Abstract
Widespread antimonial resistance in anthroponotic visceral leishmaniasis (VL) makes it critical to monitor the susceptibility of prevailing field isolates to upcoming antileishmanials in order to frame the right treatment policies to protect these drugs against development of resistance. We aimed to generate the baseline data on natural in vitro susceptibility to paromomycin and sitamaquine in Leishmania donovani field isolates from VL patients (n = 20) coming from zones of varying sodium antimony gluconate (SAG) resistance. We further monitored nitric oxide (NO) release in infected macrophages treated with these drugs. Field isolates exhibited variable sensitivity to paromomycin and sitamaquine with respective mean 50% effective dose (ED50) values ± standard error of the mean (SEM) of 3.9 ± 0.3 μM and 2.1 ± 0.2 μM at the intracellular amastigote stage and 29.8 ± 2.5 μM and 17.7 ± 1.0 μM at the promastigote stage. Susceptibilities at the two parasite stages did not correlate for either drug. Isolates from high SAG resistance zones exhibited significantly lower susceptibility to sitamaquine than those from low SAG resistance zones, while isolates from different zones showed similar susceptibilities to paromomycin. NO release was promoted in L. donovani-infected macrophages upon treatment with paromomycin/sitamaquine. NO inhibitors significantly compromised amastigote killing by sitamaquine, but not by paromomycin. In conclusion, SAG-resistant/sensitive VL isolates were susceptible to both paromomycin and sitamaquine. Paromomycin, exhibiting higher efficacy against SAG-resistant parasites and having a distinct mechanism of action, appears to be a promising drug for combination therapy.
INTRODUCTION
Visceral leishmaniasis (VL), caused by parasitic protozoa of the Leishmania donovani complex, is prevalent in over 70 countries, with an annual incidence of 500,000 and an estimated 59,000 deaths annually (http://www.who.int/Leishmaniasis/en). The heaviest burden of VL (>90%) falls mainly on 5 areas of the world, i.e., India, Nepal, Bangladesh, Brazil, and Africa (8). The Indian subcontinent alone bears 67% of the global VL burden. This has compelled the governments of India, Nepal, and Bangladesh to launch a regional VL elimination program to reduce the annual incidence to less than 1 case per 10,000 individuals by 2015 (3, 34). Presently, treatment is unsatisfactory and is therefore undergoing transition in terms of choice of the first-line treatment. Sodium antimony gluconate (SAG) monotherapy has been the treatment choice for VL for more than 6 decades. However, resistance is now especially well established in areas of endemicity in Bihar, India, where more than half of the VL patients are resistant to SAG (6, 27). The downslope of SAG efficacy has led to the use of alternative antileishmanials, such as amphotericin B and miltefosine. These drugs, although highly effective, have problems of toxicity, high cost, and risk of emergence of resistance, particularly to miltefosine, which has a long half-life (7, 19, 28).
Paromomycin and sitamaquine are new drugs with promising antileishmanial efficacy. Paromomycin is an aminoglycoside antibiotic showing activity against VL (5, 10, 31), with overall cure rates of over 90%. It has successfully completed phase III clinical trials, showing advantages of shorter treatment course, higher safety profile, and low manufacturing cost, making it an affordable therapeutic option under Indian field conditions (29). As a result, it was registered in India in August 2006 and may prove a promising public health tool in the nationwide VL elimination program (26). Sitamaquine, an 8-aminoquinoline, is an oral antileishmanial drug that has completed phase IIa clinical trials with more than 85% cure rates and is currently under phase IIb clinical trials (11, 23, 35). The major limitation for sitamaquine is the nonavailability of sufficient data on the pharmacokinetics of the drug and its metabolites and toxicity concerns, such as renal adverse events and methemoglobinemia in treated kala azar patients. Pharmacokinetic analysis of sitamaquine in dogs indicated that it has relatively high systemic clearance, a large volume of distribution, a relatively short half-life, and low systemic availability (30).
With very few chemotherapeutic alternatives in hand, the primary concern should be to protect the available drugs against development of resistance by formulating and implementing the right policies for administering these drugs. In vitro SAG efficacy is reported to correlate with the clinical outcome (15, 25). The current first-line treatment choices for VL are miltefosine and amphotericin B. However, our study revealed that SAG-resistant isolates have lower in vitro susceptibility to miltefosine and amphotericin B (14). The future of VL chemotherapy now depends on combinatorial treatment that could improve efficacy by reducing the duration or the total drug dosage (and therefore delay resistance) or lower the toxicity and cost, leading to higher patient compliance. Combination therapy using amphotericin B or its liposomal formulation, along with miltefosine or paromomycin, has shown promising results (32). The determination of the in vitro susceptibility of natural Leishmania parasite populations to new treatment options will help to develop effective control measures. In the present work, we attempted to investigate the susceptibility of L. donovani isolates derived from Indian VL patients hailing from zones with varying degrees of SAG resistance to paromomycin and sitamaquine. Further, in order to understand how these drugs produce their cytotoxic effects on intracellular parasites, we evaluated the effect of paromomycin/sitamaquine treatment on the release of nitric oxide (NO) by L. donovani-infected macrophages (1).
MATERIALS AND METHODS
Patients.
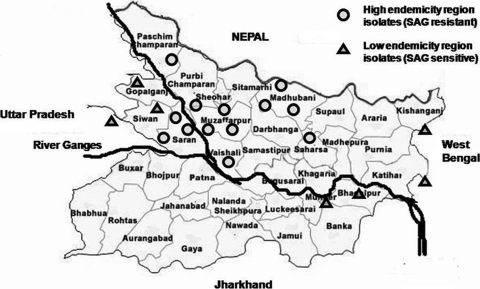
To study drug responses, clinical isolates of Leishmania were prepared as described elsewhere (25) from bone marrow aspirates of VL patients reporting to the Department of Medicine, Safdarjung Hospital (SJH), New Delhi, India, between 2001 and 2008. The patients came from Bihar, India, from zones of low and high VL endemicity, which represent, respectively, zones of low resistance (LR) and high resistance (HR) to antimony (Fig. 1) (25). Three patients originated from the neighboring state of West Bengal or Uttar Pradesh (Table 1). All patients received either 20 mg SAG/kg of body weight/day intramuscularly for 30 days or amphotericin B treatment (infusions of 1 mg/kg on alternate days) for 1 month. This work was conducted with the approval of the Ethics Committee of SJH, and informed consent was obtained from all patients.
Fig. 1.
Map of Bihar and adjoining states showing origins of parasite isolates from areas of high and low endemicity. Clinical isolates of L. donovani were prepared from patients originating from the states of Bihar, West Bengal, and Uttar Pradesh, India, from zones of low and high endemicity for visceral leishmaniasis, which represent, respectively, zones of LR and HR to antimony. Modified from the district map of Bihar available at http://www.biharonline.gov.in.
Table 1.
In vitro susceptibilities of field isolates of L. donovani to paromomycin and sitamaquine
| Parasite identifier | Sex/age (yr)a | District in Bihar (endemicity zone)b | Treatment (response)c | SAG Susceptibilityd ED50 (μg/ml), amastigote | Paromomycin susceptibilityd ED50 (μM) |
Sitamaquine susceptibilityd ED50 (μM) |
||
|---|---|---|---|---|---|---|---|---|
| Amastigote | Promastigote | Amastigote | Promastigote | |||||
| MHOM/IN/83/AG83 | —e | —e | 02.1 ± 0.2 | 2.2 ± 0.1 | 29.9 ± 1.8 | 1.7 ± 0.1 | 21.8 ± 1.7 | |
| MHOM/IN/1999/K59 | F/21 | Vaishali/HR | SAG (NR) | 14.7 ± 3.3 | 4.5 ± 0.2 | 50.7 ± 0.6 | 3.7 ± 0.8 | 18.9 ± 1.6 |
| MHOM/IN/2000/K131 | M/22 | Saharsha/HR | SAG (NR) | 19.4 ± 1.7 | 5.1 ± 0.1 | 20.9 ± 1.3 | 0.9 ± 0.1 | 12.3 ± 0.8 |
| MHOM/IN/2001/K149 | M/20 | Saran/HR | AmB (R) | 15.7 ± 4.0 | 2.6 ± 0.2 | 27.8 ± 2.5 | 1.7 ± 0.1 | 16.49 ± 0.1 |
| MHOM/IN/2002/K192 | M/24 | Saran/HR | AmB (R) | 20.3 ± 0.8 | 4.3 ± 0.4 | 21.6 ± 1.2 | 1.4 ± 0.2 | 16.7 ± 1.2 |
| MHOM/IN/2003/K251 | M/11 | Saran/HR | ND | 11.8 ± 1.3 | 5.2 ± 0.1 | 50.4 ± 3.4 | 3.5 ± 0.1 | 20.1 ± 2.3 |
| MHOM/IN/2006/K417 | F/8 | Muzaffarpur/HR | AmB (R) | 14.7 ± 0.7 | 5.5 ± 0.2 | 23.4 ± 0.6 | 3.2 ± 0.4 | 16.4 ± 0.1 |
| MHOM/IN/2006/K429 | M/26 | Saharsha/HR | AmB (R) | 13.8 ± 0.8 | 4.9 ± 0.6 | 19.6 ± 0.9 | 2.2 ± 0.5 | 18.7 ± 0.6 |
| MHOM/IN/2006/K439 | M/16 | Muzaffarpur/HR | AmB (R) | 12.9 ± 0.1 | 1.2 ± 0.01 | 23.7 ± 1.9 | 2.8 ± 0.2 | 13.6 ± 1.4 |
| MHOM/IN/2007/K498 | F/55 | Madhubani/HR | AmB (R) | 15.8 ± 0.2 | 4.1 ± 0.2 | 17.1 ± 1.2 | 2.6 ± 0.5 | 22.6 ± 1.2 |
| MHOM/IN/2007/K508 | M/50 | Muzaffarpur/HR | AmB (R) | 15.3 ± 0.3 | 4.7 ± 0.2 | 35.8 ± 0.9 | 1.7 ± 0.4 | 20.3 ± 0.8 |
| MHOM/IN/2007/K509 | F/4 | Madhubani/HR | AmB (R) | 16.8 ± 0.3 | 4.9 ± 0.3 | 28.4 ± 1.1 | 2.7 ± 0.4 | 32.9 ± 1.6 |
| MHOM/IN/2007/K516 | F/60 | Motihari/HR | AmB (R) | 16.5 ± 0.6 | 3.2 ± 0.2 | 23.9 ± 0.5 | 2.6 ± 0.2 | 15.9 ± 0.3 |
| MHOM/IN/2007/K518 | M/19 | Chhapra/HR | AmB (R) | 14.9 ± 0.4 | 2.3 ± 0.5 | 19.1 ± 0.8 | 3.5 ± 0.3 | 17.2 ± 0.6 |
| MHOM/IN/1999/K80 | F/40 | Bhagalpur/LR | SAG (NR) | 10.4 ± 2.2 | 3.0 ± 0.31 | 52.7 ± 3.5 | 0.9 ± 0.1 | 16.9 ± 1.2 |
| MHOM/IN/2000/K111 | F/36 | Siwan/LR | SAG (R) | 5.6 ± 0.6 | 2.2 ± 0.2 | 34.8 ± 0.3 | 2.1 ± 0.1 | 14.2 ± 0.3 |
| MHOM/IN/2000/K132 | F/24 | Munger/LR | ND | 3.9 ± 0.3 | 3.5 ± 0.7 | 31.6 ± 0.3 | 2.1 ± 0.2 | 16.0 ± 0.2 |
| MHOM/IN/2000/K133 | M/20 | West Bengalf/LR | SAG (R) | 3.5 ± 0.3 | 3.0 ± 0.3 | 31.5 ± 0.7 | 2.3 ± 0.1 | 17.5 ± 0.1 |
| MHOM/IN/2000/K135 | F/45 | Gopalganj/LR | SAG (R) | 4.2 ± 0.4 | 4.7 ± 0.8 | 34.8 ± 0.3 | 0.9 ± 0.1 | 13.9 ± 0.1 |
| MHOM/IN/2001/K155 | M/14 | West Bengalf/LR | SAG (R) | 2.1 ± 0.3 | 5.9 ± 0.2 | 31.6 ± 0.3 | 0.6 ± 0.1 | 20.7 ± 1.3 |
| MHOM/IN/2006/K435 | M/17 | Uttar Pradeshf/LR | AmB (R) | 11.8 ± 1.4 | 2.8 ± 0.1 | 15.7 ± 2.2 | 1.7 ± 0.3 | 12.7 ± 2.9 |
M, male; F, female.
LR and HR, zones of low resistance and high resistance to antimony, respectively.
Responses were noted 30 days after treatment with SAG infusions (20 mg/kg) or with amphotericin B (AmB) infusions (1 mg/kg) on alternate days for 1 month. Patients with an absence of fever and with a reduction in spleen size were designated responders (R); others were considered nonresponders (NR); ND, not determined.
Mean ED50 ± standard error from three separate assays. The ED50 values for SAG at the amastigote stage were reported previously (14).
—, MHOM/IN/83/AG83 is the L. donovani reference strain from India.
Neighboring states of Bihar.
Parasite and cell culture.
Parasites were routinely grown as promastigotes in Medium 199 (Sigma) with 10% heat-inactivated fetal calf serum (FCS) (Biological Industries, Israel) at 26°C. The drug susceptibilities of the isolates were determined in less than 6 passages after isolation from patients. Stationary-stage promastigotes harvested on day 6 were used for the promastigote assay and for infection of macrophages. A standard strain of L. donovani (LdAG83) from India (MHOM/IN/83/AG83) was used as the reference parasite throughout the study. All VL isolates were characterized as L. donovani on the basis of species-specific PCR (20).
The murine macrophage cell line J774 A.1 was maintained in RPMI medium (Sigma) containing 1.5 g/liter sodium bicarbonate, 2 g/liter glucose, 12.5 mM l-glutamine, penicillin (100 U/ml), and streptomycin (100 U/ml) supplemented with 10% FCS (Biological Industries, Israel) at 37°C in a humidified atmosphere with 5% CO2.
Antileishmanial drugs.
The antileishmanial drugs, viz., paromomycin sulfate (Sigma Aldrich), sitamaquine (Glaxo Smith Kline, United Kingdom), and SAG (Albert David, India) were employed in this study. Stock solutions of paromomycin and sitamaquine (10 mM each) were freshly prepared in deionized water and filter sterilized. All subsequent dilutions were prepared in the respective culture medium supplemented with 10% FCS on the day of the assay.
Drug susceptibility assay at the promastigote stage.
The stationary-phase promastigotes were subjected to a drug-screening assay by plating them at 1 × 106 parasites/well in 24-well plates (TPP, Switzerland) containing either paromomycin (10, 20, 40, 80, 120, and 200 μM) or sitamaquine (10, 15, 20, 30, 50, and 70 μM), along with untreated cells (containing only M199 with 10% FCS) used as a control. The cells were incubated for 72 h at 26°C, and the inhibition of promastigote growth in vitro was assessed by direct counting using a Neubauer chamber under the microscope at ×20 magnification. All the concentrations were tested in triplicate, and experiments were performed in duplicate. The 50% effective dose (ED50) and ED90 values were then calculated in comparison to an untreated control by sigmoidal regression analysis (Origin 6.0; Origin Laboratory).
Drug susceptibility assay at the intracellular amastigote stage.
The sensitivity of L. donovani parasites as intracellular amastigotes to paromomycin and sitamaquine was assessed as described elsewhere (24). Briefly, J774A.1 cells (1 × 105 cells/ml) were infected with stationary-stage promastigotes at a ratio of 10:1 (parasites to macrophages) and plated on an 8-chamber Labtek tissue culture slide (Nunc). The slides were incubated for 4 h at 37°C in 5% CO2. Excess, nonadhered promastigotes were removed by washing the slide, and the macrophages were incubated for 18 to 24 h. Infected cells were reincubated for 48 h, with paromomycin (1, 3, 10, 30, and 50 μM) or sitamaquine (1, 3, 10, and 20 μM) in duplicate. These drugs did not show any cytotoxicity to J774A.1 macrophages at the above concentrations. Macrophages were examined at 48 h for intracellular amastigotes after being stained with Diff-Quik solution (Dade Behring, Switzerland). The L. donovani amastigotes in 100 macrophages were counted at ×100 magnification. The percent killing relative to untreated macrophages was calculated, and the ED50 and the ED90 were determined by sigmoidal regression analysis. These assays were repeated at least in triplicate.
Measurement of nitric oxide accumulation.
J774 A.1 macrophages were plated at 2 × 105 cells per well in 24-well plates and infected with LdAG83 (1 macrophage to 10 parasites). After 24 h, noninternalized parasites were removed by washing the plates with chilled RPMI medium. Uninfected and infected macrophages were then cultured in medium alone or medium containing lipopolysaccharide (LPS) from Escherichia coli at 1 μg/ml or different doses of paromomycin (1 to 30 μM) or sitamaquine (0.5 to 10 μM) at 37°C under 5% CO2 for 48 h (in triplicate) with or without prior treatment with 1 mM N-methyl-l-arginine monoacetate (L-NMMA) (Calbiochem), a competitive inhibitor of nitric oxide synthase (NOS) that inhibits the conversion of l-arginine to NO. SAG was used as the reference drug to serve as a positive control. After 48 h, the cell supernatants were collected and stored at −70°C until nitrite estimation was performed. The NO levels were estimated by reducing the nitrate accumulated over 48 h to nitrite with nitrate reductase and measuring the nitrite concentration colorimetrically by Griess reaction. Briefly, the plates were centrifuged at 1,300 rpm for 5 min at room temperature and the supernatants were collected. One hundred microliters of 40-mg/ml Griess reagent was added to 100 μl of cell supernatant in a 96-well plate, along with the medium blank, and absorbance was measured at 540 nm following incubation for 15 min at room temperature. The amount of nitrite accumulated was calculated from a standard curve constructed with different concentrations of sodium nitrite (in a linear range between 10 and 80 μM).
Statistical analysis.
All experiments were conducted at least in triplicate, and the results were expressed as the mean ± standard error of the mean (SEM). The Mann-Whitney U test was used to determine the statistical significance of the mean ED50 values obtained (GraphPad Prism software version 5.0; GraphPad Software Inc., CA). Pearson's correlation coefficient was calculated to determine the correlation between the in vitro sensitivities of promastigotes and amastigotes. Spearman's rank correlation coefficient was calculated to determine the correlation between the endemicity zone and in vitro susceptibility. Student's t test was applied to determine the statistical significance of the mean ED50 in NO inhibition assays. Statistical significance was accepted as a P value of <0.05.
RESULTS
Susceptibilities of L. donovani isolates to antileishmanial drugs.
The susceptibilities of 20 VL isolates (7 from LR and 13 from HR regions) to paromomycin and sitamaquine were determined. The clinical profiles of VL patients and the in vitro susceptibilities of the isolates to paromomycin and sitamaquine in comparison to SAG are summarized in Table 1.
(i) Susceptibility to paromomycin.
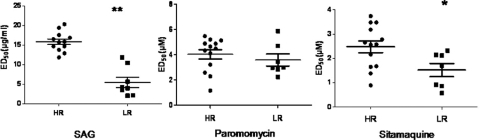
The field isolates showed a wide range of susceptibilities to paromomycin at both the amastigote and promastigote stages. The ED50 (mean ± SEM) at the promastigote stage ranged from 15.7 ± 2.2 to 52.7 ± 3.5 μM, while the ED90 (mean ± SEM) ranged from 25.0 ± 0.8 to 153.9 ± 3.1 μM. The mean and median ED50s for paromomycin at the promastigote stage were 29.8 ± 2.5 μM and 28.3 μM, while the mean and median ED90s were 71.6 ± 7.7 and 69.8 μM, respectively. The amastigotes showed higher susceptibility, with ED50s ranging from 1.2 ± 0.01 to 5.9 ± 0.3 μM (mean = 3.9 ± 0.3 μM; median = 4.2 μM) (Table 1). The mean ED50s of LR region parasites (3.2 ± 0.5 μM) and HR region parasites (3.4 ± 0.3 μM) were comparable (P = 0.47) (Fig. 2).
Fig. 2.
Representative plots of susceptibilities of parasite isolates from high- and low-resistance zones to SAG, paromomycin, and sitamaquine. The ED50s of L. donovani isolates at the intracellular amastigote stage were determined by infection in the murine macrophage cell line J774A.1 as described in Materials and Methods. The ED50s of VL isolates from HR and LR zones to these drugs are represented by circles and squares, respectively. The horizontal bars represent the means of HR/LR, and the vertical bars represent the standard errors. Each individual value represents the mean ED50 of the results from three separate assays. *, P < 0.05; **, P < 0.001, as determined by the Mann Whitney U test.
The antileishmanial activity of paromomycin did not correlate (r = 0.13; P > 0.05) at the amastigote and promastigote stages, indicating that intracellular amastigotes are the ideal stage for determining sensitivity to paromomycin.
(ii) Susceptibility to sitamaquine.
VL field isolates displayed variable susceptibility to sitamaquine at the promastigote stage, with ED50s (mean ± SEM) ranging from 12.3 ± 0.8 to 32.9 ± 1.6 μM and ED90s ranging from 27.9 ± 1.0 to 62.5 ± 0.7 μM. The mean ED50 for sitamaquine was 17.7 ± 1.0 μM, and the mean ED90 was 39.9 ± 2.6 μM (Table 1). The median ED50 was determined to be 16.9 μM and the median ED90 35.8 μM.
Susceptibilities at the amastigote stage ranged from 0.6 ± 0.1 to 3.7 ± 0.8 μM, with a mean ED50 of 2.1 ± 0.2 μM and a median ED50 of 2.2 μM. Again, the amastigote stage of the parasite was more susceptible than the promastigote stage, with promastigotes displaying 5- to >35-fold-higher ED50s (Table 1). There was no significant correlation between the susceptibilities at the two stages (r = 0.24; P > 0.05). In vitro studies on the antiparasitic effect of total sitamaquine are assumed to be representative of its metabolites, which are largely unknown.
The isolates from the HR region displayed mean ED50s for sitamaquine (2.5 ± 0.2 μM) significantly higher (P = 0.0195) than those from the LR region (1.5 ± 0.3 μM) (Fig. 2).
Effects of paromomycin and sitamaquine on the production of NO by infected macrophages.
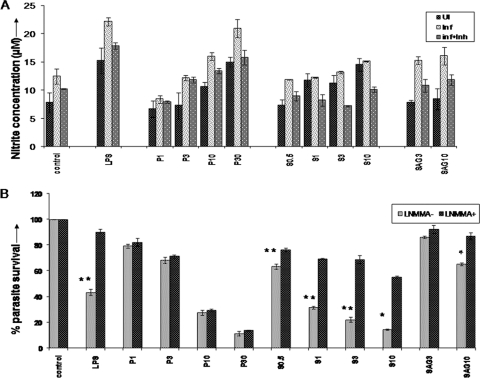
An increase in NO levels upon paromomycin or sitamaquine treatment was observed in both uninfected and infected macrophages, with the increase higher in infected cells than in uninfected cells (Fig. 3A). Sodium nitrite dilutions (0 to 80 μM) were prepared using RPMI medium with and without phenol red. One hundred microliters of the standard dilutions was mixed with an equal volume of Griess reagent, followed by 15 min of incubation at room temperature, and the absorbance at 540 nm was recorded. Similar experiments were performed for nitrite estimation in uninfected J774 cells exposed to paromomycin/sitamaquine. The presence of phenol red in the medium did not interfere significantly with nitrite determination by the Griess method (not shown).
Fig. 3.
Effects of paromomycin and sitamaquine on production of nitric oxide by macrophages and parasite survival. (A) Production of nitric oxide in parasite-infected macrophages upon treatment with paromomycin or sitamaquine. J774.A.1 murine macrophages that were uninfected (UI) or infected with L. donovani LdAG83 (1:10 infection ratio) (Inf) or infected macrophages treated with L-NMMA 1 h prior to drug exposure (inf+Inh) were incubated with medium alone (control) or medium containing increasing concentrations of paromomycin (P1, P3, P10, and P30, denoting 1, 3, 10, and 30 μM, respectively) or sitamaquine (S0.5, S1, S3, and S10, denoting 0.5, 1, 3, and 10 μM, respectively) for 48 h at 37°C. LPS (1 μg/ml) from E. coli was used as a positive control, while SAG was taken as the reference drug (SAG3 and SAG10, denoting 3 and 10 μg/ml, respectively). Nitrite concentrations were measured in supernatants by the Griess method. The graphs represent the means of triplicates from two independent experiments ± standard error of the mean (SEM). *, P < 0.05; **, P < 0.001, as determined by a t test. (B) Effects of nitric oxide inhibitor on parasite survival in L. donovani-infected macrophages treated with paromomycin/sitamaquine. The percent parasite survival in infected macrophages upon treatment with different drugs in the presence (L-NMM+) or absence (L-NMMA−) of inhibitor is shown. Other details are as in panel A.
(i) Effect of paromomycin on NO release.
Paromomycin-activated macrophages produced NO in a dose-dependent manner, and the percent parasite killing correlated significantly with NO release (r = 0.70; P < 0.05) (Fig. 3A). Further, upon addition of L-NMMA (a NOS inhibitor), a decrease in NO production comparable to that of the control infected cells was observed, while the percent parasite killing did not alter significantly (P = 0.186), suggesting that the activity of paromomycin against L. donovani is not dependent on NO (Fig. 3B).
(ii) Effect of sitamaquine on NO release.
Sitamaquine treatment induced NO production in a dose-dependent manner in infected macrophages. The percent parasite killing correlated positively with NO release (r = 0.58; P < 0.05). Addition of L-NMMA resulted in a decrease in NO production comparable to that of the control infected cells in sitamaquine-treated macrophages, with a significant decrease in parasite killing (P = 0.019), suggesting that the cytotoxic activity of sitamaquine against L. donovani involves the NO pathway (Fig. 3B).
DISCUSSION
The present investigation shows for the first time the natural susceptibility of L. donovani from Indian VL cases to upcoming antileishmanial drugs, paromomycin and sitamaquine. The study reports the scenario of drug susceptibility in the parasite population currently prevailing in the field and as yet unexposed to these drugs. In the present study, more than 5-fold variations in in vitro susceptibility to these two drugs were observed within field isolates. The variability in drug susceptibility observed among field isolates reflects differences in intrinsic susceptibility that could have an important impact on clinical outcomes in the future.
Sensitivities to paromomycin and sitamaquine have been reported previously only in reference strains of Leishmania (2, 12, 16, 21). The ED50 of L. donovani for sitamaquine has been reported to range from 19.8 to 29.2 μM in the promastigote form (9, 16) and 4.78 to 5.41 μM in intracellular amastigotes (21). Similarly, susceptibility to paromomycin in L donovani ranged from 6 to 50 μM at the promastigote stage and from 8 to 48.81 μM at the intracellular stage (12, 22).
All VL isolates examined in the study, whether from HR or LR regions, were found to be susceptible to both paromomycin and sitamaquine. In the case of sitamaquine, HR region isolates showed significantly lower susceptibilities than LR isolates, similar to previous observations with miltefosine and amphotericin B (14). On the other hand, parasite isolates from HR and LR zones exhibited similar sensitivity to paromomycin, indicating its potential efficacy in areas where VL is endemic. Paromomycin is a polycationic hydrophilic sugar that specifically possesses high affinities for certain portions of RNAs and interferes with protein synthesis (13). Its antileishmanial action involves a change in the mitochondrial membrane potential, ribosomal and respiratory dysfunction, and decreased membrane fluidity, leading to altered drug uptake (12, 17). Comparable paromomycin sensitivities in parasite populations from HR and LR regions further indicates that it has a mechanism of action distinct from those of other antileishmanial drugs currently being used.
The leishmanicidal action of antimonials involves generation of reactive oxygen and NO species and affects the mitogen-activated protein (MAP) kinase pathway (18). Insight into the mechanism of action of paromomycin highlights the role of mitochondrial membrane potential, ribosomes, and respiratory dysfunction. However, much less is known about the mechanism of antileishmanial action of sitamaquine. The present study revealed an increase in NO levels in L. donovani-infected macrophages upon sitamaquine and paromomycin treatment, coupled with a marked decrease in the number of amastigotes. The presence of NO inhibitor compromised amastigote killing by sitamaquine, suggesting that its cytotoxic activity against L. donovani amastigotes involves the NO pathway. Another well-established aminoquinoline, chloroquine, was shown to induce the expression of inducible nitric oxide synthase and NO production in C6 glioma cells (4).
We observed that amastigotes were invariably more sensitive to paromomycin and sitamaquine, up to 4- to 40-fold and 5- to 35-fold, respectively. The higher paromomycin and sitamaquine susceptibility of intracellular amastigotes than of promastigotes, as in the case of SAG, is suggestive of a stage-specific antileishmanial action of these drugs. Although previous studies have mainly used a Leishmania promastigote model for determining drug sensitivity in vitro, lack of correlation between the promastigote and amastigote susceptibilities to both paromomycin and sitamaquine observed in the present study established the intracellular amastigote model as more appropriate for susceptibility studies, as has been reported for other antileishmanial reference drugs (33).
Paromomycin appears promising, since (i) SAG-resistant and -sensitive field isolates were equally susceptible and (ii) it has a mechanism of action distinct from those of other currently available antileishmanials. In this study, we established that isolates from SAG-responsive and unresponsive patients are susceptible to paromomycin and sitamaquine. Additionally, paromomycin, which has a mechanism of action different from those of existing antileishmanials, would be a useful candidate for combination therapy.
ACKNOWLEDGMENTS
We thank GlaxoSmithkline (Greenford, United Kingdom) for providing sitamaquine.
Financial support from the Indian Council of Medical Research, New Delhi, India, is gratefully acknowledged. A.K. and D.K. are grateful to the Council for Scientific and Industrial Research, India, for fellowships. R.S. received financial support from UNESCO (L'Oreal for Women in Science Program).
We have no conflict of interest to declare.
Footnotes
Published ahead of print on 4 April 2011.
REFERENCES
- 1. Bogdan C., Rollinghoff M., Diefenbach A. 2000. The role of nitric oxide in innate immunity. Immunol. Rev. 173:17–26 [DOI] [PubMed] [Google Scholar]
- 2. Bories C., Cojean S., Huteau F., Loiseau P. M. 2008. Selection and phenotype characterization of sitamaquine-resistant promastigotes of Leishmania donovani. Biomed. Pharmacother. 62:164–167 [DOI] [PubMed] [Google Scholar]
- 3. Chappuis F., et al. 2007. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol. 5:873–882 [DOI] [PubMed] [Google Scholar]
- 4. Chen T. H., Chang P. C., Chang M. C., Lin Y. F., Lee H. M. 2005. Chloroquine induces the expression of inducible nitric oxide synthase in C6 glioma cells. Pharmacol. Res. 51:329–336 [DOI] [PubMed] [Google Scholar]
- 5. Croft S. L., Yardley V. 2002. Chemotherapy of leishmaniasis. Curr. Pharm. Des. 8:319–342 [DOI] [PubMed] [Google Scholar]
- 6. Das V. N., et al. 2005. Magnitude of unresponsiveness to sodium stibogluconate in the treatment of visceral leishmaniasis in Bihar. Natl. Med. J. India 18:131–133 [PubMed] [Google Scholar]
- 7. den Boer M. D., Davidson R. N. 2006. Treatment options for visceral leishmaniasis. Expert Rev. Anti Infect. Ther. 4:187–197 [DOI] [PubMed] [Google Scholar]
- 8. Desjeux P. 2004. Leishmaniasis: current situation and new perspectives. Comp. Immnunol. Microbiol. Infect. Dis. 27:305–318 [DOI] [PubMed] [Google Scholar]
- 9. Dueñas-Romero A. M., Loiseau P. M., Saint-Pierre-Chazalet M. 2007. Interaction of sitamaquine with membrane lipids of Leishmania donovani promastigotes. Biochim. Biophys. Acta 1768:246–252 [DOI] [PubMed] [Google Scholar]
- 10. Jha T. K., et al. 1998. Randomised controlled trial of aminosidine (paromomycin) vs sodium stibogluconate for treating visceral leishmaniasis in North Bihar, India. Br. Med. J. 316:1200–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jha T. K., et al. 2005. A phase II dose-ranging study of sitamaquine for the treatment of visceral leishmaniasis in India. Am. J. Trop. Med. Hyg. 73:1005–1011 [PubMed] [Google Scholar]
- 12. Jhingran A., Chawla B., Saxena S., Barrett M. P., Madhubala R. 2009. Paromomycin: uptake and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 164:111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kotra L. P., Haddad J., Mobashery S. 2000. Aminoglycosides: perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrob. Agents Chemother. 44:3249–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumar D., Kulshrestha A., Singh R., Salotra P. 2009. In vitro susceptibility of field isolates of Leishmania donovani to miltefosine and amphotericin B: correlation with sodium antimony gluconate susceptibility and implications for treatment in areas of endemicity. Antimicrob. Agents Chemother. 53:835–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lira R., et al. 1999. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J. Infect. Dis. 180:564–567 [DOI] [PubMed] [Google Scholar]
- 16. López-Martín C., Pérez-Victoria J. M., Carvalho L., Castanys S., Gamarro F. 2008. Sitamaquine sensitivity in Leishmania species is not mediated by drug accumulation in acidocalcisomes. Antimicrob. Agents Chemother. 52:4030–4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maarouf M., Lawrence F., Brown S., Robert-Gero M. 1997. Biochemical alterations in paromomycin-treated Leishmania donovani promastigotes. Parasitol. Res. 83:198–202 [DOI] [PubMed] [Google Scholar]
- 18. Mookerjee Basu J., et al. 2006. Sodium antimony gluconate induces generation of reactive oxygen species and nitric oxide via phosphoinositide 3-kinase and mitogen-activated protein kinase activation in Leishmania donovani-infected macrophages. Antimicrob. Agents Chemother. 50:1788–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pérez-Victoria F. J., et al. 2006. Mechanisms of experimental resistance of Leishmania to miltefosine: implications for clinical use. Drug Resist. Updat. 9:26–39 [DOI] [PubMed] [Google Scholar]
- 20. Salotra P., et al. 2001. Development of a species-specific PCR assay for detection of Leishmania donovani in clinical samples from patients with kala-azar and post-kala-azar dermal leishmaniasis. J. Clin. Microbiol. 39:849–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seifert K., Croft S. L. 2006. In vitro and in vivo interactions between miltefosine and other antileishmanial drugs. Antimicrob. Agents Chemother. 50:73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seifert K., Escobar P., Croft S. L. 2010. In vitro activity of anti-leishmanial drugs against Leishmania donovani is host cell dependent. J. Antimicrob. Chemother. 65:508–511 [DOI] [PubMed] [Google Scholar]
- 23. Sherwood J. A., et al. 1994. Phase 2 efficacy trial of an oral 8-aminoquinoline (WR6026) for treatment of visceral leishmaniasis. Clin. Infect. Dis. 19:1034–1039 [DOI] [PubMed] [Google Scholar]
- 24. Singh R., Kumar D., Duncan R. C., Nakhasi H. L., Salotra P. 2010. Overexpression of histone H2A modulates drug susceptibility in Leishmania parasites. Int. J. Antimicrob. Agents 36:50–57 [DOI] [PubMed] [Google Scholar]
- 25. Singh R., et al. 2006. Visceral leishmaniasis, or kala azar (KA): high incidence of refractoriness to antimony is contributed by anthroponotic transmission via post-KA dermal leishmaniasis. J. Infect. Dis. 194:302–306 [DOI] [PubMed] [Google Scholar]
- 26. Sundar S., Chakravarty J. 2008. Paromomycin in the treatment of leishmaniasis. Expert Opin. Investig. Drugs 17:787–794 [DOI] [PubMed] [Google Scholar]
- 27. Sundar S., et al. 2000. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the centre of the Indian epidemic. Clin. Infect. Dis. 31:1104–1107 [DOI] [PubMed] [Google Scholar]
- 28. Sundar S., et al. 2002. Low-dose liposomal amphotericin B in refractory Indian visceral leishmaniasis: a multicenter study. Am. J. Trop. Med. Hyg. 66:143–146 [DOI] [PubMed] [Google Scholar]
- 29. Sundar S., Jha T. K., Thakur C. P., Sinha P. K., Bhattacharya S. K. 2007. Injectable paromomycin for visceral leishmaniasis in India. N. Engl. J. Med. 356:2571–2581 [DOI] [PubMed] [Google Scholar]
- 30. Taylor T., Hawkins D. R., Morris G. R., Chung H. 1991. Pharmacokinetics of the anti-leishmanial agent WR 6026 in dogs. Eur. J. Drug Metab. Pharmacokinet. 3:136–139 [PubMed] [Google Scholar]
- 31. Thakur C. P., et al. 2000. A prospective randomized, comparative, open-label trial of the safety and efficacy of paromomycin (aminosidine) plus sodium stibogluconate versus sodium stibogluconate alone for the treatment of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 94:429–431 [DOI] [PubMed] [Google Scholar]
- 32. van Griensven J., et al. 2010. Combination therapy for visceral leishmaniasis. Lancet Infect. Dis. 10:184–194 [DOI] [PubMed] [Google Scholar]
- 33. Vermeersch M., et al. 2009. In vitro susceptibilities of Leishmania donovani promastigote and amastigote stages to antileishmanial reference drugs: practical relevance of stage-specific differences. Antimicrob. Agents Chemother. 53:3855–3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. World Health Organization 2004. Countries of South-East Asia region plan to eliminate kala azar, vol. 73 TDR News. [Google Scholar]
- 35. Yeates C. 2002. Sitamaquine GlaxoSmithKline/Walter Reed Army Institute. Curr. Opin. Investig. Drugs 3:1446–1452 [PubMed] [Google Scholar]