Abstract
During aging, there is an increase in neurodegenerative diseases and a decrease in cognitive performance. Postmenopausal women are more vulnerable as their estrogen levels decline, but most hormone replacement therapies do not prevent cognitive decline. One potential reason is that the timing of hormone replacement is critical and changes in estrogen receptor expression may over-ride hormonal intervention. In rodents, ERβ mRNA decreases in the cortex with age. One mechanism by which ERβ mRNA could be regulated is by epigenetic modification of ERβ promoter. Here we show an increase in methylation of ERβ promoter corresponding to decreases in ERβ mRNA in aging female cortex.
Keywords: Estrogen receptors, Aging, Cortex, Methylation, Pyrosequencing
INTRODUCTION
The importance of estrogens such as 17β-estradiol in cognitive function has been demonstrated in studies of cognition in relation to phases of the menstrual cycle, menopausal symptoms, circulating hormone levels and aging [1,2]. Estrogen exerts physiological effects on target tissues by binding to the nuclear receptors, ERα and ERβ [3,4]. ERα and ERβ are differentially expressed throughout the rodent brain in both neurons and glial cells [5]. ERβ mRNA is concentrated in the cerebral cortex and hippocampus and other brain regions that indicate non-reproductive actions of E2 [6,7]. ERα and ERβ are expressed in the hippocampus, a region of the brain involved in cognitive function [8], but only ERβ is expressed in the adult rodent cortex [6]. It has previously been hypothesized that diminished responsiveness to E2 in aging may be mediated by a decrease in the density of estrogen receptors, such as ERβ. In the cerebral cortex, ERβ mRNA expression decreases by middle age [9,10]. It is interesting to note that this decline in receptor expression correlates with the cognitive decline seen in middle-aged rats [11].
This change in ERβ mRNA expression with aging could be regulated by a variety of mechanisms. One potential mechanism of ERβ gene regulation is through epigenetic modification of the ERβ promoter. Epigenetic regulation involves post-replication and post-translational modification of DNA and histones that lead to lasting changes in chromatin structure and thus changes in gene transcription [12]. Methylation involves recruitment of several families of proteins including the DNA methyltransferases and the methyl-CpG-binding domain proteins. [13,14]. Recruitment of these proteins to the promoter region of a gene can result in gene silencing [15,16]. Methyl-CpG-binding domain proteins such as methyl-CpG-binding protein 2 (MeCP2) have been shown to play a crucial role in neural development [14]. In breast cancer cells, all have been shown to interact with the ER promoters [17]. In the current studies, we investigated the role of DNA methyltransferases and MeCP2 in regulating ERβ gene expression.
METHODS
Real Time PCR
Cortical tissue was homogenized and RNA was isolated using TriZol. 1μg of RNA was reverse transcribed (Invitrogen). For PCR, each reaction contained DEPC H20 up 25 μl, 12.5 μl of 2X SYBRGreen Brilliant Master Mix (Stratagene), 250 pmol of upstream primer, 50 pmole of downstream primer, 0.375 μl of Reference Dye and 1μg of cDNA. Each 96 well plate had a non-template control and each sample was run in triplicate. The change in threshold cycle (ΔCt) for each sample was normalized to the control gene Histone 3.1. The cycling parameters and primers used for each gene have been described previously [18].
Methylation-Specific PCR
Genomic DNA was extracted from young and middle-aged female rat cortex using previously described methods [19]. Briefly, tissue was homogenized in 250 μl of lysis solution (10 mM Tris-HCl, 150 mM EDTA, 1% SDS, 100 g/ml proteinase K) and incubated for 20 minutes at 55°C. 100 μl of RNase solution, consisting of 10 mM Tris-HCl (pH 8.0), 1 mM EDTA (TE) and 30 units of RNase was added, and the mixture was incubated at 37°C for 1 hour. DNA was purified by phenol cholroform extraction. Sodium bisulfite modification was conducted on 500 ng of genomic DNA using the EZ Methylation Gold kit (Zymo Research). PCRs were done with the FailSafe PCR system protocol (Epicenter) as previously described [20]. The primers were designed using MethPrimer with ERβ promoter sequence (gi: 83031780) used as a template.
Pyrosequencing
We examined the methylation status of specific CpG dinucleotides within the promoter region of rat ERβ. Genomic DNA was extracted from the cortex of young and middle-aged female rats (n = 6–8), as described above. Pyrosequencing was performed by EpigenDx, as previously described [21]. Briefly, sodium bisulfite modification was conducted on the genomic DNA. PCR was performed using HotStar Taq Polymerase (Qiagen). Each reaction contained the following: 1X PCR buffer, 3 mM MgCl2, 200 μM of each dNTPs, 6 pmol forward primer, 6 pmol reverse primer, 0.75 U HotStar Taq Polymerase, 1 μl of bisulfite treated DNA and water to adjust to proper volume. PCR conditions were as follows: 95°C for 15 minutes; 30 × (95°C for 30 seconds; Ta°C for 30 seconds; 72°C for 30 seconds); 72°C for 5 minutes. Universally methylated and unmethylated DNA samples were used as controls in these experiments.
Chromatin Immunoprecipitation Assay (ChIP)
For ChIP assays, we used the Upstate Biotechnologies ChIP Assay Kit (Catalog # 17–295, Temecula, CA) and methods previously described [18]. Briefly, cortex was dissected and minced. Proteins were cross-linked in 1% formaldehyde and tissue was placed in lysis buffer including protease inhibitors (Roche Applied Science). Cross-linked DNA was sheared to 500 bp and sonicated cell supernatant was diluted 10-fold in ChIP dilution buffer. A sample was removed after dilution and called the “input” sample. The remaining diluted cell supernatant was pre-cleared with Salmon Sperm DNA/Protein A agarose 50% slurry. Primary MeCP2 antibody (Upstate Biotechnology, 1:1000) was added to the remaining pre-cleared supernatant and incubated overnight. Agarose was pelleted by centrifugation and the complex was washed with Low Salt Immune Complex Wash Buffer and High Salt Immune Complex Wash Buffer, followed by LiCl Complex Wash buffer and TE Buffer and eluted in elution buffer. Cross-links were reversed and DNA was recovered. Standard PCR with 30 cycles was performed on the resulting sample as well as on the “input” incubated samples that did not undergo any pre-clearing or immunoprecipitation. Resulting sample was also immunoprecipitated with a non-immune antibody.
RESULTS
Experiment 1. ERβ mRNA and protein expression in the cortex decrease in middle age
Using real time PCR and Western Blot, we examined ERβ mRNA and protein expression in young (3–4 months) and middle-aged (9–12 months) female rats (n=7–10 per group). ERβ mRNA was significantly decreased in the cortex in middle-aged females compared to young females (Figure 1A). We also investigated changes in ERβ mRNA expression in cerebellum. ERβ mRNA expression was high in the cerebellum, but did not decrease in middle age (data not shown). Protein was collected from the cortex of young and middle-aged females and found a corresponding decrease in ERβ protein expression in middle-aged compared to young females (data not shown).
Figure 1. ERβ mRNA expression and promoter methylation.
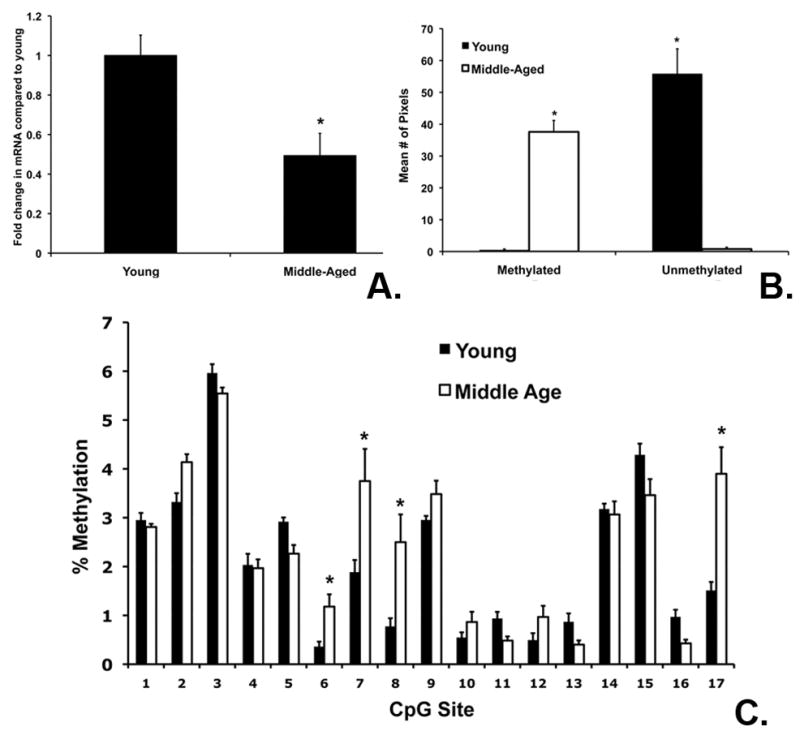
(A). ERβ mRNA was significantly decreased in middle-aged compared to young cortex. (B). There was an e-associated increase methylation at both CpG loci of the ERβ promoter Exon 1. Genomic DNA was amplified using both methylated (M) and un-methylated primers (U), as well as primers for unmethylated β-actin. The graph shows the average # of pixels for 6 replications. (C). For all graphs, asterisks indicate significant differences between young and middle-aged cortex (p< 0.05).
Experiment 2. Methylation of the ERβ Exon 1 is increased in middle-age cortex
Methylation-Specific PCR (MSP) was conducted to test the hypothesis that decreased ERβ mRNA expression during aging corresponds with changes in the methylation status of ERβ Exon 1. Genomic DNA was isolated and treated with sodium bisulfite and used for MSP using primers designed to detect the methylation status of ERβ Exon 1. There was significantly increased methylation at 2 CpG regions of the ERβ Exon 1 in middle-aged rats (One-Way ANOVA, p= 0.001) (Figure 1B). These data suggest that ERβ mRNA expression may be regulated by an increase in methylation as a result of aging.
Experiment 3. Pyrosequencing of CG sites along the ERβ promoter during aging
To further examine the methylation status of the ERβ promoter in young and middle-aged cortex, we used pyrosequencing to analyze methylation of CG sites along ERβ Exon 1. Genomic DNA was collected from young and middle-aged rats to determine % methylation of sites along the ERβ promoter 1 from −1601 to −1062 from the mRNA start site. Two-Way ANOVA comparing age and CpG site demonstrated a significant effect of age (p= 0.001), CpG site (p=0.001) and a significant interaction (p= 0.002) (Figure 1C). Differences in % methylation between young and middle age were site specific and indicate that changes in methylation are not global.
Experiment 4. DNMT mRNA expression increases in middle-aged female cortex
DNMTs are involved in the methylation of cytosine residues. DNMT 3A is the de novo methyltransferase responsible for initial methylation, while DNMT 1 is responsible for maintenance of methylation status. We investigated both DNMT 1 and DNMT 3A mRNA expression in the cortex using real time PCR. DNMT 1 and DNMT 3A mRNA levels were significantly increased in middle-aged females compared to young (p< 0.001) (Figure 2A). These data suggest that changes in DNMT mRNA expression occur at the same time that ERβ mRNA expression decreases and promoter methylation increases with middle age.
Figure 2. DNA methyl transferases and MeCP2 play a role in regulation of ERβ during aging.
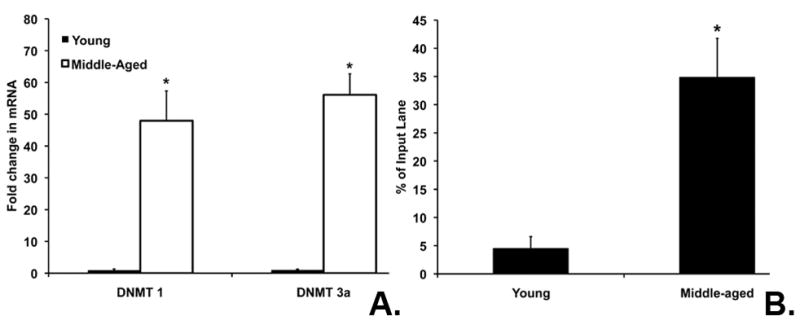
(A.) DNMT 1 and 3a mRNA expression was significantly increased in middle-aged cortex compared to young. Asterisks on the graph indicate significant differences from young (p< 0.05). (B). There was a significant increase in MeCP2 association with ERβ Exon 1 in middle-aged cortex (p= 0.001). All ChIP experiments were repeated 4 times and PCR bands on the gels were quantified using NIH Image J. The graph shows the % of the original input sample for the immunoprecipitated lane.
Experiment 5. Methyl-CpG binding protein 2 (MeCP2) is associated with the ERβ Exon1 in middle-aged females
Methyl binding proteins also play an important role in regulation of gene expression by stabilizing methylation of CpG sites. Here we investigated the direct interaction of MeCP2 with ERβ Exon 1 in the cortex. MeCP2 was associated the ERβ promoter in middle-aged cortex. MeCP2 was not associated in young animals when ERβ mRNA expression was higher (Figure 2B). The input sample was compared to demonstrate equal starting sample quantities and the graph represents the % of input sample. The association of MeCP2 with ERβ Exon 1 in middle-aged cortex suggests that methylation and recruitment of MeCP2 could account for the changes in the ERβ gene expression in middle age.
DISCUSSION
As previously described, we observed an age-associated decrease in ERβ mRNA expression in the cortex of middle-aged female rats compared to younger rats [9]. In female rats, the timing of these age-related changes in ERβ expression in the cortex is correlated with a decline in cognitive function [1–5]. This effect in the cortex appears to be ERβ-specific because there is a very low level of ERα expression in the normal adult cortex [22]. In middle-aged females, there was also an age-associated increase in methylation of the rat ERβ promoter Exon 1. This change in methylation was not global. In fact, pyrosequencing analysis revealed that changes in methylation of the ERβ promoter in the cortex were specific to certain CpG regions of the promoter. A focus of future studies will be to identify the importance of these specific regions for promoter function and regulation of ERβ mRNA expression.
We also found an aged-associated increase in DNMT mRNA expression. This increase was seen in both DNMT1, which is responsible for maintenance of methylation status and DNMT3A, the de novo methyltransferase responsible for initial methylation [13,14]. These data suggest that middle age may be the beginning of these changes in methylation. We found that MeCP2 protein expression increases with middle age in general and also becomes associated with the ERβ promoter. Interestingly, the association with ERβ occurred in a region of Exon 1 that also showed an increase in methylation as demonstrated by pyrosequencing. These data are the first to demonstrate a correlation between ERβ mRNA expression and methylation of the ERβ promoter in the cortex. Though it is highly likely that multiple epigenetic modifications occur, we have begun by establishing DNA methylation as a potential mechanism by which these changes occur.
ERβ is thought to mediate estrogen’s effects on cognition in the cortex and other brain regions. A loss of expression would render E2 inactive and any potential hormone replacement would be ineffective. These data suggest that it may be necessary to not only replace decreased hormone levels, but also understand and potentially prevent loss of the ERβ as well.
CONCLUSIONS
Here we have shown that the age-related decrease in ERβ mRNA expression is correlated with an increase in methylation of at least one region of the ERβ promoter. Epigenetic modifications in the aging brain could have significant consequences on gene expression and modify cognitive function.
Acknowledgments
Sources of funding: Grant P20 RR 15592 from the National Center for Research Resources (NCRR) and NSF 10S-0919944 to MEW.
Footnotes
The authors have no conflicts of interest.
References
- 1.Maki PM, Rich JB, Rosenbaum RS. Implicit memory varies across the menstrual cycle: estrogen effects in young women. Neuropsychologia. 2002;40:518–529. doi: 10.1016/s0028-3932(01)00126-9. [DOI] [PubMed] [Google Scholar]
- 2.Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koike S, Sakai M, Muramatsu M. Molecular cloning and characterization of rat estrogen receptor cDNA. Nucleic acids research. 1987;15:2499–2513. doi: 10.1093/nar/15.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donahue JE, Stopa EG, Chorsky RL, King JC, Schipper HM, Tobet SA, et al. Cells containing immunoreactive estrogen receptor-alpha in the human basal forebrain. Brain Res. 2000;856:142–151. doi: 10.1016/s0006-8993(99)02413-0. [DOI] [PubMed] [Google Scholar]
- 6.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. The Journal of comparative neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, et al. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 8.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 9.Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) gene expression in specific regions of the rat brain. Mech Ageing Dev. 2002;123:593–601. doi: 10.1016/s0047-6374(01)00406-7. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi-Shima N, Yuri K. Age-related changes in the expression of ER-beta mRNA in the female rat brain. Brain Res. 2007;1155:34–41. doi: 10.1016/j.brainres.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci. 1999;19:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolffe AP, Matzke MA. Epigenetics: regulation through repression. Science (New York, NY) 1999;286:481–486. doi: 10.1126/science.286.5439.481. [DOI] [PubMed] [Google Scholar]
- 13.Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, et al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 14.Nan X, Bird A. The biological functions of the methyl-CpG-binding protein MeCP2 and its implication in Rett syndrome. Brain Dev. 2001;23 (Suppl 1):S32–37. doi: 10.1016/s0387-7604(01)00333-3. [DOI] [PubMed] [Google Scholar]
- 15.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Bird AP, Wolffe AP. Methylation-induced repression--belts, braces, and chromatin. Cell. 1999;99:451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- 17.Sharma D, Blum J, Yang X, Beaulieu N, Macleod AR, Davidson NE. Release of methyl CpG binding proteins and histone deacetylase 1 from the Estrogen receptor alpha (ER) promoter upon reactivation in ER-negative human breast cancer cells. Molecular endocrinology (Baltimore, Md) 2005;19:1740–1751. doi: 10.1210/me.2004-0011. [DOI] [PubMed] [Google Scholar]
- 18.Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology. 151:731–740. doi: 10.1210/en.2009-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishino K, Hattori N, Tanaka S, Shiota K. DNA methylation-mediated control of Sry gene expression in mouse gonadal development. The Journal of biological chemistry. 2004;279:22306–22313. doi: 10.1074/jbc.M309513200. [DOI] [PubMed] [Google Scholar]
- 20.Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- 21.Kim HG, Higgins AW, Herrick SR, Kishikawa S, Nicholson L, Kutsche K, et al. Candidate loci for Zimmermann-Laband syndrome at 3p14.3. Am J Med Genet A. 2007;143:107–111. doi: 10.1002/ajmg.a.31544. [DOI] [PubMed] [Google Scholar]
- 22.Westberry JM, Prewitt AK, Wilson ME. Epigenetic regulation of the estrogen receptor alpha promoter in the cerebral cortex following ischemia in male and female rats. Neuroscience. 2008;152:982–989. doi: 10.1016/j.neuroscience.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]


