Abstract
The human gastric pathogen Helicobacter pylori steals host cholesterol, modifies it by glycosylation, and incorporates the glycosylated cholesterol onto its surface via a cholesterol glucosyltransferase, encoded by cgt. The impact of cholesterol on H. pylori antimicrobial resistance is unknown. H. pylori strain 26695 was cultured in Ham's F12 chemically defined medium in the presence or absence of cholesterol. The two cultures were subjected to overnight incubations with serial 2-fold dilutions of 12 antibiotics, six antifungals, and seven antimicrobial peptides (including LL-37 cathelicidin and human alpha and beta defensins). Of 25 agents tested, cholesterol-grown H. pylori cells were substantially more resistant (over 100-fold) to nine agents than were H. pylori cells grown without cholesterol. These nine agents included eight antibiotics and LL-37. H. pylori was susceptible to the antifungal drug pimaricin regardless of cholesterol presence in the culture medium. A cgt mutant retained cholesterol-dependent resistance to most antimicrobials but displayed increased susceptibility to colistin, suggesting an involvement of lipid A. Mutation of lpxE, encoding lipid A1-phosphatase, led to loss of cholesterol-dependent resistance to polymyxin B and colistin but not other antimicrobials tested. The cgt mutant was severely attenuated in gerbils, indicating that glycosylation is essential in vivo. These findings suggest that cholesterol plays a vital role in virulence and contributes to the intrinsic antibiotic resistance of H. pylori.
INTRODUCTION
Helicobacter pylori is a major public health problem worldwide, infecting about 50% of the worldwide population. Eradication of H. pylori leads to resolution of both gastritis and peptic ulcers (3, 4, 22, 29, 36), although antimicrobial resistance is an increasing concern (4, 13). Antibiotic treatment and previously identified resistance mechanisms in H. pylori have been heavily studied and are reviewed elsewhere (23, 30). The currently recommended treatment for H. pylori infections is triple drug therapy (4). The first arm of therapy constitutes a proton pump inhibitor to block acid secretion or a bismuth compound (ranitidine bismuth citrate or bismuth subsalicylate). The second and third arms of the therapy are two antibiotics from the list that includes clarithromycin (almost always), amoxicillin, metronidazole, tetracycline, or a fluoroquinolone (e.g., ciprofloxacin). Therapy lasts for 7 to 14 days. Resistance to metronidazole is common (∼30 to 40%), while resistance to clarithromycin is less common (10 to 15%) and resistance to amoxicillin is rare (4, 24). Quadruple therapy is necessary for treatment failures, which are becoming increasingly common. A newer sequential therapy is having clinical success (5). A subset of the H. pylori population may be intracellular (1), which could explain why some antibiotics are less effective than would be predicted based on in vitro susceptibility testing. Overall, there are limited drug options available to treat H. pylori infections, and novel anti-Helicobacter agents are sorely needed.
In the laboratory setting, H. pylori grown on blood agar is susceptible to penicillins, macrolides, cephalosporins, aminoglycosides, chloramphenicol, rifampin, and fluoroquinolones (24, 30), but the unique acidic environment where H. pylori resides acts to increase the MICs of most antibiotics (24, 30). Under these same blood agar conditions, the organism is inherently resistant to nalidixic acid, bacitracin, vancomycin, sulfonamides (e.g., trimethoprim), and polymyxins (24, 30, 31). In contrast to H. pylori cultured in rich blood agar medium, H. pylori cultured in chemically defined media lacking serum now becomes susceptible to polymyxin B and trimethoprim (31). Susceptibility to trimethoprim could be reversed by the addition of either whole serum or bovine serum albumin to H. pylori cultures, but susceptibility to polymyxin B could be reversed only with serum (31). The results illustrate that commonly used antimicrobial susceptibility tests and their corresponding profiles for H. pylori may be heavily influenced by growth conditions. Moreover, antimicrobial susceptibility results from blood agar-grown H. pylori may not be accurately predicting clinical outcomes.
The biological functions of cholesterol and cholesterol derivatives in the H. pylori membrane are not well understood. H. pylori encounters host cholesterol in vivo, where the specific source could be host membrane-bound cholesterol (44), gastric mucus cholesterol, or dietary cholesterol. H. pylori incorporates the cholesterol into its membrane and modifies the cholesterol by unique alpha-glycosylation of the free hydroxyl group on the cholesterol steroid nucleus (2, 7–9, 12, 14). Recent evidence has shown that this cholesterol incorporation and modification contribute to the pathogenicity of the organism in mice (44) and, likewise, that cholesterol is important for H. pylori initial colonization of gerbils (11). The present study is the first to address the role of cholesterol glucosyltransferase in H. pylori virulence in the gerbil model.
In the current study, using chemically defined media (31, 32), we provide compelling evidence that cholesterol substantially increases the resistance of H. pylori to antibiotics as well as the antimicrobial peptide LL-37. These findings suggest that cholesterol incorporation by H. pylori may adversely affect the ability of many antibiotics to clear H. pylori infections in patients. We also demonstrate that the cholesterol glucosyltransferase, Cgt, plays a critical role in H. pylori virulence in gerbils.
MATERIALS AND METHODS
Bacterial strains and growth media.
H. pylori strain 26695 (standard laboratory strain [33]) was used for bactericidal experiments, and strain SS1 (19) was used for animal studies. The cgt (hp0421) and lpxE mutants were described previously (11). H. pylori was routinely passaged every other day on campylobacter blood agar containing 10% defibrinated sheep blood (CBA) at 37°C in a humidified microaerobic atmosphere of 5% O2, 10% CO2, and 85% N2. H. pylori was inoculated into 7 ml Ham's F-12 in a 25-cm2 tissue culture flask containing 1 mg/ml bovine serum albumin (F12/BSA). After overnight growth, the bacteria were split into two new flasks containing 7 ml F12/BSA. To one flask, cholesterol was added to a final concentration of 50 μg/ml. Due to cholesterol insolubility in aqueous solutions, the cholesterol was prepared either in ethanol or in chloroform. The ethanol-solubilized cholesterol was added to a 10× stock of BSA (10 mg/ml BSA, 500 μg/ml cholesterol) to create a suspension, or chloroform-solubilized cholesterol was directly added to the tissue culture plastic and the chloroform allowed to evaporate prior to H. pylori inoculation. Ethanol or chloroform controls showed no adverse effects on H. pylori viability, and cholesterol solubilized by either method showed similar results (data not shown). In some experiments, cholesterol was replaced with the nonutilizable cholesteryl myristate at 50 μg/ml.
Chemicals.
Antimicrobial peptides LL-37 and human beta defensins 1, 2, 3, and 4 were from Phoenix Pharmaceuticals (Burlingame, CA), and other chemicals were from Sigma (St. Louis, MO). Bismuth was obtained from over-the-counter bismuth subsalicylate, and all master stocks were made in sterile water and filter sterilized except as noted. Rifampin, ketoconazole, nystatin, and tetracycline were dissolved in methanol; miconazole was dissolved in dimethyl sulfoxide; clarithromycin, filipin, and chloramphenicol were dissolved in 95% ethanol; nalidixic acid and amphotericin B were dissolved in 1 M NaOH; ciprofloxacin was dissolved in 0.1 M HCl; pimaricin was dissolved in glacial acetic acid; and trimethoprim was dissolved in dimethylformamide. From the master stocks, lower-concentration working stocks were prepared. Control experiments showed that the organic solvents, acid, or base had no adverse effects on H. pylori viability at the concentrations present in the drug susceptibility experiments.
Bacterial viability assays.
After overnight growth (16 to 18 h) of H. pylori in F12/BSA (7 ml) in the presence or absence of cholesterol (50 μg/ml), bacteria were subcultured into 24-well tissue culture plates (1:20 dilution) containing 1 ml F12/BSA with or without cholesterol (50 μg/ml). Antimicrobial compounds at serial 2-fold dilutions (10 μl volume) were added to 24-well plates. Equal numbers of viable bacteria (in ∼50 μl; ∼108 CFU/ml) were added to all wells. Wells lacking compounds served as positive controls. Numerous pilot experiments were done at 2-, 5-, or 10-fold dilutions of antimicrobial agent until the desired concentration ranges were identified (data not shown). After overnight growth (∼16 to 18 h) of the bacteria in the presence of antimicrobials under microaerobic conditions, 1 ml was removed, and the bacteria were pelleted and resuspended in 1 ml 1× Dulbecco's phosphate-buffered saline (pH 7.4) to avoid continued residual killing by the drug. The bacteria were serially diluted 10-fold in 1× PBS, plated in duplicate for viable counts on CBA, and incubated at 37°C microaerobically for 4 to 5 days. The processing time from removal of the bacteria to final plating for viable counts was typically 1 to 2 h. No decrease in H. pylori viability in the positive controls was observed.
Disk diffusion assays.
Sterile filter paper disks (diameter, 6 mm; Becton Dickinson, Sparks, MD) were applied to CBA containing H. pylori cultures, and 5 to 10 μl of drug solution was added. Water was the negative control. Plates were incubated in a microaerobic atmosphere for 5 days, and diameters of zones of inhibition were measured in millimeters.
Gerbil colonization.
Female Mongolian gerbils (Meriones unguiculatus; Charles River Laboratories; n = 4 per group) were used in accordance with the Institutional Animal Care and Use Committee at Louisiana State University Health Sciences Center—Shreveport. H. pylori strains were grown in 40 ml Mueller-Hinton broth containing 2% heat-inactivated fetal bovine serum overnight, harvested, and resuspended in sterile saline. Animals were inoculated orally (25 μl containing ∼1 × 109 CFU). At 1 month postinfection, animals were euthanized by carbon dioxide asphyxiation. Stomachs were removed and dissected longitudinally along the greater curvature, and the chyme was removed. The stomach was dissected into antrum, body, and fundus portions, weighed, and then homogenized (Ultra-Turrax T25, 10 to 15 s at a setting of 3; IKA Works, Inc.) in 1.0 ml sterile PBS. Stomach homogenates and dilutions thereof in PBS were plated for viable counts in duplicate on CBA plates containing 5-fluorocytosine (5 μg/ml), vancomycin (10 μg/ml), amphotericin B (5 μg/ml), bacitracin (30 μg/ml), polymyxin B (10 U/ml), and trimethoprim (10 μg/ml) to suppress normal flora. Plates were incubated for 5 days at 37°C in 5% O2, 10% CO2, and 85% N2. Data are presented as CFU/g tissue.
RESULTS
H. pylori grown in the presence of cholesterol is more resistant to many antibiotics.
The biological roles of cholesterol and cholesterol derivatives in H. pylori are not well understood. Thus, we sought to determine whether the incorporation of cholesterol alters antimicrobial resistance of H. pylori. To that end, H. pylori was grown in the chemically defined medium F12/BSA in the presence or absence of cholesterol. Under these conditions, growth rates (∼4-h doubling time) and levels of viability were similar (11). Bacteria were exposed to serial 2-fold dilutions of antimicrobial compounds overnight, and then cultures were plated for viability. Each of the antibiotic classes tested will be discussed next.
Antibiotics inhibiting folate metabolism.
Under the conditions used, H. pylori was resistant to trimethoprim when cultured in the presence or absence of cholesterol (Fig. 1A). We previously reported that H. pylori is susceptible to trimethoprim if BSA or serum is omitted from the media (31), suggesting that a serum component other than cholesterol is responsible for altered trimethoprim susceptibility.
Fig. 1.
Effect of antibiotics on the viability of H. pylori cultured in the presence or absence of cholesterol. H. pylori strain 26695 was grown in F-12/BSA in the presence or absence of cholesterol (50 μg/ml) for 16 to 18 h at the concentration of antimicrobial noted. Bacteria were harvested, washed, and then serially diluted and plated for viable counts (see Materials and Methods). Since the logarithm of zero is undefined, in those instances in which no bacteria were recovered, we set the value to one to give a logarithm of zero. (A) Trimethoprim. Representative of two experiments (independent cultures) with duplicate measurements. (B) Tetracycline. Representative of four experiments with duplicate measurements. (C) Clarithromycin. Representative of three experiments with duplicate measurements. (D) Ciprofloxacin. Representative of four experiments with duplicate measurements. (E) Amoxicillin. Representative of five experiments with duplicate measurements. (F) Bismuth. Bismuth subsalicylate was used, and the concentration of the bismuth active ingredient was determined by information on the label. Representative of three experiments with duplicate measurements.
Antibiotics inhibiting protein synthesis.
Three antibiotics inhibiting protein synthesis were tested: chloramphenicol, clarithromycin, and tetracycline. The latter two are used clinically to treat H. pylori infections. While cholesterol modestly increased the resistance of H. pylori to chloramphenicol (data not shown), cholesterol substantially increased resistance of H. pylori to tetracycline (Fig. 1B) and clarithromycin (Fig. 1C).
Antibiotics inhibiting DNA replication.
Three antibiotics inhibiting DNA replication were tested: nalidixic acid, ciprofloxacin, and metronidazole. The latter two are used clinically to treat H. pylori infections, while nalidixic acid is an agent to which H. pylori is known to be inherently resistant (24, 30). Under our culture conditions, we also observed resistance to nalidixic acid up to 60 μg/ml, with very little difference observed between cultures grown with cholesterol versus those cultured without cholesterol (data not shown). In contrast, H. pylori cultured with cholesterol was much more resistant to ciprofloxacin than H. pylori cultured without cholesterol (Fig. 1D). H. pylori cultured with cholesterol showed a modest increase in resistance to metronidazole (∼10- to 30-fold; Fig. 2 and other data not shown).
Fig. 2.
Summary of effects of antibiotics on H. pylori cultured in the presence or absence of cholesterol. H. pylori cells were cultured, harvested and plated for viable counts as described in the legend to Fig. 1. The data for 12 antimicrobial agents are summarized. Shown is the log difference in viability between H. pylori cultured with cholesterol versus H. pylori cultured without cholesterol in the presence of the antimicrobial at the concentration listed. Dose-response curves were conducted for all of these antimicrobials, and representative plots for some are shown in Fig. 1. Representative of two to five experiments with duplicate measurements. Abbreviations: Amp, ampicillin; Amox, amoxicillin; Col, colistin; Cipro, ciprofloxacin; Mtz, metronidazole; Nal, naladixic acid; Bis, bismuth; Cam, chloramphenicol; Clar, clarithromycin; Rif, rifampin; Tet, tetracycline; Trm, trimethoprim.
Antibiotics inhibiting cell wall biosynthesis.
Two antibiotics inhibiting cell wall biosynthesis were tested: ampicillin and amoxicillin. The latter antibiotic is used clinically to treat H. pylori infections. The two antibiotics gave similar results. Cholesterol-grown H. pylori was up to 1,000-fold more resistant to these antibiotics than H. pylori grown without cholesterol (Fig. 1E and 2 and data not shown). Vancomycin and bacitracin, also cell wall biosynthesis inhibitors, were not tested because H. pylori is inherently resistant to these antibiotics (24, 30).
Antimicrobial inhibiting protein function (bismuth).
Bismuth compounds are part of H. pylori treatment modalities in some countries. We therefore selected the commonly used bismuth-containing agent bismuth subsalicylate to determine its effects on H. pylori cultured in the presence or absence of cholesterol. H. pylori bacteria cultured with cholesterol were substantially (up to 107) more resistant to bismuth than H. pylori bacteria cultured without cholesterol (Fig. 1F and 2). Of all the agents tested, bismuth showed the largest difference between H. pylori bacteria cultured with or without cholesterol.
Antibiotics inhibiting transcription.
It was found that H. pylori bacteria cultured without cholesterol were more susceptible to rifampin than H. pylori bacteria cultured with cholesterol (Fig. 2 and 3A).
Antibiotics inhibiting cell membrane integrity.
H. pylori cultured with cholesterol was completely resistant to colistin, while H. pylori grown in the absence of cholesterol exhibited a dose-dependent decline in viability (Fig. 3B and data not shown).
Fig. 3.
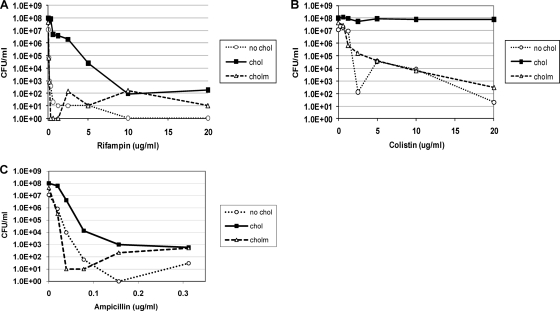
H. pylori cultured with cholesteryl myristate did not show increased resistance to antimicrobial agents. Cholesteryl myristate (CM) is a nonutilizable form of cholesterol. H. pylori cells were cultured, harvested, and plated for viable counts as described in the legend to Fig. 1, except that a third batch of cells was cultured with CM (50 μg/ml) instead of cholesterol. (A) Rifampin. (B) Colistin. (C) Ampicillin.
Cholesterol specificity of antimicrobial resistance.
To show that the increased resistance of H. pylori to antibiotics is dependent upon cholesterol uptake, we replaced cholesterol with cholesteryl myristate (CM), which is an esterified form of cholesterol that H. pylori is unable to utilize. H. pylori grown in the presence of CM did not increase its resistance to rifampin, colistin, or ampicillin compared to that in cholesterol-free control cultures (Fig. 3A, B, and C). Thus, H. pylori specifically utilizes cholesterol but not cholesteryl myristate to increase its antibiotic resistance.
Hydrophobicity of antimicrobial agents.
Five of the antimicrobials tested, clarithromycin, chloramphenicol, rifampin, tetracycline, and trimethoprim, are hydrophobic, raising the concern that the hydrophobic cholesterol was merely sequestering these antimicrobial agents. If this was the case, we should expect to see much greater cholesterol-dependent differences in drug susceptibility for the hydrophobic antimicrobials than for the water-soluble antimicrobials. A relationship between hydrophobicity and cholesterol-dependent resistance was not observed (Fig. 2). For example, H. pylori cultured with cholesterol showed a dramatically increased resistance to the water-soluble ciprofloxacin and amoxicillin, while several hydrophobic antimicrobials, chloramphenicol, and trimethoprim showed little to no difference (Fig. 2). Thus, artifactual sequestration of hydrophobic agents did not account for the cholesterol-dependent differences observed.
Effect of antifungal agents on H. pylori cultured in the presence or absence of cholesterol.
Some antifungals specifically bind to ergosterol found in fungal membranes and may also interact to some extent with cholesterol. Mycoplasma spp. contain cholesterol in their membranes, presumably to increase the rigidity of the membranes of these cell wall-free bacteria. Interestingly, Mycoplasma laidlawii grown in the presence of cholesterol is more susceptible to the sterol-binding antifungal filipin than when grown in media lacking cholesterol (42). To address whether the same is true for H. pylori, we grew H. pylori bacteria in the presence or absence of cholesterol and treated them with filipin, nystatin, amphotericin B, pimaricin, ketoconazole, or miconazole. Sensitivities to antifungals were largely similar between H. pylori cultured in the presence and that cultured in the absence of cholesterol (Fig. 4). Interestingly, filipin often stimulated growth at a concentration of about 50 to 100 μg/ml (Fig. 4). H. pylori was susceptible to low concentrations of pimaricin (1 μg/ml; data not shown), a drug not previously reported for treatment of H. pylori infections. H. pylori also was susceptible to miconazole.
Fig. 4.
Effect of antifungals on the viability of H. pylori cultured in the presence or absence of cholesterol. H. pylori cells were cultured, harvested, and plated for viable counts as described in the legend to Fig. 1, except that chloroform-solubilized cholesterol was used. (A) Amphotericin B. (B) Filipin. (C) Nystatin. (D) Ketoconazole. (E) Miconazole. Representative of two to three experiments with duplicate measurements.
The cholesterol glucosyltransferase, Cgt, is not required for cholesterol-dependent resistance of H. pylori to most antimicrobials.
The cholesterol glucosyltransferase, Cgt, is a logical candidate for the cholesterol-dependent resistance phenotypes, since this enzyme catalyzes the glycosylation of cholesterol and is the only currently known enzyme discovered in the pathway. However, bacteria with a mutation in cgt did not have altered cholesterol-dependent resistance to ciprofloxacin, tetracycline, bismuth, or clarithromycin (data not shown).
The cholesterol glucosyltransferase mutant displays increased susceptibility to colistin.
A disk diffusion assay showed that the cgt mutant had increased susceptibility to colistin and a trend toward increased susceptibility to polymyxin B, compared with results for the wild-type strain (Table 1).
Table 1.
Assessment of H. pylori wild-type, lpxE, or cgt mutant strain susceptibility to polymyxin B and colistin by disk diffusion assaya
| Strain | Zone of inhibition (mm diam)b |
|
|---|---|---|
| Polymyxin B | Colistin (mm) | |
| Wild-type 26695 | 8 | 9 |
| 26695 lpxE | 21 | 27 |
| 26695 cgt | 10 | 15 |
H. pylori wild-type, lpxE, and cgt mutant strains were plated (∼108/ml) onto campylobacter blood agar plates along with filter paper disks (6 mm) containing 15 μl of polymyxin B (1.7 mg/ml) or colistin (10 mg/ml).
Values are representative of results for two to three experiments with similar outcomes.
Lipid A 1-phosphatase (LpxE) plays an essential role in cholesterol-dependent resistance to colistin and polymyxin B.
Since the cgt knockout strain displayed elevated susceptibility to colistin and since colistin and polymyxin B (both polymyxin antibiotics) target lipid A of the lipopolysaccharide (LPS), we reasoned that a mutation leading to altered lipid A structure should change the susceptibility of H. pylori to polymyxin antibiotics. Moreover, LPS profiles of H. pylori change in a cholesterol-dependent fashion (11). To this end, we constructed a mutation in the lpxE (hp0021) gene encoding lipid A 1-phosphatase (35); recent work showed that this mutant loses some, but not all, cholesterol-dependent LPS changes (11). In contrast with results for the wild-type strain, the lpxE mutant largely lacked cholesterol-dependent resistance to both colistin and polymyxin (Fig. 5A and B) and displayed increased zones of inhibition to these antimicrobials (Table 1). However, the lpxE mutant still retained cholesterol-dependent resistance to tetracycline, clarithromycin, bismuth, and ciprofloxacin (data not shown).
Fig. 5.
The lipid A 1-phosphatase, LpxE, is required for cholesterol-dependent resistance to colistin and polymyxin B. (A) H. pylori wild-type 26695 and the isogenic lpxE mutant were grown in the absence or presence of cholesterol in different concentrations of colistin. Shown is percent survival, with the drug-free cultures being set to 100%. (B) H. pylori wild-type 26695 and the isogenic lpxE mutant were grown in the absence or presence of cholesterol in different concentrations of polymyxin B.
H. pylori grown in the presence of cholesterol is more resistant to LL-37 but not the alpha and beta defensins.
H. pylori encounters antimicrobial peptides in vivo. H. pylori induces the expression of bactericidal, cationic antimicrobial peptides in tissue culture models and in patients, including LL-37 cathelicidin, human alpha defensins (HNP-1 to HNP-3), and human beta defensins (especially human beta defensin-2 [HBD-2]) (6, 10, 15, 16, 25, 27, 37, 40, 41, 43). The beta defensins are produced by gastric epithelial cells and other cell types, while the alpha defensins are stored in azurophilic granules of neutrophils and are released following infiltration of the neutrophils into gastric tissues infected with H. pylori. LL-37 is secreted by gastric epithelial cells (10) and other cell types (26).
Under our growth conditions, H. pylori cultured in the presence of cholesterol was completely resistant to LL-37, whereas there was dose-dependent killing of H. pylori cultured in the absence of cholesterol (Fig. 6). The cgt mutant behaved similarly to wild-type H. pylori, namely, there was still similar cholesterol-dependent resistance to LL-37 with the cgt mutant compared to the wild-type strain (data not shown).
Fig. 6.
Effect of LL-37 on viability of H. pylori cultured in the presence or absence of cholesterol. H. pylori strain 26695 was cultured, harvested, and plated for viable counts as described in the legend to Fig. 1. Representative of three experiments with duplicate measurements.
At the highest concentrations tested (range, 0.5 to 10 μg/ml), H. pylori was inherently resistant to all four human beta defensins (HBD-1 to -4) and to alpha defensins (HNP-1 and HNP-2), regardless of cholesterol presence in the growth medium (data not shown). Defensin concentrations higher than 10 μg/ml were not tested due to the expense and concerns about physiologic relevance.
The cholesterol glucosyltransferase, Cgt, is essential for colonization of gerbils.
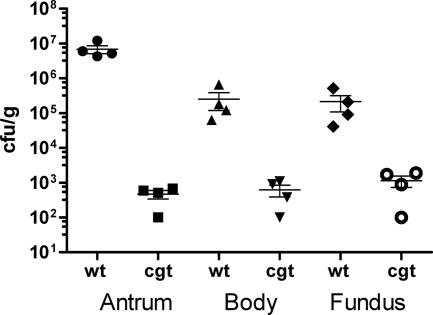
To determine the role of Cgt in vivo, we assessed the ability of the cgt knockout strain, compared with that of the isogenic SS1 wild-type strain, to colonize gerbils. While the wild type colonized the antrum, body, and fundus regions of the stomach, the cgt mutant was severely attenuated in all three regions (Fig. 7).
Fig. 7.
A cholesterol glucosyltransferase mutant, cgt, is attenuated for colonization of gerbil stomachs. Gerbils were inoculated with wild-type SS1 or the isogenic cgt (hp0421) mutant. Animals were euthanized 1 month postinfection, and the gastric tissue was processed as described in Materials and Methods. Error bars, standard error of the mean; bar, mean. Limit of detection is ∼1 × 102 CFU/g.
DISCUSSION
The results suggest that H. pylori has cholesterol-dependent resistance to some antibiotics and LL-37. The studies presented here were made possible by the development of chemically defined media (31, 32), which allow drug susceptibility to be assessed without the complications arising from the use of cholesterol-containing serum or blood. In a previous study, it was shown that serum does impact antimicrobial resistance profiles for H. pylori and some other Helicobacter species (31), suggesting that growth conditions can have profound influences on antimicrobial resistance profiles. Indeed, in the present study, we demonstrate that cholesterol enhances the resistance of H. pylori to many antimicrobial compounds. Antibiotics showing this cholesterol-dependent resistance include some that are used clinically to treat H. pylori infections, as well as the cationic antimicrobial peptide expressed in the gastric mucosa, LL-37. Additionally, recent work showed that H. pylori also displays cholesterol-dependent resistance to bile salts (34). This suggests that H. pylori may utilize cholesterol to modify its envelope so as to resist multiple stresses. Cholesterol-dependent modification of surface lipopolysaccharide was recently demonstrated (11) and is an active area of exploration in the laboratory.
H. pylori has been known for some time to steal host cholesterol, modify it by alpha-glycosylation, and incorporate the modified cholesteryl glucosides into its membrane (2, 7–9, 12, 14). However, the significance of these modifications to the bacterial membrane was largely unexplored until recently, when it was shown that these cholesteryl glucosides play an important role in colonization of mice and immune evasion (44). We demonstrate here that the cholesteryl glucosides probably play a similar role in H. pylori colonization of gerbils, because the cgt knockout mutant is severely attenuated in this animal model in the antrum, body, and fundus regions. Cholesterol glucosyltransferase, interestingly, is inhibited by alpha-1,4-N-acetylglucosamine-capped O-glycans occurring in gastric mucin, and this inhibition suppresses H. pylori growth (17, 20, 21). This observation, coupled with the important role of cgt in virulence of mice (44) and gerbils (this study), indicates that the cholesterol uptake and glycosylation constitute attractive targets for development of new anti-Helicobacter agents.
H. pylori is susceptible to azole antifungals (miconazole, ketoconazole, and others) (28, 38, 39); miconazole is a bactericidal agent (39). This is the first report, to our knowledge, that H. pylori is susceptible to nystatin, filipin, and pimaricin. These antifungals might have been expected to inhibit cholesterol-grown H. pylori more than cholesterol-free H. pylori, as is observed for M. laidlawii (42). Instead, only minor differences in antifungal susceptibility were observed between H. pylori grown in the absence and that grown in the presence of cholesterol. Perhaps the prominent glycosylation of the cholesterol in the H. pylori membrane prevents the antifungal from binding to its sterol target. Since the membranes of H. pylori grown without cholesterol lack any sterols (18), the actual mechanism of cholesterol-free H. pylori killing by some of these sterol-targeting antifungals under these conditions, especially the strong bactericidal effect by pimaricin, remains to be determined.
The mechanism(s) by which cholesterol enhances resistance of H. pylori to antimicrobials is not yet entirely understood, but the process likely involves multiple mechanisms. Cholesterol probably changes the surface properties of H. pylori. For example, cholesterol could increase cell surface hydrophobicity, decrease cell surface negative charges, decrease membrane fluidity, or alter LPS (11) or protein profiles (E. A. Trainor and D. J. McGee, unpublished observations). Such changes to the cell surface may affect the uptake of the antimicrobial compounds by H. pylori grown in the presence of cholesterol. These possibilities are under exploration. Importantly, cholesterol-dependent resistance to colistin and polymyxin B was lost in the lpxE knockout strain, consistent with the earlier work of Tran and colleagues on rich media (35). lpxE encodes a lipid A 1-phosphatase whose disruption increases the net negative charge of lipopolysaccharide (35). This would favor increased electrostatic interactions between the positively charged polymyxin antibiotics and the lipid A moiety. The lpxE gene also plays an important role in at least some of the cholesterol-dependent LPS modifications (11), thereby linking these LPS modifications to a subset of antibiotics (the polymyxins) which H. pylori resists when grown in the presence of cholesterol. The mechanism(s) of cholesterol-dependent resistance to the other antibiotics and LL-37, however, remains a mystery and may involve unidentified genes involved in the H. pylori cholesterol uptake pathway.
In summary, the data presented here suggest that cholesterol plays a critical role in some antibiotic and LL-37 resistance of H. pylori. Cholesterol uptake by H. pylori in the gastric mucosa may contribute to the difficulties encountered in treating patients infected with this human pathogen.
ACKNOWLEDGMENTS
We thank James Van Etten for alerting us to the Mycoplasma antifungal work of the 1960s. We thank Dan Shelver for suggesting antimicrobial peptides for this work and providing some of the initial stocks.
We are grateful to the Office of Multicultural Affairs for support of A.E.G. as a minority high school student in the Jump Start Enrichment Program and as an undergraduate student in the Undergraduate Research Apprenticeship Program at LSUHSC-S. This work was supported by an intramural Bridge Award from LSUHSC-S.
D.J.M. conceived the experiments, drafted the manuscript, and collected and analyzed data. T.L.T. edited the paper and analyzed data, A.E.G., K.E.H., and E.H. collected data, and E.A.T. collected and analyzed data.
Footnotes
Published ahead of print on 4 April 2011.
REFERENCES
- 1. Amieva M. R., Salama N. R., Tompkins L. S., Falkow S. 2002. Helicobacter pylori enter and survive within multivesicular vacuoles of epithelial cells. Cell. Microbiol. 4:677–690 [DOI] [PubMed] [Google Scholar]
- 2. Ansorg R., Muller K. D., von Recklinghausen G., Nalik H. P. 1992. Cholesterol binding of Helicobacter pylori. Zentralbl. Bakteriol. 276:323–329 [DOI] [PubMed] [Google Scholar]
- 3. Bazzoli F., et al. 2002. Treatment of Helicobacter pylori infection. Indications and regimens: an update. Dig. Liver Dis. 34:70–83 [DOI] [PubMed] [Google Scholar]
- 4. Bazzoli F., Pozzato P., Rokkas T. 2002. Helicobacter pylori: the challenge in therapy. Helicobacter 7(Suppl. 1):43–49 [DOI] [PubMed] [Google Scholar]
- 5. Gatta L., Vakil N., Leandro G., Di Mario F., Vaira D. 2009. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am. J. Gastroenterol. 104:3069–3079, 1080 [DOI] [PubMed] [Google Scholar]
- 6. Hamanaka Y., et al. 2001. Expression of human beta-defensin 2 (hBD-2) in Helicobacter pylori induced gastritis: antibacterial effect of hBD-2 against Helicobacter pylori. Gut 49:481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haque M., et al. 1996. Lipid profile of Helicobacter spp.: presence of cholesteryl glucoside as a characteristic feature. J. Bacteriol. 178:2065–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haque M., Hirai Y., Yokota K., Oguma K. 1995. Lipid profiles of Helicobacter pylori and Helicobacter mustelae grown in serum-supplemented and serum-free media. Acta Med. Okayama 49:205–211 [DOI] [PubMed] [Google Scholar]
- 9. Haque M., Hirai Y., Yokota K., Oguma K. 1995. Steryl glycosides: a characteristic feature of the Helicobacter spp.? J. Bacteriol. 177:5334–5337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hase K., et al. 2003. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology 125:1613–1625 [DOI] [PubMed] [Google Scholar]
- 11. Hildebrandt E., McGee D. J. 2009. Helicobacter pylori lipopolysaccharide modification, Lewis antigen expression, and gastric colonization are cholesterol-dependent. BMC Microbiol. 9:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirai Y., et al. 1995. Unique cholesteryl glucosides in Helicobacter pylori: composition and structural analysis. J. Bacteriol. 177:5327–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Houben M. H., et al. 1999. A systematic review of Helicobacter pylori eradication therapy—the impact of antimicrobial resistance on eradication rates. Aliment. Pharmacol. Ther. 13:1047–1055 [DOI] [PubMed] [Google Scholar]
- 14. Inamoto Y., et al. 1995. Lipid composition and fatty acid analysis of Helicobacter pylori. J. Gastroenterol. 30:315–318 [DOI] [PubMed] [Google Scholar]
- 15. Isomoto H., et al. 2004. Elevated concentrations of alpha-defensins in gastric juice of patients with Helicobacter pylori infection. Am. J. Gastroenterol. 99:1916–1923 [DOI] [PubMed] [Google Scholar]
- 16. Isomoto H., et al. 2005. High concentrations of human beta-defensin 2 in gastric juice of patients with Helicobacter pylori infection. World J. Gastroenterol. 11:4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawakubo M., et al. 2004. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 305:1003–1006 [DOI] [PubMed] [Google Scholar]
- 18. Lebrun A. H., et al. 2006. Cloning of a cholesterol-alpha-glucosyltransferase from Helicobacter pylori. J. Biol. Chem. 281:27765–27772 [DOI] [PubMed] [Google Scholar]
- 19. Lee A., et al. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386–1397 [DOI] [PubMed] [Google Scholar]
- 20. Lee H., et al. 2006. Expression cloning of cholesterol alpha-glucosyltransferase, a unique enzyme that can be inhibited by natural antibiotic gastric mucin O-glycans, from Helicobacter pylori. Biochem. Biophys. Res. Commun. 349:1235–1241 [DOI] [PubMed] [Google Scholar]
- 21. Lee H., et al. 2008. Alpha1,4GlcNAc-capped mucin-type O-glycan inhibits cholesterol alpha-glucosyltransferase from Helicobacter pylori and suppresses H. pylori growth. Glycobiology 18:549–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McNulty C. A., et al. 1986. Campylobacter pyloridis and associated gastritis: investigator blind, placebo controlled trial of bismuth salicylate and erythromycin ethylsuccinate. Br. Med. J. (Clin. Res. Ed.) 293:645–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Megraud F., Hazell S., Glupczynski Y. 2001. Antibiotic susceptibility and resistance. ASM Press, Washington, DC: [PubMed] [Google Scholar]
- 24. Megraud F., Lehours P. 2007. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin. Microbiol. Rev. 20:280–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishi Y., et al. 2005. Concentrations of alpha- and beta-defensins in gastric juice of patients with various gastroduodenal diseases. World J. Gastroenterol. 11:99–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nizet V., et al. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454–457 [DOI] [PubMed] [Google Scholar]
- 27. O'Neil D. A., et al. 2000. Regulation of human beta-defensins by gastric epithelial cells in response to infection with Helicobacter pylori or stimulation with interleukin-1. Infect. Immun. 68:5412–5415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rautelin H., Vaara M., Renkonen O. V., Kosunen T. U., Seppala K. 1992. In vitro activity of antifungal azoles against Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 11:273–274 [DOI] [PubMed] [Google Scholar]
- 29. Rauws E. A., Tytgat G. N. 1990. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet 335:1233–1235 [DOI] [PubMed] [Google Scholar]
- 30. Taylor D. E. 1997. Antibiotic resistance mechanisms of Helicobacter pylori, p. 101–109 In Moran A. P., O'Morain C. A. (ed.), Pathogenesis and host response in Helicobacter pylori infections. Normed Verlag, Englewood, NJ [Google Scholar]
- 31. Testerman T. L., Conn P. B., Mobley H. L., McGee D. J. 2006. Nutritional requirements and antibiotic resistance patterns of Helicobacter species in chemically defined media. J. Clin. Microbiol. 44:1650–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Testerman T. L., McGee D. J., Mobley H. L. 2001. Helicobacter pylori growth and urease detection in the chemically defined medium Ham's F-12 nutrient mixture. J. Clin. Microbiol. 39:3842–3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tomb J. F., et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539–547 [DOI] [PubMed] [Google Scholar]
- 34. Trainor E. T., Horton K. E., Savage P. B., Testerman T. L., McGee D. J. 2011. Role of the HefC efflux pump in Helicobacter pylori cholesterol-dependent resistance to ceragenins and bile salts. Infect. Immun. 79:88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tran A. X., et al. 2006. The lipid A 1-phosphatase of Helicobacter pylori is required for resistance to the antimicrobial peptide polymyxin. J. Bacteriol. 188:4531–4541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tytgat G. N., Rauws E., Langenberg W. 1986. The role of colloidal bismuth subcitrate in gastric ulcer and gastritis. Scand. J. Gastroenterol. Suppl. 122:22–29 [DOI] [PubMed] [Google Scholar]
- 37. Uehara N., et al. 2003. Human beta-defensin-2 induction in Helicobacter pylori-infected gastric mucosal tissues: antimicrobial effect of overexpression. J. Med. Microbiol. 52:41–45 [DOI] [PubMed] [Google Scholar]
- 38. von Recklinghausen G., di Maio C., Ansorg R. 1992. Activity of the antimycotic ketoconazole against Helicobacter pylori. J. Antimicrob. Chemother. 30:238–240 [DOI] [PubMed] [Google Scholar]
- 39. von Recklinghausen G., Schmid E. N., Vollmer A., Ansorg R. 1998. Morphological effects of miconazole on Helicobacter pylori. Antimicrob. Agents Chemother. 42:725–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wada A., et al. 1999. Induction of human beta-defensin-2 mRNA expression by Helicobacter pylori in human gastric cell line MKN45 cells on cag pathogenicity island. Biochem. Biophys. Res. Commun. 263:770–774 [DOI] [PubMed] [Google Scholar]
- 41. Wada A., et al. 2001. Helicobacter pylori-mediated transcriptional regulation of the human beta-defensin 2 gene requires NF-kappaB. Cell. Microbiol. 3:115–123 [DOI] [PubMed] [Google Scholar]
- 42. Weber M. M., Kinsky S. C. 1965. Effect of cholesterol on the sensitivity of Mycoplasma laidlawii to the polyene antibiotic filipin. J. Bacteriol. 89:306–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wehkamp J., et al. 2003. Defensin pattern in chronic gastritis: HBD-2 is differentially expressed with respect to Helicobacter pylori status. J. Clin. Pathol. 56:352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wunder C., et al. 2006. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nature Med. 12:1030–1038 [DOI] [PubMed] [Google Scholar]