mosquito bites) during pyrimethamine prophylaxis can elicit protective immunity, we inoculated animals (n = 20) by bites of P. berghei ANKA-infected Anopheles stephensi mosquitoes during oral administration of pyrimethamine. The infectivity rate of the mosquitoes' salivary glands was determined by epifluorescence microscopy. We used only mosquito batches where ≥70% of insects were infected. The protocol consisted of three consecutive exposures to 10 mosquitoes/mouse during pyrimethamine prophylaxis as described previously (32). The by-bite challenge in the absence of pyrimethamine prophylaxis was performed 49 days after the last by-bite exposure, again by exposure to 10 homologous P. berghei ANKA-infected mosquitoes (Fig. 2A). Five out of 20 immunized and 2 out of 5 control mice stayed malaria free, indicating that not all inoculated animals progressed to a blood-stage infection. Notably, the time to patency was not significantly higher in immunized mice than in control mice (mean prepatent period, 5.3 versus 4.3 days, respectively; P, 0.09 by the Gehan-Breslow-Wilcoxon test). All three infected animals in the control group developed symptoms of cerebral malaria, compared to 9 out of the 15 infected mice in the inoculation/prophylaxis group (P, <0.05 by the Gehan-Breslow-Wilcoxon test) (Fig. 2B).
Fig. 2.
Immunization of mice with mosquito bites (mosq.) under pyrimethamine (Pyr.) cover leads to a delay in the onset of blood-stage infection or to protection against reinfection relative to outcomes for nonimmunized mice. (A) Immunizations were carried out by bites of infectious Anopheles mosquitoes in two independent experiments. In the first experiment (Exp.), three consecutive immunizations were carried out by exposure to 10 mosquitoes/mouse, whereas in the second experiment, three immunizations with higher mosquito numbers (11 to 15 bites/mouse) were performed. (B) Times to patency for immunized and control animals after challenge by bite. (C) Percentages of immunized and control animals developing cerebral malaria.
We next tested whether three inoculation/prophylaxis immunizations with higher mosquito numbers (11 to 15 bites/mouse) would increase the degree of protection against reinfection. The challenge with 5 to 10 infected mosquitoes was carried out 40 days after the last immunization (Fig. 2A). Simultaneously increasing the immunization dose and decreasing the challenge dose resulted in substantial protection. In this experiment, 4 out of 5 animals stayed parasitemia free, whereas all the controls became patent 3 days after exposure to infected-mosquito bites and subsequently developed cerebral malaria (Fig. 2B).
Incomplete attenuation of pyrimethamine-resistant P. berghei by pyrimethamine during intrahepatocytic development delays, but does not prevent, blood-stage infection and confers limited protection against challenge.
In the next set of experiments, we aimed to find out whether pyrimethamine retains partial activity against liver-stage parasites from a P. berghei strain that has been selected for high pyrimethamine resistance during blood-stage infections (31) and whether the degree of attenuation, if any, can delay or even prevent patent blood-stage infections. Finally, in an exploratory experiment, we wanted to find out whether breakthrough blood-stage infections after sporozoite inoculation during pyrimethamine prophylaxis can alter permissiveness to subsequent sporozoite challenge (28, 30). Since drug-mediated clearance of P. berghei infections renders C57BL/6 mice refractory to a second blood-stage infection, immunity needs to be determined by measuring the parasite load in the liver shortly after challenge. The use of P. berghei sera1(−) parasites for this experiment also gave us the opportunity to specifically test the prophylactic efficacy of our sporozoite inoculation/pyrimethamine prophylaxis protocol in the context of drug resistance. We used irradiated P. berghei sporozoites for boosting potentially protective immune responses after a single inoculation/prophylaxis step. Irradiated sporozoites, the gold standard for live malaria vaccines (27), are reliably growth arrested after hepatocyte invasion (22).
We infected mice either with 25,000 wild-type (WT) sporozoites or with 25,000 sporozoites from a clonal P. berghei strain containing a chromosomally integrated Toxoplasma gondii dihydrofolate reductase/thymidylate synthase resistance cassette with two amino acid replacements (Ser36→Arg, Thr83→Asn) that, if inserted into P. falciparum, confers high-level resistance to pyrimethamine on blood-stage parasites (15). This genetically engineered parasite clone was previously selected in mice during blood-stage infections due to uninhibited growth during treatment with oral pyrimethamine (31). These parasites, referred to here as sera1(−), are indistinguishable from WT parasites during liver-stage development. We treated infected mice with oral pyrimethamine for 42 h and then determined the relative P. berghei 18S (Pb18S) rRNA transcript levels in order to assess the hepatic parasite burden (Fig. 3D). We detected a significant 80% reduction of the relative parasite burden in the livers of pyrimethamine-treated animals infected with pyrimethamine-resistant parasites from that in animals receiving WT inoculations (P, 0.03 by an unpaired t test).
We next studied the causal prophylactic activity of pyrimethamine against the sera1(−) clone by determining the time to blood-stage patency in treated (70 μg/ml) versus untreated mice (5 mice per group) (Fig. 3A). As reported previously (31), the time to patency in untreated control animals was 3.2 days for both WT and sera1(−) parasites. In pyrimethamine-treated animals, the prepatent period of sera1(−) parasites was almost double that for untreated control animals (5.8 days versus 3.3 days; P, <0.001 by two-way analysis of variance [ANOVA]). Pyrimethamine-treated animals infected with wild-type parasites remained negative for blood-stage parasites (Fig. 1A and 3A). We also confirmed this finding in vitro in cultured hepatoma cells (Fig. 3B and C). Exposure of preerythrocytic forms from the sera1(−) clone to pyrimethamine at concentrations of ≥3.3 μM led to growth inhibition. Together, our findings indicate that parasites selected for high-level pyrimethamine resistance during blood-stage infections display partial susceptibility in the brief preerythrocytic phase.
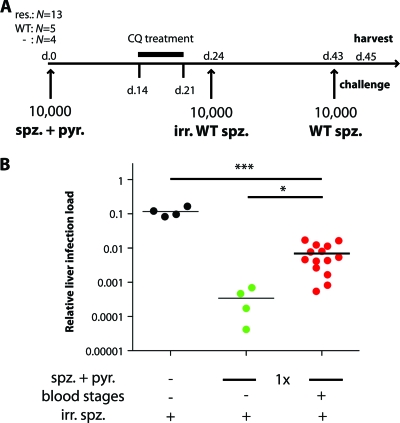
To study the role of parasite resistance and/or blood-stage breakthrough infections in the development of liver-stage immunity, we infected animals with 10,000 sporozoites from the sera1(−) clone (n = 13) or the WT ANKA strain (n = 5) during administration of subtherapeutic doses of pyrimethamine (three intraperitoneal injections of 0.3 to 5 mg pyrimethamine) (Fig. 4). Fourteen out of 18 mice developed blood-stage parasitemia [12/13 with sera1(−) and 2/5 with WT parasites]. All animals, including the four that remained blood-stage negative, received a curative treatment regimen with chloroquine between days 14 and 21, and shortly thereafter, on day 24, a boost of hepatic-stage immunity by inoculation of 10,000 irradiated WT sporozoites. On day 43, all animals were challenged by i.v. inoculation of 10,000 fully viable WT sporozoites (Fig. 4). On day 45, the liver-stage burden was significantly reduced in all animals that had received a sporozoite infection during pyrimethamine prophylaxis (P, <0.001 by an unpaired t test). However, animals that had previously developed a blood-stage infection had a higher relative liver load than animals that remained blood-stage negative (P, <0.05 by an unpaired t test).
Fig. 4.
Breakthrough blood-stage infections curtail preerythrocytic immunity. (A) Animals (n = 18) were infected with pyrimethamine (pyr.)-resistant (res.) (n = 13) or pyrimethamine-sensitive (WT) (n = 5) sporozoites (spz.) under suboptimal pyrimethamine cover. All animals were monitored for parasitemia and were cured with 1.6 mg/day chloroquine (CQ) for 7 days, starting at day 14. Fourteen animals developed parasitemia between days 3 and 10 after infection, and 4 stayed malaria free. All treated animals and untreated control animals (−) (n = 4) were immunized with 10,000 irradiated (irr.) sporozoites at day 24. Finally, all mice were challenged with 10,000 WT sporozoites at day 43. Mice were sacrificed 42 h after the WT challenge, and livers were removed for RNA extraction and cDNA synthesis. (B) Quantitative RT-PCR data for control mice (n = 4) (black circles), blood-stage-negative mice given spz. and pyr. (n = 4) (green circles), and blood-stage-positive mice given spz. and pyr. (n = 14) (red circles). Relative expression levels of the Pb18S gene were normalized to those of the mouse GAPDH gene. *, P < 0.05; ***, P < 0.001.
DISCUSSION
Attenuation of Plasmodium liver-stage parasites or complete suppression of subsequent blood-stage infections has shown that exposure of the immune system to a requisite number of intrahepatocytic parasites induces potent and long-lasting protection against challenge with sporozoites. Irradiated or genetically attenuated sporozoites that can invade hepatocytes but cannot fully mature inside them are being developed as whole-organism vaccine candidates (22, 25, 27). Interestingly, similar effects can be achieved with unaltered wild-type sporozoites delivered naturally by mosquito bites during exposure to certain drugs (6, 7, 16, 22, 32, 33). The use of pyrimethamine, a drug with causal prophylactic activity, as a component of SP in extensive IPT programs prompted us to study the tantalizing question of whether pyrimethamine-mediated attenuation of liver-stage parasites could also generate protective immune responses against reinfection. For an experimental model in which sterile protection is difficult to achieve, we chose P. berghei and the C57BL/6 mouse strain as an appropriate parasite-host pair (21).
We found that (i) pyrimethamine blocks the intrahepatocytic replication of Plasmodium berghei liver-stage parasites in vitro and in vivo; (ii) pyrimethamine retains partial activity against liver-stage parasites from a P. berghei strain that has been selected for high pyrimethamine resistance during blood-stage infections; (iii) prophylactic administration of pyrimethamine during repeated intravenous inoculation of mice with live sporozoites induces complete protection against sporozoite challenge, but this effect was less pronounced when mice were “immunized” naturally by infected mosquito bites, warranting further exploration in future experiments; and (iv) incomplete attenuation of pyrimethamine-resistant P. berghei by pyrimethamine during intrahepatocytic development delayed, but did not prevent, blood-stage infection and conferred only limited protection against challenge (Fig. 3 and 4).
Although a side-by-side comparison was not a focus of this study, the protection observed after repeated inoculations with wild-type sporozoites during the administration of oral pyrimethamine appears to be akin to the immunological mechanism and degree of protection afforded by irradiated sporozoites, by certain genetically modified sporozoites, or by primaquine/sporozoite “immunization.” Using these attenuation protocols, sporozoites are able to glide and invade, but growth arrest occurs at a relatively early stage during liver-stage development. Intrahepatic development of malaria parasites is a prerequisite for allowing the immune system to mount protective responses, since heat-killed sporozoites that cannot invade hepatocytes do not elicit robust protection against sporozoite challenge (12, 19, 37). In contrast, attenuation by pyrimethamine permits hepatocyte invasion but appears to block intrahepatocytic replication (Fig. 1 and 3). This could be explained by the inhibitory effect of the antifolate drug pyrimethamine on DNA synthesis. We do not know whether or for how long pyrimethamine-exposed liver-stage parasites may persist in this apparently growth arrested but metabolically active state in vivo. However, this pyrimethamine-mediated attenuation is clearly different from the lethal injury of intrahepatocytic parasites by primaquine.
What could be the implications of our findings for IPT with SP or potential replacement drugs with equivalent anti-liver-stage activity? More generally, are mouse model data relevant for the interpretation of IPT studies and possibly valuable for monitoring IPT programs? Even though it may be tempting to speculate on a potential added immunoprophylactic benefit for individuals who received IPT, a number of important gaps in our knowledge preclude any firm conclusions. First, only a single, though very visible, study demonstrated sustained protection during 1 year of follow-up in children who had received IPT in their first year of life (35). Subsequent studies were unable to replicate this intriguing finding and, in some instances, even suggested a rebound effect (4, 14). Second, it is controversial whether homologous challenge in the P. berghei-C57BL/6 model reflects the effect of protective immune responses in human infections with P. falciparum. Finally, we do not know whether the typically low dose inoculation of approximately 100 P. falciparum sporozoites by Anopheles spp. under natural transmission conditions can provide the requisite “immunization” dose that permits the immune system to mount protective responses, even in high-transmission areas. The inoculation dose dependency seen in this study definitely seems to suggest so. Perhaps a more practical application of our findings for a P. berghei clone that had been selected for high-level pyrimethamine resistance in the blood stage but that remained partially susceptible to pyrimethamine during liver-stage development is to help explain the somewhat surprising protective efficacy of IPT with SP in infants and children despite a high prevalence of antifolate resistance (4, 14). These findings also indicate that an adjustment of the currently inappropriate SP dose (5) may entail a disproportional increase in the chemoprophylactic efficacy after each IPT dose.
ACKNOWLEDGMENTS
We thank Diana Scheppan for excellent technical assistance. We also thank Kevin Marsh for discussions and advice on the direction of this study.
This study was partly supported by the Deutsche Forschungsgemeinschaft (SFB 544, A7, and B10, to S.B. and K.M.), the Kenya Medical Research Institute—Wellcome Trust Research Programme, Kilifi (to S.B.), and the Max Planck Society, the European Commission (BioMalPar, participant 23; EviMalaR, participant 34), the Joachim Siebeneicher Foundation, and the Chica and Heinz Schaller Foundation (to K.M.).
This study was published with the permission of the Director of KEMRI.
Footnotes
Published ahead of print on 28 March 2011.
REFERENCES
- 1. Alonso P. L., et al. 1993. A malaria control trial using insecticide-treated bed nets and targeted chemoprophylaxis in a rural area of The Gambia, West Africa. 2. Mortality and morbidity from malaria in the study area. Trans. R. Soc. Trop. Med. Hyg. 87(Suppl. 2):13–17 [DOI] [PubMed] [Google Scholar]
- 2. Amin A. A., et al. 2007. The challenges of changing national malaria drug policy to artemisinin-based combinations in Kenya. Malar. J. 6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aponte J. J., et al. 2007. Age interactions in the development of naturally acquired immunity to Plasmodium falciparum and its clinical presentation. PLoS Med. 4:e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aponte J. J., et al. 2009. Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised, placebo-controlled trials. Lancet 374:1533–1542 [DOI] [PubMed] [Google Scholar]
- 5. Barnes K. I., et al. 2006. Sulfadoxine-pyrimethamine pharmacokinetics in malaria: pediatric dosing implications. Clin. Pharmacol. Ther. 80:582–596 [DOI] [PubMed] [Google Scholar]
- 6. Beaudoin R. L., Strome C. P. A., Mitchell F., Tubergen T. A. 1977. Plasmodium berghei—immunization of mice against Anka strain using unaltered sporozoite as an antigen. Exp. Parasitol. 42:1–5 [DOI] [PubMed] [Google Scholar]
- 7. Belnoue E., et al. 2004. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J. Immunol. 172:2487–2495 [DOI] [PubMed] [Google Scholar]
- 8. Bray R. S., Burgess R. W., Fox R. M., Miller M. J. 1959. Effect of pyrimethamine upon sporogony and pre-erythrocytic schizogony of Laverania falciparum. Bull. World Health Organ. 21:233–238 [PMC free article] [PubMed] [Google Scholar]
- 9. Bruña-Romero O., et al. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 31:1499–1502 [DOI] [PubMed] [Google Scholar]
- 10. Ceesay S. J., et al. 2008. Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet 372:1545–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Certain L. K., et al. 2008. Characteristics of Plasmodium falciparum dhfr haplotypes that confer pyrimethamine resistance, Kilifi, Kenya, 1987–2006. J. Infect. Dis. 197:1743–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cockburn I. A., et al. 2010. Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites. PLoS Pathog. 6:e1000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D'Alessandro U., et al. 1995. Mortality and morbidity from malaria in Gambian children after introduction of an impregnated bednet programme. Lancet 345:479–483 [DOI] [PubMed] [Google Scholar]
- 14. Dicko A., et al. 2011. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Mali: a randomised, double-blind, placebo-controlled trial. PLoS Med. 8:e1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donald R. G., Roos D. S. 1993. Stable molecular transformation of Toxoplasma gondii: a selectable dihydrofolate reductase-thymidylate synthase marker based on drug-resistance mutations in malaria. Proc. Natl. Acad. Sci. U. S. A. 90:11703–11707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Friesen J., et al. 2010. Natural immunization against malaria: causal prophylaxis with antibiotics. Sci. Transl. Med. 2:40ra49. [DOI] [PubMed] [Google Scholar]
- 17. Fryauff D. J., et al. 1997. Malaria in a nonimmune population after extended chloroquine or primaquine prophylaxis. Am. J. Trop. Med. Hyg. 56:137–140 [DOI] [PubMed] [Google Scholar]
- 18. Greenwood B. 2007. Intermittent preventive antimalarial treatment in infants. Clin. Infect. Dis. 45:26–28 [DOI] [PubMed] [Google Scholar]
- 19. Hafalla J. C., et al. 2006. Priming of CD8+ T cell responses following immunization with heat-killed Plasmodium sporozoites. Eur. J. Immunol. 36:1179–1186 [DOI] [PubMed] [Google Scholar]
- 20. Hay S. I., et al. 2010. Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med. 7:e1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar K. A., et al. 2006. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature 444:937–940 [DOI] [PubMed] [Google Scholar]
- 22. Matuschewski K., Hafalla J. C., Borrmann S., Friesen J. 2011. Arrested Plasmodium liver stages as experimental anti-malaria vaccines. Hum. Vaccin. 7(Suppl. 1):16–21 [DOI] [PubMed] [Google Scholar]
- 23. Menendez C., et al. 1997. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet 350:844–850 [DOI] [PubMed] [Google Scholar]
- 24. Most H., Herman R., Schoenfeld C. 1967. Chemotherapy of sporozoite- and blood-induced Plasmodium berghei infections with selected antimalarial agents. Am. J. Trop. Med. Hyg. 16:572–575 [DOI] [PubMed] [Google Scholar]
- 25. Mueller A. K., Labaied M., Kappe S. H., Matuschewski K. 2005. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 433:164–167 [DOI] [PubMed] [Google Scholar]
- 26. Njagi J. K., Magnussen P., Estambale B., Ouma J., Mugo B. 2003. Prevention of anaemia in pregnancy using insecticide-treated bednets and sulfadoxine-pyrimethamine in a highly malarious area of Kenya: a randomized controlled trial. Trans. R. Soc. Trop. Med. Hyg. 97:277–282 [DOI] [PubMed] [Google Scholar]
- 27. Nussenzweig R. S., Vanderberg J., Most H., Orton C. 1967. Protective immunity produced by the injection of X-irradiated sporozoites of Plasmodium berghei. Nature 216:160–162 [DOI] [PubMed] [Google Scholar]
- 28. Ocaña-Morgner C., Mota M. M., Rodriguez A. 2003. Malaria blood stage suppression of liver stage immunity by dendritic cells. J. Exp. Med. 197:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Meara W. P., et al. 2008. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet 372:1555–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Orjih A. U., Nussenzweig R. S. 1979. Plasmodium berghei: suppression of antibody response to sporozoite stage by acute blood stage infection. Clin. Exp. Immunol. 38:1–8 [PMC free article] [PubMed] [Google Scholar]
- 31. Putrianti E. D., et al. 2010. The Plasmodium serine-type SERA proteases display distinct expression patterns and non-essential in vivo roles during life cycle progression of the malaria parasite. Cell. Microbiol. 12:725–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Putrianti E. D., Silvie O., Kordes M., Borrmann S., Matuschewski K. 2009. Vaccine-like immunity against malaria by repeated causal-prophylactic treatment of liver-stage Plasmodium parasites. J. Infect. Dis. 199:899–903 [DOI] [PubMed] [Google Scholar]
- 33. Roestenberg M., et al. 2009. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 361:468–477 [DOI] [PubMed] [Google Scholar]
- 34. Roper C., et al. 2004. Intercontinental spread of pyrimethamine-resistant malaria. Science 305:1124. [DOI] [PubMed] [Google Scholar]
- 35. Schellenberg D., et al. 2005. Intermittent preventive antimalarial treatment for Tanzanian infants: follow-up to age 2 years of a randomised, placebo-controlled trial. Lancet 365:1481–1483 [DOI] [PubMed] [Google Scholar]
- 36. Schellenberg J. R., et al. 2001. Effect of large-scale social marketing of insecticide-treated nets on child survival in rural Tanzania. Lancet 357:1241–1247 [DOI] [PubMed] [Google Scholar]
- 37. Sergent E. 1910. Sur l'immunité dans le paludisme des oiseaux. Conservation in vitro des sporozoites de Plasmodium relictum. Immunité relative obtenue par inoculation de ces sporozoites. C. R. Hebd. Seances Acad. Sci. 151:407–409 [Google Scholar]
- 38. Sutherland C. J., Drakeley C. J., Schellenberg D. 2007. How is childhood development of immunity to Plasmodium falciparum enhanced by certain antimalarial interventions? Malar. J. 6:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsuji M., Mattei D., Nussenzweig R. S., Eichinger D., Zavala F. 1994. Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol. Res. 80:16–21 [DOI] [PubMed] [Google Scholar]
- 40. Vallely A., Vallely L., Changalucha J., Greenwood B., Chandramohan D. 2007. Intermittent preventive treatment for malaria in pregnancy in Africa: what's new, what's needed? Malar. J. 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. White N. J. 2005. Intermittent presumptive treatment for malaria. PLoS Med. 2:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]