Abstract
More than two thirds of the US population are considered overweight or obese. Adipocytes are now appreciated as important endocrine organs, secreting various factors with hormonal effects. Several different adipokines have been identified, including adiponectin, which is associated with improved insulin sensitivity, a better lipoprotein profile, and lower rates of vascular inflammation and cardiovascular disease. Several studies have identified the renin-angiotensin-aldosterone system as important in the regulation of adiponectin. These studies lay the fundamental groundwork for developing targeted therapies with potential to reduce the burden of obesity-associated diseases, such as the cardiorenal metabolic syndrome.
Key Words: Adiponectin, Aldosterone, Angiotensin, Obesity-associated diseases
Introduction
Adipocytes secrete many different adipokines, which are hormonally active molecules with widespread effects throughout the body. Obesity is increasing in prevalence, underscoring the importance of understanding the pathophysiology of adipocytes in obesity and possible areas of therapeutic intervention. Adiponectin is an adipokine that is associated with the improvement in several obesity-associated disorders, such as the cardiorenal metabolic syndrome (CRS), type-2 diabetes mellitus and cardiovascular disease (CVD). Adiponectin is regulated, in part, by components of the renin-angiotensin-aldosterone system (RAAS). Studies have helped to delineate the mechanism of aldosterone-induced adiponectin suppression. Given these data, drugs that act on the angiotensin (Ang) type-1 and -2 receptors could be potential therapeutic targets to improve adiponectin levels and promote its beneficial effects.
Biology of Adiponectin
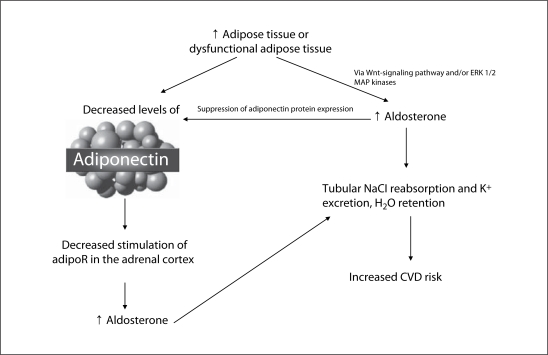
Adiponectin is a 244-amino acid, collagen-like protein produced exclusively by adipose tissue [1]. It is the product of the apM1 gene [2]. Adiponectin belongs to the collectin family and circulates in human plasma at high levels, making up 0.01% of the total plasma protein [1]. The concentration of adiponectin is three times higher than that of most other hormones [2]. It circulates in the blood in three different forms, as a low-molecular-weight trimer, a middle-molecular-weight hexamer, and a high-molecular-weight complex (HMW) [3]. There is widespread expression of adiponectin receptors (AdipoR) [4,5]. Plasma adiponectin levels tend to be higher in females, in insulin-sensitive persons, in patients with type-1 diabetes mellitus, and in patients treated with peroxisome proliferator-activated receptor (PPAR)-γ agonists. Conversely, adiponectin levels tend to be lower in males and in patients with obesity, insulin resistance and type-2 diabetes mellitus, high aldosterone levels, CRS, lipodystrophy, and CVD (fig. 1) [6].
Fig. 1.
Relationship between adiponectin and the RAAS axis: impact on renal function and CVD risk.
Adiponectin and Obesity-Related Diseases
Adiponectin is produced exclusively by adipocytes; however, levels of adiponectin tend to be lower in subjects with increased fat mass. Levels of adiponectin have been found to be lower in obese individuals and individuals with type-2 diabetes [7,8]. Hypoadiponectemia has been shown to be a risk factor for developing type-2 diabetes and coronary artery disease (CAD), and it is an independent risk factor for the CRS [9,10,11]. A study of 780 diabetic men in the Health Professionals’ Follow-Up Study indicated an inverse relationship between adiponectin levels and consumption of food with a high glycemic index [12]. Oxidative stress has also been associated with lower levels of adiponectin, which could explain the mechanism of hypoadiponectinemia in obesity, which has high levels of oxidative stress [13]. A group of 12 subjects that underwent laparoscopic banding or gastric bypass with significant weight loss also had significant increases in plasma total and HMW adiponectin levels 24 months after surgery [14]. This reveals a link between weight loss and increased adiponectin levels. Després and Lemieux [15] compared adiponectin levels in different fat depots. They found plasma adiponectin levels are more closely correlated to visceral rather than total body fat.
Adiponectin is also linked to CVD. A study comparing the levels of adiponectin in diabetic patients with and without CAD revealed lower levels of adiponectin in subjects with CAD [1]. Another group of investigators evaluated the effect of HMW and non-HMW adiponectin on endothelial function in healthy young men without CVD. They found that measurements of flow-mediated dilatation of the forearm (an endothelium-dependent response) were positively correlated with levels of HMW adiponectin. Their data also suggested that HMW adiponectin levels were a more important factor in predicting endothelial function than lipid profiles [16]. An investigative team observed that adiponectin reduced levels of blood free fatty acids. Additionally, they noted an association with improved lipid profiles, better glycemic control, and reduced inflammation in diabetic patients [17].
Aldosterone and Adiponectin
Given the potential benefits of adiponectin, it is important to understand the mechanisms involved in adiponectin regulation. Several studies point to the RAAS as a potential mediator of adiponectin activity. Prior studies have demonstrated that adipocytes secrete other factors that stimulate aldosterone release from the adrenal cortex. In vitro studies have specifically identified the involvement of the Wnt-signaling pathway and mitogen-activated protein kinases in adipocyte-stimulated aldosterone release [18,19,20,21]. Several studies have demonstrated a clear inverse relationship between the level of plasma aldosterone and the level of plasma adiponectin. A study on Sprague-Dawley rats that were fed high-salt diets resulted in an increase in blood pressure, a decrease in aldosterone, and an increase in plasma adiponectin levels [18]. A similar study in humans investigated the relationship between the RAAS and adiponectin. A high-salt diet given to healthy men suppressed plasma renin activity, plasma Ang II and plasma aldosterone, which correlated with a significant rise in plasma adiponectin [19]. Another study comparing the level of adiponectin in a group of subjects with primary hyperaldosteronism versus low-renin essential hypertension found significantly lower levels of adiponectin in the primary hyperaldosteronism group. Their analysis also showed an inverse relationship between adiponectin levels and the HOMA index (a measure of insulin resistance) [20]. Another investigative team also evaluated the association between primary aldosteronism and adiponectin. Compared to subjects with essential hypertension, they found a significantly higher level of adiponectin in patients with primary aldosteronism [21]. Studies in both rats and humans infused with Ang II resulted in reduced levels of plasma adiponectin [19,22].
To further investigate the relationship between aldosterone and adiponectin, Li et al. [23] looked for the expression of adiponectin receptors in mouse adrenal glands. They detected both AdipoR1 and AdipoR2 mRNAs in mouse adrenal cells. This group of investigators also treated the mouse adrenocortical cell line Y-1 with adiponectin for 3 h, which significantly decreased the expression of aldosterone and cortisol. Given the identification of adiponectin receptors in the adrenal glands of mice, Guo et al. [24] evaluated adiponectin gene expression from retroperitoneal and heart adipose tissue in obese, diabetic db/db mice. This group compared those findings to lean, non-diabetic mice after treatment with the mineralocorticoid receptor blocker, eplerenone. They found that treatment with eplerenone blocked the reduction in adiponectin that occurred in obese mice. This group also treated undifferentiated pre-adipocytes with aldosterone, which reduced mRNA and protein levels of adiponectin and PPAR-γ [24]. These studies support a direct effect of aldosterone on adiponectin gene expression. Human studies have also identified AdipoR1 and AdipoR2 mRNA expression in the human adrenal cortex and aldosterone-producing adenomas [25].
Given the benefits of increased levels of adiponectin, it is important to explore potential therapies that can raise adiponectin levels. Ang type-1 receptor blockers (ARBs) prevent the adverse effects of Ang II on the cardiovascular system. Ran et al. [22] treated Ang-II-infused rats with the Ang type-1 receptor antagonist, olmesartan, which returned the levels of adiponectin to pre-Ang II infusion levels. The insulin sensitivity index of the Ang-II-infused rats was also decreased compared to saline-infused rats, and treatment with olmesartan increased the insulin sensitivity index in the Ang-II-infused rats [22]. Some of the ARBs (telmisartan and irbesartan) have been identified as ligands of PPAR-γ [26]. Furthermore, PPAR-γ activation by thiazolidinediones increases the level of adiponectin. A study in humans with primary hypertension investigated the effects of an Ang-converting enzyme (ACE) inhibitor or an ARB on plasma levels of aldosterone as well as adiponectin. Plasma levels of aldosterone remained suppressed in the ARB (telmisartan) group, but not in the ACE inhibitor (perindopril) group at 24 weeks. Plasma adiponectin levels were significantly increased only in the telmisartan-treated group [27]. This study points to an induction in adiponectin expression with sustained aldosterone suppression by an ARB. A further study provided evidence that ARB-induced adiponectin stimulation is obtained through PPAR-γ activation [26]. In this study, obese Zucker rats were also treated with irbesartan, which prevented cellular adiponectin protein depletion [26]. Conversely, stimulation of the Ang type-2 receptor (AT2R) by Ang II results in beneficial actions, such as vasodilatation and tissue regeneration [28,29], and, in fact, stimulation of AT2R induces adiponectin expression [26]. These studies point to a potential therapy with ARBs, via PPAR-γ activation, or AT2R agonists in raising adiponectin levels [30].
Conclusion
Obesity-related diseases contribute to the high morbidity and mortality in the USA. The function of adipocytes and adipokines in obesity is important to understand. Adiponectin is a unique adipokine given its high concentration in the plasma and its beneficial effects in obesity-associated conditions. These studies have identified important mechanisms of adiponectin regulation and potential targets for therapy. Further research investigating the use of ARBs and aldosterone antagonists [30] in treating hypoadiponectinemia is needed.
References
- 1.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 2.Diez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148:293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 3.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 4.Psilopanagioti A, Papadaki H, Kranioti EF, Alexandrides TK, Varakis JN. Expression of adiponectin and adiponectin receptors in human pituitary gland and brain. Neuroendocrinology. 2009;89:38–47. doi: 10.1159/000151396. [DOI] [PubMed] [Google Scholar]
- 5.Kos K, Harte AL, da Silva NF, Tonchev A, Chaldakov G, James S, Snead DR, Hoggart B, O'Hare JP, McTernan PG, Kumar S. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J Clin Endocrinol Metab. 2007;92:1129–1136. doi: 10.1210/jc.2006-1841. [DOI] [PubMed] [Google Scholar]
- 6.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto Y, Hirose H, Saito I, Nishikai K, Saruta T. Adiponectin, an adipocyte-derived protein, predicts future insulin resistance: two-year follow-up study in Japanese population. J Clin Endocrinol Metab. 2004;89:87–90. doi: 10.1210/jc.2003-031163. [DOI] [PubMed] [Google Scholar]
- 8.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 9.Krakoff J, Funahashi T, Stehouwer CD, Schalkwijk CG, Tanaka S, Matsuzawa Y, Kobes S, Tataranni PA, Hanson RL, Knowler WC, Lindsay RS. Inflammatory markers, adiponectin, and risk of type 2 diabetes in the Pima Indian. Diabetes Care. 2003;26:1745–1751. doi: 10.2337/diacare.26.6.1745. [DOI] [PubMed] [Google Scholar]
- 10.Matsushita K, Yatsuya H, Tamakoshi K, Wada K, Otsuka R, Takefuji S, Sugiura K, Kondo T, Murohara T, Toyoshima H. Comparison of circulating adiponectin and proinflammatory markers regarding their association with metabolic syndrome in Japanese men. Arterioscler Thromb Vasc Biol. 2006;26:871–876. doi: 10.1161/01.ATV.0000208363.85388.8f. [DOI] [PubMed] [Google Scholar]
- 11.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 12.Qi L, Rimm E, Liu S, Rifai N, Hu FB. Dietary glycemic index, glycemic load, cereal fiber, and plasma adiponectin concentration in diabetic men. Diabetes Care. 2005;28:1022–1028. doi: 10.2337/diacare.28.5.1022. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linscheid P, Christ-Crain M, Stoeckli R, Reusch CE, Lutz TA, Muller B, Keller U. Increase in high molecular weight adiponectin by bariatric surgery-induced weight loss. Diabetes Obes Metab. 2008;10:1266–1270. doi: 10.1111/j.1463-1326.2008.00899.x. [DOI] [PubMed] [Google Scholar]
- 15.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 16.Torigoe M, Matsui H, Ogawa Y, Murakami H, Murakami R, Cheng XW, Numaguchi Y, Murohara T, Okumura K. Impact of the high-molecular-weight form of adiponectin on endothelial function in healthy young men. Clin Endocrinol (Oxf) 2007;67:276–281. doi: 10.1111/j.1365-2265.2007.02876.x. [DOI] [PubMed] [Google Scholar]
- 17.Mantzoros CS, Li T, Manson JE, Meigs JB, Hu FB. Circulating adiponectin levels are associated with better glycemic control, more favorable lipid profile, and reduced inflammation in women with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:4542–4548. doi: 10.1210/jc.2005-0372. [DOI] [PubMed] [Google Scholar]
- 18.Kamari Y, Shimoni N, Koren F, Peleg E, Sharabi Y, Grossman E. High-salt diet increases plasma adiponectin levels independent of blood pressure in hypertensive rats: the role of the renin-angiotensin-aldosterone system. J Hypertens. 2010;28:95–101. doi: 10.1097/HJH.0b013e3283325eee. [DOI] [PubMed] [Google Scholar]
- 19.Lely AT, Krikken JA, Bakker SJ, Boomsma F, Dullaart RP, Wolffenbuttel BH, Navis G. Low dietary sodium and exogenous angiotensin II infusion decrease plasma adiponectin concentrations in healthy men. J Clin Endocrinol Metab. 2007;92:1821–1826. doi: 10.1210/jc.2006-2092. [DOI] [PubMed] [Google Scholar]
- 20.Fallo F, Della MP, Sonino N, Bertello C, Ermani M, Vettor R, Veglio F, Mulatero P. Adiponectin and insulin sensitivity in primary aldosteronism. Am J Hypertens. 2007;20:855–861. doi: 10.1016/j.amjhyper.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Ronconi V, Turchi F, Rilli S, Di MD, Agostinelli L, Boscaro M, Giacchetti G. Metabolic syndrome in primary aldosteronism and essential hypertension: relationship to adiponectin gene variants. Nutr Metab Cardiovasc Dis. 2010;20:93–100. doi: 10.1016/j.numecd.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Ran J, Hirano T, Fukui T, Saito K, Kageyama H, Okada K, Adachi M. Angiotensin II infusion decreases plasma adiponectin level via its type 1 receptor in rats: an implication for hypertension-related insulin resistance. Metabolism. 2006;55:478–488. doi: 10.1016/j.metabol.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Li P, Sun F, Cao HM, Ma QY, Pan CM, Ma JH, Zhang XN, Jiang H, Song HD, Chen MD. Expression of adiponectin receptors in mouse adrenal glands and the adrenocortical Y-1 cell line: adiponectin regulates steroidogenesis. Biochem Biophys Res Commun. 2009;390:1208–1213. doi: 10.1016/j.bbrc.2009.10.122. [DOI] [PubMed] [Google Scholar]
- 24.Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, Li J, Williams GH, Adler GK. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117:2253–2261. doi: 10.1161/CIRCULATIONAHA.107.748640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossi GP, Sticchi D, Giuliani L, Bernante P, Zavattiero S, Pessina AC, Nussdorfer GG. Adiponectin receptor expression in the human adrenal cortex and aldosterone-producing adenomas. Int J Mol Med. 2006;17:975–980. [PubMed] [Google Scholar]
- 26.Clasen R, Schupp M, Foryst-Ludwig A, Sprang C, Clemenz M, Krikov M, Thone-Reineke C, Unger T, Kintscher U. PPARγ-activating angiotensin type-1 receptor blockers induce adiponectin. Hypertension. 2005;46:137–143. doi: 10.1161/01.HYP.0000168046.19884.6a. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura T, Kawachi K, Saito Y, Saito T, Morishita K, Hoshino J, Hosoi T, Iwasaki T, Ohyama Y, Kurabayashi M. Effects of ARB or ACE-inhibitor administration on plasma levels of aldosterone and adiponectin in hypertension. Int Heart J. 2009;50:501–512. doi: 10.1536/ihj.50.501. [DOI] [PubMed] [Google Scholar]
- 28.Pulakat L, DeMarco VG, Whaley-Connell A, Sowers JR. The impact of overnutrition on insulin metabolic signaling in the heart and kidney. CardioRen Med. 2011;2:102–112. doi: 10.1159/000327140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sowers JR. Endocrine functions of adipose tissue: focus on adiponectin. Clin Cornerstone. 2008;9:32–38. doi: 10.1016/s1098-3597(08)60026-5. discussion 39–40. [DOI] [PubMed] [Google Scholar]
- 30.Sowers JR, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150:776–783. doi: 10.7326/0003-4819-150-11-200906020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]