Abstract
Ureteropelvic junction (UPJ) obstruction is the most frequently observed cause of obstructive nephropathy in children. Neonatal and foetal animal models have been developed that mimic closely what is observed in human disease. The purpose of this review is to discuss how obstructive nephropathy alters kidney histology and function and describe the molecular mechanisms involved in the progression of the lesions, including inflammation, proliferation/apoptosis, renin–angiotensin system activation and fibrosis, based on both human and animal data. Also we propose that during obstructive nephropathy, hydrodynamic modifications are early inducers of the tubular lesions, which are potentially at the origin of the pathology. Finally, an important observation in animal models is that relief of obstruction during kidney development has important effects on renal function later in adult life. A major short-coming is the absence of data on the impact of UPJ obstruction on long-term adult renal function to elucidate whether these animal data are also valid in humans.
Keywords: fibrosis, mechanical stress, neonatal models
Introduction
Congenital obstructive nephropathy is the main cause of end stage renal disease (ESRD) in children (Benfield et al. 2003). This contrasts sharply with adult ESRD which for the greater part originates from ageing and type II diabetes. Hydronephrosis, defined clinically by an enlargement of the kidney as a result of urine accumulation in the renal pelvis or calyces, is a consequence of obstructive nephropathy. The most frequently found cause of hydronephrosis is ureteropelvic junction (UPJ) obstruction with an estimated incidence of 1 in 1000–1500 (Chang et al. 2004). UPJ obstruction is mostly considered as a functional obstruction originating from abnormalities in the smooth muscle of the pelvis and ureter (Mendelsohn 2004). Physical obstruction of the ureter, e.g. compression of the UPJ by crossing vessels, is also observed but whether the vessel alone causes obstruction or whether there is also a functional component is still debated (Yiee et al. 2010). Although surgery in UPJ obstruction is efficient in protecting against short term detectable renal lesions, increasing support obtained in both experimental- and human-studies is available suggesting that UPJ obstruction induces permanent modifications of the renal parenchyma.
Other causes of congenital obstructive nephropathy include disorders of the urethra such as posterior valves or disorders of the bladder such as trigonal cysts (Roth et al. 2002). However, these are all rare diseases. Therefore, we will focus in this review on unilateral UPJ obstruction as it is the most frequently encountered obstructive nephropathy in children and therefore the majority of the insight of human obstructive nephropathy comes from this pathology. In addition, excellent neonatal rodent and foetal models for partial and complete UPJ obstruction have been developed (Peters 2001; Thornbill et al. 2005). We have attempted to combine all the available knowledge obtained in human UPJ obstruction, animal models and in vitro studies with the purpose of identifying concepts that hold both in the models and in human pathology and to support future experiments aiming at the better understanding of obstructive nephropathy and drug testing.
As the response of the adult kidney to obstruction clearly differs from the response of the foetal kidney (Chevalier et al. 2009), all work described in this review concerns responses to foetal or neonatal obstruction. Any reference to adult obstruction is clearly indicated and foetal/neonatal obstruction is the default situation.
Causes UPJ obstruction
Causes of human UPJ obstruction
Effective urine transport depends on formation of proper connections and functioning between the kidney and the ureter. In UPJ obstruction attention has been drawn on the development of smooth muscle cells that line the pelvis and the ureter and conduct peristaltic waves to expel urine (Chang et al. 2004; Lye et al. 2010). Failure in development of the renal pelvis or impaired smooth muscle differentiation can, indeed, lead to functional obstruction and hydronephrosis in experimental models (Miyazaki et al. 1998). Also, analysis of tissue obtained from the ureteropelvic junction of patients which have undergone pyeloplasty (i.e. surgical reconstruction or revision of the renal pelvis to drain and decompress the kidney) have shown that the defective urinary tracts present pathological changes in both smooth muscle rearrangement and pyeloureteral innervation (Zhang et al. 2000). An interesting hypothesis has recently been proposed to explain the role of smooth muscle cells in kidney maturation during late human gestation. In utero, the head of the embryo usually rests in the mother's pelvis (Lye et al. 2010). In this position urine must work against gravity and when peristalsis fails, this will lead to functional obstruction of the maturing kidney.
Although the molecular mechanisms underlying this smooth muscle cell maldevelopment are still largely unknown this seems, to date, to be the most probable cause of UPJ obstruction (Mendelsohn 2004). As this review focuses on the renal consequences of UPJ obstruction, we will not discuss the (genetic) details of failure of the normal smooth muscle development in the ureter and in the pelvis. For more information in this field readers should refer to other excellent reviews (Mendelsohn 2004; Lye et al. 2010).
Animal models of UPJ obstruction
Different animal models of UPJ obstruction exist, either spontaneous or specifically developed, that mimic human UPJ obstruction, each with specific merits and drawbacks. Specific rodent strains have been identified that present reproducible lesions of spontaneous congenital hydronephrosis (Peters 2001). Although the underlying causes responsible for this spontaneous UPJ obstruction phenotype remain poorly understood, these models are interesting in that they are naturally occurring and reflect the human pathology more rationally. However, the often observed infertility or low reproduction status of these strains limits the use of such models (Peters 2001).
Unilateral ureteral obstruction (UUO) is a well-known surgical model to study tubulointerstitial fibrosis in adult rodents (Bascands & Schanstra 2005). However, the effects of obstruction in the mature adult kidney differ widely of what happens in the developing kidney. Indeed, in the latter, the obstruction can interfere with kidney morphogenesis, growth and maturation. For this reason, different models of prenatal obstruction have been developed in many species (Peters 1997). Compared to the congenital models, they offer the advantages of controlling the onset, the duration and the severity of the pathology. In sheep, primate and guinea pig, in which renal nephrogenesis, like in humans, is completed before birth but where renal maturation continues after birth, UUO must be performed during foetal life (Chevalier et al. 2009). However intrauterine surgery is a complex procedure. An alternative to this approach is the use of the opossum, which is a marsupial with extrauterine foetal development. The surgery can thus be performed in the mother's pouch (Peters 1997; Chevalier et al. 2009). The other alternative is to study the effect of UUO in the neonate mouse, rat, rabbit or pig. In mice and rats, only 10% of the nephrogenesis is completed at birth, corresponding to the midtrimester in the human foetus and continues 10 days after birth followed by a period of renal maturation. The advantage of rodent models is the potential use of knockout or transgenic animals to study the role of a specific molecule in the pathophysiology of obstruction and the relatively easy access to these animals to increase statistical power. In rabbits and pigs, as in mice and rats, nephrogenesis is incomplete at birth and continues for 2 and 3 weeks after birth, respectively. The advantage in pigs is that the kidney anatomy is close to the human anatomy. As opposed to the unipapillary structure of the rodent kidney, pigs have multipapillary kidneys and predominantly short-looped nephrons similar to humans (Eskild-Jensen et al. 2007). Although very useful, the remaining question of these postnatal models is whether the neonatal kidney reacts differently to the obstruction compared to the foetal kidney. Indeed, the demand on renal function is much higher in neonates than in foetuses where placenta is partly involved in foetal blood dialysis (Peters 1997).
Another issue is the technique employed to perform the obstruction. Basically, there are two major ways to study the effect of obstruction in foetuses and neonates: complete or partial obstruction of the ureter. Complete obstruction is most often performed by a simple suture ligation of the ureter. This model mimics severe UPJ obstruction and evolves rapidly towards hydronephrosis and loss of renal parenchyma (1–2 weeks after UUO) (Chevalier et al. 2009). But, since in the clinical situation most of the UPJ cases present partial rather than complete obstruction, partial UUO models have been developed. One technique consists in wrapping the ureter into the underlying psoas muscle which allows the obstruction to evolve with the growing animal but leads to variable results. Another method consists in placing a stainless steel wire of known diameter parallel to the ureter, followed by ligature of both the ureter and the stainless steel wire. Subsequent withdrawing of the wire results in a well calibrated partial obstruction. Not only this technique is more reproducible than the psoas muscle approach but also, by choosing the different diameters of the stainless steel wire, the severity of the obstruction can be controlled (Chevalier et al. 2009).
In conclusion, a number of animal models have been used to understand the pathophysiology of UPJ obstruction. Clearly none of the individual models is perfect, but combining data obtained in the different species and by different techniques will help to better characterize the mechanisms involved in this pathology.
Renal consequences of UPJ obstruction
Histological alterations
The spectrum of renal abnormalities varies greatly in UPJ obstruction. In human renal biopsies, one observes all lesions found in renal disease including subtle changes such as modified proximal or tubular size, chronic tubulointerstitial injury, glomerulosclerosis, fibrosis, aberration of nephron development and in severe cases (<1%) renal dysplasia (Rosen et al. 2008).
Studies have attempted to link the histological state of renal biopsy to the function of the obstructed kidney. However, no clear correlation between differential renal function and the gross histological status of the kidney was observed (Elder et al. 1995; Stock et al. 1995; Han et al. 1998; Zhang et al. 2000). The only observation that seems to hold is that severe UPJ obstruction (differential function of <30%) is associated with a high degree of parenchymal damage (Zhang et al. 2000). Also renal histology in humans was found not to be related to the duration of obstruction (Han et al. 1998). This absence of a clear correlation between renal function and gross renal histology might be the cause of the observed high variability in the outcome of UPJ obstruction and the persisting difficulties in the clinical assessment of UPJ obstruction (Csaicsich et al. 2004). However, detailed analysis of kidney histology in UPJ obstruction showed that subtle histological changes might be associated to impaired function of the obstructed kidney (Huang et al. 2006). It was observed that in biopsies with little tubulointerstitial changes, the sizes of both proximal and distal tubules were significantly larger in UPJ obstruction patients with significant functional obstruction (Huang et al. 2006).
In contrast to these observations in humans, alterations of renal parenchyma have been intensively studied in animal models of UPJ obstruction (Figure 1). Although these lesions can differ depending on the species or the severity of the pathology, a general observation is that the earlier obstruction occurs during in utero nephrogenesis, the more severe the associated histopathological changes (Matsell & Tarantal 2002). Alterations affect renal growth, nephron number, glomerular and tubulointerstitial histology, as well as the contralateral (i.e. non-obstructed) kidney.
Figure 1.
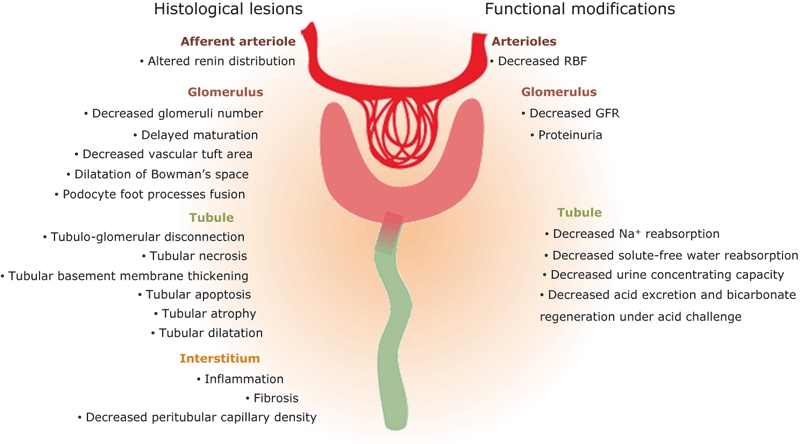
Histological lesions and functional modifications induced by obstruction.
Renal growth
Partial and complete UUO in newborn rodents is characterized by an enlarged pelvic diameter and increased kidney volume (Shi et al. 2004; Thornhill et al. 2005; Topcu et al. 2007; Guerin et al. 2008). UUO severely affects kidney growth, as shown by decreased renal mass, protein or DNA content and parenchymal atrophy (Chevalier et al. 1996; Chung & Chevalier 1996; Wen et al. 2002; Shi et al. 2004; Thornhill et al. 2005; Topcu et al. 2007; Guerin et al. 2008). UUO also inhibits nephrogenesis thereby reducing the number of functional nephrons (Josephson 1983; Chevalier et al. 1999; Wen et al. 2002; Thornhill et al. 2005; Burt et al. 2007). Many of these effects are also observed in neonatal pig model (Eskild-Jensen et al. 2002). In addition, and in contrast to human, variable partial UUO in the neonatal rat shows that these lesions increase with the severity of the UUO (Thornhill et al. 2005).
Glomerular changes
In the neonatal model, partial or complete UUO results in a decrease in the number of glomeruli, not only by halting nephrogenesis but also by destruction of previously formed glomeruli via apoptosis (Cachat et al. 2003; Eskild-Jensen et al. 2007) or phenotypic transformation of glomerular cells into mesenchymal cells (Thornhill et al. 2005; Chevalier 2008). Glomerular maturation, evaluated by the number of capillary loops and the phenotype of podocytes, is delayed (Chevalier et al. 1999a, 2000a; Wen et al. 2002; Cachat et al. 2003; Burt et al. 2007; Chen et al. 2007) and vascular tuft area is reduced (Chevalier et al. 1999a; Burt et al. 2007). Complete UUO increases the number of renin secreting cells (Norwood et al. 1994) and maintains foetal renin distribution along the afferent arteriole rather confining renin expression to the juxtaglomerular apparatus (el-Dahr et al. 1991; Chung & Chevalier 1996). In oppossum pups and in foetal lambs, a similar UUO-induced decrease in glomerular number is observed (Liapis et al. 2000; Mure et al. 2006a), associated to podocyte foot processes fusion (Fenghua et al. 2009) and cystic-like changes such as dilatation of Bowman's space and collapsed capillary loops (Liapis et al. 2000; Mure et al. 2006a; Fenghua et al. 2009).
Tubulointerstitial injury
Tubular damage is an important characteristic of UPJ obstruction, which is observed following obstruction in neonatal rodents, foetal sheep and opossum pups. They include tubular apoptosis (Chevalier et al. 1999a, 2000a; Liapis et al. 2000; Cachat et al. 2003; Thornhill et al. 2005; Lange-Sperandio et al. 2006, 2007; Yoo et al. 2006a; Burt et al. 2007; Eskild-Jensen et al. 2007a), tubular dilatation (Steinhardt et al. 1995; Chevalier et al. 1999a; Liapis et al. 2000, 2001; Cachat et al. 2003), tubular atrophy (Chevalier et al. 1999a, 2000a; Fern et al. 1999; Lange-Sperandio et al. 2002, 2006, 2007; Thornhill et al. 2005), tubular basement membrane thickening (Cachat et al. 2003) and necrosis (Cachat et al. 2003). Progressive apoptosis/necrosis of tubules may promote glomerulotubular disconnection leading to non functional nephrons (Thornhill et al. 2005). Interestingly, each tubular segment responds differently to complete UUO: apoptosis and dilatation are most consistently observed in collecting ducts and distal tubules, the tubular segments adjacent to the obstructed pelvis, whereas basement membrane thickening and necrosis predominate in the proximal tubule (Cachat et al. 2003). However, no overall changes in either proximal- or distal-tubule length are found in the obstructed kidney of newborn pigs, explained by a combination of a reduction in length in damaged tubules and increased length in compensating nephrons (Eskild-Jensen et al. 2002, 2007a). Finally, effect of UUO on tubular proliferation is not clear because both increase (Cachat et al. 2003; Thornhill et al. 2005) and decrease (Chevalier et al. 1999a, 2000a) in proliferation were observed in the obstructed kidney.
Partial and complete UUO also modify the peritubular space of the obstructed kidney. The main interstitial modifications are enhanced inflammation (Chevalier et al. 1996; Lange-Sperandio et al. 2002, 2006, 2007; Burt et al. 2007; Chen et al. 2007; Guerin et al. 2008), tubulointerstitial fibrosis (Steinhardt et al. 1995; Chevalier et al. 1999a,b, 2000a; Fern et al. 1999; Liapis et al. 2000, 2001; Lange-Sperandio et al. 2002, 2006, 2007; Cachat et al. 2003; Kiley et al. 2005; Mure et al. 2006a; Chen et al. 2007; Eskild-Jensen et al. 2007b; Guerin et al. 2008) and decreased peritubular capillary density. The latter has been shown to be particularly prominent in the medulla during complete UUO (Burt et al. 2007).
Tubular and interstitial changes are closely correlated. The number of apoptotic tubular cells was found to parallel fibrosis (Cachat et al. 2003; Guerin et al. 2008) and macrophage infiltration (Lange-Sperandio et al. 2002; Guerin et al. 2008). Interestingly, while alterations are rapidly induced following complete UUO in foetal sheep, inflammatory cells are not visible. However, a marked inflammatory infiltrate, associated with increased fibrosis, is observed if foetal obstruction continues after the birth (Mure et al. 2006a). Consequently, the inflammatory response has probably not a decisive role in the occurrence of renal alterations during foetal life (Mure et al. 2006a) and UUO induced-inflammation in newborn rodents may be influenced by the renal functional demand imposed by extrauterine life (Matsell & Tarantal 2002).
The contralateral kidney
In response to partial and complete UUO, the non-obstructed contralateral kidney develops compensating hypertrophy (Norwood et al. 1994; Chevalier 1996; Chevalier et al. 1996, 1999a,b; Fern et al. 1999; Malik et al. 2001; Chung & Wen et al. 2002; Cachat et al. 2003; Shi et al. 2004b; Topcu et al. 2007), the adaptative growth-rate increasing with the severity and duration of obstruction (Yoo et al. 2006a). Neither the number of glomeruli (Chevalier et al. 1999a; Cachat et al. 2003; Thornhill et al. 2005; Burt et al. 2007) nor their maturation index are modified (Chevalier et al. 2000a; Cachat et al. 2003). However, the mean glomerular area is higher than in the control kidney (Chevalier et al. 1999a; Yoo et al. 2006a) and renin expression is inhibited (Norwood et al. 1994; Chevalier et al. 1996) while the number and the localization of renin cells are normal (Norwood et al. 1994; Chevalier et al. 1999a). No particular modification is described in tubules excepted proliferation which is sometimes observed (Cachat et al. 2003) and the number of peritubular capillaries is not changed (Burt et al. 2007).
In conclusion, nearly all histological alterations found in humans in response to UPJ obstruction are found in the prenatal and neonatal animal UUO models suggesting that the observations in animal models have fair chance to be valid in human UPJ obstruction.
Functional modifications
It is well established that UPJ obstruction induces mild to severe impairment of kidney function, including glomerular filtration and tubular exchanges of water and solutes. Studies in humans are lacking but how these mechanisms are modified has been extensively studied in animal models of obstruction (Figure 1). Changes in the obstructed kidney, in the contralateral kidney and in the whole animal are variable, depending on species, the duration and the severity of obstruction, but also depend on the diet and the hydration status.
Renal blood flow
Limited data are available on the response of renal blood flow (RBF) to foetal/neonatal UUO. In the neonatal rat, 4–24 weeks of partial UUO induces a progressive reduction in RBF in the obstructed kidney (Shi et al. 2004b; Topcu et al. 2007; Wen et al. 2009). In foetal lamb, chronic complete UUO also decreases RBF, this effect being less severe when UUO is partial (Nguyen & Kogan 1998; Mure et al. 2006b). This diminution in RBF leads most probably to ischemia, thus contributing in part to the observed lesions of renal tissue.
Glomerular filtration
In general, neonatal UUO results in decreased glomerular filtration rate (GFR) in the obstructed kidney as seen in rats (Josephson 1983; Shi et al. 2004a,b; Thornhill et al. 2005; Topcu et al. 2007) and pigs (Eskild-Jensen et al. 2000, 2001). When partial UUO persists, the fall in GFR becomes more pronounced in the rat (Shi et al. 2004a) whereas it is attenuated in the pig model (Eskild-Jensen et al. 2000, 2001). In the non-obstructed contralateral kidney, GFR may either increase (Josephson 1983; Eskild-Jensen et al. 2001, 2007b) or remain unchanged (Shi et al. 2004a; Thornhill et al. 2005; Topcu et al. 2007) and the compensatory response of contralateral kidney depends also on the duration of obstruction (Eskild-Jensen et al. 2001). In parallel proteinuria develops in the obstructed kidney and contralateral kidney following partial or complete UUO, respectively (Thornhill et al. 2005). Finally, total GFR in obstructed animals can reach (Eskild-Jensen et al. 2001; Wang et al. 2009) or not (Shi et al. 2004a; Topcu et al. 2007) the same level as GFR in the sham operated animals.
Tubular function
Modification of tubular function in response to UUO in neonatal rats was extensively studied and showed profound effects of UUO on the tubular handling of water and solutes. Partial UUO is clearly associated with a defect in sodium reabsorption since the fractional excretion of sodium is increased in the obstructed kidney. As a result, and in the absence of a compensatory change in the non-obstructed kidney, animals display natriuria in spite of a decrease in the filtered sodium load (Shi et al. 2004a,b; Eskild-Jensen et al. 2007a; Topcu et al. 2007). In addition, the solute-free water reabsorption (TcH2O) is markedly decreased in the obstructed kidney from partially obstructed rats, indicating that the ability of these kidneys to reabsorb water in collecting ducts is reduced. TcH2O does not seem to increase in the contralateral kidney and finally, urine osmolarity decreases, showing an impaired urinary concentrating capacity in UUO animals (Shi et al. 2004a,b; Topcu et al. 2007). These effects can be explained by looking at the UUO-induced changes in transporters expression and activity. Neonatal complete or partial UUO induces a reduction in expression and distribution of major sodium transporters and water channels including Na,K-ATPase, NHE1, NHE3 and aquaporins (AQP1, AQP2, AQP3, AQP7), associated to a down-regulation of the vasopressin 2 receptor (Table 1) (Silverstein et al. 2003a; Shi et al. 2004a; Manucha et al. 2007; Topcu et al. 2007; Wang et al. 2009). This decrease of transporters expression could be regulated by dynamin, a key component of the endocytic machinery involved in internalization of several transporters, as it was found frequently stimulated in UUO (Table 1) (Silverstein et al. 2003a; Padovano et al. 2009; Moeller et al. 2010; Ramstrom et al. 2010). In parallel to the regulation of transporter expression, it seems also that the activity of the transporters can be modified. Indeed, the mechano-sensitive gap-junction protein connexin 30 participates in ATP release into the tubular fluid and this inhibits salt and water reabsorption by the transporters (Sipos et al. 2009). Interestingly, connexin 30 expression is increased in the obstructed kidney (Table 1) (Silverstein et al. 2003b). Altogether, these results provide the molecular mechanism for the observed modified urine osmolality in neonatal UUO animals.
Table 1.
Literature data about tubular transport and glomerular function-related genes and proteins that have been altered during obstructive nephropathy. We have reported here only molecules of which activity or expression has been significantly modified compared to sham-animals (animal model) or control cells (in vitro)
| Animal model | In vitro | ||||||
|---|---|---|---|---|---|---|---|
| Gene symbol | Gene name | PUUO | CUUO | Ref | Stretch | Fluid flow | Ref. |
| AQP1 | Aquaporin 1 | Down (24w) | Shi et al. (2004a) | ||||
| AQP2 | Aquaporin 2 | Down (10w) | Topcu et al. (2007) | ||||
| AQP3 | Aquaporin 3 | Down (24w) | Shi et al. (2004a) | ||||
| AQP7 | Aquaporin 7 | Down (2w) | Silverstein et al. (2003a) | ||||
| ATP1a1 | Na+/K+ ATPase | Down (7w to 24w) | Shi et al. (2004a), Topcu et al. (2007), Wang et al. (2009) | ||||
| Avpr2 | Vasopressin V2 receptor | Down (2w) | Silverstein et al. (2003a) | ||||
| Calb1 | Calbindin D28 | Down (2w) | Silverstein et al. (2003a) | ||||
| Dnm1 | Dynamin | Up (2w) | Silverstein et al. (2003a) | ||||
| Gjb6 | Connexin 30 | Up (2w) | Silverstein et al. (2003b) | ||||
| Itga3 | Integrin, alpha 3 | Down | Dessapt et al. (2009) | ||||
| Itgb1 | Integrin, beta 1 | Down | Dessapt et al. (2009) | ||||
| Nphs1 | Nephrin; nephrosis 1 homolog | Down | Miceli et al. (2010) | ||||
| Scnn1a | ENaC; epithelial Na+ channel | Up* | Satlin et al. (2001) | ||||
| Slc12a3 | Thiazide-sensitive sodium-chloride cotransporter | Down (2w) | Silverstein et al. (2003a) | ||||
| Slc20a1 | Sodium-dependent phosphate transporter 1 | Down (2w) | Silverstein et al. (2003a) | ||||
| Slc21a4 | OAT-K1; kidney-specific anion transporter | Down (2w) | Silverstein et al. (2003a) | ||||
| Slc26a4 | Pendrin; sodium-independent chloride/iodide transporter | Up (7w) | Wang et al. (2009) | ||||
| Slc4a4 | NBC1; anion exchanger, member 4 | Up (7w) Down (14w) | Wang et al. (2009) | ||||
| Slc4a7 | NBCn1; sodium bicarbonate cotransporter, member 7 | Down (14w) | Wang et al. (2009) | ||||
| Slc9a1 | NHE1; sodium/hydrogen exchanger, member 1 | Down (2w) | Manucha et al. (2007) | ||||
| Slc9a3 | NHE3; sodium/hydrogen exchanger, member 3 | Up (7w) | Down (2w) | Silverstein et al. (2003a), Wang et al. (2009) | |||
u: urine; t: tissue; p: plasma; w: weeks of obstruction; d: days of obstruction; PUUO: partial UUO; CUUO: complete UUO; *activity; Ref.: references.
Uremia, hydromineral and acido-basic status
Plasma urea levels are identical in neonatal UUO and control rats (Shi et al. 2004a), indicating that the UUO-induced filtration defect is either not severe enough to induce hyperuremia or counterbalanced by a defect in urea reabsorption. Moreover, in spite of the reduced ability to concentrate urine, animals with neonatal UUO have normal natremia and osmolality (Shi et al. 2004a,b; Topcu et al. 2007) and are probably also not dehydrated because arterial blood pressure is either unchanged (el-Dahr et al. 1991; Fern et al. 1999; Eskild-Jensen et al. 2007a) or increased (Topcu et al. 2007), but not decreased.
Partial UUO induces a transient increase in the expression of transporters involved in the acid/base balance such as NHE3, NBC1, Pendrin and Na,K-ATPase after 7 weeks of obstruction, as a compensatory mechanism to maintain systemic acid-base balance (Table 1) (Wang et al. 2009). However, at later stages, this compensatory mechanism is failing and the expression of the transporters is dramatically decreased. Interestingly in these animals, the acid–base equilibrium is not modified as no changes in blood pH, bicarbonates and CO2 pressure were observed (Wang et al. 2009). The consequences of the transporter down-regulation are observed only when animals are subjected to acid loading. During acid challenge, UUO rats fail to regulate acid excretion and bicarbonate regeneration and develop metabolic acidosis whereas control rats maintain normal blood pH (Wang et al. 2009).
Finally, it is interesting to point out that other proteins or transporters involved in the hydromineral balance such as the sodium dependent phosphate transporter, the thiazide sensitive sodium chloride transporter, the kidney specific anion transporter OAT-K1 and calbindin D28, implicated in calcium transport regulation, are also down-regulated during experimental neonatal UUO (Table 1) (Silverstein et al. 2003a).
Renal recovery after UPJ obstruction release
Although it has been observed that even delayed pyeloplasty leads to recovery of short term renal function (Chertin et al. 2006), what is the consequence of the UPJ-induced renal lesions later in life? One recent study reports that surgically corrected UPJ obstruction leads to improved renal function compared to preoperative function in patients followed just after puberty (Chertin et al. 2009). Unfortunately in this study the control group without pyeloplasty is missing. Moreover, since long-term studies in humans are not available, the consequence of UPJ on adult renal function is not known.
Once again, the use of experimental animal models has shed light on the renal consequences of UUO after surgical release. Studies in neonatal rodents have shown that after 5 days of complete UUO and although primary stimulus for injury was removed, structural lesions persist 1 month after release: (i) glomerular number was still low (Chevalier et al. 1999a), indicating that the loss of nephrons is definitive; (ii) renin expression was not confined to the juxtaglomerular apparatus thus conserving its immature expression pattern (Chevalier et al. 1999a); (iii) tubular deterioration was attenuated, but not reversed, since tubular atrophy with significant apoptotic lesions was still observed (Chevalier et al. 1999a,b;); (iv) fibrotic lesions remained; and (v) cytokine/growth factor expression did not returned to basal levels (Chevalier et al. 1999a,b;). An exception is in neonatal mice, where the process of glomerulotubular disconnection is arrested by release of obstruction (Thornhill et al. 2007).
While most of the initial UUO lesions did not disappear 1 month after release of the obstruction, glomerular function was strongly improved with a normalized GFR (Chevalier et al. 1999a). However, tubular function was still perturbed. Indeed, urine flow rate and sodium excretion were increased in the post-obstructed kidney with normal GFR, indicating that reduced reabsorption of water and sodium persisted (Chevalier et al. 1999a). This suggests that modified renal transporter expression is not corrected after the release. Surprisingly, although glomerular function 1 month after release was improved, this amelioration was only transient as both GFR and tubular reabsorption were profoundly impaired in the postobstructed kidney 3 months or 1 year after release (Chevalier et al. 2000b, 2002). The contralateral kidney maintained a stable GFR, but hypertrophy, glomerulosclerosis, tubular atrophy, inflammation and interstitial fibrosis were observed. In addition the contralateral kidney produced proteinuric urine (Chevalier et al. 2000b). Thus there was progressive loss of renal function due to glomerular and tubulointerstitial damage at the level of remnant nephrons (Chevalier et al. 2000b). Partial UUO led to a less severe phenotype since 6 months after 1 week-partial UUO, neither GFR nor sodium and solute-free water reabsorption are decreased in the postobstructed kidney (Shi et al. 2004b).
As seen above for ‘the earlier the obstruction the worse the outcome’, also ‘the earlier the release the better the outcome’ holds. Experiments where partial or complete UUO were induced during nephrogenesis and released at different time-points showed that early release of neonatal obstruction provided a better protection of renal structure and function than late release (Chevalier et al. 1999b; Shi et al. 2004b). Moreover, when renal outcome was analysed 1 month after temporary complete UUO performed either during nephrogenesis or during maturation in neonatal rats, it appears that maturing kidneys are more sensitive to obstructive injury immediately following the completion of nephrogenesis than during ongoing nephrogenesis (Chevalier et al. 2002).
Overall, in humans the follow-up on UPJ obstruction patients, either surgically corrected or spontaneously resolving UPJ obstruction shows that renal function is unaffected upto at least shortly after puberty in the majority of the UPJ obstruction population. However the gross and fine renal histology in the human UPJ obstruction kidney and animal experiments suggest that renal lesions are present at the moment of severe obstruction that potentially have important effects on adult renal function as it has been shown in animals (Chevalier et al. 2000b). Whether these animal data are also valid in human UPJ obstruction remains to be studied.
Hydrodynamic modifications as potentially primary inducer of lesions
As tubular cells are first in line during the early phases of neonatal UUO, what are the different types of stress encountered by these cells? The literature points out to two potential candidates that can be involved in the primary induction of the renal lesions: tubular ischemia resulting from renal hypoperfusion and pressure-induced compression or stretching of tubular cells. However, these hypotheses mainly originate from knowledge obtained from the adult UUO model. In this section, we will discuss whether these hypotheses are still valid in neonatal UUO and we propose a new candidate-process involved in the early phases of UUO-induced lesions.
Tubular ischemia induced by renal hypoperfusion
The first candidate, often proposed to be an early inducer of renal lesions following obstruction, is tubular ischemia. In adult animals, UUO induces a rapid increase in renal blood flow (RBF) caused by local prostaglandin E2/NO generation, which decreases the resistance of afferent arterioles. However, this increase is transient as only 2 h after UUO RBF declines due to activation of the renin–angiotensin system (RAS) and/or vasoconstrictor thromboxane. This decreased RBF is associated with a prompt reduction in medullar tissue oxygen tension (Wilson 1980; Nguyen & Kogan 1998; Le Normand et al. 2005; Quinlan et al. 2008; Jensen et al. 2009).
In contrast, UUO in the foetal lamb leads to a delayed (1 week after UUO) and less dramatic fall in RBF than in adult UUO (Nguyen & Kogan 1998). Thus ischemia and related insults like hypoxia, nutrient depletion and waste accumulation are indeed potentially involved in the pathophysiology of both adult and foetal/neonatal obstruction. However, due to differences in timing, it seems less relevant to consider ischemia as a primary and early inducer of renal lesions in foetal than in adult UUO.
Tubular compression and stretch
In adult animals, UUO induces profound and rapid elevation of intrapelvic pressure during the first hours following UUO, which is immediately transmitted to renal tubules. Indeed, micropuncture experiments indicate that during the first 2 h following complete UUO, hydrostatic pressure is increased in the proximal tubule (Gottschalk & Mylle 1956; Dal Canton et al. 1977). Elevated intra-tubular pressure may have two consequences on the tubular cell. Either the tubular cell is exposed to compression, due to increased transmural pressure or renal tubule is subjected to pressure-induced deformation, leading to increased stretch of the cells (Quinlan et al. 2008). This increased pressure is only transient. Indeed, the combination of pelvic dilatation, reduction of RBF, reduction of glomerular filtration and continuous urine drainage by lymphatic and venous circulation, allows pelvic pressure to rapidly decline until it reaches baseline levels (Wilson 1980; Nguyen & Kogan 1998; Quinlan et al. 2008). This is accompanied by normalisation of intra-tubular pressure (Gottschalk & Mylle 1956; Dal Canton et al. 1979; Wilson 1980). Thus, although transitory, it is often considered that stretch and compression of the tubular cells are primary inducer of UUO-induced tubular lesions in adults.
Complete UUO in foetal lamb also induces elevated pressure as measured in the ureteral segment above the ligature. Interestingly, this increased pressure is not transient like in adults, but persists even 10 days after obstruction (Nguyen & Kogan 1998). This suggests that compression of tubular cells and stretch may also be considered as good candidates for primary inducers of UUO-induced lesions. Different tubular segments potentially respond differently to these mechanical stimuli. The low compliance of the proximal tubule (Cortell et al. 1973) and the lack of tubular proximal dilatation in the newborn mouse after chronic complete UUO (Cachat et al. 2003) suggest that proximal cells are resistant to stretch. In contrast, distal tubules and collecting ducts have shown to be highly compliant (Cortell et al. 1973) and dilate without marked cellular proliferation after foetal/neonatal UUO (Liapis et al. 2000; Cachat et al. 2003) resulting potentially in tubular stretching. Thus, increased transmural pressure and mechanical stretch may be considered as primary inducers of lesions in foetal/neonatal model of UUO for proximal and distal parts of the renal tubule, respectively.
Modification of urinary shear stress
As discussed above, modifications of intra-tubular hydrodynamic forces induce compression and stretch of epithelial cells. However, it has been recently suggested that another candidate can lead to renal lesion following urinary flow modifications (Essig et al. 2001; Rohatgi & Flores 2010). Indeed, renal tubules are continually subjected to fluid shear stress corresponding to the frictional forces exerted by flowing urine on the tubular wall. The intensity of shear stress depends on fluid viscosity, fluid flow rate (i.e. glomerular filtration rate) and tubular diameter (i.e. level of tubular dilatation).
Little is known about early changes of GFR during foetal and neonatal obstruction. Indeed, although it is known that neonatal or foetal chronic UUO induces GFR fall (Josephson 1983; Eskild-Jensen et al. 2000, 2001; Shi et al. 2004a,b; Thornhill et al. 2005; Topcu et al. 2007), variations of GFR during the first phases of the pathology were not studied. However in adults, GFR is shortly maintained in the first hours of obstruction, most probably since the increase in intra-tubular pressure is compensated by increased RBF and glomerular capillary pressure (Dal Canton et al. 1977). Then, a marked decrease of GFR occurs, due to reduction of both glomerular capillary pressure and RBF induced by vasoconstriction of afferent arterioles (Gottschalk & Mylle 1956; Dal Canton et al. 1979). Altogether these results suggest that the potential reduction of GFR, as seen in adults, associated to the tubular dilatation can lead to UUO-induced modifications of urinary shear stress and that shear stress may therefore participate to pathophysiology of obstructive nephropathy.
In conclusion, hydrodynamic fluid changes can potentially lead to processes including tubular compression, stretch and urinary shear stress that contribute to the early induction and progression of obstructive nephropathy (Figure 2). These issues have been studied on appropriate in vitro models and will be discussed within the following section. In contrast, because ischaemia seems to be involved in the later phases of neonatal and foetal UUO, the in vitro effects of ischaemia on tubular cells will not be reported. For this the reader is referred to other manuscripts (Heyman et al. 2008; Tanaka & Nangaku 2010).
Figure 2.
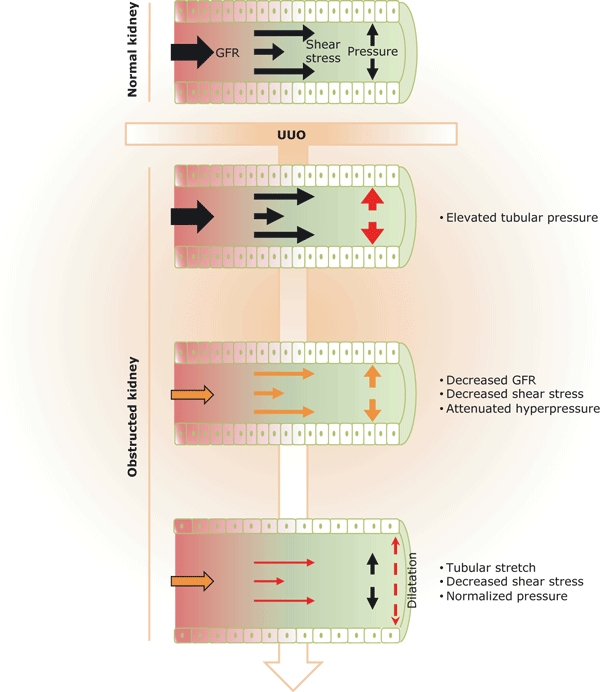
Renal tubular exposure to hydrodynamic modifications following obstruction. Following intra-pelvic urine accumulation, UUO induces rapid elevation of hydrostatic pressure into the renal tubular lumina, leading to stretch and compression of the tubular cells and thus to tubular dilatation. As GFR declines and tubular dilatation increases, hyperpressure progressively returns to basal levels. However in the mean time, fluid shear stress, which depends on both GFR and tubular diameter, is strongly reduced. Thus modifications of renal hydrodynamics can induce mechanical stimuli such as pressure, shear stress and stretch. These three factors are potential insults for tubular cells and can be involved in the primary induction of the UUO-induced renal lesions.
Molecular mechanisms: lessons from human studies and experimental models
The majority of the research efforts in human UPJ obstruction have focused on inflammatory and profibrotic responses as these were clearly identified events in UPJ obstruction (Elder et al. 1995; Zhang et al. 2000). Although data is scarce, it is confirming the general pathological mechanisms observed in renal pathology associated to renal fibrosis including inflammation, activation of the RAS, profibrotic cytokine induction, modification of tubular enzyme activity and extracellular matrix accumulation. This information has been largely complemented and confirmed by data obtained in a variety of animal models of obstruction. Moreover, the effects of intra-tubular hydrodynamic forces modifications have been studied in vitro. Some limits of these experiments exist. Indeed, pressure studies are limited and the effect of stretch has mainly been investigated on podocytes and proximal tubular cells with only a few studies describing the effects of stretch on distal tubule cells. Moreover, in all these studies, cells are not derived from foetal/neonatal animals. However, these in vitro experiments can give some clues to understand the molecular mechanisms involved in the progression of obstructive nephropathy.
Inflammation
Chronic neonatal obstructive nephropathy is associated with massive interstitial macrophage infiltration. The recruitment of inflammatory cells is mediated in part by increased secretion of chemokines by stressed tubular cells. The chemokine CCL2 (MCP-1) is a powerful chemotactic agent and is involved in monocyte activation (Leonard & Yoshimura 1990). Patients with UPJ obstruction had fourfold higher CCL2 concentrations in their urine than healthy controls. This was correlated with a significant increase in MCP-1 expression on renal biopsies as shown by in situ hybridization (Table 2) (Grandaliano et al. 2000). Four months after pyeloplasty, urinary MCP-1 concentrations decreased close to levels of control individuals. In experimental models, complete neonatal obstruction also induced up-regulation of CCL2 together with other chemokines including CCL3 (MIP-1α), CCL4 (MIP-1β) and CCL5 (RANTES) (Table 2) (Silverstein et al. 2003a; Lange-Sperandio et al. 2007). Macrophage infiltration is also induced by up-regulation of adhesion molecules on endothelial and inflammatory cells. It has been shown that neonatal UUO in mice induced renal expression of intercellular adhesion molecule-1 (ICAM-1), receptor for advanced glycation end-products (RAGE) and junction adhesion molecule-C (JAM-C) adhesion molecules (Table 2) (Lange-Sperandio et al. 2006). Moreover, Mac-1 (αMβ2, CD11b/CD18) β2 integrin knockout, LFA-1 (αLβ2, CD11a/CD18) β2 integrin knockout, L-selectin knockout or L-, P-, and E-selectin triple knockout mice are protected from inflammation induced by neonatal UUO (Table 2) (Lange-Sperandio et al. 2002, 2006). To our knowledge, no data are available on adhesion molecules during UPJ in children. Finally, obstruction is also characterized by induced expression of transcription factors known to be involved in inflammation, such as interferon regulatory factor-1 (IRF-1) and nuclear factor-κB (NF-κB) (Table 2) (Silverstein et al. 2003a; Topcu et al. 2007) and of members of the pro-inflammatory tumour necrosis factor superfamily. In children, expression of the cytokine tumour necrosis factor-α (TNFα) is increased in urine during UPJ obstruction (Valles et al. 2003) whereas in neonatal rats, mRNA expression of Fas and Fas ligand is induced in the obstructed kidney compared to the sham (Silverstein et al. 2003a) (Table 2).
Table 2.
Literature data about inflammation-related genes and proteins that have been altered during obstructive nephropathy. We have reported here only molecules of which expression has been significantly modified compared to healthy control (human), sham-animals (animal model) or control cells (in vitro)
| Animal model | In vitro | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene symbol | Gene name | Human | Ref. | PUUO | CUUO | Ref. | Stretch | Ref. |
| Ager | RAGE; receptor for advanced glycosylation end products | Up (1d) | Lange-Sperandioet al. (2006) | |||||
| Ccl2 | Chemokine (C-C motif) ligand 2; MCP-1 | Up (u, t) | Grandaliano et al. (2000) | Up (2w) | Silversteinet al. (2003a) | |||
| Ccl3 | Chemokine (C-C motif) ligand 3; MIP-1α | Up (5d) | Lange-Sperandioet al. (2007) | |||||
| Ccl4 | Chemokine (C-C motif) ligand 4; MIP-1β | Up (5d) | Lange-Sperandioet al. (2007) | |||||
| Ccl5 | Chemokine (C-C motif) ligand 5; RANTES | Up (5d) | Lange-Sperandioet al. (2007) | |||||
| Cd14 | Monocyte differentiation antigen CD14 | Up (2w) | Silversteinet al. (2003a) | |||||
| Fas | TNF receptor superfamily, member 6 | Up (2w) | Silversteinet al. (2003a) | |||||
| Faslg | Fas ligand | Up (2w) | Silversteinet al. (2003a) | |||||
| Icam1 | Intercellular adhesion molecule 1 | Up (5d) | Lange-Sperandioet al. (2006) | Up | Hu et al. (2008) | |||
| IL8 | Interleukin 8; CXCL8 | Up | Vlahakis et al. (1999), Yamamoto et al. (2001), Oudin and Pugin (2002) | |||||
| Irf1 | Interferon regulatory factor 1 | Up (2w) | Silversteinet al. (2003a) | |||||
| Jam3 | JAM-C; junction adhesion molecule 3 | Up(5d, 2w) | Lange-Sperandioet al. (2006) | |||||
| Nfkb1 | NF-κB | Up (10w) | Topcu et al. (2007) | |||||
| Tnf | Tumor necrosis factor; TNF-α | Up (u) | Valles et al. (2003) | Up | Dixon et al. (2008) | |||
u: urine; t: tissue; p: plasma; w: weeks of obstruction; d: days of obstruction; PUUO: partial UUO; CUUO: complete UUO; Ref.: references.
Effects of mechanical stretch on inflammatory mediators have been studied in vitro, mainly in pulmonary epithelial cells but not in tubular epithelial cells. Nevertheless, the fact that stretch modifies adhesion molecules expression, such as ICAM-1 (Hu et al. 2008) and cytokine production, such as TNFα (Dixon et al. 2008) and interleukin-8 (Vlahakis et al. 1999; Yamamoto et al. 2001; Oudin & Pugin 2002) (Table 2) in other epithelial cell types suggests that it may also trigger inflammatory signalling in renal tubules and thus participate to progression of obstructive nephropathy.
Fibrosis
In almost all form of renal diseases, chronic inflammation is associated with the development of tubulointerstitial lesions of fibrosis. Fibrosis is defined by increased extracellular matrix (ECM) accumulation, due to both enhanced synthesis and decreased degradation of matrix proteins such as collagens and fibronectin. Myofibroblasts are the major extracellular matrix (ECM) producing cells. They play an important role in the fibrotic process and their renal accumulation in UPJ obstruction was evidenced by increased interstitial α-smooth muscle actin and vimentin staining (Murer et al. 2006). In the kidney, myofibroblasts are supposed classically to arise from the proliferation and activation of resident fibroblasts, but also from a process known as epithelial to mesenchymal transition (EMT). During EMT, tubular epithelial cells loose progressively their epithelial phenotype, detach from the surrounding cells, digest the basal membrane and migrate into the interstitium where they acquire mesenchymal properties (Liu 2010). Well-described in vitro, the existence of EMT in vivo and its real participation to the fibrotic process is still under debate. However, some characteristic features of EMT have been found during experimental UUO (Table 3): increased expression of essential signalling proteins such as Snail1, Snail2/Slug and beta-catenin (Lange-Sperandio et al. 2007); decreased expression of E-Cadherin, an epithelial protein involved in cell-cell adhesion (Lange-Sperandio et al. 2007); increased expression of MMP2 (matrix metalloprotease 2) and MMP9 (matrix metalloprotease 9), two enzymes involved in the degradation of the tubular basal membrane (Mure et al. 2006a); and increased expression of mesenchymal markers such as desmin, calponin, vimentin and α-smooth muscle actin (Silverstein et al. 2003a; Lange-Sperandio et al. 2007). Some markers have also been observed in renal tubular cells submitted to mechanical stretch (Sato et al. 2003).
Table 3.
Literature data about fibrosis-related genes and proteins that have been altered during obstructive nephropathy. We have reported here only molecules of which expression has been significantly modified compared to healthy control (human), sham-animals (animal model) or control cells (in vitro)
| Animal model | In vitro | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene symbol | Gene name | Human | Ref. | CUUO | Ref. | Stretch | Shear stress | Ref. |
| Acta2 | α-SMA; actin, alpha, vascularsmooth muscle | Up (t) | Murer et al. (2006) | Up (5d) | Lange-Sperandio et al. (2007) | |||
| Cdh1 | Cadherin 1; E-cadherin | Down (5d) | Lange-Sperandio et al. (2007) | |||||
| Cnn1 | Calponin 1 | Up (2w) | Silverstein et al. (2003a) | |||||
| Col5a2 | Collagen, type V, alpha 2 (fragments) | Up (u) | Decramer et al. (2006) | |||||
| Col9a3 | Collagen, type IX, alpha 3 (fragments) | Up (u) | Decramer et al. (2006) | |||||
| Ctnnb1 | β-Catenin; catenin (cadherinassociated protein), beta 1 | Up (5d) | Lange-Sperandio et al. (2007) | |||||
| Dcn | Decorin | Up (2w) | Silverstein et al. (2003a) | |||||
| Des | Desmin | Up (2w) | Silverstein et al. (2003a) | |||||
| Egr1 | Early growth response 1; Krox24 | Up (2w) | Silverstein et al. (2003a) | |||||
| Mmp1 | Matrix metalloproteinase 1 | Up (6w) | Mure et al. (2006a) | |||||
| Mmp2 | Matrix metallopeptidase 2 | Up (6w) | Mure et al. (2006a) | |||||
| Mmp9 | Matrix metallopeptidase 9 | Up (6w) | Mure et al. (2006a) | |||||
| Pdgfa | PDGF-A | Up (10d, 20d) | Liapis et al. (2000) | |||||
| Plat | tPA; plasminogen activator, tissue | Up | Essig et al. (2001) | |||||
| Plau | uPA; plasminogen activator, urokinase | Up | Essig et al. (2001) | |||||
| Snai1 | Snail1; snail homolog 1(Drosophila) | Up (5d) | Lange-Sperandio et al. (2007) | |||||
| Snai2 | Snail2; Slug; snail Homolog 2(Drosophila) | Up (5d) | Lange-Sperandio et al. (2007) | |||||
| Tgfb1 | TGFβ; transforminggrowth factor, beta 1 | Up(u, t) | Furness et al. (1999), El-Sherbiny et al. (2002), Murer et al. (2006), Yang et al. (2006), Taha et al. (2007a) | Up (5d to 4w) | Chung and Chevalier (1996), Yang et al. (2001), Silverstein et al. (2003a), Lange-Sperandio et al. (2007) | Up | Miyajima et al. (2000a,b);, Dessapt et al. (2009) | |
| Tgfbr1 | TGFβRI; transforminggrowth factor, beta receptor I | Up (10d) | Yang et al. (2001) | Up | Miyajima et al. (2000a,b);, Durvasula et al. (2004), Dessapt et al. (2009), | |||
| Tgfbr2 | TGFβRII; transforminggrowth factor, beta receptor II | Up (10d) | Yang et al. (2001) | Up | Miyajima et al. (2000a,b);, Durvasula et al. (2004), Dessapt et al. (2009) | |||
| Timp1 | Tissue inhibitor of metallopeptidase 1 | Up (6w) | Mure et al. (2006a) | |||||
| Timp2 | Tissue inhibitor of metalloproteinase 2 | Up (6w) | Mure et al. (2006a) | |||||
| Vim | Vimentin | Up (t) | Murer et al. (2006) | Up (5d) | Lange-Sperandio et al. (2007) | |||
u: urine; t: tissue; p: plasma; w: weeks of obstruction; d: days of obstruction; CUUO: complete UUO; Ref.: references.
Renal collagen accumulation, as a consequence of myofibroblast appearance, has been frequently observed during human UPJ obstruction, but only using classical histology (H&E and Masson-trichrome) therefore lacking the identification of the specific ECM components (Elder et al. 1995; Zhang et al. 2000). We have observed using urinary proteome analysis increased secretion of collagen Vα and IXα3 fragments (Decramer et al. 2006) in UPJ obstruction patients but the origin, renal or urinary tract, of these fragments is currently unknown. Collagen accumulation is also described during experimental UUO (Chevalier et al. 2009) and experiments of UUO in the foetal lamb have shown increased expression of tissue inhibitors of metalloproteases (TIMP-1 and TIMP-2) (Table 3), which inhibit ECM degradation (Mure et al. 2006a). Interestingly, exposing cultured proximal cells to shear stress modifies both expression and activity of tPA (tissue type-plasminogen activator) and uPA (urokinase), two proteases strongly involved in ECM remodelling suggesting that modification of shear stress during obstruction could be involved in the development of fibrosis (Table 3) (Essig et al. 2001).
Renal fibrosis is regulated by different cytokines and growth factor. Among them, transforming growth factor beta (TGFβ) is the most potent pro-fibrogenic cytokine involved in renal diseases and is clearly associated to human and experimental obstruction. A number of studies showed that urinary TGFβ levels are increased in human UPJ obstruction (Table 3) (Furness et al. 1999; El-Sherbiny et al. 2002; Taha et al. 2007a). In addition, urinary TGFβ concentrations from UPJ obstruction patients were 2–4-fold higher in the pelvis than in the bladder (Furness et al. 1999; El-Sherbiny et al. 2002), suggesting that the urinary TGFβ found in the bladder is mainly coming from the obstructed kidney. This is further corroborated in renal biopsy studies where increased TGFβ expression was observed in UPJ obstruction (Murer et al. 2006; Yang et al. 2006). A decline of TGFβ urinary levels is detected after surgery, but the fact that TGFβ urinary levels decrease more slowly that urinary MCP-1 concentrations (Taha et al. 2007b) suggests that the inflammatory response resolves more rapidly than the fibrotic response. In experimental UUO, both TGFβ and TGFβ receptors (TGFβRI and TGFβRII, Table 3) are up-regulated in the obstructed kidney compared to sham (Chung & Chevalier 1996; Yang et al. 2001; Silverstein et al. 2003a; Lange-Sperandio et al. 2007). Decorin is an endogenous inhibitor of TGFβ expression and activity (Mogyorosi & Ziyadeh 1999; Wu et al. 2007). It is assumed that increased expression of decorin associated with increased TGFβ expression may provide a regulatory mechanism to limit the TGFβ induced injury (Diamond et al. 1997; Mogyorosi & Ziyadeh 1999). Very interestingly, it has been shown that during complete UUO in neonatal rats, the up-regulation of TGFβ mRNA parallels decorin expression (Table 3) (Silverstein et al. 2003a) and even after release of UUO, as in humans, overexpression of TGFβ-1 persists (Chevalier et al. 1999a). In vitro experiments on podocytes or proximal cells indicate that under certain conditions, mechanical stretch stimulates TGFβ signalling with increased TGFβ and TGFβ receptors expression and TGFβ secretion (Table 3) (Miyajima et al. 2000a,b; Durvasula et al. 2004; Dessapt et al. 2009). Moreover in non-renal cells, decorin mRNA expression is modified by mechanical stretch suggesting that it may also be the case in renal tubular cells (Table 3) (Ludwig et al. 2004; Ozaki et al. 2005). Altogether, these data suggest that TGFβ is a major mediator of renal injury (i.e. fibrosis) during UUO.
Nitric oxide and oxidative stress
Nitric oxide produced by the various forms of nitric oxide (NO) synthase (NOS) has been shown to be involved in the evolution of experimental obstructive nephropathy. Increased NOS activity was also observed in humans with UPJ obstruction originating from both endothelial NOS (eNOS) and inducible NOS (iNOS) (Table 4) (Miyajima et al. 2000b; Valles et al. 2003, 2007). NOS activity is involved in a number of important processes in the kidney including glomerular haemodynamics and sodium and water excretion (Kone & Baylis 1997) that are strongly modified in the different phases of UPJ obstruction (see above). Modification of NOS activity has also been studied during experimental UUO in neonates (Table 4). Very interestingly, NO expression differs depending on the severity (i.e. complete vs. partial) and on the duration of the obstruction. For example, increased renal NO was associated with iNOS up-regulation 1 week after complete UUO in the neonatal rat (Manucha & Valles 2008), whereas both the NO level and eNOS and iNOS expression were reduced at a later stage (2 weeks) (Silverstein et al. 2003a; Manucha & Valles 2008). Decreased NO levels were associated with decreased anti-apoptotic Bcl2 expression and increased pro-apoptotic caspase 3 activity leading to the conclusion that NO is protective during complete neonatal UUO. In mice, while the protective and anti-apoptotic role of iNOS activity has been also demonstrated during complete obstruction (Yoo et al. 2010), it has been shown that NO has opposite effects during partial obstruction and seems to aggravate renal morphological alterations. After 1 week, partially obstructed iNOS knockout mice showed reduced renal pelvic dilatation and preserved renal papilla compared to wild-type mice although they displayed similar tubular apoptosis (Yoo et al. 2010). This discrepancy between complete or partial obstruction could be explained by the differential action of iNOS and NO on renal parenchyma (anti-apoptotic) and on the ureteropelvic junction (inhibition of smooth muscle contraction). While inhibition on ureteral contractile activity in complete UUO is without effect on urine retention, it can modify the response to partial UUO. This is exemplified by the fact that, in partial neonatal obstruction, iNOS knockout mice showed less hydronephrosis compared to wild-type mice because pelvic urine drainage is increased (Yoo et al. 2010).
Table 4.
Literature data about NO and oxidative stress-related genes and proteins that have been altered during obstructive nephropathy. We have reported here only molecules of which activity or expression has been significantly modified compared to healthy control (human), sham-animals (animal model) or control cells (in vitro).
| Animal model | In vitro | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene symbol | Gene name | Human | Ref. | CUUO | Ref. | Stretch | Shear stress | Pressure | Ref. |
| Cat | Catalase | Down | Ricardo et al. (1997) | ||||||
| Nos2 | iNOS; nitric oxide synthase 2, inducible | Up* (t) | Valleset al. (2003) | Up (5d) Down (2w) | Manucha andValles (2008) | Up*/ =* | Up* | Miyajima et al. (2000a), Hegarty et al. (2002), Broadbelt et al. (2007) | |
| Nos3 | eNOS; nitric oxide synthase, inducible | Up* (t) | Valleset al. (2003), Valleset al. (2007) | Down (2w) | Silversteinet al. (2003a) | ||||
| Nox1 | NADPH oxidase | Down* (5d) Up* (2w) | Manucha andValles (2008) | ||||||
| Sod1 | SOD; superoxide dismutase | Down* (2w) | Manucha andValles (2008) | ||||||
u: urine; t: tissue; p: plasma; w: weeks of obstruction; d: days of obstruction; CUUO: complete UUO; *activity; Ref.: references.
In vitro experiments indicate that renal NO pathway is controlled by mechanical forces. Indeed, increased luminal flow stimulates NO release and induces eNOS activation and translocation to apical membrane in the rat microperfused ascending limb (Ortiz et al. 2004). However, in order to determinate if this effect is caused by stretch, transmural pressure or shear stress modifications, experiments have been carried out studying individual change of each parameter. First, in vitro application of elevated hydrostatic pressure (Broadbelt et al. 2007) or stretch (Miyajima et al. 2000b; Hegarty et al. 2002) to proximal cells increases iNOS expression and NO production (Table 4). Interestingly, the effect of stretch seems to be species-dependent as the NO increase is induced in rat but not in human cells (Miyajima et al. 2000b; Hegarty et al. 2002). Moreover, NO donors attenuate both stretch-induced apoptosis and proliferation inhibition whereas NO inhibitors amplify them. These in vitro results confirm that NO exerts a protective effect for the severely obstructed stretched kidney (Miyajima et al. 2001; Hegarty et al. 2002). Second, increased shear stress has been shown to increase NO production in medullary collecting duct cells (Cai et al. 2000). This latter result is more difficult to directly transpose to the in vivo UUO situation where the shear stress is not increased but decreased due to GFR decline and tubular dilatation (see above). However it suggests that tubular NO production could be also sensitive to urinary shear stress variations.
Another consequence of chronic UUO in newborns is the increase of oxidative stress. In vitro, cellular stretch increases superoxide production and down-regulates catalase mRNA levels (Table 4) (Ricardo et al. 1997; Garvin & Hong 2008). In vivo, 2 weeks of complete UUO in neonatal rats induced NADPH oxidase activity and decreased superoxide dismutase activity (Table 4) (Manucha & Valles 2008). The role of NADPH oxidase-mediated ROS generation has been extensively studied in vivo and in vitro. Studies have shown that it can promote apoptosis or proliferation of renal cells, such as mesangial cells, podocytes and tubular cells (Jiang 2009). Moreover, NADPH oxidase mediates proximal tubular cell death induced by angiotensin II (Ang II) (Jiang 2009). Consistently with these results up-regulation of NADPH oxidase activity paralleled the increase of apoptosis during experimental UUO supporting the deleterious role of oxidative stress during neonatal obstructive nephropathy (Manucha & Valles 2008).
Apoptosis and proliferation
During obstruction, tubular stretch, inflammation and oxidative stress induce a severe apoptotic response of both tubular and interstitial cells. Indeed urinary tubular enzymes potentially released by those cells including N-acetyl-β-d-glucosaminidase (Carr et al. 1994), alkaline phosphatase and γ-glutamyl transferase (Shokeir & Taha 2009) have been found increased in human UPJ obstruction (Table 5). The role of other intracellular mediators of apoptosis or cell survival in obstruction has been investigated in animal models. In rats, mice and opossums complete and partial UUO stimulated expression and activity of pro-apoptotic molecules such as Bad, Caspase 3, Fas, Fas ligand and p53, together with down-regulation of anti-apoptotic Bcl2 and heat shock protein 70 (HSP70) (Table 5) (Steinhardt et al. 1995; Kiley et al. 2003; Silverstein et al. 2003a; Manucha et al. 2007; Guerin et al. 2008; Manucha & Valles 2008). Ceramide, an endogenous lipid also considered as an intracellular mediator of cell death (Saba et al. 1996) was induced during neonatal UUO and paralleled apoptosis (Malik et al. 2001). Moreover, clusterin, which is a small heat shock protein-like involved in pro-apoptotic mechanisms induced by oxidative stress (Chevalier et al. 1996, 1999a; Silverstein et al. 2003a; Trougakos & Gonos 2006), is induced during experimental UUO (Table 5). Interestingly, clusterin over-expression persists even after the release of obstruction (Chevalier et al. 1999a). Some other molecules that are modified during UUO have been described to be involved in the regulation of apoptosis (Table 5): in addition to its inhibitory effect on TGFβ activity, decorin has been found to induce apoptosis on mesangial cells in vitro (Wu et al. 2008); heat shock protein 27 (HSP27) is a crucial chaperone protein activated in response of renal epithelial cellular stress to limit apoptosis (de Graauw et al. 2005); JunD, one of the components of the AP-1 transcription factor complex has been shown to promote cell survival in epithelial renal cells in vitro (Silverstein et al. 2003a; Lu et al. 2009); the sodium/proton exchanger-1 (NHE1) is involved in renal sodium transport but is also a caspase substrate and its proteolytic cleavage promotes progression towards apoptosis (Manucha et al. 2007; Schelling & Abu Jawdeh 2008).
Table 5.
Literature data about apoptosis and proliferation-related genes and proteins that have been altered during obstructive nephropathy. We have reported here only molecules of which activity or expression has been significantly modified compared to healthy control (human), sham-animals (animal model) or control cells (in vitro)
| Animal model | In vitro | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene symbol | Gene name | Human | Ref. | PUUO | CUUO | Ref. | Stretch | Ref. |
| Alpl | Alkaline phosphatase | Up* (u) | Shokeir and Taha (2009) | |||||
| Anxa5 | Annexin V | Up | Dessapt et al. (2009) | |||||
| Bad | BCL2-associated agonist of cell death | Up* (7d) | Kiley et al. (2003) | Up* | Kiley et al. (2003) | |||
| Bcl2 | B-cell CLL/lymphoma 2 | Down (2w) | Steinhardt et al. (1995), Manucha et al. (2007), Manucha and Valles (2008), | |||||
| Casp3 | Caspase 3 | Up (2w, 4w) | Up* (2w, 4w) | Manucha et al. (2007), Guerin et al. (2008), Manucha and Valles (2008) | Up* | Dessapt et al. (2009) | ||
| Clu | Clusterin | Up (2w) | Chevalier et al. (1996), Silverstein et al. (2003a) | |||||
| Cycs | Cytochrome C | Up | Kiley et al. (2003) | |||||
| Dcn | Decorin | Up (2w) | Silverstein et al. (2003a) | |||||
| Fas | TNF receptor superfamily, member 6 | Up (2w) | Silverstein et al. (2003a) | |||||
| Faslg | Fas ligand | Up (2w) | Silverstein et al. (2003a) | |||||
| Ggt1 | g-Glutamyl transferase | Up* (u) | Shokeir and Taha (2009) | |||||
| Hexa/Hexb | N-acetyl-β-D-glucosaminidase; Hexoaminidase | Up* (u) | Carr et al. (1994), Shokeir and Taha (2009) | |||||
| Hspa4 | HSP70; heat shock protein 4 | Up (5d, 2w) | Manucha and Valles (2008) | |||||
| Hspb1 | HSP27; heat shock protein 1 | Up (2w) | Silverstein et al. (2003a) | |||||
| JunD | jun D proto-oncogene | Up (2w) | Silverstein et al. (2003a) | |||||
| Nfkb1 | NF-κB | Up (10w) | Topcu et al. (2007) | |||||
| Slc9a1 | NHE1; Sodium/hydrogen exchanger, member 1 | Down (2w) | Manucha et al. (2007) | |||||
| Sparc | Secreted protein, acidic, cysteine-rich (osteonectin) | Up | Durvasula and Shankland (2005) | |||||
| Tp53 | p53; tumor protein p53 | Up (2w) | Silverstein et al. (2003a) | |||||
u: urine; t: tissue; p: plasma; w: weeks of obstruction; d: days of obstruction; PUUO: partial UUO; CUUO: complete UUO; *: activity; Ref.: references.
The apoptotic response to mechanical stretch has been extensively studied in vitro. Stretch increases apoptosis or susceptibility to apoptosis in murine, human and canine epithelial renal cells (Miyajima et al. 2000a, 2001; Nguyen et al. 2000; Hegarty et al. 2002; Cachat et al. 2003; Kiley et al. 2003, 2005; Durvasula et al. 2004; Dessapt et al. 2009). This effect is associated with elevated markers of early apoptosis such as dephosphorylated BAD, released mitochondrial cytochrome C, caspase-3 activity and annexin V (Table 5) (Kiley et al. 2003; Dessapt et al. 2009). In addition, the sodium/proton exchanger NHE is also sensitive to mechanical stress, as described in cardiomyocytes but not yet in renal cells (Yamazaki et al. 1998). Underlying mechanisms seem to be dependent on the cell type since stretch-induced apoptosis is mediated by TGFβ in rat proximal cells (Miyajima et al. 2000a) whereas it is driven by type 1 angiotensin II receptor in mouse podocytes (Durvasula et al. 2004). In addition stretch dependent apoptosis is greater in the collecting duct than in proximal tubules (Cachat et al. 2003). This result can explain why in neonatal experimental UUO, apoptosis is more severe in collecting ducts than in proximal tubules (Cachat et al. 2003). Stretch not only promotes apoptosis, it also decreases proliferation as demonstrated in cultured mouse podocytes and HK-2 or MDCK cells (Hegarty et al. 2002, 2003; Petermann et al. 2002, 2005). In mice, this anti-proliferative effect is mediated through the reduction of cyclins levels and their partner CDKs associated to increment in CDK-inhibitors expression (Petermann et al. 2002). It is also associated to increased SPARC (secreted protein acidic and rich in cystein) levels (Table 5), a protein which in vivo diminishes proliferative capacity of various tissues via inhibition of mitogenic growth factors (Durvasula & Shankland 2005).
The renin–angiotensin system and associated partners
Activation of the renin-angiotensin system (RAS) has been observed in many renal diseases. Also UPJ obstruction does not escape this fate. In mice and rats, renal expression of renin is up-regulated in neonatal models of complete UUO compared to the sham operated kidney (Table 6) (el-Dahr et al. 1991; Chevalier et al. 1996; Chung & Chevalier 1996; Yoo et al. 1997; Silverstein et al. 2003a) and this is correlated with increased angiotensin II (AngII) content in the obstructed kidney (Yoo et al. 1997). In children, plasma renin activity was observed to gradually increase with the time of UPJ obstruction and showed to precede parameters of actual renal injury including split renal function and glomerular filtration rate. In addition plasma renin activity was reduced postoperatively (Bajpai et al. 2007). We have observed decreased urinary excretion of proprotein convertase subtilisin/kexin type 1 inhibitor (proSAAS) in UPJ-children that can be linked to the observed increase in renin activity (Table 6) (Decramer et al. 2007). proSAAS can inhibit prohormone convertase-1 (PC1) activity (Basak et al. 2001) and PC1 was shown to efficiently convert prorenin into renin (Benjannet et al. 1992). We therefore speculate that the decline of proSAAS levels in UPJ-obstruction lower PC1 inhibition and thus increase processing of renin from prorenin leading to increased activation of the RAS. Another interesting finding is the effect of UUO on connexins 37 and 40 expression. Connexins 37 and 40 are two GAP junction proteins, which are expressed in the renin-secreting cells of the juxtaglomerular apparatus and control in part the tubulo-glomerular feedback (Takenaka et al. 2008; Just et al. 2009). Intra-renal infusion of blocking peptides for Connexin 37 and 40 increased plasma renin activity and AngII levels (Takenaka et al. 2008). On the another hand, it has been shown that mRNA expression of connexin 37 and 40 is increased during obstruction in neonatal rats (Table 6) (Silverstein et al. 2003b). Taken together, these results seem controversial although one can speculate that the increased renin expression during UUO can induce a positive feedback on connexin expression to limit the hyperreninemia.
Table 6.
Literature data about renin–angiotensin system (and associated)-related genes and proteins that have been altered during obstructive nephropathy. We have reported here only molecules of which activity or expression has been significantly modified compared to healthy control (human), sham-animals (animal model) or control cells (in vitro)
| Animal model | In vitro | ||||||
|---|---|---|---|---|---|---|---|
| Gene symbol | Gene name | Human | Ref. | CUUO | Ref. | Stretch | Ref. |
| Agtr1 | Angiotensin II receptor, type 1 | = (t) | Murer et al. (2006), Valles et al. (2007) | Down (1d) Up (4w) | Yoo et al. (1997) | Up | Durvasula et al. (2004),Kolb et al. (2004) |
| Agtr2 | Angiotensin II receptor, type 2 | = (t) | Murer et al. (2006), Valles et al. (2007) | Down(1d; 4w) | Yoo et al. (1997) | = | Durvasula et al. (2004) |
| Bdkrb2 | Bradykinin receptor B2 | Up (4w) | Chen et al. (2007) | ||||
| Gja4 | Connexin 37 | Up (2w) | Silverstein et al. (2003b) | ||||
| Gja5 | Connexin 40 | Up (2w) | Silverstein et al. (2003b) | ||||
| Pcsk1n | proSAAS;proprotein convertase1 inhibitor | Down(u) | Decrameret al. (2006) | ||||
| Ren | Renin | Up*(p) | Bajpaiet al. (2007) | Up(2w, 4w) | el-Dahr et al. (1991), Chevalier et al. (1996), Chung and Chevalier (1996), Yoo et al. (1997), Silverstein et al. (2003a) | Up | Ricardo et al. (2000) |
u: urine; t: tissue; p: plasma; w: weeks of obstruction; d: days of obstruction; CUUO: complete UUO; *activity; Ref.: references.
One major difference between animal models and human findings is the effect of obstruction on angiotensin receptors (Table 6). In rats with neonatal UUO, renal expression of type 1 and type 2 angiotensin receptors (AT1-R and AT2-R respectively) was decreased after 1 day of obstruction. However after 4 weeks AT1-R was overexpressed whereas AT2-R was still down-regulated (Yoo et al. 1997). In human, renal angiotensin receptor expression is not modified by severe UPJ obstruction (Murer et al. 2006; Valles et al. 2007). Nevertheless, caveolin-1, which is colocalized with the AT1-R in renal tubular cells, was induced in UPJ obstruction both in renal biopsies and urine (Table 6) (Valles et al. 2007) suggesting that, although expression is not modified, AT1-R activation is increased. Altogether, these data are in favour of activation of the RAS in UPJ obstruction.
In vitro data show that renal RAS is activated by mechanical stretch. This has been shown for podocytes or proximal tubule cells where AngII production, renin mRNA and AT1-R, but not AT2-R, expression are increased in response to stretch (Table 6) (Ricardo et al. 2000; Durvasula et al. 2004; Miceli et al. 2010). In addition, part of the stretch-induced cellular effects including apoptosis, down-regulation of nephrin (see below ‘tubular transport and glomerular function’) or stimulation of osteopontin expression (see below ‘other cytokines and growth factors’) are mediated by the AT1-R (Diamond et al. 1998; Ricardo et al. 2000; Durvasula et al. 2004; Miceli et al. 2010). Interestingly, Caveolin-1 is also a stretch sensitive protein, as demonstrated in non-renal murine cells, where translocation of caveolin-1 from caveolar to non-caveolar sites within the plasma membrane or from the plasma membrane to cytoplasm can be observed in response to stretch (Kawabe et al. 2004; Wang et al. 2010). As stretch, shear stress alters the RAS. Indeed, application of fluid shear stress on proximal cells results in relocation of AT1-R out of apical recycling endosomes into the apical surface membrane (Kolb et al. 2004). Thus, tubular mechanical forces can account for modifications of the RAS status in the obstructed kidney.
In adults, blocking the RAS is a well-admitted target to block the progression of renal diseases. AngII is involved in many, if not all, pathological mechanisms of renal fibrosis. It participates in the inflammatory process by stimulating expression of adhesion molecules such as VCAM-1 and ICAM-1, expression of chemokines such as CCL2 and CCL5 (Mezzano et al. 2001; Ruster & Wolf 2006; Wynn 2008). It is involved in oxidative stress by stimulating NADPH oxidase activity and the production of reactive oxygen species (Mezzano et al. 2001; Ruster & Wolf 2006; Wynn 2008). It is also involved in EMT and fibroblast activation (Mezzano et al. 2001; Ruster & Wolf 2006; Wynn 2008). Finally, AngII has been shown to induce collagen synthesis and TIMP-1 expression (Mezzano et al. 2001; Ruster & Wolf 2006; Wynn 2008). Most of the effects of AngII are mediated by the modulation of cytokine and growth factor expression such as TGFα (Transforming Growth Factor-α) and TGFβ (Mezzano et al. 2001; Ruster & Wolf 2006; Wynn 2008). It is interesting to point out that most of these mechanisms and molecules are also induced in the neonatal model of UUO. However, targeting the RAS in infants is no longer considered to be a valuable therapeutic strategy. Blocking the RAS during renal development in human or rodents induces severe damage to the kidney and worsens the renal lesions induced by obstruction. Administration of angiotensin converting enzyme (ACE) inhibitors or AT1-R antagonists to pregnant women lead to severe renal malformations in the foetus (Sekine et al. 2009). Moreover, a polymorphism into AT2-R gene involved in efficient splicing of the mRNA has been shown to be associated with UPJ in two human cohorts (Nishimura et al. 1999). In mouse, AT1-R, AT2-R and angiotensinogen gene knockout led to renal malformations (Sekine et al. 2009). In piglets with partial UUO, AT1-R blockade by candesartan prevents interstitial and glomerular apoptosis but neither fibrosis nor tubular dysfunction (Eskild-Jensen et al. 2007a,b;). In rats, losartan, another AT1-R antagonist, aggravates lesions of partial UUO when administered during the first 10 days of life, which corresponds to the period of nephrogenesis. However, losartan was without effect when administered 10 days after birth, which corresponds to the renal maturation period (Coleman et al. 2007). Inhibition of AT2-R was without effect at any time (Coleman et al. 2007). Other studies have shown that enalapril, an ACE inhibitor, induced functional and histological renal alterations when administered during nephrogenesis (Guron et al. 1999), but did not exert additional deleterious effect in partial UUO (Chen et al. 2007). Conversely, administration during the maturation period had no effect in control rats (Guron et al. 1999) but worsened renal lesions induced by partial UUO (Chen et al. 2007). These results seem controversial. However it is important to keep in mind that ACE inhibition or AT1-R blockade is not equivalent. ACE activity not only generates AngII but also other AngII related peptides such as Ang1-7, which exerts its biological effect through AT1-R independent mechanisms (Ruster & Wolf 2006). Moreover AngII can be generated by other serine proteases than ACE, such as chymase, which is not affected by ACE inhibition (Ruster & Wolf 2006).
In conclusion, the RAS is induced during obstruction and seems to be related to most of the deleterious mechanisms involved in this pathology. However, the role of RAS during kidney development is also crucial and cannot be targeted easily. One alternative could be to target the RAS-associated kinin-kallikrein system (KKS). KKS is composed by two receptors, the B1 and the B2 receptors and by their respective ligands, des-arg9bradykinin and bradykinin (Leeb-Lundberg et al. 2005). KKS is linked to the RAS through the ACE since ACE is not only involved into the conversion of AngI in AngII but also in the degradation of bradykinin (Leeb-Lundberg et al. 2005).
The B2 receptor has been shown to exert a renal protective anti-fibrotic effect in different adult rodents models by promoting extracellular matrix degradation (Schanstra et al. 2002; Okada et al. 2004; Seccia et al. 2006) and its expression was increased during UUO in rat neonates (Table 6) (Chen et al. 2007). One can thus speculate that exogenous administration of B2 receptor ligand during neonatal obstructive nephropathy can be an interesting therapeutic strategy. However the protective effects of ACE inhibitors in adults are known to be mediated, at least in part, by increased bradykinin expression or B2 receptor activation (Okada et al. 2004; Seccia et al. 2006). Therefore the severe renal alterations in rodents and humans during the neonatal or the foetal period upon ACE inhibition might also be, at least partially, mediated by bradykinin or its B2 receptor.
On the another hand, we have recently demonstrated that kinin B1 receptor blockade significantly improves both renal inflammation and fibrosis in two models of renal disease (Klein et al. 2009, 2010). Contrarily to the B2 receptor, which is constitutively expressed, the B1 receptor is not detectable under physiological conditions but over-expressed at the site of injury during inflammation (Leeb-Lundberg et al. 2005). Moreover, B1 receptor knockout mice showed no renal alterations (Pesquero et al. 2000). All these arguments support the hypothesis that targeting the B1 receptor during UUO in neonates should have limited impact on non-inflamed contralateral kidney and on renal development and thus be potentially beneficial in obstructive nephropathy. Further studies need to investigate whether this therapeutic strategy is a valuable approach during congenital obstructive nephropathy.
Tubular transport and glomerular podocytes
As described above, UUO induces severe impairment of kidney function including profound modification of glomerular filtration and tubular function. Little information about the regulation of renal transporters by mechanical stimuli is available. Nevertheless, it appears that NHE and ENac, the apical sodium transporters in proximal tubule and collecting duct respectively, are activated by fluid flow (Table 1) (Preisig 1992; Satlin et al. 2001). If the changes result from hydrostatic pressure, membrane stretch or shear stress are however conflicting (Satlin et al. 2001; Carattino et al. 2004). Besides, fluid flow augments apical and basal release of nucleotides which subsequently activates purinergic receptors in isolated perfused renal tubules (Jensen et al. 2007). This effect is also observed in polarized MDCK cells subjected to transepithelial pressure changes (Praetorius et al. 2005). Altogether, these results suggest that mechanical aggression could modify salt and water reabsorption in the obstructed kidney (Sipos et al. 2009).
To our knowledge, the effect of UUO on the function of glomerular podocytes has never been investigated in vivo, neither in human nor in animals. However, numerous stretching experiments have been performed in vitro using podocytes. Indeed, these cells are probably subjected to stretch in vivo during obstruction. First, when elevated hydrostatic pelvis pressure due to urine accumulation is transmitted to Bowman's capsule, it could generate podocyte deformation. Moreover, when hydrostatic pressure increases in the glomerular capillary, as a result of afferent arteriole vasodilatation, it induces capillary distention, which may be transmitted to podocytes, leading to their stretching. As mentioned above, stretched-podocytes show activation of RAS and TGFβ axis, stimulation of apoptosis and inhibition of cellular proliferation. In addition to these effects, stretch induces morphologic alterations such as podocyte hypertrophy (Petermann et al. 2005) and stress fibers reorganization (Endlich et al. 2001). It down-regulates α3β1 integrin (Table 1), which allows podocyte ancrage to glomerular basement membrane in vivo, thus leading to reduced cellular adhesion (Dessapt et al. 2009). Finally, stretch reduces expression of nephrin in podocyte foot process (Table 1), a key protein of the slit diaphragm, thereby undermining the integrity of the filtration barrier. This effect is dependent of angiotensin-AT1-R system (Miceli et al. 2010) and could be mediated by nephrin internalization. Indeed it was recently demonstrated that nephrin trafficking depends on dynamin (Qin et al. 2009), a protein which is up-regulated in the obstructed kidney after neonatal complete UUO (Silverstein et al. 2003a). Altogether, these results suggest that stretch injury may induce podocyte dysfunction, thus leading to proteinuria and ultimately glomerulosclerosis, as observed in adult rats having undergone UUO during nephogenesis (Chevalier et al. 2000b). To test this hypothesis, further studies on podocyte function and in particular on nephrin status should be undertaken in human biopsies and animal models of UPJ obstruction.
Other cytokines and growth factors
Epidermal growth factor family
Members of the epidermal growth factor (EGF) family are suggested to be mediators of normal tubulogenesis and tubular regeneration (Zeng et al. 2009). In children studies on the urinary EGF secretion during UPJ obstruction have been contradictory. UPJ obstruction was found without effect on urinary EGF excretion (Yang et al. 2006) while others have observed reduced urinary EGF expression (Grandaliano et al. 2000). However additional evidence on renal biopsies tends to support the decreased renal EGF expression (Table 7) (Grandaliano et al. 2000; Yang et al. 2006).
Table 7.
Literature data about cytokine and growth factor-related genes and proteins that have been altered during obstructive nephropathy. We have reported here only molecules of which activity or expression has been significantly modified compared to healthy control (human), sham-animals (animal model) or control cells (in vitro)
| Animal model | In vitro | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene symbol | Gene name | Human | Ref. | PUUO | CUUO | Ref. | Stretch | Pressure | Ref. |
| Egf | Epidermal growth factor | = / Down(u, t) | Grandaliano et al. (2000), Yang et al. (2006), Taha et al. (2007a) | Down (2w, 4w) | Chung and Chevalier (1996) | ||||
| Egfr | Epidermal growth factor receptor; Erbb1 | Up (5d to 4w) | Nguyen et al. (1999a) | Up* | (Broadbelt et al. (2009) | ||||
| Tgfa | TGFα; transforming growth factor α | Up (5d to 4w) | Nguyen et al. (1999a) | ||||||
| Vegfa | Vascular endothelial growth factor A | Up/Down (2w, 4w) | Down (2w, 4w) | Burt et al. (2007), Fenghua et al. (2009) | |||||
| Edn1 | Endothelin 1 | Up (u) | Taha et al. (2007a) | ||||||
| Spp1 | Osteopontin; secreted phosphoprotein 1 | Up | Diamond et al. (1998), Endlich et al. (2002) | ||||||
u: urine; t: tissue; p: plasma; w: weeks of obstruction; d: days of obstruction; CUUO: complete UUO; *activity; Ref.: references.
Results in animal models are also complicated. In neonatal rat subjected to complete UUO, a marked suppression of EGF is observed in the obstructed kidney (Chung & Chevalier 1996) whereas TGFα (Transforming Growth Factor-α), another ligand of the EGF family, and the EGF-receptor Erb1 are up-regulated (Nguyen et al. 1999a). Moreover, in rat neonatal partial UUO, although up-regulation of the TGFα and the EGF receptor was still observed, no significant effect was shown in EGF and heparin-binding EGF (HB-EGF, a ligand for EGF-receptor) expression, after 24 weeks of obstruction (Table 7) (Bor et al. 2006).
Both EGF and TGFα are known to be involved in kidney development and alteration in their expression can thus be associated with modified nephrogenesis or renal maturation (Carev et al. 2008; Zeng et al. 2009). But many in vivo and in vitro observations indicate that EGF is mainly involved in modulation of apoptosis during obstructive nephropathy. Interestingly, EGF can be a protector or a deleterious factor depending on the disease severity and the species. Indeed, EGF administration attenuates UUO-induced renal injury by increasing tubular proliferation and reducing apoptosis, tubular dilation, tubular atrophy and interstitial fibrosis in rat neonates (Chevalier et al. 1998; Wen et al. 2009). Moreover, while expression of EGF is not normalized after release of UUO (Chevalier et al. 1999a), EGF treatment improves recovery following relief (Chevalier et al. 1999a,c;). In parallel, treatment with EGF decreases stretch-induced apoptosis in rat proximal tubular cell in vitro (Nguyen et al. 2000; Kiley et al. 2003). Conversely, EGF administration during neonatal mouse obstruction does not correct UUO-induced apoptosis in vivo (Kiley et al. 2005) and aggravates stretch-induced apoptosis in mouse proximal tubular cell in vitro (Kiley et al. 2005). In addition, obstructed kidney of neonatal mice lacking EGF or with diminished EGF receptor activity elicit less apoptosis than wild-type mice (Kiley et al. 2005). It was proposed that constitutive Src activity in mouse is the underlying cause of EGF receptor dysregulation and susceptibility to EGF-induced cell death (Kiley & Chevalier 2007).
Because of these striking species differences, it is hard to speculate whether EGF administration could be benificial or deleterious in humans. However it is interesting to point out that application of elevated pressure activates the EGF receptor in human proximal tubular cells in vitro (Table 7) and consecutively leads to iNOS and NO expression which has been shown to attenuate experimental obstructive renal injury (see above) (Broadbelt et al. 2009).
Vascular endothelial growth factor
Vascular endothelial growth factor (VEGF) is a pleiotropic cytokine known to be involved in many processes in kidney physiology and pathology such as glomerular capillary development and permeability regulation (Robert & Abrahamson 2001; Schrijvers et al. 2004), growth and proliferation of glomerular and peritubular capillary endothelial cells (Schrijvers et al. 2004) or inhibition of apoptosis in tubular epithelial cells (Villegas et al. 2005). It has been often proposed that during renal diseases increased VEGF levels can prevent the loss of peritubular capillaries, thus reducing hypoxia and subsequent development of renal fibrosis. However, a number of studies have shown that VEGF can be either deleterious or beneficial, depending on the form of renal disease (Schrijvers et al. 2004). In neonates, relationships between experimental UUO and VEGF are not fully understood. While complete UUO induced a complete loss of renal VEGF expression in foetal lambs and neonatal rats (Burt et al. 2007; Fenghua et al. 2009), partial UUO in neonatal rats led to strong inter-individual heterogeneity, with VEGF expression varying between down- to up-regulation (Table 7) (Burt et al. 2007). Moreover, exogenous VEGF administration tended to aggravate the UUO-induced loss of peritubular capillaries (Burt et al. 2007). All these results suggest a complex role of VEGF on the developing kidney during obstructive nephropathy, depending on the severity of the disease and probably on the global pathological context.
Insulin-like growth factor
Different studies have shown that insulin-like growth factor 1 (IGF-1) could play a protective role in the pathology of obstructive nephropathy. As stated above, mechanical stretch of proximal cells induced Bad-mediated cell death in vitro (Kiley et al. 2003). Interestingly, treatment with IGF-1 during stretch strongly decreased apoptosis by restoring bad phosphorylation level (Kiley et al. 2003). Altough IGF-1 and IGF-1 receptors expression is not modified during neonatal UUO in rats exogenous IGF-1 administration significantly decreased the UUO-induced apoptosis as well as tubular atrophy and interstitial fibrosis (Chevalier et al. 2000a). Similarly, while increasing inflammation, IGF-1 administration protected against the loss of renal architecture and decreased interstitial fibrosis during UUO in opossum pups (Steinhardt et al. 1995). Studies measuring the effect of IGF-1 administration on UUO-induced loss of renal function are absent. However these results suggest that IGF-1 may be a potential therapeutic target in the pathology of obstructive nephropathy.
Endothelin-1
Endothelin-1 (ET-1) is a potent vasoconstrictor that has been implicated in the tissue damage and dysfunction associated with UUO (Josephson & Hemsen 1994; Feldman et al. 2000) and UPJ obstruction (Taha et al. 2007a). It has been shown that urinary ET-1 levels were 4-fold higher in UPJ obstruction patients than in healthy controls, patients with vesicoureteral reflux and patients with renal stones (Table 7). Surgery decreased urinary ET-1 levels that slowly declined over 1 year (Taha et al. 2007a). This shows that, as observed for urinary TGFβ, that relief of obstruction does not rapidly decrease the concentrations of molecules that are potentially involved in disease progression.
Osteopontin
Osteopontin is a secreted glycosylated phosphoprotein, which has been shown to be up-regulated in a number of experimental models of renal disease and in human nephropathy (Xie et al. 2001). Osteopontin seems to have two opposite roles in the kidney. On one hand, osteopontin has been shown to promote macrophage chemotaxis and renal fibrosis (Xie et al. 2001; Tian et al. 2006). On another hand, osteopontin is strongly involved in renal protection by inhibiting oxidative stress, decreasing interstitial and tubular cell apoptosis and participating in the regeneration of tubular cells (Xie et al. 2001). Many factors have been shown to be involved in increased osteopontin expression, such as TNF-α, PDGF, TGFβ and EGF and it has been shown that osteopontin could modulate AngII-induced renal injury (Xie et al. 2001; Wolak et al.2009). Following mechanical stretch, rat proximal cells and mouse podocytes exhibit an increase in osteopontin mRNA levels, which is normalized following pretreatment with an AT1-R antagonist (Table 7) (Diamond et al. 1998; Endlich et al. 2002). Experimental UUO in osteopontin knockout neonatal mice showed that osteopontin blockade exerted a deleterious role on tubular and interstitial apoptosis (Yoo et al. 2006b). However this result was counterbalanced by an attenuation of fibrotic lesions in osteopontin knockout compared to WT mice, although no significant effect was shown on macrophage accumulation (Yoo et al. 2006b). In this study, the effect of osteopontin blockade on functional parameters has not been investigated. Future experiments should explore whether the protective (i.e. anti-apoptotic) or deleterious (i.e. pro-fibrotic) role of osteopontin dominates on kidney function during obstructive nephropathy.
Renal development
Finally, as stated previously, the major difference between adult and neonatal UUO models is the impairment of immature kidney development. Very interestingly, some studies have shown that UUO induced up-regulation of some genes involved in kidney embryogenesis, such as decorin and lumican (Silverstein et al. 2003a) involved in connective tissue organization during organ differentiation (Wilda et al. 2000), p53 (Silverstein et al. 2003a) which regulates metanephric development (Saifudeen et al. 2009) or Pax-2 (Fenghua et al. 2009) and Wnt4 (Nguyen et al. 1999b), two molecules involved in the mesenchymal to epithelial transition of the condensing mesenchyme (Table 8) (Lindoso et al. 2009).
Table 8.
Literature data about renal development-related genes and proteins that have been altered during obstructive nephropathy. We have reported here only molecules of which expression has been significantly modified compared to sham-animals (animal model)
| Animal model | |||
|---|---|---|---|
| Gene symbol | Gene name | CUUO | Ref. |
| Dcn | Decorin | Up (2w) | Silverstein et al. (2003a) |
| Lum | Lumican | Up (2w) | Silverstein et al. (2003a) |
| Pax2 | Paired box 2 | Up (10w) | Fenghua et al. (2009) |
| Tp53 | p53; tumor protein p53 | Up (2w) | Silverstein et al. (2003a) |
| Wnt4 | Wingless-related MMTV integration site 4 | Up (4w) | Nguyen et al. (1999a) |
w: weeks of obstruction; d: days of obstruction; CUUO: complete UUO; Ref.: references.
Conclusion
Summarizing, review of the literature both on human UPJ obstruction and the neonatal- and foetal-obstruction models strongly suggest that those models closely mimic human pathology. This observation therefore justifies the use of these models to test the effects of pharmacological interventions on evolution of the nephropathy associated to ureteropelvic junction obstruction. The molecular mechanisms involved in the pathogenesis of obstructive nephropathy follow closely what is observed in a number of nephropathies including inflammation, proliferation/apoptosis, growth factor induction, RAS activation and fibrosis. A major exception of obstructive nephropathy is the important fall in expression of many transporters. Also we have pointed in this review to the potential inducers of aggression of the tubular cell that is first in-line in obstructive nephropathy. Therapeutics aiming the blockade of this early tubular aggression might have a good future. Finally one important question remains for the long-term effects of obstructive nephropathy in humans. Does UPJ-obstruction induce permanent lesions, as suggested by the animal models? Longer follow-up studies in humans should shed light on this important remaining question.
Acknowledgments
JK and JPS acknowledge the support from the European FP7 programme e-LICO.
References
- Bajpai M, Bal CS, Tripathi M, Kalaivani M, Gupta AK. Prenatally diagnosed unilateral hydronephrosis: prognostic significance of plasma renin activity. J. Urol. 2007;178:2580–2584. doi: 10.1016/j.juro.2007.08.058. [DOI] [PubMed] [Google Scholar]
- Basak A, Koch P, Dupelle M, et al. Inhibitory specificity and potency of proSAAS-derived peptides toward proprotein convertase 1. J. Biol. Chem. 2001;276:32720–32728. doi: 10.1074/jbc.M104064200. [DOI] [PubMed] [Google Scholar]
- Bascands JL, Schanstra JP. Obstructive nephropathy: insights from genetically engineered animals. Kidney Int. 2005;68:925–937. doi: 10.1111/j.1523-1755.2005.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfield MR, McDonald RA, Bartosh S, Ho PL, Harmon W. Changing trends in pediatric transplantation: 2001 Annual Report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr. Transplant. 2003;7:321–335. doi: 10.1034/j.1399-3046.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- Benjannet S, Reudelhuber T, Mercure C, Rondeau N, Chretien M, Seidah NG. Proprotein conversion is determined by a multiplicity of factors including convertase processing, substrate specificity, and intracellular environment. Cell type-specific processing of human prorenin by the convertase PC1. J. Biol. Chem. 1992;267:11417–11423. [PubMed] [Google Scholar]
- Bor MV, Shi Y, Sorensen BS, et al. Increased TGF-alpha and EGF Receptor mRNA expression in response to neonatal unilateral partial ureter obstruction in rats. Nephron Exp. Nephrol. 2006;104:e76–e82. doi: 10.1159/000094000. [DOI] [PubMed] [Google Scholar]
- Broadbelt NV, Stahl PJ, Chen J, et al. Early upregulation of iNOS mRNA expression and increase in NO metabolites in pressurized renal epithelial cells. Am. J. Physiol. Renal Physiol. 2007;293:F1877–F1888. doi: 10.1152/ajprenal.00238.2007. [DOI] [PubMed] [Google Scholar]
- Broadbelt NV, Chen J, Silver RB, Poppas DP, Felsen D. Pressure activates epidermal growth factor receptor leading to the induction of iNOS via NFkappaB and STAT3 in human proximal tubule cells. Am. J. Physiol. Renal Physiol. 2009;297:F114–F124. doi: 10.1152/ajprenal.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt LE, Forbes MS, Thornhill BA, Kiley SC, Minor JJ, Chevalier RL. Renal vascular endothelial growth factor in neonatal obstructive nephropathy. II. Exogenous VEGF. Am. J. Physiol. Renal Physiol. 2007;292:F168–F174. doi: 10.1152/ajprenal.00294.2005. [DOI] [PubMed] [Google Scholar]
- Cachat F, Lange-Sperandio B, Chang AY, et al. Ureteral obstruction in neonatal mice elicits segment-specific tubular cell responses leading to nephron loss. Kidney Int. 2003;63:564–575. doi: 10.1046/j.1523-1755.2003.00775.x. [DOI] [PubMed] [Google Scholar]
- Cai Z, Xin J, Pollock DM, Pollock JS. Shear stress-mediated NO production in inner medullary collecting duct cells. Am. J. Physiol. Renal Physiol. 2000;279:F270–F274. doi: 10.1152/ajprenal.2000.279.2.F270. [DOI] [PubMed] [Google Scholar]
- Carattino MD, Sheng S, Kleyman TR. Epithelial Na+ channels are activated by laminar shear stress. J. Biol. Chem. 2004;279:4120–4126. doi: 10.1074/jbc.M311783200. [DOI] [PubMed] [Google Scholar]
- Carev D, Saraga M, Saraga-Babic M. Expression of intermediate filaments, EGF and TGF-alpha in early human kidney development. J. Mol. Histol. 2008;39:227–235. doi: 10.1007/s10735-007-9157-7. [DOI] [PubMed] [Google Scholar]
- Carr MC, Peters CA, Retik AB, Mandell J. Urinary levels of the renal tubular enzyme N-acetyl-beta-D-glucosaminidase in unilateral obstructive uropathy. J. Urol. 1994;151:442–445. doi: 10.1016/s0022-5347(17)34983-2. [DOI] [PubMed] [Google Scholar]
- Chang CP, McDill BW, Neilson JR, et al. Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J. Clin. Invest. 2004;113:1051–1058. doi: 10.1172/JCI20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CO, Park MH, Forbes MS, et al. Angiotensin-converting enzyme inhibition aggravates renal interstitial injury resulting from partial unilateral ureteral obstruction in the neonatal rat. Am. J. Physiol. Renal Physiol. 2007;292:F946–F955. doi: 10.1152/ajprenal.00287.2006. [DOI] [PubMed] [Google Scholar]
- Chertin B, Pollack A, Koulikov D, et al. Conservative treatment of ureteropelvic junction obstruction in children with antenatal diagnosis of hydronephrosis: lessons learned after 16 years of follow-up. Eur. Urol. 2006;49:734–738. doi: 10.1016/j.eururo.2006.01.046. [DOI] [PubMed] [Google Scholar]
- Chertin B, Pollack A, Koulikov D, et al. Does renal function remain stable after puberty in children with prenatal hydronephrosis and improved renal function after pyeloplasty? J. Urol. 2009;182:1845–1848. doi: 10.1016/j.juro.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Chevalier RL. Chronic partial ureteral obstruction and the developing kidney. Pediatr. Radiol. 2008;38(Suppl. 1):S35–S40. doi: 10.1007/s00247-007-0585-z. [DOI] [PubMed] [Google Scholar]
- Chevalier RL, Chung KH, Smith CD, Ficenec M, Gomez RA. Renal apoptosis and clusterin following ureteral obstruction: the role of maturation. J. Urol. 1996;156:1474–1479. [PubMed] [Google Scholar]
- Chevalier RL, Goyal S, Wolstenholme JT, Thornhill BA. Obstructive nephropathy in the neonatal rat is attenuated by epidermal growth factor. Kidney Int. 1998;54:38–47. doi: 10.1046/j.1523-1755.1998.00966.x. [DOI] [PubMed] [Google Scholar]
- Chevalier RL, Kim A, Thornhill BA, Wolstenholme JT. Recovery following relief of unilateral ureteral obstruction in the neonatal rat. Kidney Int. 1999a;55:793–807. doi: 10.1046/j.1523-1755.1999.055003793.x. [DOI] [PubMed] [Google Scholar]
- Chevalier RL, Thornhill BA, Wolstenholme JT, Kim A. Unilateral ureteral obstruction in early development alters renal growth: dependence on the duration of obstruction. J. Urol. 1999b;161:309–313. [PubMed] [Google Scholar]
- Chevalier RL, Goyal S, Thornhill BA. EGF improves recovery following relief of unilateral ureteral obstruction in the neonatal rat. J. Urol. 1999c;162:1532–1536. [PubMed] [Google Scholar]
- Chevalier RL, Goyal S, Kim A, Chang AY, Landau D, LeRoith D. Renal tubulointerstitial injury from ureteral obstruction in the neonatal rat is attenuated by IGF-1. Kidney Int. 2000a;57:882–890. doi: 10.1046/j.1523-1755.2000.057003882.x. [DOI] [PubMed] [Google Scholar]
- Chevalier RL, Thornhill BA, Chang AY. Unilateral ureteral obstruction in neonatal rats leads to renal insufficiency in adulthood. Kidney Int. 2000b;58:1987–1995. doi: 10.1111/j.1523-1755.2000.00371.x. [DOI] [PubMed] [Google Scholar]
- Chevalier RL, Thornhill BA, Chang AY, Cachat F, Lackey A. Recovery from release of ureteral obstruction in the rat: relationship to nephrogenesis. Kidney Int. 2002;61:2033–2043. doi: 10.1046/j.1523-1755.2002.00359.x. [DOI] [PubMed] [Google Scholar]
- Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75:1145–1152. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- Chung KH, Chevalier RL. Arrested development of the neonatal kidney following chronic ureteral obstruction. J. Urol. 1996;155:1139–1144. doi: 10.1097/00005392-199603000-00095. [DOI] [PubMed] [Google Scholar]
- Coleman CM, Minor JJ, Burt LE, Thornhill BA, Forbes MS, Chevalier RL. Angiotensin AT1-receptor inhibition exacerbates renal injury resulting from partial unilateral ureteral obstruction in the neonatal rat. Am. J. Physiol. Renal Physiol. 2007;293:F262–F268. doi: 10.1152/ajprenal.00071.2007. [DOI] [PubMed] [Google Scholar]
- Cortell S, Gennari FJ, Davidman M, Bossert WH, Schwartz WB. A definition of proximal and distal tubular compliance. Practical and theoretical implications. J. Clin. Invest. 1973;52:2330–2339. doi: 10.1172/JCI107422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csaicsich D, Greenbaum LA, Aufricht C. Upper urinary tract: when is obstruction obstruction? Curr. Opin. Urol. 2004;14:213–217. doi: 10.1097/01.mou.0000135075.19968.d9. [DOI] [PubMed] [Google Scholar]
- el-Dahr SS, Gomez RA, Gray MS, Peach MJ, Carey RM, Chevalier RL. Renal nerves modulate renin gene expression in the developing rat kidney with ureteral obstruction. J. Clin. Invest. 1991;87:800–810. doi: 10.1172/JCI115083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canton A, Stanziale R, Corradi A, Andreucci VE, Migone L. Effects of acute ureteral obstruction on glomerular hemodynamics in rat kidney. Kidney Int. 1977;12:403–411. doi: 10.1038/ki.1977.131. [DOI] [PubMed] [Google Scholar]
- Dal Canton A, Corradi A, Stanziale R, Maruccio G, Migone L. Effects of 24-hour unilateral ureteral obstruction on glomerular hemodynamics in rat kidney. Kidney Int. 1979;15:457–462. doi: 10.1038/ki.1979.61. [DOI] [PubMed] [Google Scholar]
- Decramer S, Wittke S, Mischak H, et al. Predicting the clinical outcome of congenital unilateral ureteropelvic junction obstruction in newborn by urinary proteome analysis. Nat. Med. 2006;12:398–400. doi: 10.1038/nm1384. [DOI] [PubMed] [Google Scholar]
- Decramer S, Bascands JL, Schanstra JP. Non-invasive markers of ureteropelvic junction obstruction. World J. Urol. 2007;25:457–465. doi: 10.1007/s00345-007-0201-8. [DOI] [PubMed] [Google Scholar]
- Dessapt C, Baradez MO, Hayward A, et al. Mechanical forces and TGFbeta1 reduce podocyte adhesion through alpha3beta1 integrin downregulation. Nephrol. Dial. Transplant. 2009;24:2645–2655. doi: 10.1093/ndt/gfp204. [DOI] [PubMed] [Google Scholar]
- Diamond JR, Levinson M, Kreisberg R, Ricardo SD. Increased expression of decorin in experimental hydronephrosis. Kidney Int. 1997;51:1133–1139. doi: 10.1038/ki.1997.156. [DOI] [PubMed] [Google Scholar]
- Diamond JR, Kreisberg R, Evans R, Nguyen TA, Ricardo SD. Regulation of proximal tubular osteopontin in experimental hydronephrosis in the rat. Kidney Int. 1998;54:1501–1509. doi: 10.1046/j.1523-1755.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- Dixon DL, Barr HA, Bersten AD, Doyle IR. Intracellular storage of surfactant and proinflammatory cytokines in co-cultured alveolar epithelium and macrophages in response to increasing CO2 and cyclic cell stretch. Exp. Lung Res. 2008;34:37–47. doi: 10.1080/01902140701807928. [DOI] [PubMed] [Google Scholar]
- Durvasula RV, Shankland SJ. Mechanical strain increases SPARC levels in podocytes: implications for glomerulosclerosis. Am. J. Physiol. Renal Physiol. 2005;289:F577–F584. doi: 10.1152/ajprenal.00393.2004. [DOI] [PubMed] [Google Scholar]
- Durvasula RV, Petermann AT, Hiromura K, et al. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65:30–39. doi: 10.1111/j.1523-1755.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- Elder JS, Stansbrey R, Dahms BB, Selzman AA. Renal histological changes secondary to ureteropelvic junction obstruction. J. Urol. 1995;154:719–722. doi: 10.1097/00005392-199508000-00102. [DOI] [PubMed] [Google Scholar]
- El-Sherbiny MT, Mousa OM, Shokeir AA, Ghoneim MA. Role of urinary transforming growth factor-beta1 concentration in the diagnosis of upper urinary tract obstruction in children. J. Urol. 2002;168:1798–1800. doi: 10.1097/01.ju.0000027231.84450.8f. [DOI] [PubMed] [Google Scholar]
- Endlich N, Kress KR, Reiser J, et al. Podocytes respond to mechanical stress in vitro. J. Am. Soc. Nephrol. 2001;12:413–422. doi: 10.1681/ASN.V123413. [DOI] [PubMed] [Google Scholar]
- Endlich N, Sunohara M, Nietfeld W, et al. Analysis of differential gene expression in stretched podocytes: osteopontin enhances adaptation of podocytes to mechanical stress. FASEB J. 2002;16:1850–1852. doi: 10.1096/fj.02-0125fje. [DOI] [PubMed] [Google Scholar]
- Eskild-Jensen A, Christensen H, Lindvig M, et al. Renal functional outcome in unilateral hydronephrosis in newborn pigs. J. Urol. 2000;163:1896–1900. [PubMed] [Google Scholar]
- Eskild-Jensen A, Jacobsen L, Christensen H, et al. Renal function outcome in unilateral hydronephrosis in newborn pigs. II. Function and volume of contralateral kidneys. J. Urol. 2001;165:205–209. doi: 10.1097/00005392-200101000-00059. [DOI] [PubMed] [Google Scholar]
- Eskild-Jensen A, Frokiaer J, Djurhuus JC, Jorgensen TM, Nyengaard JR. Reduced number of glomeruli in kidneys with neonatally induced partial ureteropelvic obstruction in pigs. J. Urol. 2002;167:1435–1439. [PubMed] [Google Scholar]
- Eskild-Jensen A, Paulsen LF, Wogensen L, et al. AT1 receptor blockade prevents interstitial and glomerular apoptosis but not fibrosis in pigs with neonatal induced partial unilateral ureteral obstruction. Am. J. Physiol. Renal Physiol. 2007a;292:F1771–F1781. doi: 10.1152/ajprenal.00479.2006. [DOI] [PubMed] [Google Scholar]
- Eskild-Jensen A, Thomsen K, Rungo C, et al. Glomerular and tubular function during AT1 receptor blockade in pigs with neonatal induced partial ureteropelvic obstruction. Am. J. Physiol. Renal Physiol. 2007b;292:F921–F929. doi: 10.1152/ajprenal.00407.2006. [DOI] [PubMed] [Google Scholar]
- Essig M, Terzi F, Burtin M, Friedlander G. Mechanical strains induced by tubular flow affect the phenotype of proximal tubular cells. Am. J. Physiol. Renal Physiol. 2001;281:F751–F762. doi: 10.1152/ajprenal.2001.281.4.F751. [DOI] [PubMed] [Google Scholar]
- Feldman DL, Mogelesky TC, Chou M, Jeng AY. Enhanced expression of renal endothelin-converting enzyme-1 and endothelin-A-receptor mRNA in rats with interstitial fibrosis following ureter ligation. J. Cardiovasc. Pharmacol. 2000;36:S255–S259. doi: 10.1097/00005344-200036051-00075. [DOI] [PubMed] [Google Scholar]
- Fenghua W, Junjie S, Gaoyan D, Jiacong M. Does intervention in utero preserve the obstructed kidneys of fetal lambs? A histological, cytological, and molecular study. Pediatr. Res. 2009;66:145–148. doi: 10.1203/PDR.0b013e3181aa42f6. [DOI] [PubMed] [Google Scholar]
- Fern RJ, Yesko CM, Thornhill BA, Kim HS, Smithies O, Chevalier RL. Reduced angiotensinogen expression attenuates renal interstitial fibrosis in obstructive nephropathy in mice. J. Clin. Invest. 1999;103:39–46. doi: 10.1172/JCI4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness PD, 3rd, Maizels M, Han SW, Cohn RA, Cheng EY. Elevated bladder urine concentration of transforming growth factor-beta1 correlates with upper urinary tract obstruction in children. J. Urol. 1999;162:1033–1036. doi: 10.1016/S0022-5347(01)68056-X. [DOI] [PubMed] [Google Scholar]
- Garvin JL, Hong NJ. Cellular stretch increases superoxide production in the thick ascending limb. Hypertension. 2008;51:488–493. doi: 10.1161/HYPERTENSIONAHA.107.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk CW, Mylle M. Micropuncture study of pressures in proximal tubules and peritubular capillaries of the rat kidney and their relation to ureteral and renal venous pressures. Am. J. Physiol. 1956;185:430–439. doi: 10.1152/ajplegacy.1956.185.2.430. [DOI] [PubMed] [Google Scholar]
- de Graauw M, Tijdens I, Cramer R, Corless S, Timms JF, van de Water B. Heat shock protein 27 is the major differentially phosphorylated protein involved in renal epithelial cellular stress response and controls focal adhesion organization and apoptosis. J. Biol. Chem. 2005;280:29885–29898. doi: 10.1074/jbc.M412708200. [DOI] [PubMed] [Google Scholar]
- Grandaliano G, Gesualdo L, Bartoli F, et al. MCP-1 and EGF renal expression and urine excretion in human congenital obstructive nephropathy. Kidney Int. 2000;58:182–192. doi: 10.1046/j.1523-1755.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- Guerin F, Azoulay R, Berrebi D, et al. Partial unilateral ureteral obstruction in newborn mice: magnetic resonance imaging and pathology studies. J. Urol. 2008;179:1553–1563. doi: 10.1016/j.juro.2007.11.088. [DOI] [PubMed] [Google Scholar]
- Guron G, Marcussen N, Nilsson A, Sundelin B, Friberg P. Postnatal time frame for renal vulnerability to enalapril in rats. J. Am. Soc. Nephrol. 1999;10:1550–1560. doi: 10.1681/ASN.V1071550. [DOI] [PubMed] [Google Scholar]
- Han SW, Lee SE, Kim JH, Jeong HJ, Rha KH, Choi SK. Does delayed operation for pediatric ureteropelvic junction obstruction cause histopathological changes? J. Urol. 1998;160:984–988. doi: 10.1097/00005392-199809020-00004. [DOI] [PubMed] [Google Scholar]
- Hegarty NJ, Watson RW, Young LS, O'Neill AJ, Brady HR, Fitzpatrick JM. Cytoprotective effects of nitrates in a cellular model of hydronephrosis. Kidney Int. 2002;62:70–77. doi: 10.1046/j.1523-1755.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- Hegarty NJ, Young LS, O'Neill AJ, Watson RW, Fitzpatrick JM. Endothelin in unilateral ureteral obstruction: vascular and cellular effects. J. Urol. 2003;169:740–744. doi: 10.1097/01.ju.0000036813.52746.89. [DOI] [PubMed] [Google Scholar]
- Heyman SN, Khamaisi M, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia response and the progression of chronic kidney disease. Am. J. Nephrol. 2008;28:998–1006. doi: 10.1159/000146075. [DOI] [PubMed] [Google Scholar]
- Hu X, Zhang Y, Cheng D, et al. Mechanical stress upregulates intercellular adhesion molecule-1 in pulmonary epithelial cells. Respiration. 2008;76:344–350. doi: 10.1159/000137509. [DOI] [PubMed] [Google Scholar]
- Huang WY, Peters CA, Zurakowski D, et al. Renal biopsy in congenital ureteropelvic junction obstruction: evidence for parenchymal maldevelopment. Kidney Int. 2006;69:137–143. doi: 10.1038/sj.ki.5000004. [DOI] [PubMed] [Google Scholar]
- Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J. Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J. Am. Soc. Nephrol. 2007;18:2062–2070. doi: 10.1681/ASN.2006070700. [DOI] [PubMed] [Google Scholar]
- Jensen AM, Norregaard R, Topcu SO, Frokiaer J, Pedersen M. Oxygen tension correlates with regional blood flow in obstructed rat kidney. J. Exp. Biol. 2009;212:3156–3163. doi: 10.1242/jeb.029249. [DOI] [PubMed] [Google Scholar]
- Jiang F. NADPH oxidase in the kidney: a Janus in determining cell fate. Kidney Int. 2009;75:135–137. doi: 10.1038/ki.2008.478. [DOI] [PubMed] [Google Scholar]
- Josephson S. Experimental obstructive hydronephrosis in newborn rats. III. Long-term effects on renal function. J. Urol. 1983;129:396–400. doi: 10.1016/s0022-5347(17)52125-4. [DOI] [PubMed] [Google Scholar]
- Josephson S, Hemsen A. Renal tissue endothelin in long-term complete ureteric obstruction in the young rat. Urol. Int. 1994;53:57–61. doi: 10.1159/000282636. [DOI] [PubMed] [Google Scholar]
- Just A, Kurtz L, de Wit C, Wagner C, Kurtz A, Arendshorst WJ. Connexin 40 mediates the tubuloglomerular feedback contribution to renal blood flow autoregulation. J. Am. Soc. Nephrol. 2009;20:1577–1585. doi: 10.1681/ASN.2008090943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe J, Okumura S, Lee MC, Sadoshima J, Ishikawa Y. Translocation of caveolin regulates stretch-induced ERK activity in vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1845–H1852. doi: 10.1152/ajpheart.00593.2003. [DOI] [PubMed] [Google Scholar]
- Kiley SC, Chevalier RL. Species differences in renal Src activity direct EGF receptor regulation in life or death response to EGF. Am. J. Physiol. Renal Physiol. 2007;293:F895–F903. doi: 10.1152/ajprenal.00227.2007. [DOI] [PubMed] [Google Scholar]
- Kiley SC, Thornhill BA, Tang SS, Ingelfinger JR, Chevalier RL. Growth factor-mediated phosphorylation of proapoptotic BAD reduces tubule cell death in vitro and in vivo. Kidney Int. 2003;63:33–42. doi: 10.1046/j.1523-1755.2003.00706.x. [DOI] [PubMed] [Google Scholar]
- Kiley SC, Thornhill BA, Belyea BC, et al. Epidermal growth factor potentiates renal cell death in hydronephrotic neonatal mice, but cell survival in rats. Kidney Int. 2005;68:504–514. doi: 10.1111/j.1523-1755.2005.00428.x. [DOI] [PubMed] [Google Scholar]
- Klein J, Gonzalez J, Duchene J, et al. Delayed blockade of the kinin B1 receptor reduces renal inflammation and fibrosis in obstructive nephropathy. FASEB J. 2009;23:134–142. doi: 10.1096/fj.08-115600. [DOI] [PubMed] [Google Scholar]
- Klein J, Gonzalez J, Decramer S, et al. Protective effects of kinin B1 receptor blockade in glomerulonephritis. J. Am. Soc. Nephrol. 2010 doi: 10.1681/ASN.2009090887. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb RJ, Woost PG, Hopfer U. Membrane trafficking of angiotensin receptor type-1 and mechanochemical signal transduction in proximal tubule cells. Hypertension. 2004;44:352–359. doi: 10.1161/01.HYP.0000136645.90116.1a. [DOI] [PubMed] [Google Scholar]
- Kone BC, Baylis C. Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am. J. Physiol. 1997;272:F561–F578. doi: 10.1152/ajprenal.1997.272.5.F561. [DOI] [PubMed] [Google Scholar]
- Lange-Sperandio B, Cachat F, Thornhill BA, Chevalier RL. Selectins mediate macrophage infiltration in obstructive nephropathy in newborn mice. Kidney Int. 2002;61:516–524. doi: 10.1046/j.1523-1755.2002.00162.x. [DOI] [PubMed] [Google Scholar]
- Lange-Sperandio B, Schimpgen K, Rodenbeck B, et al. Distinct roles of Mac-1 and its counter-receptors in neonatal obstructive nephropathy. Kidney Int. 2006;69:81–88. doi: 10.1038/sj.ki.5000017. [DOI] [PubMed] [Google Scholar]
- Lange-Sperandio B, Trautmann A, Eickelberg O, et al. Leukocytes induce epithelial to mesenchymal transition after unilateral ureteral obstruction in neonatal mice. Am. J. Pathol. 2007;171:861–871. doi: 10.2353/ajpath.2007.061199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Normand L, Buzelin JM, Bouchot O, Rigaud J, Karam G. Upper urinary tract: physiology, pathophysiology of obstructions and function assessment. Ann. Urol. (Paris) 2005;39:30–48. doi: 10.1016/j.anuro.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol. Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- Leonard EJ, Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1) Immunol. Today. 1990;11:97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- Liapis H, Yu H, Steinhardt GF. Cell proliferation, apoptosis, Bcl-2 and Bax expression in obstructed opossum early metanephroi. J. Urol. 2000;164:511–517. [PubMed] [Google Scholar]
- Liapis H, Barent B, Steinhardt GF. Extracellular matrix in fetal kidney after experimental obstruction. J. Urol. 2001;166:1433–1438. [PubMed] [Google Scholar]
- Lindoso RS, Verdoorn KS, Einicker-Lamas M. Renal recovery after injury: the role of Pax-2. Nephrol. Dial. Transplant. 2009;24:2628–2633. doi: 10.1093/ndt/gfp307. [DOI] [PubMed] [Google Scholar]
- Liu Y. New insights into epithelial–mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. 2010;21:212–222. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Ren W, Su XM, Chen JQ, Wu SH, Zhou GP. EGF-recruited JunD/c-fos complexes activate CD2AP gene promoter and suppress apoptosis in renal tubular epithelial cells. Gene. 2009;433:56–64. doi: 10.1016/j.gene.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Ludwig MS, Ftouhi-Paquin N, Huang W, Page N, Chakir J, Hamid Q. Mechanical strain enhances proteoglycan message in fibroblasts from asthmatic subjects. Clin. Exp. Allergy. 2004;34:926–930. doi: 10.1111/j.1365-2222.2004.01980.x. [DOI] [PubMed] [Google Scholar]
- Lye CM, Fasano L, Woolf AS. Ureter myogenesis: putting Teashirt into context. J. Am. Soc. Nephrol. 2010;21:24–30. doi: 10.1681/ASN.2008111206. [DOI] [PubMed] [Google Scholar]
- Malik RK, Thornhill BA, Chang AY, Kiley SC, Chevalier RL. Renal apoptosis parallels ceramide content after prolonged ureteral obstruction in the neonatal rat. Am. J. Physiol. Renal Physiol. 2001;281:F56–F61. doi: 10.1152/ajprenal.2001.281.1.F56. [DOI] [PubMed] [Google Scholar]
- Manucha W, Valles PG. Cytoprotective role of nitric oxide associated with Hsp70 expression in neonatal obstructive nephropathy. Nitric Oxide. 2008;18:204–215. doi: 10.1016/j.niox.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Manucha W, Carrizo L, Ruete C, Valles PG. Apoptosis induction is associated with decreased NHE1 expression in neonatal unilateral ureteric obstruction. BJU Int. 2007;100:191–198. doi: 10.1111/j.1464-410X.2007.06840.x. [DOI] [PubMed] [Google Scholar]
- Matsell DG, Tarantal AF. Experimental models of fetal obstructive nephropathy. Pediatr. Nephrol. 2002;17:470–476. doi: 10.1007/s00467-002-0910-6. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C. Functional obstruction: the renal pelvis rules. J. Clin. Invest. 2004;113:957–959. doi: 10.1172/JCI21402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzano SA, Ruiz-Ortega M, Egido J. Angiotensin II and renal fibrosis. Hypertension. 2001;38:635–638. doi: 10.1161/hy09t1.094234. [DOI] [PubMed] [Google Scholar]
- Miceli I, Burt D, Tarabra E, Camussi G, Perin PC, Gruden G. Stretch reduces nephrin expression via an angiotensin II-AT(1)-dependent mechanism in human podocytes: effect of rosiglitazone. Am. J. Physiol. Renal Physiol. 2010;298:F381–F390. doi: 10.1152/ajprenal.90423.2008. [DOI] [PubMed] [Google Scholar]
- Miyajima A, Chen J, Lawrence C, et al. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int. 2000a;58:2301–2313. doi: 10.1046/j.1523-1755.2000.00414.x. [DOI] [PubMed] [Google Scholar]
- Miyajima A, Chen J, Kirman I, Poppas DP, Darracott Vaughan EJ, Felsen D. Interaction of nitric oxide and transforming growth factor-beta1 induced by angiotensin II and mechanical stretch in rat renal tubular epithelial cells. J. Urol. 2000b;164:1729–1734. [PubMed] [Google Scholar]
- Miyajima A, Chen J, Poppas DP, Vaughan ED, Jr, Felsen D. Role of nitric oxide in renal tubular apoptosis of unilateral ureteral obstruction. Kidney Int. 2001;59:1290–1303. doi: 10.1046/j.1523-1755.2001.0590041290.x. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Tsuchida S, Nishimura H, et al. Angiotensin induces the urinary peristaltic machinery during the perinatal period. J. Clin. Invest. 1998;102:1489–1497. doi: 10.1172/JCI4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller HB, Praetorius J, Rutzler MR, Fenton RA. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc. Natl Acad. Sci. USA. 2010;107:424–429. doi: 10.1073/pnas.0910683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogyorosi A, Ziyadeh FN. What is the role of decorin in diabetic kidney disease? Nephrol. Dial. Transplant. 1999;14:1078–1081. doi: 10.1093/ndt/14.5.1078. [DOI] [PubMed] [Google Scholar]
- Mure PY, Gelas T, Dijoud F, et al. Complete unilateral ureteral obstruction in the fetal lamb. Part II: Long-term outcomes of renal tissue development. J. Urol. 2006a;175:1548–1558. doi: 10.1016/S0022-5347(05)00654-3. [DOI] [PubMed] [Google Scholar]
- Mure PY, Gelas T, Benchaib M, et al. Complete unilateral ureteral obstruction in the fetal lamb. Part I: long-term outcomes of renal hemodynamics and anatomy. J. Urol. 2006b;175:1541–1547. doi: 10.1016/S0022-5347(05)00655-5. [DOI] [PubMed] [Google Scholar]
- Murer L, Benetti E, Centi S, et al. Clinical and molecular markers of chronic interstitial nephropathy in congenital unilateral ureteropelvic junction obstruction. J. Urol. 2006;176:2668–2673. doi: 10.1016/j.juro.2006.08.055. discussion 2673. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Kogan BA. Renal hemodynamic changes after complete and partial unilateral ureteral obstruction in the fetal lamb. J. Urol. 1998;160:1063–1069. doi: 10.1097/00005392-199809020-00026. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Thomson AA, Kogan BA, Baskin LS, Cunha GR. Growth factor expression in the obstructed developing and mature rat kidney. Lab. Invest. 1999a;79:171–184. [PubMed] [Google Scholar]
- Nguyen HT, Thomson AA, Kogan BA, Baskin LS, Cunha GR. Expression of the Wnt gene family during late nephrogenesis and complete ureteral obstruction. Lab. Invest. 1999b;79:647–658. [PubMed] [Google Scholar]
- Nguyen HT, Bride SH, Badawy AB, et al. Heparin-binding EGF-like growth factor is up-regulated in the obstructed kidney in a cell- and region-specific manner and acts to inhibit apoptosis. Am. J. Pathol. 2000;156:889–898. doi: 10.1016/S0002-9440(10)64958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Yerkes E, Hohenfellner K, et al. Role of the angiotensin type 2 receptor gene in congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Mol. Cell. 1999;3:1–10. doi: 10.1016/s1097-2765(00)80169-0. [DOI] [PubMed] [Google Scholar]
- Norwood VF, Carey RM, Geary KM, Jose PA, Gomez RA, Chevalier RL. Neonatal ureteral obstruction stimulates recruitment of renin-secreting renal cortical cells. Kidney Int. 1994;45:1333–1339. doi: 10.1038/ki.1994.174. [DOI] [PubMed] [Google Scholar]
- Okada H, Watanabe Y, Kikuta T, et al. Bradykinin decreases plasminogen activator inhibitor-1 expression and facilitates matrix degradation in the renal tubulointerstitium under angiotensin-converting enzyme blockade. J. Am. Soc. Nephrol. 2004;15:2404–2413. doi: 10.1097/01.ASN.0000136132.20189.95. [DOI] [PubMed] [Google Scholar]
- Ortiz PA, Hong NJ, Garvin JL. Luminal flow induces eNOS activation and translocation in the rat thick ascending limb. Am. J. Physiol. Renal. Physiol. 2004;287:F274–F280. doi: 10.1152/ajprenal.00382.2003. [DOI] [PubMed] [Google Scholar]
- Oudin S, Pugin J. Role of MAP kinase activation in interleukin-8 production by human BEAS-2B bronchial epithelial cells submitted to cyclic stretch. Am. J. Respir. Cell Mol. Biol. 2002;27:107–114. doi: 10.1165/ajrcmb.27.1.4766. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Kaneko S, Podyma-Inoue KA, Yanagishita M, Soma K. Modulation of extracellular matrix synthesis and alkaline phosphatase activity of periodontal ligament cells by mechanical stress. J. Periodontal. Res. 2005;40:110–117. doi: 10.1111/j.1600-0765.2004.00782.x. [DOI] [PubMed] [Google Scholar]
- Padovano V, Massari S, Mazzucchelli S, Pietrini G. PKC induces internalization and retention of the EAAC1 glutamate transporter in recycling endosomes of MDCK cells. Am. J. Physiol. Cell Physiol. 2009;297:C835–C844. doi: 10.1152/ajpcell.00212.2009. [DOI] [PubMed] [Google Scholar]
- Pesquero JB, Araujo RC, Heppenstall PA, et al. Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors. Proc. Natl Acad. Sci. USA. 2000;97:8140–8145. doi: 10.1073/pnas.120035997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann AT, Hiromura K, Blonski M, et al. Mechanical stress reduces podocyte proliferation in vitro. Kidney Int. 2002;61:40–50. doi: 10.1046/j.1523-1755.2002.00102.x. [DOI] [PubMed] [Google Scholar]
- Petermann AT, Pippin J, Durvasula R, et al. Mechanical stretch induces podocyte hypertrophy in vitro. Kidney Int. 2005;67:157–166. doi: 10.1111/j.1523-1755.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- Peters CA. Obstruction of the fetal urinary tract. J. Am. Soc. Nephrol. 1997;8:653–663. doi: 10.1681/ASN.V84653. [DOI] [PubMed] [Google Scholar]
- Peters CA. Animal models of fetal renal disease. Prenat. Diagn. 2001;21:917–923. doi: 10.1002/pd.211. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Frokiaer J, Leipziger J. Transepithelial pressure pulses induce nucleotide release in polarized MDCK cells. Am. J. Physiol. Renal Physiol. 2005;288:F133–F141. doi: 10.1152/ajprenal.00238.2004. [DOI] [PubMed] [Google Scholar]
- Preisig PA. Luminal flow rate regulates proximal tubule H-HCO3 transporters. Am. J. Physiol. 1992;262:F47–F54. doi: 10.1152/ajprenal.1992.262.1.F47. [DOI] [PubMed] [Google Scholar]
- Qin XS, Tsukaguchi H, Shono A, Yamamoto A, Kurihara H, Doi T. Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J. Am. Soc. Nephrol. 2009;20:2534–2545. doi: 10.1681/ASN.2009010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan MR, Docherty NG, Watson RW, Fitzpatrick JM. Exploring mechanisms involved in renal tubular sensing of mechanical stretch following ureteric obstruction. Am. J. Physiol. Renal Physiol. 2008;295:F1–F11. doi: 10.1152/ajprenal.00576.2007. [DOI] [PubMed] [Google Scholar]
- Ramstrom C, Chapman H, Viitanen T, et al. Regulation of HERG (KCNH2) potassium channel surface expression by diacylglycerol. Cell. Mol. Life Sci. 2010;67:157–169. doi: 10.1007/s00018-009-0176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo SD, Ding G, Eufemio M, Diamond JR. Antioxidant expression in experimental hydronephrosis: role of mechanical stretch and growth factors. Am. J. Physiol. 1997;272:F789–F798. doi: 10.1152/ajprenal.1997.272.6.F789. [DOI] [PubMed] [Google Scholar]
- Ricardo SD, Franzoni DF, Roesener CD, Crisman JM, Diamond JR. Angiotensinogen and AT(1) antisense inhibition of osteopontin translation in rat proximal tubular cells. Am. J. Physiol. Renal Physiol. 2000;278:F708–F716. doi: 10.1152/ajprenal.2000.278.5.F708. [DOI] [PubMed] [Google Scholar]
- Robert B, Abrahamson DR. Control of glomerular capillary development by growth factor/receptor kinases. Pediatr. Nephrol. 2001;16:294–301. doi: 10.1007/s004670000534. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Flores D. Intratubular hydrodynamic forces influence tubulointerstitial fibrosis in the kidney. Curr. Opin. Nephrol. Hypertens. 2010;19:65–71. doi: 10.1097/MNH.0b013e32833327f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S, Peters CA, Chevalier RL, Huang WY. The kidney in congenital ureteropelvic junction obstruction: a spectrum from normal to nephrectomy. J. Urol. 2008;179:1257–1263. doi: 10.1016/j.juro.2007.11.048. [DOI] [PubMed] [Google Scholar]
- Roth KS, Koo HP, Spottswood SE, Chan JC. Obstructive uropathy: an important cause of chronic renal failure in children. Clin. Pediatr. (Phila) 2002;41:309–314. doi: 10.1177/000992280204100503. [DOI] [PubMed] [Google Scholar]
- Ruster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J. Am. Soc. Nephrol. 2006;17:2985–2991. doi: 10.1681/ASN.2006040356. [DOI] [PubMed] [Google Scholar]
- Saba JD, Obeid LM, Hannun YA. Ceramide: an intracellular mediator of apoptosis and growth suppression. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996;351:233–240. doi: 10.1098/rstb.1996.0021. discussion 240–241. [DOI] [PubMed] [Google Scholar]
- Saifudeen Z, Dipp S, Stefkova J, Yao X, Lookabaugh S, El-Dahr SS. p53 regulates metanephric development. J. Am. Soc. Nephrol. 2009;20:2328–2337. doi: 10.1681/ASN.2008121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na(+) channels are regulated by flow. Am. J. Physiol. Renal Physiol. 2001;280:F1010–F1018. doi: 10.1152/ajprenal.2001.280.6.F1010. [DOI] [PubMed] [Google Scholar]
- Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Invest. 2003;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanstra JP, Neau E, Drogoz P, et al. In vivo bradykinin B2 receptor activation reduces renal fibrosis. J. Clin. Invest. 2002;110:371–379. doi: 10.1172/JCI15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelling JR, Abu Jawdeh BG. Regulation of cell survival by Na+/H+ exchanger-1. Am. J. Physiol. Renal Physiol. 2008;295:F625–F632. doi: 10.1152/ajprenal.90212.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- Seccia TM, Belloni AS, Guidolin D, et al. The renal antifibrotic effects of angiotensin-converting enzyme inhibition involve bradykinin B2 receptor activation in angiotensin II-dependent hypertension. J. Hypertens. 2006;24:1419–1427. doi: 10.1097/01.hjh.0000234124.94013.ac. [DOI] [PubMed] [Google Scholar]
- Sekine T, Miura K, Takahashi K, Igarashi T. Children's toxicology from bench to bed--Drug-induced renal injury (1): The toxic effects of ARB/ACEI on fetal kidney development. J. Toxicol. Sci. 2009;34(Suppl. 2):SP245–SP250. doi: 10.2131/jts.34.sp245. [DOI] [PubMed] [Google Scholar]
- Shi Y, Li C, Thomsen K, et al. Neonatal ureteral obstruction alters expression of renal sodium transporters and aquaporin water channels. Kidney Int. 2004a;66:203–215. doi: 10.1111/j.1523-1755.2004.00721.x. [DOI] [PubMed] [Google Scholar]
- Shi Y, Pedersen M, Li C, et al. Early release of neonatal ureteral obstruction preserves renal function. Am. J. Physiol. Renal Physiol. 2004b;286:F1087–F1099. doi: 10.1152/ajprenal.00201.2003. [DOI] [PubMed] [Google Scholar]
- Shokeir AA, Taha MA. Role of urinary tubular enzymes in evaluation of children with ureteropelvic junction narrowing under conservative management. Urology. 2009;73:1016–1020. doi: 10.1016/j.urology.2008.12.038. [DOI] [PubMed] [Google Scholar]
- Silverstein DM, Travis BR, Thornhill BA, et al. Altered expression of immune modulator and structural genes in neonatal unilateral ureteral obstruction. Kidney Int. 2003a;64:25–35. doi: 10.1046/j.1523-1755.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- Silverstein DM, Thornhill BA, Leung JC, et al. Expression of connexins in the normal and obstructed developing kidney. Pediatr. Nephrol. 2003b;18:216–224. doi: 10.1007/s00467-002-1065-1. [DOI] [PubMed] [Google Scholar]
- Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J. Am. Soc. Nephrol. 2009;20:1724–1732. doi: 10.1681/ASN.2008101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt GF, Liapis H, Phillips B, Vogler G, Nag M, Yoon KW. Insulin-like growth factor improves renal architecture of fetal kidneys with complete ureteral obstruction. J. Urol. 1995;154:690–693. doi: 10.1097/00005392-199508000-00093. [DOI] [PubMed] [Google Scholar]
- Stock JA, Krous HF, Heffernan J, Packer M, Kaplan GW. Correlation of renal biopsy and radionuclide renal scan differential function in patients with unilateral ureteropelvic junction obstruction. J. Urol. 1995;154:716–718. doi: 10.1097/00005392-199508000-00101. [DOI] [PubMed] [Google Scholar]
- Taha MA, Shokeir AA, Osman HG, Abd el-Aziz Ael A, Farahat SE. Diagnosis of ureteropelvic junction obstruction in children: role of endothelin-1 in voided urine. Urology. 2007a;69:560–564. doi: 10.1016/j.urology.2006.09.070. discussion 564–565. [DOI] [PubMed] [Google Scholar]
- Taha MA, Shokeir AA, Osman HG, Abd El-Aziz Ael A, Farahat SE. Pelvi-ureteric junction obstruction in children: the role of urinary transforming growth factor-beta and epidermal growth factor. BJU Int. 2007b;99:899–903. doi: 10.1111/j.1464-410X.2006.06641.x. [DOI] [PubMed] [Google Scholar]
- Takenaka T, Inoue T, Kanno Y, et al. Expression and role of connexins in the rat renal vasculature. Kidney Int. 2008;73:415–422. doi: 10.1038/sj.ki.5002673. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Nangaku M. The role of hypoxia, increased oxygen consumption, and hypoxia-inducible factor-1 alpha in progression of chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2010;19:43–50. doi: 10.1097/MNH.0b013e3283328eed. [DOI] [PubMed] [Google Scholar]
- Thornhill BA, Burt LE, Chen C, Forbes MS, Chevalier RL. Variable chronic partial ureteral obstruction in the neonatal rat: a new model of ureteropelvic junction obstruction. Kidney Int. 2005;67:42–52. doi: 10.1111/j.1523-1755.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- Thornhill BA, Forbes MS, Marcinko ES, Chevalier RL. Glomerulotubular disconnection in neonatal mice after relief of partial ureteral obstruction. Kidney Int. 2007;72:1103–1112. doi: 10.1038/sj.ki.5002512. [DOI] [PubMed] [Google Scholar]
- Tian S, Ding G, Jia R, Chu G. Tubulointerstitial macrophage accumulation is regulated by sequentially expressed osteopontin and macrophage colony-stimulating factor: implication for the role of atorvastatin. Mediators Inflamm. 2006;2006:12919. doi: 10.1155/MI/2006/12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topcu SO, Pedersen M, Norregaard R, et al. Candesartan prevents long-term impairment of renal function in response to neonatal partial unilateral ureteral obstruction. Am. J. Physiol. Renal Physiol. 2007;292:F736–F748. doi: 10.1152/ajprenal.00241.2006. [DOI] [PubMed] [Google Scholar]
- Trougakos IP, Gonos ES. Regulation of clusterin/apolipoprotein J, a functional homologue to the small heat shock proteins, by oxidative stress in ageing and age-related diseases. Free Radic. Res. 2006;40:1324–1334. doi: 10.1080/10715760600902310. [DOI] [PubMed] [Google Scholar]
- Valles PG, Pascual L, Manucha W, Carrizo L, Ruttler M. Role of endogenous nitric oxide in unilateral ureteropelvic junction obstruction in children. Kidney Int. 2003;63:1104–1115. doi: 10.1046/j.1523-1755.2003.00833.x. [DOI] [PubMed] [Google Scholar]
- Valles PG, Manucha W, Carrizo L, Vega Perugorria J, Seltzer A, Ruete C. Renal caveolin-1 expression in children with unilateral ureteropelvic junction obstruction. Pediatr. Nephrol. 2007;22:237–248. doi: 10.1007/s00467-006-0290-4. [DOI] [PubMed] [Google Scholar]
- Villegas G, Lange-Sperandio B, Tufro A. Autocrine and paracrine functions of vascular endothelial growth factor (VEGF) in renal tubular epithelial cells. Kidney Int. 2005;67:449–457. doi: 10.1111/j.1523-1755.2005.67101.x. [DOI] [PubMed] [Google Scholar]
- Vlahakis NE, Schroeder MA, Limper AH, Hubmayr RD. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am. J. Physiol. 1999;277:L167–L173. doi: 10.1152/ajplung.1999.277.1.L167. [DOI] [PubMed] [Google Scholar]
- Wang G, Topcu SO, Ring T, et al. Age-dependent renal expression of acid-base transporters in neonatal ureter obstruction. Pediatr. Nephrol. 2009;24:1487–1500. doi: 10.1007/s00467-009-1193-y. [DOI] [PubMed] [Google Scholar]
- Wang Y, Maciejewski BS, Drouillard D, et al. A role for caveolin-1 in mechanotransduction of fetal type II epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;298:775–783. doi: 10.1152/ajplung.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen JG, Frokiaer J, Zhao JB, Ringgaard S, Jorgensen TM, Djurhuus JC. Severe partial ureteric obstruction in newborn rats can produce renal dysplasia. BJU Int. 2002;89:740–745. doi: 10.1046/j.1464-410x.2002.02747.x. [DOI] [PubMed] [Google Scholar]
- Wen JG, Wang GX, Chen Y, et al. Long-term EGF treatment partially prevents reduction of renal blood flow in response to neonatally induced partial unilateral ureteral obstruction. Nephron. Exp. Nephrol. 2009;111:e51–e59. doi: 10.1159/000191105. [DOI] [PubMed] [Google Scholar]
- Wilda M, Bachner D, Just W, et al. A comparison of the expression pattern of five genes of the family of small leucine-rich proteoglycans during mouse development. J. Bone Miner. Res. 2000;15:2187–2196. doi: 10.1359/jbmr.2000.15.11.2187. [DOI] [PubMed] [Google Scholar]
- Wilson DR. Pathophysiology of obstructive nephropathy. Kidney Int. 1980;18:281–292. doi: 10.1038/ki.1980.138. [DOI] [PubMed] [Google Scholar]
- Wolak T, Kim H, Ren Y, Kim J, Vaziri ND, Nicholas SB. Osteopontin modulates angiotensin II-induced inflammation, oxidative stress, and fibrosis of the kidney. Kidney Int. 2009;76:32–43. doi: 10.1038/ki.2009.90. [DOI] [PubMed] [Google Scholar]
- Wu F, Yao H, Bader A, et al. Decorin gene transfer inhibited the expression of TGFbeta1 and ECM in rat mesangial cells. Eur. J. Med. Res. 2007;12:360–368. [PubMed] [Google Scholar]
- Wu H, Wang S, Xue A, et al. Overexpression of decorin induces apoptosis and cell growth arrest in cultured rat mesangial cells in vitro. Nephrology (Carlton) 2008;13:607–615. doi: 10.1111/j.1440-1797.2008.00961.x. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Nishi S, Iguchi S, et al. Expression of osteopontin in gentamicin-induced acute tubular necrosis and its recovery process. Kidney Int. 2001;59:959–974. doi: 10.1046/j.1523-1755.2001.059003959.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Teramoto H, Uetani K, Igawa K, Shimizu E. Stretch induces a growth factor in alveolar cells via protein kinase. Respir. Physiol. 2001;127:105–111. doi: 10.1016/s0034-5687(01)00244-4. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Komuro I, Kudoh S, et al. Role of ion channels and exchangers in mechanical stretch-induced cardiomyocyte hypertrophy. Circ. Res. 1998;82:430–437. doi: 10.1161/01.res.82.4.430. [DOI] [PubMed] [Google Scholar]
- Yang SP, Woolf AS, Quinn F, Winyard PJ. Deregulation of renal transforming growth factor-beta1 after experimental short-term ureteric obstruction in fetal sheep. Am. J. Pathol. 2001;159:109–117. doi: 10.1016/s0002-9440(10)61678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hou Y, Wang CL, Ji SJ. Renal expression of epidermal growth factor and transforming growth factor-beta1 in children with congenital hydronephrosis. Urology. 2006;67:817–821. doi: 10.1016/j.urology.2005.10.062. discussion 821–822. [DOI] [PubMed] [Google Scholar]
- Yiee JH, Johnson-Welch S, Baker LA, Wilcox DT. Histologic Differences Between Extrinsic and Intrinsic Ureteropelvic Junction Obstruction. Urology. 2010;76:181–184. doi: 10.1016/j.urology.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Yoo KH, Norwood VF, el-Dahr SS, Yosipiv I, Chevalier RL. Regulation of angiotensin II AT1 and AT2 receptors in neonatal ureteral obstruction. Am. J. Physiol. 1997;273:R503–R509. doi: 10.1152/ajpregu.1997.273.2.R503. [DOI] [PubMed] [Google Scholar]
- Yoo KH, Thornhill BA, Forbes MS, Chevalier RL. Compensatory renal growth due to neonatal ureteral obstruction: implications for clinical studies. Pediatr. Nephrol. 2006a;21:368–375. doi: 10.1007/s00467-005-2119-y. [DOI] [PubMed] [Google Scholar]
- Yoo KH, Thornhill BA, Forbes MS, et al. Osteopontin regulates renal apoptosis and interstitial fibrosis in neonatal chronic unilateral ureteral obstruction. Kidney Int. 2006b;70:1735–1741. doi: 10.1038/sj.ki.5000357. [DOI] [PubMed] [Google Scholar]
- Yoo KH, Thornhill BA, Forbes MS, Chevalier RL. Inducible nitric oxide synthase modulates hydronephrosis following partial or complete unilateral ureteral obstruction in the neonatal mouse. Am. J. Physiol. Renal Physiol. 2010;298:F62–F71. doi: 10.1152/ajprenal.00234.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Singh AB, Harris RC. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp. Cell Res. 2009;315:602–610. doi: 10.1016/j.yexcr.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PL, Peters CA, Rosen S. Ureteropelvic junction obstruction: morphological and clinical studies. Pediatr. Nephrol. 2000;14:820–826. doi: 10.1007/s004679900240. [DOI] [PubMed] [Google Scholar]


