Abstract
Obesity has reached epidemic proportions with far-reaching health care and economic implications. Overnutrition, characterized by excess intake of carbohydrates and fats, has been associated with end-organ damage in several tissues, including the heart and the kidney. Furthermore, overnutrition is one of the most important modifiable and preventable causes of morbidity and mortality associated with cardiovascular and kidney diseases. Insulin resistance and compensatory hyperinsulinemia as well as associated mechanisms, including enhanced renin-angiotensin-aldosterone system activity, inflammation, and oxidative stress, have been implicated in obesity-related cardiorenal injury. In this review, the effect of overnutrition on heart and kidney disease is assessed in a rodent model of overnutrition and obesity, the Zucker obese rat.
Key Words: Cardiorenal syndrome, Heart/kidney disease, Obesity, Overnutrition, Zucker rat model
Introduction
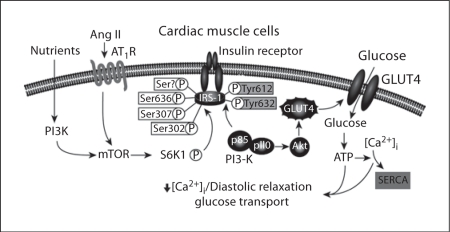
Rates of overweight and obesity have increased strikingly over the past 3 decades, especially in minority and socioeconomically disadvantaged populations [1,2,3,4,5,6,7,8,9,10,11]. Overnutrition (especially when characterized by excessive intake of carbohydrates and fat) is a major contributor to increases in the incidence rates of hypertension, diabetes, and heart and kidney disease. These overweight-/obesity-related comorbidities appear to be driven, in part, by decreases in insulin metabolic signaling in cardiac and renal tissue (fig. 1) [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. In addition to insulin resistance, several other mechanisms, such as enhanced activation of the renin-angiotensin-aldosterone system (RAAS), inflammation and oxidative stress, may help explain the linkage between overnutrition and heart and kidney disease. In this review, the effect of overnutrition on heart and kidney disease is assessed in a rodent model of overnutrition and obesity.
Fig. 1.
Impact of overnutrition and Ang II on insulin metabolic signaling in the heart.
A Rodent Model of Overnutrition and Heart Disease: The Zucker Obese Rat
The Zucker obese (ZO) rat has been widely employed as a model of obesity-related heart and kidney injury and therefore represents a potentially important tool to investigate the cardiorenal syndrome [17]. Our laboratory and others have shown that the young ZO rat heart exhibits impaired insulin metabolic signaling (fig. 1) as well as abnormal cardiomyocyte and cardiac interstitial architecture (fig. 2a, b), and increased oxidative stress (fig. 2c, d) in conjunction with increased systemic insulin resistance (by homeostasis model assessment of insulin resistance) compared to the Zucker lean (ZL) rat [17]. The increased oxidative stress in the young ZO rat heart [17] is an important observation as the balance between the production and the elimination of reactive oxygen species (ROS) is critical in the preservation of normal cardiac function, especially for diastolic relaxation. Indeed, excessive myocardial ROS lead to abnormal myocardial structures and function [12,17,25,38,39,40,41]. These sources of excess ROS have been reported to result from increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity [17] and mitochondrial electron transport chain dysfunction [38,39], as well as from mitochondrial antioxidant dysfunction [39]. These increases in oxidative stress and inflammation may help explain the increase in interstitial and perivascular fibrosis observed in young ZO rat hearts (fig. 2a, b). Impairments in diastolic relaxation depend, in part, on abnormalities in the passive properties of the ventricular wall that affect chamber compliance, such as excess accumulation of collagen and elastin fibers in the myocardium. Indeed, studies conducted in young ZO and ZL rats using high-resolution cine magnetic resonance imaging showed that, compared to the ZL rat heart, the ZO rat heart exhibits left ventricular diastolic dysfunction due to a prolonged diastolic relaxation time and a reduced initial filling rate [17]. These abnormalities are associated with reductions in myocardial glucose uptake (fig. 3), insulin metabolic signaling and endothelial cell nitric oxide (NO) synthase activity, as well as increased activation of the mammalian target of the rapamycin (mTOR)/S6 kinase 1 (S6K-1) signaling pathway (fig. 1). Indeed, there is evolving evidence that overnutrition and enhanced RAAS activation may promote reduced tissue metabolic signaling through activation of this pathway [42,43,44].
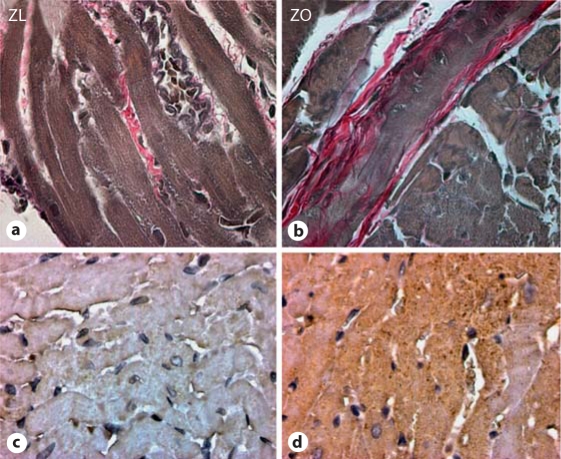
Fig. 2.
The ZO rat heart manifests increased interstitial fibrosis (a, b) compared to the ZL rat heart due to increases in oxidative stress, i.e. 3-nitrotyrosine (c, d). The ZO rat heart (a) displays increased intensity of staining compared to the ZL rat heart (b) with Verhoeff-Van Gieson stain, which stains collagen pink. The ZO rat heart (c) displays increased intensity of immunostaining for 3-nitrotyrosine compared to the ZL rat heart (d).
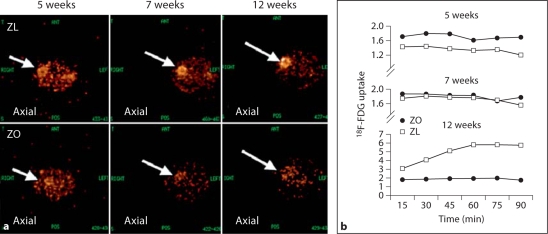
Fig. 3.
Time course of micro-PET determination of insulin/glucose uptake in ZO rats. a Representative images of decreased 18F-FDG uptake in the ZO (bottom panel) compared to the ZL rat (top panel) at 5, 7 and 12 weeks of age. b Time course of 18F-FDG uptake over 90 min. At 12 weeks, there is a trend to decreased uptake in ZO compared to ZL rats.
A Model of Obesity-Related Kidney Disease
Obesity and insulin resistance are increasingly recognized as independent risk factors for chronic kidney disease [45,46]. Mechanisms by which obesity and insulin resistance lead to kidney disease have been investigated in numerous models; however, the ZO rat is one of the best-characterized models for obesity-related kidney injury [47,48]. Observations from the ZO rat kidney suggest that activation of the renin-angiotensin system (RAS) in juxtaglomerular cells (fig. 4a) and proximal tubule cells (PTC) (fig. 4c) promotes a pro-inflammatory and pro-oxidative milieu (fig. 4b, d) and triggers obesity-induced mechanical forces which impair natriuresis and contribute to kidney injury [47,48,49]. These abnormalities, in turn, result in reductions in bioavailable NO, thus enhancing renal injury and progressive kidney disease [50,51]. Importantly, increased renal NO appears to counter-regulate the effects of both the sympathetic nervous system and the RAAS in the renal regulation of salt and fluid homeostasis, as well as renal injury [52]. Treatment strategies which reduce oxidative stress and increase bioavailable NO are renoprotective in several rodent models of RAAS- and sympathetic nervous system-mediated renal injury, including the ZO rat model [49,50,51,52,53,54].
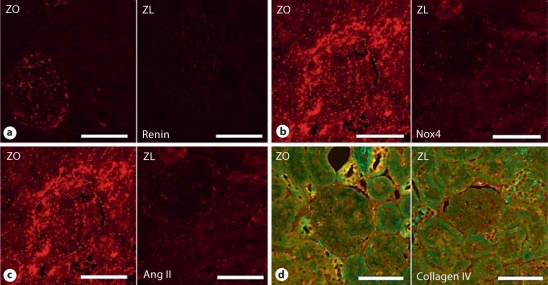
Fig. 4.
ZO rat kidney manifests increased RAS activation with increases in juxtaglomerular renin (a) as well as tissue Ang II in the proximal tubule (c) compared to ZL controls. Tissue-based RAS increased vascular enzyme complex NADPH oxidase and subunits such as renal gp91-phox (Nox4) in the kidney (b). Activation of NADPH oxidase is critical in the generation of oxidative stress as it relates to structural remodeling and abnormal collagen deposition in the ZO rat kidney, as shown by staining with Verhoeff-Van Gieson stain, which stains collagen pink (d).
RAAS activation and decreased activity of natriuretic peptides are both involved in obesity and contribute to impaired natriuresis with increased sodium (Na+) reabsorption and resultant volume expansion [55]. Obesity has been implicated in altered intrarenal physical forces that play a role in abnormal pressure natriuresis and Na+ retention. Observations from animal models of obesity and insulin resistance as well as studies in humans have demonstrated an increase in kidney weight attributable to endothelial cell proliferation and intrarenal lipid deposition, which can lead to altered intrarenal mechanical forces. This increased weight and the resultant elevated interstitial hydrostatic pressure lead to parenchymal collapse, followed by urinary outflow obstruction due to tubular collapse and increased sodium reabsorption due to reduced tubular flow. The increased Na+ reabsorption produces a feedback-mediated renal vasodilatation, elevation of the glomerular filtration rate, and RAAS stimulation despite a state of relative volume expansion.
Evidence suggests that ROS are an important mediator of adverse RAAS-induced renal injury in models of obesity (fig. 4b, d) [52]. ROS are highly reactive molecules that oxidize lipids and proteins, cause cellular injury, and induce glomerular podocyte (fig. 5a) and renal epithelial PTC injury (fig. 5c, d) and associated proteinuria. ROS also promote uncoupling of endothelial NO synthase, thereby suppressing its activity, resulting in reductions in bioavailable NO, and cause impairments in vasodilation. Increased tissue levels of ROS can also diminish the bioactivity of NO by conversion of locally released NO to peroxynitrite (ONOO−), which itself contributes to tissue injury.
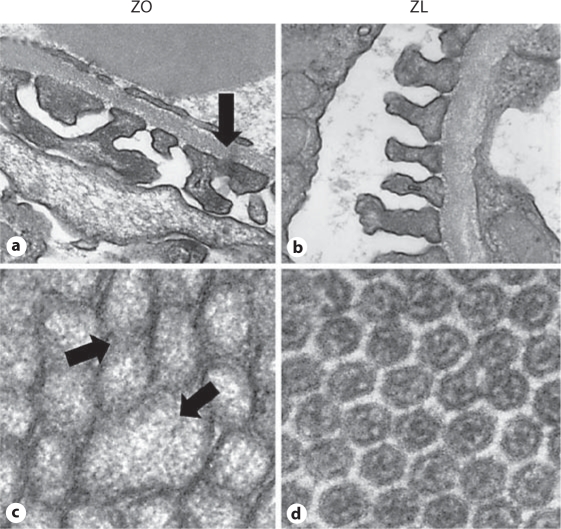
Fig. 5.
Podocyte foot process and PTC remodeling in the ZO compared to the ZL rat. a Images of ultrastructural analysis of the glomerular filtration barrier utilizing transmission electron microscopy. The ZO rat (a) displays podocyte foot process effacement and fusion (arrow) with loss of the slit-pore diaphragm compared to the ZL rat (b), findings consistent with the development of proteinuria. The development of proteinuria is coupled with parallel findings of structural remodeling of proximal tubule microvilli in ZO (c) compared to ZL rats (d). The ZO rat demonstrates enlarged microvilli with multiple abnormal forms (arrows) which are not found in ZL rats.
Recently, our group has reported that treatments which reduce NADPH oxidase activity and increase bioavailable NO attenuate proteinuria and maladaptive glomerular and proximal tubular remodeling in models of insulin resistance, hypertension and proteinuria, effects which are largely due to RAAS-mediated oxidative stress [54,56,57,58,59,60,61]. In these studies, treatment with an agent that reduced NADPH oxidase activity substantially reduced tubulointerstitial oxidative stress and fibrosis in concert with reductions in urinary N-acetyl-β-D-glucosamine and kidney injury molecule-1 (KIM-1), which are both markers for PTC dysfunction and/or injury. Our collective data support a shift in our understanding of the origins of proteinuria in models of obesity and insulin resistance as a function of early diabetic kidney disease, wherein proteinuria is now thought to have a PTC as well as a glomerular origin [56].
To evaluate in how far PTC injury, in addition to glomerular alterations, contributes importantly to proteinuria in the early stages of diabetic kidney disease, we have examined structural and functional properties of the proximal tubule in relation to glomerular abnormalities in the ZO rat model (fig. 4, 5) [54,56,57,58]. In young ZO rats (9–10 weeks of age), glomerular injury has been attributed to alterations in intraglomerular hemodynamics due to obesity and associated insulin resistance/hyperinsulinemia and impairments in vasodilation derived from reductions in bioavailable NO [50,51,57,58]. Our recent data further support a glomerular origin of proteinuria in the ZO rat. These glomerular abnormalities include reductions in podocyte-specific proteins (nephrin and synaptopodin) and podocyte foot process effacement/reduction in slit-pore diaphragm integrity, coupled with thickening of the glomerular basement membrane (fig. 5a). Observed ultrastructural changes are consistent with previous reports of changes in size and charge selectivity of the filtration barrier and loss of the slit-pore diaphragm contributing to a glomerular origin of proteinuria. However, our current data as well as recent work from other groups suggest that, at early stages of metabolic/diabetic kidney disease, we need to consider contributions from impairments in either the retrieval or degradation process in the proximal tubule as well (fig. 5b) [57,58].
In keeping with PTC injury contributing to proteinuria in the young insulin-resistant ZO rat model, urinary levels of γ-glutamyl transferase, a specific urinary marker for injury to the PTC brush border, are increased [57]. Our findings of increased urinary levels of γ-glutamyl transferase suggest a potential mechanism of impaired retrieval/degradation in this obese insulin-resistant rodent model. This notion is supported by modest reductions in the PTC-specific proteins megalin and lysosomal-associated membrane protein (LAMP-2) in the ZO rat. These PTC proteins are responsible for the endocytotic/lysosomal mechanism and further substantiate impairments in protein reabsorption/degradation concomitant with PTC injury [59]. Our ultrastructural observations additionally support maladaptive PTC remodeling with loss of basal polarity due to mitochondrial remodeling, loss of invaginating canalicular plasma membrane infoldings, and PTC thickening. Collectively, these data suggest that there is a proximal tubular origin of proteinuria with impaired tubular endocytosis of protein and a functionally impaired retrieval mechanism in models of obesity-induced renal injury.
Megalin is a critical protein which is directly and indirectly involved in the retrieval mechanism of albumin reabsorption: directly as a receptor and indirectly by its effects on the expression of cubilin, which is co-expressed with megalin in the brush border and the endocytic apparatus. Recent data highlight the impact of obesity on RAS activation and disruption of the retrieval mechanism in PTC [57,59] and, specifically, the impact angiotensin II (Ang II) has on the disruption of cytoskeletal organization. This endocytic pathway is especially susceptible to metabolic factors and growth factors such as Ang II and aldosterone. Data from PTC culture models as well as small animal models of obesity, such as the ZO rat model, support the concept that increased AT1 receptor (AT1R) pathway signaling reduces megalin expression and that blockade of AT1R improves megalin expression and lysosomal degradation of albumin. Consistent with our ultrastructural observations in the ZO rat and other insulin-resistant models, reduced megalin expression has been associated with loss of PTC endocytic invaginations/vesicles, reduced lysosomes and loss of canilicular integrity [56,57,58]. Our findings of markers of increased renal RAAS activation in the proximal tubule region suggest the possibility that RAAS-dependent reductions in megalin expression are closely associated with the impairment in the retrieval mechanism. However, the precise mechanisms in obesity-induced renal injury have yet to be elucidated.
To conclude, the data obtained from rodent models of obesity and insulin resistance, such as the ZO rat model, support the notion that obesity contributes to the activation of circulating RAAS but also tissue RAS components and to reduced insulin metabolic signaling that lead to NADPH oxidase generation of ROS and subsequent heart and kidney injury.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Sturm R. Increases in morbid obesity in the USA: 2000–2005. Public Health. 2007;121:492–496. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell AC, Adair LS, Popkin BM. Ethnic differences in the association between body mass index and hypertension. Am J Epidemiol. 2002;155:346–353. doi: 10.1093/aje/155.4.346. [DOI] [PubMed] [Google Scholar]
- 4.Sowers JR. Obesity as a cardiovascular risk factor. Am J Med. 2003;115(suppl 8A):37S–41S. doi: 10.1016/j.amjmed.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Alley DE, Chang VW. The changing relationship of obesity and disability, 1988–2004. JAMA. 2007;298:2020–2027. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- 6.Minkler M, Fuller-Thomson E, Guralnik JM. Gradient of disability across the socioeconomic spectrum in the United States. N Engl J Med. 2006;355:695–703. doi: 10.1056/NEJMsa044316. [DOI] [PubMed] [Google Scholar]
- 7.Goodpaster BH, DeLany JP, Otto AD, Kuller L, Vockley J, South-Paul JE, Thomas SB, Brown J, McTigue K, Hames KC, Lang W, Jakicic JM. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults. JAMA. 2010;304:1795–1802. doi: 10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr, International Diabetes Federation Task Force on Epidemiology and Prevention, National Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 9.McGee DL. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol. 2005;15:87–97. doi: 10.1016/j.annepidem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Ghandehari H, Le V, Kamal-Bahl S, Bassin SL, Wong ND. Abdominal obesity and the spectrum of global cardiometabolic risks in US adults. Int J Obes (Lond) 2009;33:239–248. doi: 10.1038/ijo.2008.252. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Differences in prevalence of obesity among black, white, and Hispanic adults – United States, 2006–2008. MMWR Morb Mortal Wkly Rep. 2009;58:740–744. [PubMed] [Google Scholar]
- 12.Govindarajan G, Hayden MR, Cooper SA, Figueroa SD, Ma L, Hoffman TJ, Stump CS, Sowers JR. Metabolic derangements in the insulin-resistant heart. J Cardiometab Syndr. 2006;1:102–106. doi: 10.1111/j.1559-4564.2006.05683.x. [DOI] [PubMed] [Google Scholar]
- 13.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris EM, Whaley-Connell AT, Thyfault JP, Britton SL, Koch LG, Wei Y, Ibdah JA, Sowers JR. Low aerobic capacity and high-fat diet contribute to oxidative stress and IRS-1 degradation in the kidney. Am J Nephrol. 2009;30:112–119. doi: 10.1159/000204362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whaley-Connell A, Sowers JR. Hypertension and insulin resistance. Hypertension. 2009;54:462–464. doi: 10.1161/HYPERTENSIONAHA.109.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X, Ma L, Habibi J, Whaley-Connell A, Hayden MR, Tilmon RD, Brown AN, Kim JA, DeMarco VG, Sowers JR. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the Zucker obese rat. Hypertension. 2010;55:880–888. doi: 10.1161/HYPERTENSIONAHA.109.145136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira AJ, Santos RA, Bradford CN, Mecca AP, Sumners C, Katovich MJ, Raizada MK. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension. 2010;55:207–213. doi: 10.1161/HYPERTENSIONAHA.109.140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motley ED, Eguchi K, Gardner C, Hicks AL, Reynolds CM, Frank GD, Mifune M, Ohba M, Eguchi S. Insulin-induced Akt activation is inhibited by angiotensin II in the vasculature through protein kinase C-alpha. Hypertension. 2003;41(3 Pt 2):775–780. doi: 10.1161/01.HYP.0000051891.90321.12. [DOI] [PubMed] [Google Scholar]
- 20.Carvalheira JB, Calegari VC, Zecchin HG, Nadruz W, Jr, Guimarães RB, Ribeiro EB, Franchini KG, Velloso LA, Saad MJ. The cross-talk between angiotensin and insulin differentially affects phosphatidylinositol 3-kinase- and mitogen-activated protein kinase-mediated signaling in rat heart: implications for insulin resistance. Endocrinology. 2003;144:5604–5614. doi: 10.1210/en.2003-0788. [DOI] [PubMed] [Google Scholar]
- 21.Andreozzi F, Laratta E, Sciacqua A, Perticone F, Sesti G. Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ Res. 2004;94:1211–1218. doi: 10.1161/01.RES.0000126501.34994.96. [DOI] [PubMed] [Google Scholar]
- 22.Wei Y, Whaley-Connell AT, Chen K, Habibi J, Uptegrove GM, Clark SE, Stump CS, Ferrario CM, Sowers JR. NADPH oxidase contributes to vascular inflammation, insulin resistance, and remodeling in the transgenic (mRen2) rat. Hypertension. 2007;50:384–391. doi: 10.1161/HYPERTENSIONAHA.107.089284. [DOI] [PubMed] [Google Scholar]
- 23.Ren J, Samson WK, Sowers JR. Insulin-like growth factor 1 as a cardiac hormone: physiological and pathophysiological implications in heart disease. J Mol Cell Cardiol. 1999;31:2049–2061. doi: 10.1006/jmcc.1999.1036. [DOI] [PubMed] [Google Scholar]
- 24.Ren J, Sowers JR, Walsh MF. Reduced contractile response to insulin and IGF-I in ventricular myocytes from genetically obese Zucker rats. Am J Physiol Heart Circ Physiol. 2000;279:H1708–H1714. doi: 10.1152/ajpheart.2000.279.4.H1708. [DOI] [PubMed] [Google Scholar]
- 25.Privratsky JR, Wold LE, Sowers JR, Ren J. AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension. 2003;42:206–212. doi: 10.1161/01.HYP.0000082814.62655.85. [DOI] [PubMed] [Google Scholar]
- 26.Gropler RJ. Methodology governing the assessment of myocardial glucose metabolism by positron emission tomography and fluorine 18-labeled fluorodeoxyglucose. J Nucl Cardiol. 1994;1(2 Pt 2):S4–S14. doi: 10.1007/BF02940063. [DOI] [PubMed] [Google Scholar]
- 27.Dutka DP, Pitt M, Pagano D. Myocardial glucose transport and utilization in patients with type 2 diabetes mellitus, left ventricular dysfunction, and coronary artery disease. J Am Coll Cardiol. 2006;48:2225–2231. doi: 10.1016/j.jacc.2006.06.078. [DOI] [PubMed] [Google Scholar]
- 28.Ouwens DM, Boer C, Fodor M, de Galan P, Heine RJ, Maassen JA. Cardiac dysfunction induced by high-fat diet is associated with altered myocardial insulin signalling in rats. Diabetologia. 2005;48:1229–1237. doi: 10.1007/s00125-005-1755-x. [DOI] [PubMed] [Google Scholar]
- 29.Scognamiglio R, Negut C, De Kreutzenberg SV, Tiengo A, Avogaro A. Postprandial myocardial perfusion in healthy subjects and in type 2 diabetic patients. Circulation. 2005;112:179–184. doi: 10.1161/CIRCULATIONAHA.104.495127. [DOI] [PubMed] [Google Scholar]
- 30.Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52:434–441. doi: 10.2337/diabetes.52.2.434. [DOI] [PubMed] [Google Scholar]
- 31.Sundell J, Knuuti J. Insulin and myocardial blood flow. Cardiovasc Res. 2003;57:312–319. doi: 10.1016/s0008-6363(02)00718-6. [DOI] [PubMed] [Google Scholar]
- 32.Peterson LR, Herrero P, Schechtman KB. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 33.Warnock DG, Muntner P, McCullough PA, Zhang X, McClure LA, Zakai N, Cushman M, Newsome BB, Kewalramani R, Steffes MW, Howard G, McClellan WM. Kidney function, albuminuria, and all-cause mortality in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. Am J Kidney Dis. 2010;56:861–871. doi: 10.1053/j.ajkd.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toto RD, Greene T, Hebert LA, Hiremath L, Lea JP, Lewis JB, Pogue V, Sika M, Wang X, AASK Collaborative Research Group Relationship between body mass index and proteinuria in hypertensive nephrosclerosis: results from the African American Study of Kidney Disease and Hypertension (AASK) cohort. Am J Kidney Dis. 2010;56:896–906. doi: 10.1053/j.ajkd.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sowers JR, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150:776–783. doi: 10.7326/0003-4819-150-11-200906020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayasi R, Akamine EH, Davel AP, Rodrigues MAM, Carvalho CRO, Rossoni LV. Oxidative stress and inflammatory mediators contribute to endothelial dysfunction in high-fat diet-induced obesity in mice. J Hypertens. 2010;28:2111–2119. doi: 10.1097/HJH.0b013e32833ca68c. [DOI] [PubMed] [Google Scholar]
- 37.Stehr CB, Mellado R, Ocaranza MP, Carvajal CA, Mosso L, Becerra E, Solis M, García L, Lavandero S, Jalil J, Fardella CE. Increased levels of oxidative stress, subclinical inflammation, and myocardial fibrosis markers in primary aldosteronism patients. J Hypertens. 2010;28:2120–2126. doi: 10.1097/HJH.0b013e32833d0177. [DOI] [PubMed] [Google Scholar]
- 38.Serpillon S, Floyd BC, Gupte RS, George S, Kozicky M, Neito V, Recchia F, Stanley W, Wolin MS, Gupte SA. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am J Physiol Heart Circ Physiol. 2009;297:H153–H162. doi: 10.1152/ajpheart.01142.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med. 2010;88:993–1001. doi: 10.1007/s00109-010-0663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young ME, Guthrie PH, Razeghi P, Leighton B, Abbasi S, Patil S, Youker KA, Taegtmeyer H. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes. 2002;51:2587–2595. doi: 10.2337/diabetes.51.8.2587. [DOI] [PubMed] [Google Scholar]
- 41.Coort SL, Hasselbaink DM, Koonen DP, Willems J, Coumans WA, Chabowski A, van der Vusse GJ, Bonen A, Glatz JF, Luiken JJ. Enhanced sarcolemmal FAT/CD36 content and triacylglycerol storage in cardiac myocytes from obese Zucker rats. Diabetes. 2004;53:1655–1663. doi: 10.2337/diabetes.53.7.1655. [DOI] [PubMed] [Google Scholar]
- 42.Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Tremblay F, BrÛlé S, Um SH, Li Y, Masuda K, Roden M, Sun XJ, Brebs M, Polakiewicz RD, Thomas G, Marette A. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2007;104:14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayer CM, Belsham DD. Central insulin signaling is attenuated by long-term insulin exposure via insulin receptor substrate-1 serine phosphorylation, proteasomal degradation, and lysosomal insulin receptor degradation. Endocrinology. 2010;151:75–84. doi: 10.1210/en.2009-0838. [DOI] [PubMed] [Google Scholar]
- 45.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 46.Ejerblad E, Fored M, Lindblad P, Fryzek J, McLaughlin JK, Nyren O. Obesity and risk of chronic renal failure. J Am Soc Nephrol. 2006;17:1695–1702. doi: 10.1681/ASN.2005060638. [DOI] [PubMed] [Google Scholar]
- 47.Aleixandre de Artiñano A, Miguel Castro M. Experimental rat models to study the metabolic syndrome. Br J Nutr. 2009;102:1246–1253. doi: 10.1017/S0007114509990729. [DOI] [PubMed] [Google Scholar]
- 48.Baynes J, Murrray DB. Cardiac and renal function are progressively impaired with aging in Zucker diabetic fatty type II diabetic rats. Oxid Med Cell Longev. 2009;2:328–334. doi: 10.4161/oxim.2.5.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coimbra TM, Janssen U, Gröne HJ, Ostendorf T, Kunter U, Schmidt H, Brabant G, Floege J. Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int. 2000;57:167–182. doi: 10.1046/j.1523-1755.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 50.Trujillo J, Ramírez V, Pérez J, Torre-Villalvazo I, Torres N, Tovar AR, Muñoz RM, Uribe N, Gamba G, Bobadilla NA. Renal protection by a soy diet in obese Zucker rats is associated with restoration of nitric oxide generation. Am J Physiol Renal Physiol. 2005;288:F108–F116. doi: 10.1152/ajprenal.00077.2004. [DOI] [PubMed] [Google Scholar]
- 51.Erdely A, Freshour G, Maddox DA, Olson JL, Samsell L, Baylis C. Renal disease in rats with type 2 diabetes is associated with decreased renal nitric oxide production. Diabetologia. 2004;47:1672–1676. doi: 10.1007/s00125-004-1509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nistala R, Whaley-Connell A, Sowers JR. Redox control of renal function and hypertension. Antioxid Redox Signal. 2008;10:2047–2089. doi: 10.1089/ars.2008.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blanco S, Bonet J, López D, Casas I, Romero R. ACE inhibitors improve nephrin expression in Zucker rats with glomerulosclerosis. Kidney Int Suppl. 2005;93:S10–S14. doi: 10.1111/j.1523-1755.2005.09303.x. [DOI] [PubMed] [Google Scholar]
- 54.Hayden MR, Habibi J, Whaley-Connell A, Sowers D, Johnson M, Tilmon R, Jain D, Ferrario CM, Sowers JR. Nebivolol attenuates maladaptive proximal tubule remodeling in transgenic rats. Am J Nephrol. 2010;31:262–272. doi: 10.1159/000278757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci. 2002;324:127–137. doi: 10.1097/00000441-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Whaley-Connell A, Rehmer N, Habibi J, Rehmer J, Ferrario CM, Sowers JR. Nebivolol reduces proteinuria and renal NADPH oxidase-generated reactive oxygen species in the transgenic Ren2 rat. Am J Nephrol. 2009;30:354–360. doi: 10.1159/000229305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Habibi J, Hayden MR, Sowers JR, Pulakat L, Elliott D, Tilmon RD, Manrique C, Lastra G, DeMarco VG, Whaley-Connell A. Nebivolol attenuates redox-sensitive glomerular and tubular mediated proteinuria in obese rats. Endocrinology. 2010 doi: 10.1210/en.2010-1038. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whaley-Connell A, Lastra G, Manrique C, Nistala R, Cooper SA, Westerly B, Wiedmeyer C, Miner J, Chowdhury N, Stump CS, Sowers JR. Insulin resistance, oxidative stress, and podocyte injury: role of rosuvastatin modulation of filtration barrier injury. Am J Nephrol. 2008;28:67–75. doi: 10.1159/000109394. [DOI] [PubMed] [Google Scholar]
- 59.Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol. 2009;20:489–494. doi: 10.1681/ASN.2008050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alonso-Galicia M, Brands MW, Zappe DH, Hall JE. Hypertension in obese Zucker rats. Role of angiotensin II and adrenergic activity. Hypertension. 1996;28:1047–1054. doi: 10.1161/01.hyp.28.6.1047. [DOI] [PubMed] [Google Scholar]
- 61.Kuwabara A, Satoh M, Tomita N, Sasaki T, Kashihara N. Deterioration of glomerular endothelial surface layer induced by oxidative stress is implicated in altered permeability of macromolecules in Zucker fatty rats. Diabetologia. 2010;53:2056–2065. doi: 10.1007/s00125-010-1810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]