Abstract
Infections caused by Acinetobacter baumannii are of increasing concern, largely due to the multidrug resistance of many strains. Here we show that insertion sequence ISAba11 movement can result in inactivation of the A. baumannii lipid A biosynthesis genes lpxA and lpxC, resulting in the complete loss of lipopolysaccharide production and high-level colistin resistance.
TEXT
The Gram-negative pathogen Acinetobacter baumannii is a leading cause of hospital-acquired infections, including septicemia, pneumonia, and urinary tract infections. The treatment of infections caused by A. baumannii is significantly hampered by an increase in multidrug resistance (MDR), including resistance to last-line antibiotics such as colistin (9). Recently, colistin resistance in the A. baumannii type strain ATCC 19606 was shown to result from the loss of lipopolysaccharide (LPS), the initial binding target of colistin and the major component of the outer leaflet of the Gram-negative outer membrane (7).
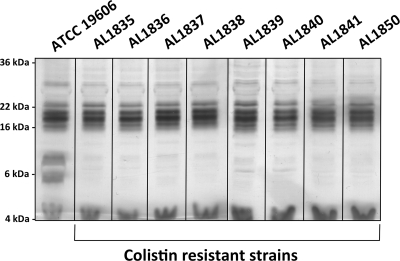
We have previously shown that we can select for colistin-resistant mutants of A. baumannii strain ATCC 19606 by growth on Mueller-Hinton agar containing 10 μg/ml colistin sulfate (7). Our initial study reported the characterization of a group of 13 colistin-resistant mutants which each contained point mutations or deletions in one of the first three genes in the lipid A biosynthesis pathway, lpxA, lpxC, or lpxD, resulting in the loss of LPS production and high-level colistin resistance (MIC > 128 μg/ml) (7). In the present study, we report the characterization of a second group of eight colistin-resistant mutants of A. baumannii strain ATCC 19606. Carbohydrate-specific silver staining (7) of the proteinase K-treated whole-cell lysates of these strains indicated that they also produced no LPS (Fig. 1), which was confirmed by Limulus amebocyte lysate assay (7) (data not shown). However, sequencing analysis of the lpxA, lpxC, and lpxD genes in these strains showed that they contained no point mutations or deletions but rather contained an insertion sequence (IS) element, ISAba11 (GenBank accession number JF309050), in either lpxA or lpxC.
Fig. 1.
Colistin-resistant derivatives of ATCC 19606 do not produce LPS. SDS-PAGE separation and carbohydrate-specific silver staining of proteinase K-treated whole-cell lysates of the colistin-sensitive strain ATCC 19606 and eight colistin-resistant derivatives. The positions of standard molecular mass markers are shown on the left.
In seven of the strains, the ISAba11 element had inserted into lpxC, while in the remaining strain, it had inserted into lpxA (Table 1). In six of the colistin-resistant strains, ISAba11 was bracketed by 5-bp direct repeats, indicating target site duplication. However, in strain AL1838, it was bracketed by 34-bp direct repeats, and in strain AL1837, no direct repeats were observed, but rather, ISAba11 was associated with a 27-bp deletion within lpxC (Table 1). In four of the colistin-resistant strains, ISAba11 had inserted between nucleotides 390 and 393 of the lpxC gene, while in three of the strains, it inserted at either nucleotide 420 or 421 of the lpxC gene, indicating that these regions may represent hot spots for ISAba11 insertion.
Table 1.
Positions of ISAba11 insertions and target site duplications in the colistin-resistant, LPS-deficient derivatives of ATCC 19606
| Strain | Gene | Insertion (nucleotide) | Target site duplication |
|---|---|---|---|
| AL1835 | lpxC | 393 | AAATA |
| AL1836 | lpxC | 393 | AAATA |
| AL1837 | lpxC | 391 | No duplicationa |
| AL1838 | lpxC | 420 | AAAAATATTAAAGCCAGTTGAGGCTTTAATTGAT |
| AL1839 | lpxC | 421 | TGATG |
| AL1840 | lpxC | 420 | TTGAT |
| AL1841 | lpxC | 390 | TAAAA |
| AL1850 | lpxA | 588 | GTATA |
Deletion (27 bp) within lpxC following insertion of ISAba11.
ISAba11 has previously been identified only as part of the transposon Tn6021 (http://www-is.biotoul.fr/index.html?is_special_name=ISAba11) from A. baumannii ATCC 17978 (10). The 1.1-kb element ISAba11 is flanked by perfect 13-bp inverted repeats and is predicted to encode a transposase with a conserved DDE motif, considered essential for transposition (8). ISAba11 shows the greatest similarity to insertion sequence elements from the IS701 and IS4 families; these elements have inverted repeats of 14 to 24 bp and 13 to 26 bp, respectively, which contain conserved nucleotides and target site duplications of 4 bp (IS701) and 10 to 13 bp (IS4) (2). The absence of conserved nucleotides in the inverted repeats and the different target site duplication sizes observed here for ISAba11 do not fit the defining characteristics of either family (2), and given these key differences, we propose that ISAba11 represents an emerging insertion sequence (IS) family.
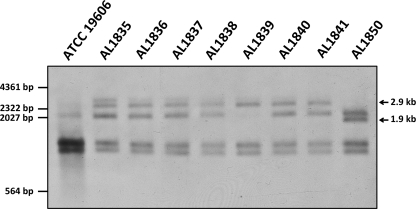
The number of ISAba11 copies present in the ATCC 19606 genome, and the ability of the IS element to replicate and mobilize in this strain, was assessed by Southern hybridization. We compared the number of copies and the position of the element in the parent strain ATCC 19606 with the numbers of copies and the positions of the elements in the genomes of the colistin-resistant, LPS-deficient derivatives. Genomic DNA from the parent strain ATCC 19606 and the colistin-resistant, LPS-deficient derivative strains was digested with DraI (ISAba11 does not contain any DraI sites) and separated by gel electrophoresis, and the resulting DNA fragments were transferred to a nylon membrane. The membrane was hybridized under high-stringency conditions using a probe specific for the ISAba11 transposase gene (Fig. 2). The 760-bp probe was amplified from ATCC 19606 genomic DNA using the primers BAP6531 (GAAGACTACACACCGCACGA) and BAP6532 (TCCGCTCAAACTGGTTCTTT), with digoxigenin (DIG)-11-dUTP (Roche) incorporation. At least three DNA fragments of approximately 2.1, 1.5, and 1.3 kb in size hybridized with the ISAba11 probe in the ATCC 19606 genomic DNA, indicating that this strain contains multiple copies of ISAba11. However, in the lanes containing genomic DNA isolated from the colistin-resistant, LPS-deficient derivatives of ATCC 19606, up to two additional bands hybridized with the probe, indicating that the insertion element had mobilized and that, in most instances, mobilization involved replicative movement of the element (Fig. 2). For the seven mutants in which ISAba11 was identified by DNA sequencing as present in lpxC (AL1835 to AL1841) (Table 1), one hybridizing fragment of 2.9 kb (Fig. 2) was consistently observed across all strains. This fragment corresponded to the expected size of the lpxC DraI fragment containing ISAba11. For the mutant in which ISAba11 was inserted into lpxA (AL1850) (Table 1), a unique hybridizing fragment that corresponded to the predicted size of the lpxA DraI fragment containing ISAba11 was observed (1.9 kb) (Fig. 2). In summary, these data indicate that ISAba11 in ATCC 19606 is mobile and replicative (Fig. 2).
Fig. 2.
The insertion sequence ISAba11 is mobile and replicative in ATCC 19606. Southern hybridization using a probe specific for the ISAba11 transposase gene against DraI-digested genomic DNA isolated from ATCC 19606, AL1835 to AL1841 (lpxC::ISAba11 mutants), and AL1850 (lpxA::ISAba11 mutant). The positions of DNA size markers are shown on the left. Arrows shown on the right indicate the positions and sizes of the novel hybridizing fragments present in the LPS-deficient, colistin-resistant strains.
Further bioinformatic analyses indicated that ISAba11 is present in the A. baumannii ATCC 17978 genome (11) and in the unclosed genome sequences of A. haemolyticus, A. lwoffii, and A. johnsonii. However, there is no evidence that the element is present in any of the recently sequenced clinical A. baumannii isolates, suggesting that ISAba11 is not present in the global European clone I and clone II lineages. However, we have recently shown the involvement of a separate novel IS element in the disruption of the lipid A biosynthesis gene lpxD of a colistin-resistant clinical A. baumannii isolate, B0707-070 (7), thus supporting our hypothesis that IS elements may play a significant role in the mechanism of colistin resistance in A. baumannii. Indeed, 15 different IS elements have been described in A. baumannii to date, and the mobilization of many of these elements is associated with an increase in antibiotic resistance (1, 3–6). This study highlights the significant role that IS element movement and insertion play in the genome plasticity and increasing antibiotic resistance profile of A. baumannii and shows that ISAba11 movement can directly result in colistin resistance in ATCC 19606.
Acknowledgments
This work was supported by an Australian National Health and Medical Research Council project grant. J.H.M. is supported by an Australian Postgraduate Award scholarship. J.L. is an Australian National Health and Medical Research Council R. Douglas Wright Research Fellow.
Footnotes
Published ahead of print on 14 March 2011.
REFERENCES
- 1. Corvec S., et al. 2003. AmpC cephalosporinase hyperproduction in Acinetobacter baumannii clinical strains. J. Antimicrob. Chemother. 52:629–635 [DOI] [PubMed] [Google Scholar]
- 2. De Palmenaer D., Siguier P., Mahillon J. 2008. IS4 family goes genomic. BMC Evol. Biol. 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Figueiredo S., Poirel L., Papa A., Koulourida V., Nordmann P. 2009. Overexpression of the naturally occurring blaOXA-51 gene in Acinetobacter baumannii mediated by novel insertion sequence ISAba9. Antimicrob. Agents Chemother. 53:4045–4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heritier C., Poirel L., Nordmann P. 2006. Cephalosporinase over-expression resulting from insertion of ISAba1 in Acinetobacter baumannii. Clin. Microbiol. Infect. 12:123–130 [DOI] [PubMed] [Google Scholar]
- 5. Jeoung O. Y., et al. 2009. Prevalence and spread of integron-IS26 in imipenem-resistant Acinetobacter baumannii clinical isolates in South Korea. Int. J. Antimicrob. Agents 34:612–614 [DOI] [PubMed] [Google Scholar]
- 6. Lee Y., et al. 2011. A novel insertion sequence, ISAba10, inserted into ISAba1 adjacent to the blaOXA-23 gene and disrupting the outer membrane protein carO gene in Acinetobacter baumannii. Antimicrob. Agents Chemother. 55:361–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moffatt J. H., et al. 2010. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 54:4971–4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nesmelova I. V., Hackett P. B. 2010. DDE transposases: structural similarity and diversity. Adv. Drug Deliv. Rev. 62:1187–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peleg A. Y., Seifert H., Paterson D. L. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Post V., White P. A., Hall R. M. 2010. Evolution of AbaR-type genomic resistance islands in multiply antibiotic-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 65:1162–1170 [DOI] [PubMed] [Google Scholar]
- 11. Smith M. G., et al. 2007. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 21:601–614 [DOI] [PMC free article] [PubMed] [Google Scholar]