Abstract
We studied the penetration of raltegravir and HIV shedding in the genital tract among 14 HIV-1-infected women receiving a raltegravir-containing regimen who had <40 copies/ml blood plasma (BP) HIV RNA. None of the cervicovaginal fluid (CVF) samples showed detectable HIV RNA. Median raltegravir concentrations were 235 ng/ml in BP and 93 ng/ml in CVF, with a CVF/BP ratio of approximately 2.3. This good penetration of raltegravir may contribute to the control of viral replication in the female genital tract.
TEXT
Penetration of antiretrovirals into the genital tracts of HIV-infected women may have an impact on controlling viral replication in this compartment (3), with potential implications for the prevention of sexual and mother-to-child transmission, as well as the acquisition of drug-resistant HIV strains (4, 12, 18). Wide differences in distribution between drugs with regard to their physicochemical and pharmacokinetic characteristics have been reported (5, 6, 13). Raltegravir is the first HIV-1 integrase inhibitor with potent in vitro activity against HIV-1 (95% inhibitory concentration [IC95] = 33 nM, ∼15 ng/ml in 50% human serum) and HIV-2 (15, 17). The penetration of raltegravir into the genital compartments of HIV-infected women receiving long-term therapy has not been reported to date, although a phase 1 study was conducted in HIV-negative volunteers (10). Our objectives were to study the penetration of raltegravir into the female genital tract and concurrent genital HIV-1 shedding.
DIVA (Diffusion Intra-Vaginale des Antirétroviraux) is a multicenter study assessing the penetration of antiretrovirals into the genital tracts of women under therapy for their own health. DIVA 01 was a substudy of women treated with raltegravir. Patients recruited in three French hospitals were eligible if they were more than 18 years old, HIV-1 infected, and on a stable raltegravir (400 mg twice a day)-containing regimen and had had good self-reported adherence and plasma HIV RNA copy numbers below 40 copies/ml for at least 3 months. Menstruating women and women reporting sexual intercourse or intravaginal treatment in the previous 2 days or a genital infection were excluded. The study was approved by the Comité de Protection des Personnes, and all women gave written informed consent.
Paired blood plasma (BP) and cervicovaginal fluid (CVF) samples were collected, and the time between the last drug intake and sampling was recorded. CVF sampling was performed as follows, after speculum placement. A 2.5-cm-diameter confetti of blotting paper (with an adsorption capacity of approximately 50 μl of fluid [Schleicher & Schuell grade 2992]) was first soaked for 1 min in the posterior fornix for pharmacological testing, and then a cervicovaginal lavage was performed with 3 ml of sterile phosphate-buffered saline (PBS) for virological assaying. Finally, exocervical secretions were sampled with a sterile cotton swab to screen for genital tract infection.
Raltegravir was measured using the UltraPerformance LC–tandem-mass spectrometry (UPLC-MS/MS) method (Acquity UPLC-Acquity tandem quadrupole detector [TQD]) after sample pretreatment (limit of quantification [LOQ], ∼1 ng/ml) (11). HIV-1 RNA was quantified using the Cobas TaqMan Roche HIV-1 assay (Roche, Meylan, France), with lower limits of detection of 40 copies/ml for BP and 200 copies/ml for CVF (16).
Fourteen patients were enrolled, and results are presented as medians (interquartile ranges [IQR] from 25% to 75%) (Table 1). The median age of the patients was 45.5 years (43 to 52 years), and their median CD4 cell count was 497 CD4 cells/μl (404 to 712 cells/μl). Antiretroviral regimens included, in addition to raltegravir, at least one nucleoside reverse transcriptase inhibitor (NRTI) for 10/14 patients, darunavir-ritonavir for 7 patients, etravirine for 6 patients, and maraviroc for 3 patients. Two patients were taking omeprazole, known to increase raltegravir's oral bioavailability (8).
Table 1.
Baseline characteristics of patients
| Patient | Age (yr) | No. of CD4 cells/mm3 (% of all cells) | Duration of raltegravir-containing regimen (days) | Associated regimena | Menopause |
|---|---|---|---|---|---|
| 1 | 49 | 421 (29) | 290 | ZDV-3TC-ABC + TDF + DRV-r | Yes |
| 2 | 45 | 927 (34) | 266 | ABC + MVC | No |
| 3 | 53 | 226 (11) | 65 | MVC | Yes |
| 4 | 21 | 488 (32) | 230 | TDF-FTC | No |
| 5 | 44 | 598 (24) | 427 | ABC-3TC + TDF + DRV-r + MVC + ETR | No |
| 6 | 46 | 268 (25) | 265 | TDF-FTC + DRV-r + ETR | No |
| 7 | 43 | 742 (26) | 365 | TDF-FTC + DRV-r | No |
| 8 | 33 | 418 (21) | 133 | ETR + omeprazole | Yes |
| 9 | 51 | 661 (43) | 49 | ABC-3TC + omeprazole | No |
| 10 | 43 | 729 (27) | 132 | TDF-FTC | No |
| 11 | 47 | 399 (25) | 223 | ABC-3TC + MVC | No |
| 12 | 26 | 122 (11) | 94 | TDF-FTC + DRV-r + ETR | No |
| 13 | 29 | 1325 (29) | 756 | DRV-r + ETR | Yes |
| 14 | 54 | 505 (31) | 204 | DRV-r + ETR | No |
| Median | 45.5 | 497 (27) | 227 | ||
| IQR | 15–52 | 404–712 (24) | 132–340 |
ZDV, zidovudine; 3TC, lamivudine; ABC, abacavir; TDF, tenofovir; DRV-r, darunavir boosted with ritonavir; MVC, maraviroc; FTC, emtricitabine; ETR, etravirine.
No patient reported missing a treatment dose in the previous 4 days. None of the patients were pregnant, and 4 were postmenopausal. The duration of raltegravir treatment was 227 days (IQR, 132 to 340 days). The time between the last drug intake and sampling was 13.4 h (13 to 14.3 h), so concentrations could be considered the minimum concentrations except for 2 patients: patient 2, for which data are missing, and patient 6, whose sampling was performed 5 h 45 min after the last drug intake and therefore could be considered a trough concentration.
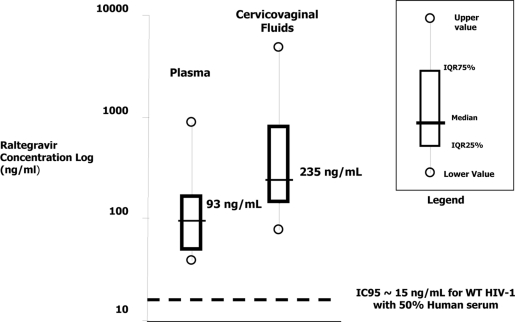
The concentration of raltegravir was 235 ng/ml (IQR, 135 to 775 ng/ml) in CVF and 93 ng/ml (48 to 167 ng/ml) in BP, with a CVF/BP concentration ratio of 2.3 (1.4 to 4.1). The median ratio was nearly the same (2.4) when patients 2 and 5 were excluded (Fig. 1; Table 2). In both compartments, all raltegravir concentrations were above the protein-corrected IC95 for wild-type HIV-1 (9). The interindividual variabilities were 176% and 127% in CVF and BP, respectively. Neither the duration of therapy nor the BP concentrations were correlated with CVF concentrations of raltegravir (Spearman coefficients were 0.386 and 0.045, respectively). Levels of CVF HIV-1 RNA were below 200 copies/ml in all samples, despite asymptomatic vaginosis in 3 women (Table 2). Patients 1 and 5 had detectable BP viral loads, and these results were considered blips, as the patients were reassessed as having fewer than 40 copies/ml thereafter (data not shown).
Fig. 1.
Distribution of raltegravir concentrations in blood plasma and cervicovaginal fluids (n = 14).
Table 2.
Concentrations of raltegravir in blood plasma and cervicovaginal fluids and concurrent viral loads in both compartmentsa
| Patientb | Time (h:min) between last drug intake and sampling of: |
BP RAL concn (ng/ml) | CVF RAL concn (ng/ml) | Ratio of RAL to CVF in BP | BP HIV-1 RNA (copies/ml) | CVF HIV-1 RNA (copies/ml) | |
|---|---|---|---|---|---|---|---|
| BP | CVF | ||||||
| 1 | 16:28 | 16:39 | 90 | 112 | 1.2 | 169 | <200 |
| 2 | Data missing | Data missing | 46 | 113 | 2.5 | <40 | <200 |
| 3 | 15:00 | 15:30 | 47 | 904 | 19.2 | <40 | <200 |
| 4* | 13:17 | 13:32 | 38 | 77 | 2.0 | <40 | <200 |
| 5 | 12:00 | 14:15 | 96 | 127 | 1.3 | 88 | <200 |
| 6 | 05:45 | 06:00 | 873 | 273 | 0.3 | <40 | <200 |
| 7 | 14:20 | 15:00 | 358 | 197 | 0.6 | <40 | <200 |
| 8 | 12:05 | 13:15 | 609 | 1,062 | 1.7 | <40 | <200 |
| 9* | 14:00 | 14:30 | 117 | 1,154 | 9.9 | <40 | <200 |
| 10* | 12:20 | 13:00 | 130 | 4,782 | 36.8 | <40 | <200 |
| 11 | 14:20 | 14:50 | 73 | 273 | 3.7 | <40 | <200 |
| 12 | 14:00 | 13:00 | 179 | 389 | 2.2 | <40 | <200 |
| 13 | 13:45 | 13:10 | 51 | 160 | 3.1 | <40 | <200 |
| 14 | 14:00 | 13:15 | 46 | 193 | 4.2 | <40 | <200 |
| Median | 13:38 | 93 | 235 | 2.3 | |||
| IQR 25% | 13:00 | 48 | 135 | 1.4 | |||
| IQR 75% | 14:27 | 167 | 775 | 4.1 | |||
The interindividual variabilities for the raltegravir concentrations in BP and in CVF and for the ratio of the raltegravir concentration in CVF to that in BP are 127%, 176%, and 159%, respectively. RAL, raltegravir.
*, patient with bacterial vaginosis.
This study shows that raltegravir accumulates in the female genital tract, confirming previous findings in 7 HIV-negative volunteers (10). Similar penetration into the male genital tract was reported with a semen/BP raltegravir concentration ratio of 1.42 (1).
Good penetration of raltegravir is consistent with its low molecular weight (482.51 Da) and its fraction not bound to BP protein (17%). The penetration of other antiretrovirals into the genital tract is highest for nucleoside reverse transcriptase inhibitors and nevirapine, which have low protein binding, and lowest for the protease inhibitors, which are strongly protein bound (13). The accumulation of raltegravir suggests either active transport to (2) or low clearance from the genital tract. Of the three patients with bacterial vaginosis, two had high CVF/BP raltegravir concentration ratios, possibly suggesting that local inflammation may increase the accumulation of raltegravir in the genital tract. The BP concentration of raltegravir, the duration of therapy, and menopause were not found to be related to raltegravir concentration in CVF. However, this study lacked the power to analyze the impact of factors for intragenital concentration, such as the menstrual cycle.
No HIV RNA was detected in any of the CVF samples. Therefore, raltegravir may be beneficial to avoid any local virus replication. However, strong data are lacking to date to confirm the hypothesis that the penetration of antiretroviral drugs into the genital tract is required. For instance, the lack of intragenital HIV replication has been reported in men receiving monotherapy with lopinavir-ritonavir, which showed very low penetration into semen (7). On the other hand, darunavir has demonstrated good penetration into the genital tracts of men, and when used as monotherapy, it was correlated with HIV suppression in most but not all cases studied (14).
The mechanism of antiretroviral action (integrase strand transfer inhibition) and the favorable physicochemical and pharmacokinetic characteristics, such as rapidity of distribution and possible accumulation in the genital compartment, may lead us to consider the future use of raltegravir in pre- and postexposure prophylaxis. Moreover, regarding the relative polarity of the drug, it could be easily coformulated with other microbicide agents for a genital application.
In conclusion, we show that raltegravir penetrates well into the genital tracts of HIV-1-infected women, reaching concentrations that are well above those required to inhibit HIV replication.
Acknowledgments
We thank the patients for their participation. We thank Evelyne Herinomenjanahary for coordinating the study and Christine Blanc for her assistance with the inclusion of patients, as well as Sidonie Lambert and Samia Mehadjri.
Grants were provided by Merck Sharp & Dohme-Chibret, Paris, France, and Roche, Paris, France.
Footnotes
Published ahead of print on 28 March 2011.
REFERENCES
- 1. Barau C., et al. 2010. High concentration of raltegravir in semen of HIV-infected men: results from a substudy of the EASIER-ANRS 138 trial. Antimicrob. Agents Chemother. 54:937–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cianfriglia M., et al. 2007. HIV-1 integrase inhibitors are substrates for the multidrug transporter MDR1-P-glycoprotein. Retrovirology 4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cu-Uvin S., et al. 2000. Effect of highly active antiretroviral therapy on cervicovaginal HIV-1 RNA. AIDS 14:415–421 [DOI] [PubMed] [Google Scholar]
- 4. De Pasquale M. P., et al. 2003. Differences in HIV-1 pol sequences from female genital tract and blood during antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 34:37–44 [DOI] [PubMed] [Google Scholar]
- 5. Dumond J. B., et al. 2009. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J. Acquir. Immune Defic. Syndr. 51:546–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dumond J. B., et al. 2007. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS 21:1899–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghosn J., et al. 2008. Absence of HIV-1 shedding in male genital tract after 1 year of first-line lopinavir/ritonavir alone or in combination with zidovudine/lamivudine. J. Antimicrob. Chemother. 61:1344–1347 [DOI] [PubMed] [Google Scholar]
- 8. Iwamoto M., et al. 2009. Effects of omeprazole on plasma levels of raltegravir. Clin. Infect. Dis. 48:489–492 [DOI] [PubMed] [Google Scholar]
- 9. Iwamoto M., et al. 2008. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin. Pharmacol. Ther. 83:293–299 [DOI] [PubMed] [Google Scholar]
- 10. Jones A. E., et al. 2009. First-dose and steady-state pharmacokinetics of raltegravir in the genital tract of HIV negative women, abstr. 0-06. 10th Int. Workshop Clin. Pharmacol. HIV Ther., Amsterdam, Netherlands, 15 to 17 April 2009 [Google Scholar]
- 11. Jung B. H., Rezk N. L., Bridges A. S., Corbett A. H., Kashuba A. D. 2007. Simultaneous determination of 17 antiretroviral drugs in human plasma for quantitative analysis with liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 21:1095–1104 [DOI] [PubMed] [Google Scholar]
- 12. Kemal K. S., et al. 2007. HIV-1 drug resistance in variants from the female genital tract and plasma. J. Infect. Dis. 195:535–545 [DOI] [PubMed] [Google Scholar]
- 13. Kwara A., et al. 2008. Antiretroviral drug concentrations and HIV RNA in the genital tract of HIV-infected women receiving long-term highly active antiretroviral therapy. Clin. Infect. Dis. 46:719–725 [DOI] [PubMed] [Google Scholar]
- 14. Lambert-Niclot S., et al. 2010. Low frequency of intermittent HIV-1 semen excretion in patients treated with darunavir-ritonavir at 600/100 milligrams twice a day plus two nucleoside reverse transcriptase inhibitors or monotherapy. Antimicrob. Agents Chemother. 54:4910–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Markowitz M., et al. 2006. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J. Acquir. Immune Defic. Syndr. 43:509–515 [DOI] [PubMed] [Google Scholar]
- 16. Pasquier C., et al. 2009. Determining seminal plasma human immunodeficiency virus type 1 load in the context of efficient highly active antiretroviral therapy. J. Clin. Microbiol. 47:2883–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roquebert B., et al. 2008. HIV-2 integrase gene polymorphism and phenotypic susceptibility of HIV-2 clinical isolates to the integrase inhibitors raltegravir and elvitegravir in vitro. J. Antimicrob. Chemother. 62:914–920 [DOI] [PubMed] [Google Scholar]
- 18. Si-Mohamed A., et al. 2000. Selection of drug-resistant variants in the female genital tract of human immunodeficiency virus type 1-infected women receiving antiretroviral therapy. J. Infect. Dis. 182:112–122 [DOI] [PubMed] [Google Scholar]