Abstract
Aims
Lack of chronic kidney disease (CKD) awareness is common. Recent data suggest that the presence of concurrent diabetes may heighten CKD awareness, but current data have not supported the hypothesis that healthcare delivery or insurance status improves awareness in the diabetic population. Diabetes is associated with high cardiovascular disease (CVD) morbidity, especially in patients with CKD. We hypothesized that a highly prevalent co-morbid condition such as CVD in patients with diabetes would predict CKD awareness.
Methods
We utilized data from the National Kidney Foundation-Kidney Early Evaluation Program (KEEPTM), a large screening program designed to identify high-risk individuals for CKD and promote awareness.
Results
Among 77,077 participants, CKD was identified in 20,200 and diabetes in 23,082. Prevalence of CVD was higher in participants with than without diabetes (39.5 vs. 22.0%) and in stage 3–5 compared to stage 1–2 CKD (43.3 vs. 34.4%). Patients with diabetes and CVD had a higher level of awareness than those without diabetes (8.2 vs. 2.2%). Among patients with diabetes and CVD, the presence of congestive heart failure was a better predictor of awareness [odds ratio (OR) 1.84; 95% confidence interval (CI) 1.40–2.43] than endpoints such as myocardial infarction or stroke [OR 1.35 (95% CI 1.04–1.73) and OR 1.34 (95% CI 1.04–1.72), respectively].
Conclusions
While prevalence of CKD awareness remained low, our data suggest that in patients with diabetes the presence of CVD was associated with increased awareness in a targeted screening program for CKD awareness.
Key Words: Cardiovascular disease, Chronic kidney disease, Diabetes mellitus, KEEP
Introduction
Chronic kidney disease (CKD) is often silent until advanced stages of disease, and many individuals are unaware of the presence of CKD. Indeed, detection often occurs only shortly before the onset of symptomatic uremia. Previous population-based surveys conducted in the United States have suggested that CKD awareness is extremely low and most patients have not been effectively informed on their kidney disease by their medical professionals [1,2,3]. Although recent data from the National Health and Nutrition Examination Survey (NHANES) suggest that certain co-morbidities such as hypertension and diabetes may heighten awareness of CKD [1], the heightened awareness in these studies was not associated with an increased access to health care and insurance, suggesting other factors might increase awareness in this population.
Diabetes mellitus is the leading cause of incident and prevalent CKD, accounting for about 30–40% of CKD and up to 45% of end-stage renal disease [4,5]. Diabetes mellitus is also associated with several traditional Framingham risk factors for cardiovascular disease (CVD), such as hypertension, obesity, dyslipidemia, tobacco use, and increasing age, which collectively predict a very high CVD morbidity and mortality [6,7,8]. Recent data suggest that co-morbid conditions might increase control rates for certain disease states [9]. Therefore, we hypothesized that the presence of co-morbid CVD in patients with diabetes would predict increased CKD awareness. To evaluate this, we utilized the National Kidney Foundation (NKF)-Kidney Early Evaluation Program (KEEPTM), a large-scale, community-based screening program to promote awareness in a targeted population at risk for CKD [10,11].
Methods
KEEP Participants
The study population included only eligible KEEP participants from August 2000 through December 31, 2007, from 47 NKF affiliates and 1,966 screening programs in 49 states and the District of Columbia. Our KEEP study cohort includes 77,077 eligible participants with non-missing values for CKD stage and diabetic status. A complete description of the KEEP has been published previously [3].
Definitions
We applied common definitions for co-morbid conditions across analyses and data sets, with some exceptions (table 1, 2). In the KEEP data, diabetes was primarily defined as a history of diabetes (self-report or retinopathy), use of medications to treat diabetes, or newly diagnosed diabetes defined as fasting blood glucose ≥126 mg/dl or non-fasting blood glucose ≥200 mg/dl in the absence of self-report or medication use.
Table 1.
Distribution of demographic characteristics of KEEP participants with diabetes stratified by CKD stage and awareness
| Characteristics | Diabetics, % |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| all CKD |
stage 1–2 CKD |
stage 3–5 CKD |
|||||||
| all (7,853) | unaware (7,117) | aware (736) | all (2,548) | unaware (2,394) | aware (154) | all (5,305) | unaware (4,723) | aware (582) | |
| Age | |||||||||
| 18–30 years | 1.2 | 1.2 | 1.2 | 3.3 | 3.4 | 2.6 | 0.2 | 0.1 | 0.9 |
| 31–45 years | 7.0 | 7.0 | 7.3 | 14.6 | 14.5 | 14.9 | 3.4 | 3.1 | 5.3 |
| 46–60 years | 27.3 | 27.4 | 26.1 | 38.2 | 38.3 | 37.7 | 22.0 | 21.9 | 23.0 |
| 61–75 years | 45.1 | 44.8 | 48.0 | 34.3 | 34.0 | 37.7 | 50.3 | 50.2 | 50.7 |
| >75 years | 19.4 | 19.6 | 17.4 | 9.6 | 9.8 | 7.1 | 24.1 | 24.6 | 20.1 |
| Sex | |||||||||
| Male | 33.5 | 32.8 | 40.5 | 37.3 | 37.4 | 35.7 | 31.6 | 30.4 | 41.8 |
| Female | 66.5 | 67.2 | 59.5 | 62.7 | 62.6 | 64.3 | 68.4 | 69.6 | 58.2 |
| Race | |||||||||
| White | 53.4 | 53.3 | 54.3 | 40.9 | 40.5 | 47.4 | 59.4 | 59.8 | 56.2 |
| African American | 27.9 | 28.3 | 23.6 | 35.2 | 36.0 | 23.4 | 24.3 | 24.4 | 23.7 |
| Others | 18.7 | 18.4 | 22.0 | 23.8 | 23.5 | 29.2 | 16.3 | 15.8 | 20.1 |
| Ethnicity | |||||||||
| Hispanic | 9.4 | 9.0 | 13.6 | 13.5 | 12.8 | 24.0 | 7.5 | 7.1 | 10.8 |
| Non-Hispanic | 90.6 | 91.0 | 86.4 | 86.5 | 87.2 | 76.0 | 92.5 | 92.9 | 89.2 |
| Education | |||||||||
| <High school | 22.6 | 22.5 | 23.7 | 22.8 | 22.4 | 29.1 | 22.5 | 22.5 | 22.3 |
| ≥High school | 77.4 | 77.5 | 76.3 | 77.2 | 77.6 | 70.9 | 77.5 | 77.5 | 77.7 |
| Health insurance coverage | |||||||||
| Yes | 85.4 | 85.7 | 82.5 | 77.9 | 78.5 | 68.7 | 89.0 | 89.4 | 86.2 |
| No | 14.6 | 14.3 | 17.5 | 22.1 | 21.5 | 31.3 | 11.0 | 10.6 | 13.8 |
| Risk factors | |||||||||
| Current smoker | 8.6 | 8.7 | 8.1 | 13.5 | 13.5 | 14.1 | 6.3 | 6.3 | 6.5 |
| Obesity | 56.6 | 56.6 | 56.6 | 59.6 | 59.5 | 60.3 | 55.2 | 55.2 | 55.7 |
| Hypertension | 93.9 | 93.8 | 94.8 | 92.8 | 93.0 | 89.6 | 94.4 | 94.2 | 96.2 |
| Self-reported CVD | 40.4 | 39.0 | 54.2 | 34.5 | 34.0 | 42.2 | 43.3 | 41.5 | 57.4 |
| Dyslipidemia | 37.3 | 36.4 | 46.3 | 38.7 | 38.2 | 46.1 | 36.7 | 35.5 | 46.4 |
Numbers of patients are given in parentheses. Diabetes was defined as self-reported history of diabetes mellitus or treatment for diabetes.
Table 2.
CVD predicts CKD awareness in participants with and without diabetes mellitus (DM)
| CVD (n = 18,868) | Multivariate analysis, OR (95% CI)1 |
||
|---|---|---|---|
| aware with DM (n = 11,601) | aware without DM (n = 24,405) | all (n = 36,006) | |
| MI (n = 5,618) | 1.35 (1.04–1.73) | 1.21 (0.91–1.61) | 1.30 (1.08–1.57) |
| Stroke (n = 4,329) | 1.34 (1.04–1.72) | 1.19 (0.90–1.57) | 1.28 (1.06–1.55) |
| PCI (n = 2,009) | 1.39 (1.06–1.83) | 1.51 (1.11–2.05) | 1.44 (1.17–1.77) |
| CABG(n = 1,754) | 0.73 (0.52–1.01) | 1.22 (0.86–1.73) | 0.91 (0.72–1.16) |
| CHF(n = 1,605) | 1.84 (1.40–2.43) | 1.71 (1.22–2.39) | 1.81 (1.46–2.24) |
Diabetes is defined as self-reported history of DM, treatment for DM, fasting blood glucose ≥126 mg/ dl, or non-fasting blood glucose ≥200 mg/dl.
Logistic regressions for persons with diabetes, without diabetes, and all cohorts, respectively. Awareness of CKD is the dependent variable (1 = yes, 0 = otherwise). Predictors are MI, stroke, PCI, CABG, and CHF; controlled variables are: risk factors (hypertension, dyslipidemia, proteinuria, and obesity), age, gender, and race.
Estimated glomerular filtration rate (eGFR) was calculated using the isotope dilution mass spectrometry (IDMS)-traceable version of the Modification of Diet in Renal Disease (MDRD) 4-variable study formula [12], and serum creatinine was calibrated according to the Cleveland Clinic Research Laboratory [13]. CKD stages were defined as follows: stage 1, eGFR ≥90 ml/min/1.73 m2 and albumin-creatinine ratio ≥30 mg/g; stage 2, eGFR 60–89 ml/min/1.73 m2 and albumin-creatinine ratio ≥30 mg/g; stage 3, eGFR 30–59 ml/min/1.73 m2; stage 4, eGFR 15–29 ml/min/1.73 m2, and stage 5, eGFR <15 ml/min/1.73 m2. Target blood glucose was defined as fasting blood glucose >126 mg/dl or non-fasting blood glucose >140 mg/dl.
Hypertension was defined as self-reported history of hypertension, use of medications to treat hypertension, or newly diagnosed hypertension (Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; JNC 7) [14], defined as systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥80 mm Hg for patients with a history of diabetes or CKD, and in the remainder defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg.
CVD was defined as self-reported history of heart attack, heart angioplasty, bypass surgery, heart failure, abnormal heart rhythm, or stroke. We defined obesity as body mass index ≥30, and dyslipidemia as cholesterol >200 mg/dl or triglycerides >150 mg/dl (fasting or non-fasting). Awareness of CKD was defined as an affirmative response to the KEEP questionnaire question, ‘Have you ever been told you have kidney disease?’ (the questionnaire includes additional yes/no questions about kidney stones, bladder infections, and polycystic kidney disease).
Statistical Analysis
Demographic characteristics of the participants were described in the KEEP cohorts according to CKD and diabetic status. Diabetic KEEP participants with CKD were also characterized by CKD stage and awareness. Among participants with diabetes, demographic characteristics, risk factors (including smoking status, obesity, hypertension, CVD, proteinuria, and dyslipidemia), and measurements of kidney function, including albumin-creatinine ratio and eGFR, were reported by proportion stratified by CKD stages and CKD awareness. In univariate analysis, awareness of CKD was calculated by prevalence of CVD and proteinuria stratified by diabetes status.
We performed multivariate logistic analyses with awareness of CKD as the dependent variable for persons with and without diabetes, and for all cohorts. Predictors were myocardial infarction (MI), stroke, percutaneous intervention (PCI), coronary artery bypass graft (CABG), and congestive heart failure (CHF) controlled for variables such as age, gender, race, hypertension, dyslipidemia, proteinuria, and body mass index. A p value <0.05 indicated statistical significance.
Results
The demographics of this population have been described previously [3]. Briefly, among the 77,077 KEEP participants, 20,200 with CKD and 23,082 with diabetes were identified. The age distribution was comparable for KEEP participants with and without diabetes. Prevalence of diabetes was higher among CKD than non-CKD participants (40.7 and 26.1%, respectively). Educational achievement was higher among diabetics versus non-diabetics. Insurance coverage was similar between diabetic and non-diabetic participants with similar access to providers in the two groups.
There were 7,853 KEEP participants with diabetes and CKD (table 1). Awareness among diabetic patients with CKD was low at 9.4% (736 of 7,853 participants), being 6% in patients with CKD stages 1 and 2 (154 of 2,548 participants), and 10.9% in those with later-stage CKD (582 of 5,305 participants). While KEEP participants with diabetes and CKD had a higher level of education and insurance coverage, education and health insurance status were similar based on the level of CKD awareness. However, in CKD stages 1 and 2, there was a higher prevalence of education among those with high CKD awareness, which was not observed in later stages. In all diabetic CKD participants, the presence of co-morbid conditions was similar in those with and without CKD awareness, except for the presence of increased self-reported CVD.
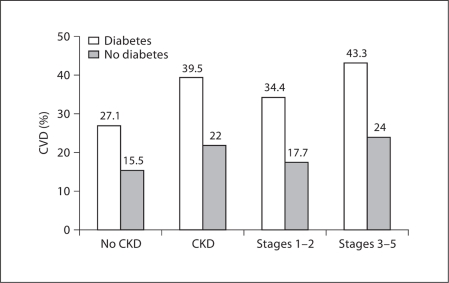
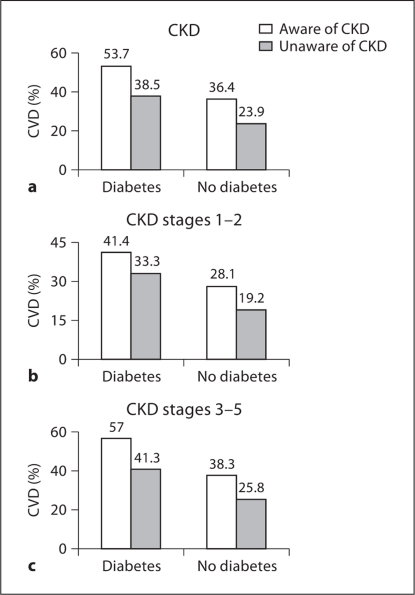
Consistent with previous reports on CVD prevalence, the prevalence of CVD was higher in participants with than without CKD (39.5 vs. 27.1%), and a similar pattern was observed in those with and without diabetes (39.5 vs. 22%; fig. 1). We explored this relationship in the context of CKD awareness (fig. 2). The presence of diabetes was associated with an increased prevalence of CVD in both those with and without CKD awareness. Prevalence of CVD was increased in participants aware compared to those unaware of CKD (53.7 vs. 38.5%; fig. 2a). The increased CVD prevalence among those with CKD awareness was apparent in both early- (fig. 2b) and late-stage CKD (fig. 2c). However, the association was stronger in late- compared to early-stage CKD (57 vs. 41%).
Fig. 1.
Prevalence of CVD according to CKD stage and diabetes status.
Fig. 2.
Prevalence of CVD in patients with CKD according to awareness and diabetic status.
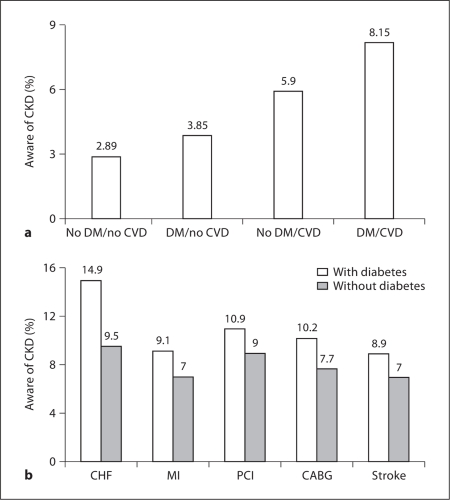
Among KEEP participants with diabetes and CVD, there was heightened CKD awareness compared to those without diabetes and without CVD (8.2 vs. 2.2%; fig. 3a). Indeed, although diabetes increased awareness in those with CVD, there was a higher prevalence of awareness in diabetics with CHF (14.9%) compared to those with self-reported MI (9.1%), PCI (10.9%), CABG (10.2%), or even stroke (8.9%; fig. 3b). Similarly, prevalent CVD predicted CKD awareness in diabetics in multivariate analyses, with CHF being the strongest predictor of CKD awareness (odds ratio 1.84; 95% confidence interval 1.40–2.43; table 2).
Fig. 3.
Prevalence of CKD awareness among KEEP participants stratified by diabetes mellitus (DM) status and CVD status (a) and DM status and individual CVD endpoints (b).
Discussion
In this report of the KEEP, as in other reports of epidemiologic studies of CKD patients, awareness of CKD was low [1,2]. Two factors, however, appear to increase CKD awareness: prevalent diabetes and prevalent CVD. Indeed, we found that among individuals with diabetes and CKD, prevalent CVD was associated with an increased CKD awareness. As the presence of diabetes was not associated with an increased delivery of healthcare or insurance status, our data suggest that co-morbid CVD explains why diabetes predicts an increased level of awareness.
Consistent with other reports, we found that CKD awareness was disturbingly low, namely <10%, in the general KEEP population [2,3]. Recent work to validate identification of CKD stages 1 and 2 may enhance the ability to effectively detect, identify, and/or communicate the necessity of screening for CKD or referral to a nephrologist [15]. Furthermore, it has been suggested that the extremely high CVD mortality rate associated with CKD negates the need for management (and, hence, communication of diagnoses) of CKD at earlier stages. Nonetheless, the awareness of CKD was only slightly higher in this population at advanced stages of CKD, with only 10.9% of those with an eGFR <60 ml/min/1.73 m2 being aware of any kidney dysfunction.
In this analysis of the KEEP data, CKD awareness was higher in patients with prevalent CVD and approached 15% in patients with heart failure. In fact, about half of the individuals in this cohort aware of their CKD status had concomitant CVD. This higher level of awareness is especially noteworthy given that healthcare delivery did not seem to increase awareness in the general diabetic population [3]. The data presented here suggest that, despite numerous programs designed to increase CKD awareness in the United States, awareness is only achieved in the presence of a serious co-morbidity. In this cohort, individuals were more likely to be aware of CKD if they were diabetic, and the level of awareness among diabetics increased significantly if they also had previous diagnoses of heart disease.
As we and others believe that CKD awareness is unacceptably low given the high morbidity and mortality associated with kidney disease [1,2,3], we should seek to understand why diabetes and CVD diagnoses heightened awareness. One possible explanation is that individuals with diabetes and CVD may be more likely to have family members with similar illnesses, including kidney disease. A family history of CKD should increase individuals’ knowledge about kidney disease and their desire to communicate with healthcare professionals about the status of their kidney function. Looking specifically at prevalent CVD and its impact on CKD awareness, we speculate that many individuals with CVD diagnoses are informed by their caregivers about the potential risk of CVD-associated diagnostic tests (e.g. cardiac catheterization) and treatments (e.g. renin angiotensin system blockade) to their kidneys, especially if renal function is already impaired. Both of these possible explanations highlight the importance of the communication between caregiver and patient to increase CKD awareness.
This study has several limitations. While we have data on participants’ overall awareness of CKD, we do not know how these participants became aware of their diagnoses and at what stage of the disease this awareness developed. We and others believe that disease awareness should improve long-term outcomes; for example, an individual who is aware of his or her CKD diagnosis may be more likely to avoid nephrotoxic medications or to adopt a low-salt diet. However, we do not have follow-up data on these participants to determine if those with CKD awareness actually fared better than those without awareness regarding kidney function, cardiovascular health, and overall mortality. Finally, because the data collection was not repeated, we were forced to assume that abnormalities in serum creatinine and urinary albumin excretion were measured appropriately and represent chronic disease states. In clinical practice, such tests should be repeated and shown to be persistent for at least 3 months to make a diagnosis of CKD. Therefore, it is conceivable that participants who were unaware of CKD in this analysis would not, on repeat testing, have chronic insufficiency.
In conclusion, this report from the KEEP reinforces the overall low awareness of CKD among individuals with early- and late-stage kidney disease. A diagnosis of diabetes increased awareness of CKD, as did prevalent heart disease. Awareness approached 15% in individuals with concomitant diagnoses of diabetes and CHF. While this is still unacceptably low, this higher awareness suggests that a high level of co-morbidity does facilitate caregiver-patient and/or provider-patient discussion about kidney function.
Disclosure Statement
KEEP is a program of the National Kidney Foundation, Inc., and is supported by Amgen, Abbott, Novartis, Siemens, Genentech, Genzyme, Nephroceuticals, Pfizer, LifeScan, and Suplena. The authors have no conflict of interest.
Acknowledgements
KEEP investigators other than the authors of this study include: Mike Shlipak, MD, Wendy Weinstock Brown, MD, MPH, Lesley Stevens, MD, Dennis Andress, MD, David Calhoun, MD, Bruce Johnson, MD, Claudine Jurkovitz, MD, MPH, and Chamberlain Obialo, MD. The authors would like to thank Monica R. Gannon (KEEP director) for regulatory assistance.
References
- 1.Plantinga LC, Boulware LE, Coresh J, Stevens LA, Miller ER, 3rd, Saran R, Messer KL, Levey AS, Powe NR. Patient awareness of chronic kidney disease: trends and predictors. Arch Intern Med. 2008;168:2268–2275. doi: 10.1001/archinte.168.20.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saab G, Whaley-Connell A, McCullough P, Bakris GL. Chronic kidney disease awareness: the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2008;52:382–383. doi: 10.1053/j.ajkd.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Whaley-Connell A, Sowers JR, McCullough PA, Roberts T, McFarlane SI, Chen SC, Li S, Wang C, Collins AJ, Bakris GL, KEEP Investigators Diabetes mellitus and CKD awareness: the Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) Am J Kidney Dis. 2009;53(S4):S11–S21. doi: 10.1053/j.ajkd.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 4.KDOQI KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49(2 suppl 2):S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Renal Data System . USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2006. [Google Scholar]
- 6.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 7.Stamler J, Vaccaro O, Neaton JD, Wetworth D. Diabetes, other risk factors, and 12-year cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 8.Fox CS, Larson MG, Leip EP, Meigs JB, Wilson PW, Levy D. Glycemic status and development of kidney disease: the Framingham Heart Study. Diabetes Care. 2005;28:2436–2440. doi: 10.2337/diacare.28.10.2436. [DOI] [PubMed] [Google Scholar]
- 9.Leoncini G, Viazzi F, Rosei EA, Ambrosioni E, Costa FV, Leonetti G, Pessina AC, Trimarco B, Volpe M, Deferrari G, Pontremoli R. Chronic kidney disease in hypertension under specialist care: the I-DEMAND study. J Hypertens. 2010;28:156–162. doi: 10.1097/HJH.0b013e328332038c. [DOI] [PubMed] [Google Scholar]
- 10.Brown WW, Peters RM, Ohmit SE, Keane WF, Collins A, Chen SC, King K, Klag MJ, Molony DA, Flack JM. Early detection of kidney disease in community settings: the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2003;42:22–35. doi: 10.1016/s0272-6386(03)00405-0. [DOI] [PubMed] [Google Scholar]
- 11.Jurkovitz CT, Qiu Y, Wang C, Gilbertson DT, Brown WW. The Kidney Early Evaluation Program (KEEP): program design and demographic characteristics of the population. Am J Kidney Dis. 2008;51(4 suppl 2):S3–S12. doi: 10.1053/j.ajkd.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration Using standardized serum creatinine values in the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 13.Stevens LA, Stoycheff N. Standardization of serum creatinine and estimated GFR in the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2008;51(4 suppl 2):S77–S82. doi: 10.1053/j.ajkd.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National High Blood Pressure Education Program Coordinating Committee The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 15.Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, Powe NR. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med. 2002;137:479–486. doi: 10.7326/0003-4819-137-6-200209170-00007. [DOI] [PubMed] [Google Scholar]