Abstract
Once-daily administration of 300 mg of lamivudine in combination with other antiretroviral agents has been proposed as a possible way to optimize anti-human immunodeficiency virus (HIV) treatment and to facilitate adherence. A single-center, randomized, two-way, crossover study was conducted in 60 healthy subjects to compare the steady-state pharmacokinetics of lamivudine in plasma and its putative active anabolite, lamivudine 5′-triphosphate (lamivudine-TP), in peripheral blood mononuclear cells (PBMCs) following 7 days of treatment with lamivudine at 300 mg once daily and 7 days of the standard regimen of 150 mg twice daily. Serial blood samples were collected over 24 h for determination of plasma lamivudine concentrations by liquid chromatography-mass spectrometry and intracellular lamivudine-TP concentrations in peripheral blood mononuclear cells by high-performance liquid chromatography/radioimmunoassay methods. Pharmacokinetic parameters were calculated based on lamivudine and lamivudine-TP concentration-time data. Regimens were considered bioequivalent if 90% confidence intervals (CI) for the ratio (once daily/twice daily) of geometric least-squares (GLS) means for lamivudine and lamivudine-TP pharmacokinetic values fell within the acceptance range of 0.8 to 1.25. Steady-state plasma lamivudine pharmacokinetics following the once- and twice-daily regimens were bioequivalent with respect to the area under the drug concentration-time curve from 0 to 24 h at steady state (AUC24,ss) (GLS mean ratio, 0.94; 90% CI, 0.92, 0.97) and average plasma lamivudine concentration over the dosing interval (Cave,ss) (GLS mean ratio, 0.94; 90% CI, 0.92, 0.97). Steady-state intracellular lamivudine-TP pharmacokinetics after the once- and twice-daily regimens were bioequivalent with respect to AUC24,ss (GLS mean ratio, 0.99; 90% CI, 0.88, 1.11), Cave,ss (GLS mean ratio, 0.99; 90% CI, 0.88, 1.11), and maximum lamivudine concentration (Cmax,ss) (GLS mean ratio, 0.93; 90% CI, 0.81, 1.07). Lamivudine-TP trough concentrations were modestly lower (by 18 to 24%) during the once-daily regimen; the clinical importance of this is unclear, given the large intersubject variability in values that was observed (coefficient of variation, 48 to 124%). Once-daily lamivudine was as well tolerated as the twice-daily regimen. Overall, the results of this study suggest that for key AUC-related parameters, lamivudine at 300 mg once daily is pharmacokinetically equivalent to lamivudine at 150 mg twice daily.
Lamivudine is a nucleoside reverse transcriptase inhibitor (NRTI) that is frequently used as a core component of highly active antiretroviral therapy (HAART) regimens in the treatment of human immunodeficiency virus (HIV) infection (13). In clinical trials conducted over 48 weeks, twice-daily regimens combining lamivudine at 150 mg with the NRTIs zidovudine (300 mg) and abacavir (300 mg) have proved equivalent to lamivudine-zidovudine-protease inhibitor combinations in reducing HIV-1 RNA levels and elevating CD4 cell counts (19; S. Matheron, F. Brun-Vezinet, R. Viraben, J.-E. Malkin, D. Troisvallets, and A. Lafeuillade, Abstr. XIII Int. AIDS Conf., 2000; P. Cahn, Abstr. XIII Int. AIDS Conf., abstr. B606, 2000). Lamivudine is generally well tolerated, even at supratherapeutic doses over many months (14, 20), with adverse events (primarily headache, nausea, and malaise/fatigue) being infrequent and usually mild and transient (13).
The antiretroviral activity of lamivudine is due to its active intracellular anabolite, lamivudine 5′-triphosphate (lamivudine-TP) (3). For this anabolite to be formed, lamivudine undergoes stepwise intracellular phosphorylation, first by deoxycytidine kinase to lamivudine monophosphate (lamivudine-MP), then by CMP kinase and dCMP kinase to lamivudine diphosphate (lamivudine-DP), and finally by pyrimidine nucleoside diphosphate kinase to the TP anabolite (3). Lamivudine-TP is eliminated slowly from cells, with a long intracellular half-life (15 to 16 h) (10) that supports once-daily usage. In clinical trials of up to 1 year's duration, 300 mg of lamivudine administered once daily in combination with other antiretroviral agents has reduced viral load and elevated CD4+ cell counts as well as has been reported with HAART regimens that include 150 mg of lamivudine twice daily (18; F. Maggiolo, M. Migliorino, R. Maserati et al., 8th Conf. Retrovir. Opportunistic Infect., p. 320, 2001; W. C. Woodward, J. Lash, and R. E. Doerfler, Abstr. Infect. Dis. Soc. Am. 37th Annu. Meet., abstr. 372, p. 104, 1997; R. Landman, R. Schiemann, S. Thiam et al., XIII Int. AIDS Conf., poster LbPp102, 2000; M. Sension, N. Bellos, J. Johnson et al., 8th Conf. Retrovir. Opportunistic Infect., abstr. 317, 2001; A. Rieger, U. Hein, H. Schon et al., 5th Conf. Retrovir. Opportunistic Infect., abstr. 697, p. 209, 1998; A. Haberl, P. Gute, A. Carlebach et al., Abstr. 12th World AIDS Conf., abstr. 22398, 1998; G. Skowron, D. Kuritzkes, M. Thompson et al., Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 537, 2000; C. Bowonwatanuwong, K. Supparatponyo, P. Moosikapun et al., 1st Int. AIDS Soc. Conf. HIV Pathogenesis Treatment, abstr. 4, 2001).
To date, studies of lamivudine phosphorylation have proved valuable in determining differences in lamivudine anabolism in different cell types (3, 6, 8), assessing steady-state lamivudine-TP concentrations following lamivudine at 150 mg twice daily (10; J. D. Moore, E. P. Acosta, G. Valette et al., Abstr. 7th Conf. Retrovir. Opportunistic Infect., abstr. 96, 2000; B. L. Robbins, M. Harris, T. Tran et al., Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1168, p. 300, 2000) and 300 mg twice daily (10), and establishing the absence of a linear relationship between concentrations of lamivudine-TP and lamivudine in serum (10). However, there have been no studies to investigate the intracellular lamivudine-TP concentrations that result from a 300-mg once-daily lamivudine regimen. The purpose of this study was to compare the steady-state pharmacokinetics of lamivudine in plasma and lamivudine-TP in peripheral blood mononuclear cells (PBMCs) in healthy subjects following 7-day courses of treatment with lamivudine at 300 mg once daily and 150 mg twice daily.
(Data from this study were presented in part as abstract 269 at the 5th International Congress on Drug Therapy in HIV Infection, Glasgow, Scotland, 22 to 26 October 2000.)
MATERIALS AND METHODS
Patient selection.
Male and nonpregnant female subjects could be included in this study if they were 18 to 55 years of age; weighed 50 to 100 kg; had a body mass index within 19 to 29 kg/m2; had laboratory values within normal limits or not clinically significantly different from normal; and, if female, were either of non-childbearing potential (e.g., sterile) or were receiving contraception. Subjects were excluded if they were HIV positive, as confirmed by antibody enzyme-linked immunosorbent assay (ELISA) and Western blot test; were positive for hepatitis C antibody or hepatitis B surface antigen at screening; had a history of hypersensitivity to lamivudine; had participated in another drug trial within 60 days prestudy; had a clinically significant electrocardiographic finding at screening; were in poor general health or had been acutely ill within 7 days prestudy; had donated blood (≥1 pint) or experienced significant blood loss within 60 days prestudy; had a medical condition that could interfere with the absorption, distribution, metabolism, or excretion of drugs; or had a history of substance abuse (detectable in urine at screening) or ethanol abuse. Subjects were not allowed to use tobacco products, xanthines, or ethanol for at least 24 h before and until 24 h after each dosing period. All females had a β-human chorionic gonadotropin (β-HCG) pregnancy test at screening (serum) and prior to each treatment period (urine) to rule out pregnancy.
Study design.
This was a single-center, open-label, randomized, two-period, crossover phase I study that was conducted between September 1999 and February 2000 at the clinical research facility within PPD Development in Austin, Texas. The study protocol was approved by the Human Investigations Committee at the study site, and written informed consent was obtained from each subject.
The subjects were randomly assigned to receive each of two lamivudine regimens: 150 mg twice daily and 300 mg once daily for 7 days. Twice daily was defined as every 12 h, and once daily was defined as every 24 h. No washout period was allowed between the regimens. The 150-mg dose of lamivudine was administered as a 150-mg tablet of Epivir (Glaxo Wellcome, Inc., Research Triangle Park, N.C.; batch no. 9ZP0828), and the 300-mg dose was administered as an experimental tablet containing said amount of lamivudine (batch no. A98B104; also supplied by Glaxo Wellcome, Inc.). All subjects received each dose of their regimen under direct supervision of an investigator.
Measurements and assays.
Blood samples were collected from fasted subjects on day 7 of each regimen period. During the twice-daily regimen, sampling was done before the morning dose (0 h) and at 1, 2, 4, 8, 12, 13, 14, 16, 18, 20, and 24 h postdose for determination of plasma lamivudine and intracellular lamivudine-TP. During the once-daily regimen, blood sampling was done at all of the times pertinent to the twice-daily period, except at 13, 14, 18, and 20 h postdose.
For sampling, 6 to 8 ml of blood was collected in three 8-ml Vacutainer (Becton Dickinson, Franklin Lakes, N.J.) cell preparation tubes (CPT; Becton Dickinson), mixed gently, and then stored upright at room temperature (15 min or less) until centrifugation. Immediately before centrifugation, the tubes were inverted six to eight times, spun for 30 min at 3,600 rpm and 18 to 25°C in a swing-out rotor, and inverted again. The two upper layers above the gel separation were then gently mixed. These layers were poured out of all of the CPTs into a 50-ml centrifuge tube. A small aliquot (0.5 ml or less) was taken and used with a Coulter counter to determine an accurate PBMC count in the entire sample. The samples were centrifuged for 10 min at 3,600 rpm and 4°C to separate the cells from the plasma. An aliquot of 1.5 ml of plasma was taken and dispensed into a fresh 3.6-ml internally threaded cryo-storage vial and stored at −20°C until plasma lamivudine bioanalysis was performed. The remaining plasma was removed, and the cell pellet was suspended with 0.6 ml of 60% methanol and incubated on ice for 15 min to ensure cell lysis. The lysate was stored at −20°C until lamivudine-TP bioanalysis was performed. Prior to analysis, the lysate was centrifuged to remove cellular debris.
Plasma lamivudine bioanalysis.
Plasma samples were analyzed for lamivudine concentrations by a bioanalytical method in which plasma samples were prepared by dilution with an equal volume of 25 mM ammonium acetate buffer (pH 5) and 100 μl of labeled internal standard followed by membrane filtration with an Amicon Centricon-30 filter to remove proteins and then analysis by liquid chromatography-tandem mass spectrometry (LC/MS/MS) using turbo ionspray. Interday precision (percent coefficient of variation [CV]) of the method of analysis based on quality control (QC) samples was <12.2.2%, and accuracy (percent bias) was ±5.3% (2.5 to 5,000 μg/ml). All samples were stored at approximately −30°C prior to analysis.
Intracellular lamivudine-TP bioanalysis.
PBMC samples were analyzed for determination of intracellular lamivudine-TP concentrations by a validated solid-phase extraction (SPE) method coupled with LC/MS/MS. Methanolic cellular extracts (200 μl each) were combined with stable isotopically labeled lamivudine-TP internal standard (15N3, 13C2-lamivudine) and loaded onto a Waters 100-mg QMA Accel 96-well SPE plate (Waters Corp., Milford, Mass.). The SPE plate was rinsed with increasing concentrations of KCl (1.0 ml each of 60, 70, 80, and 90 mM KCl [pH 5.0]) to remove lamivudine, lamivudine-MP, and lamivudine-DP. Lamivudine-TP was eluted and collected by using 2 volumes (0.75 ml each) of 750 mM KCl and incubated at room temperature for at least 1 h with 5 U of alkaline phosphatase (bovine intestinal mucosa; Sigma, St. Louis, Mo.) to dephosphorylate lamivudine-TP. After dephosphorylation, KCl and alkaline phosphatase were removed by applying the sample to additional SPE (Isolute-96; 100 mg, C18) with a water wash. Dephosphorylated lamivudine was eluted with methanol, evaporated to dryness, reconstituted with 200 μl of water, and analyzed by LC/MS/MS (9). Calibration standards and QC samples, spiked with lamivudine-TP, were processed along with the unknown sample through the entire sample preparation. The lamivudine-TP calibration range was 10 to 1,000 ng/ml and the intra- and interassay precision (percent CV) and accuracy (percent bias) were <15%. The specificity of the assay for lamivudine-TP was determined to be good, with percent contributions of molar equivalents of lamivudine, lamivudine-MP, and lamivudine-DP of 0, 0.1, and 5.9%, respectively. Due to the inadequate biological matrix supply (control 60% methanol-extracted PBMCs) to support this study, a surrogate matrix was used for preparation of calibration standards and the QC sample. The surrogate matrix was prepared by precipitating 800 ml of human plasma with 1,200 ml of methanol and centrifuging and retaining the supernatant. Assay performance using surrogate matrix was validated and similar to that of the study matrix (60% methanol cell lysate).
Pharmacokinetic analysis.
Single-dose pharmacokinetic evaluations of lamivudine in plasma and of lamivudine-TP in PBMCs were made at steady state (on day 7) of each regimen after the subjects had fasted overnight. The following pharmacokinetic parameters were derived by noncompartmental methods from lamivudine plasma concentration-time data: area under the lamivudine concentration versus time curve from 0 to 24 h after dosing at steady state (AUC24,ss), as determined by the linear trapezoidal method; the maximum measured lamivudine concentration at steady state (Cmax,ss); the lamivudine concentration just prior to the next administered dose at steady state (steady-state trough concentration; C0,ss); the lamivudine concentration at 24 h at steady state (C24,ss); the average lamivudine concentration over the dosing interval at steady state (Cave,ss), determined by dividing AUC24,ss by 24 h; and the first time to reach Cmax (tmax).
The following pharmacokinetic parameters were derived from PBMC lamivudine-TP concentration-time data at steady state: AUC24,ss, Cmax,ss, C0,ss, and C24,ss.
Safety analysis.
Safety was evaluated by monitoring adverse events and vital signs, physical examinations, clinical laboratory tests, and electrocardiograms. Each subject was questioned periodically throughout the study regarding possible adverse events. The date, time of onset after administration of the study drug, severity (mild, moderate, or severe), duration, and potential relationship to study drug (unrelated or possibly, probably, or almost certainly related [as per the investigator]) of adverse events were recorded. Changes from baseline to discharge in 12-lead electrocardiograms, vital signs, and laboratory data were summarized.
Statistical analysis.
Statistical analysis of AUC24,ss, Cmax,ss, Cave,ss, C0,ss and C24,ss for lamivudine and lamivudine-TP was performed after log transformation, whereas analysis of tmax was performed by analysis of variance without transformation. All concentration data were listed and summarized by regimen. Carryover effect was examined through predose concentration and sequence effect. The pharmacokinetic comparison of lamivudine at 300 mg once daily and 150 mg twice daily was considered equivalent with respect to AUC24,ss, Cmax,ss, and Cavg,ss if the 90% confidence interval (CI) for the 300-mg once-daily/150-mg twice-daily ratio fell entirely within the acceptance range of 0.80 to 1.25 for the loge-transformed parameters. With regard to plasma lamivudine pharmacokinetics, only 18 evaluable subjects were required to demonstrate equivalence (80 to 125%) with greater than 80% power based on an intrasubject standard deviation (SD) of 0.17 for plasma lamivudine AUC. With regard to lamivudine-TP pharmacokinetics, using a sample size of 60 and an intrasubject SD of 0.37 for lamivudine-TP AUC (based on findings from an earlier lamivudine intracellular pharmacokinetics study, NUCB4001) (10), this study had 80% power to demonstrate the equivalence (80 to 125%) of lamivudine-TP AUC24,ss between the two regimens.
No formal statistical comparison of the safety results was performed due to the relatively small number of events expected. The safety data are summarized in the tables by subject and treatment.
RESULTS
Subject disposition.
Sixty-three subjects enrolled in the study, and 60 completed it. Of the three subjects who were discontinued prematurely, one withdrew consent, one discontinued because he had experienced a non-study drug-related grand mal seizure, and one discontinued because he had experienced a nonserious, possibly study drug-related pruritus. The subject population included 46 Caucasians, 9 Hispanics, 6 African-Americans, 1 Asian, and 1 “other.” Seventy-three percent (46 subjects) were male, and 27% (17 subjects) were female. Subjects ranged in age from 18 to 54 years (mean ± SD, 31.2 ± 10.4 years) and averaged 175.3 ± 7.8 cm in height and 73.9 ± 11.5 kg in weight.
Plasma lamivudine pharmacokinetics.
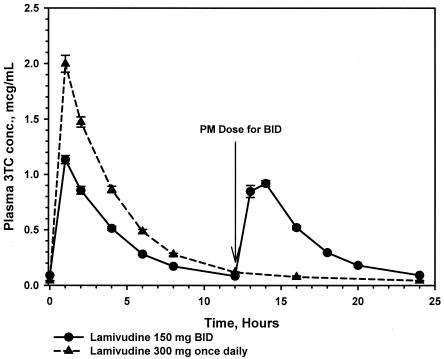
A total of 1,333 plasma samples were assayed for lamivudine concentrations. Adequate plasma lamivudine concentration-time data for pharmacokinetic analysis were obtained from 60 subjects. Steady-state plasma lamivudine pharmacokinetics following the once- and twice-daily regimens were bioequivalent with respect to AUC24,ss (geometric least-squares [GLS] mean ratio, 0.94; 90% CI, 0.92, 0.97) and Cave, ss (GLS mean ratio, 0.94; 90% CI, 0.92, 0.97) (Table 1). The 300-mg once-daily regimen produced 53% lower plasma lamivudine trough concentrations (C0,ss and C24,ss) and a 66% higher Cmax than the 150-mg twice-daily regimen (Fig. 1). A period effect was observed, as evidenced by the slightly higher plasma lamivudine concentrations seen during the period of the second dosing regimen. The GLS mean plasma half-life (95% CI) was 8.65 h (8.53 to 9.80 h), based on the once-daily regimen. Due to the abbreviated sampling schedule within the dosing interval, an accurate calculation of the plasma half-life for the twice-daily regimen could not be obtained. No difference between the two regimens was observed in tmax, which occurred at approximately 1 h postdose.
TABLE 1.
Plasma lamivudine pharmacokinetic parameters for 60 patients
| Parametera | Lamivudine regimen
|
B/A ratio | |
|---|---|---|---|
| A (150 mg twice daily) | B (300 mg once daily) | ||
| AUC24,SS (μg · h/ml) | |||
| GLS mean (95% CI) | 9.21b (8.81, 9.63) | 8.70b (8.28, 9.14) | |
| GLS mean ratio (90% CI) | 0.94 (0.92, 0.97) | ||
| Arithmetic mean ± SD | 9.35 ± 1.64 | 8.87 ± 1.83 | |
| % CV | 18 | 21 | |
| Median | 9.06 | 8.44 | |
| Range | 6.02-14.10 | 5.64-14.86 | |
| Cmax,ss (μg/ml) | |||
| GLS mean (95% CI) | 1.19b (1.12, 1.26) | 1.97b (1.84, 2.11) | |
| GLS mean ratio (90% CI) | 1.66 (1.57, 1.74) | ||
| Arithmetic mean ± SD | 1.22 ± 0.29 | 2.04 ± 0.54 | |
| % CV | 24 | 26 | |
| Median | 1.17 | 1.98 | |
| Range | 0.62-1.91 | 0.97-3.40 | |
| C0,ss (μg/ml) | |||
| GLS mean (95% CI) | 0.09b (0.08, 0.09) | 0.04b (0.04, 0.04) | |
| GLS mean ratio (90% CI) | 0.47 (0.44, 0.50) | ||
| Arithmetic mean ± SD | 0.09 ± 0.03 | 0.04 ± 0.02 | |
| % CV | 29 | 38 | |
| Median | 0.09 | 0.04 | |
| Range | 0.03-0.16 | 0.02-0.11 | |
| C24,ss (μg/ml) | |||
| GLS mean (95% CI) | 0.09b (0.08, 0.09) | 0.04b (0.04, 0.04) | |
| GLS mean ratio (90% CI) | 0.47 (0.45, 0.49) | ||
| Arithmetic mean ± SD | 0.09 ± 0.02 | 0.04 ± 0.01 | |
| % CV | 25 | 30 | |
| Median | 0.09 | 0.04 | |
| Range | 0.05-0.16 | 0.02-0.09 | |
| Cave,ss (μg/ml) | |||
| GLS mean (95% CI) | 0.38b (0.37, 0.40) | 0.36b (0.34, 0.38) | |
| GLS mean ratio (90% CI) | 0.94 (0.92, 0.97) | ||
| Arithmetic mean ± SD | 0.39 ± 0.07 | 0.37 ± 0.08 | |
| % CV | 18 | 21 | |
| Median | 0.38 | 0.35 | |
| Range | 0.25-0.59 | 0.24-0.62 | |
| tmax (h) | |||
| GLS mean (95% CI) | 1.19b (1.05, 1.22) | 1.09 (1.03, 1.17) | |
| GLS mean ratio (90% CI) | −0.05 (−0.15, 0.04) | ||
| Arithmetic mean ± SD | 1.19 ± 0.40 | 1.13b ± 0.34 | |
| % CV | 34 | 30 | |
| Median | 1.00 | 1.00 | |
| Range | 0.83-2.00 | 1.00-2.00 | |
AUC24,ss area under the plasma lamivudine concentration versus time curve from 0 to 24 h after dosing at steady state; Cmax,ss, maximum measured plasma lamivudine concentration at steady state; C0,ss, plasma lamivudine concentration just prior to the next administered dose (steady-state trough concentration; C24,ss, the plasma lamivudine concentration at 24 h after dosing at steady state; Cave,ss, average plasma lamivudine concentration over the dosing interval; tmax, time at which Cmax occurred.
Number is log-transformed.
FIG. 1.
Mean serum lamivudine (3TC) concentrations (micrograms per milliliter) plus standard errors following administration of lamivudine at 150 mg twice daily (BID) and 300 mg once daily for 7 days (n = 60).
Intracellular (PBMC) lamivudine pharmacokinetics.
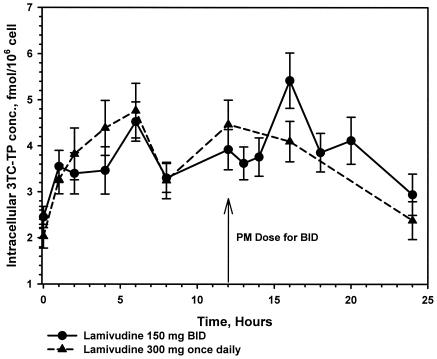
A total of 1,333 PBMC intracellular samples were assayed for intracellular lamivudine-TP concentrations. Adequate intracellular lamivudine-TP concentration data for pharmacokinetic analysis were obtained from 55 subjects during the twice-daily regimen and from 54 subjects during the once-daily regimen. Steady-state intracellular lamivudine-TP pharmacokinetics after the once- and twice-daily regimens were bioequivalent with respect to AUC24,ss (GLS mean ratio, 0.99; 90% CI, 0.88,1.11), Cave, ss (GLS mean ratio, 0.99; 90% CI, 0.88, 1.11), and maximum lamivudine concentration (Cmax,ss) (GLS mean ratio, 0.93; 90% CI, 0.81, 1.07) (Table 2). Lamivudine-TP trough concentrations were modestly lower (by 18 to 24%) during the once-daily regimen (Fig. 2). A period effect was observed, as evidenced by the slightly higher lamivudine-TP concentrations seen during the period of the second dosing regimen. The terminal half-life of intracellular lamivudine-TP was 18.4 h, based on a median naïve pool of the terminal data.
TABLE 2.
Intracellular lamivudine-TP pharmacokinetic parameters
| Parametera | Lamivudine regimen
|
B/A ratio | |
|---|---|---|---|
| A (150 mg twice daily) | B (300 mg once daily) | ||
| AUC24,SS (pmol · h/106 cells) | |||
| n | 55 | 54 | |
| GLS mean (95% CI) | 85.38b (73.71, 98.89) | 86.71b (74.38, 101.08) | |
| GLS mean ratio (90% CI) | 0.99 (0.88, 1.11) | ||
| Arithmetic mean ± SD | 96.99 ± 46.88 | 99.58 ± 51.48 | |
| % CV | 48 | 52 | |
| Median | 95.51 | 92.39 | |
| Range | 15.35-222.28 | 14.86-251.32 | |
| Cmax,ss (pmol/106 cells) | |||
| n | 55 | 54 | |
| GLS mean (95% CI) | 8.15b (6.99, 9.51) | 7.74b (6.56, 9.14) | |
| GLS mean ratio (90% CI) | 0.93 (0.81, 1.07) | ||
| Arithmetic mean ± SD | 9.45 ± 5.09 | 9.20 ± 5.55 | |
| % CV | 54 | 60 | |
| Median | 8.25 | 7.77 | |
| Range | 1.81-24.77 | 1.86-25.47 | |
| C0,ss (pmol/108 cells) | |||
| n | 54 | 53 | |
| GLS mean (95% CI) | 1.84b (1.43, 2.38) | 1.42b (1.10, 1.85) | |
| GLS mean ratio (90% CI) | 0.76 (0.58, 1.00) | ||
| Arithmetic mean ± SD | 2.48 ± 1.72 | 2.11 ± 1.95 | |
| % CV | 69 | 93 | |
| Median | 2.11 | 1.61 | |
| Range | 0-6.69 | 0.13-8.80 | |
| C24,SS (pmol/106 cells) | |||
| n | 55 | 53 | |
| GLS mean (95% CI) | 2.40b (1.79, 3.23) | 1.97b (1.43, 2.72) | |
| GLS mean ratio (90% CI) | 0.82 (0.58, 1.14) | ||
| Arithmetic mean ± SD | 3.17 ± 3.54 | 2.62 ± 3.25 | |
| % CV | 112 | 124 | |
| Median | 2.71 | 1.90 | |
| Range | 0-17.08 | 0-17.56 | |
| Cave,ss (pmol/106 cells) | |||
| n | 55 | 54 | |
| GLS mean (95% CI) | 3.55b (3.07, 4.12) | 3.61b (3.10, 4.21) | |
| GLS mean ratio (90% CI) | 0.99 (0.88, 1.11) | ||
| Arithmetic mean ± SD | 4.04 ± 1.95 | 4.15 ± 2.15 | |
| % CV | 48 | 52 | |
| Median | 3.98 | 3.85 | |
| Range | 0.64-9.26 | 0.62-10.47 | |
AUC24,ss, area under the intracellular lamivudine concentration-versus-time curve from 0 to 24 h after dosing at steady state; Cmax,SS, maximum measured intracellular lamivudine concentration at steady state; C0,ss, intracellular lamivudine concentration just prior to the next administered dose (steady-state trough concentration; C24,ss, intracellular lamivudine concentration at 24 h after dosing at steady state; Cave,ss, average intracellular lamivudine concentration over the dosing interval; tmax, time at which Cmax occurred.
Number is log-transformed.
FIG. 2.
Mean lamivudine-TP (3TC-TP) concentrations plus standard errors in PBMCs following administration of lamivudine at 150 mg twice daily (BID) (n = 55) and 300 mg once daily for 7 days (n = 54).
Safety.
The once- and twice-daily regimens were equally well tolerated. Adverse events possibly related to lamivudine were reported in 11 (18%) and 9 (15%) subjects following the once-daily and twice-daily dosing periods, respectively. These events included headache (4 versus 2 patients), disturbance of concentration (2 versus 0 patients), fatigue (2 versus 0 patients), abdominal pain (1 versus 1 patient), dyspepsia (1 versus 1 patient), musculoskeletal pain (1 versus 0 patient), pruritus (0 versus 1 patient), and rash (0 versus 1 patient). No clinically significant changes in laboratory safety parameters occurred.
DISCUSSION
The results of this study suggest that, based on key AUC-related parameters, lamivudine at 300 mg once daily is pharmacokinetically equivalent to the standard 150-mg twice-daily regimen. This at least partly explains why in comparative clinical trials, the once-daily regimen, in combination with other antiretroviral agents, has reduced plasma HIV RNA levels and elevated CD4 cell counts as well as lamivudine at 150 mg twice daily in HIV-1-infected patients receiving the same concurrent antiretroviral therapy (Sension et al., 8th Conf. Retrovir. Opportunistic Infect., p. 317; Bonwonwatanuwong et al., 1st Int. AIDS Soc. Conf. HIV Pathogenesis Treatment, abstr. 4).
The steady-state plasma lamivudine pharmacokinetic parameters observed in this study were similar to those previously reported in HIV-infected patients (7). A higher steady-state plasma Cmax in the absence of a significant change in lamivudine exposure (AUC) occurred following the 300-mg once-daily regimen compared to the 150-mg twice-daily regimen, a pattern that was also seen when these two dosage regimens were compared over a 15-day period in patients with hepatitis B infection (7). Although lamivudine plasma trough concentrations (C0,ss, 0.02 to 0.11 μg/ml; C24,ss, 0.02 to 0.09 μg/ml) were 53% lower with the 300-mg once-daily regimen than the 150-mg twice-daily regimen, they nevertheless remained within the 90% inhibitory concentration (IC90) range of lamivudine against HIV-1 in various cell lines (0.0087 to 0.464 μg/ml) (4, 17). In a crossover study of 7-day regimens of lamivudine at 300 mg once daily and 150 mg twice daily in 12 HIV-infected patients, Bruno (R. Bruno, 1st Int. AIDS Soc. Conf. HIV Pathogenesis Treatment, abstr. 342, 2001) reported similarly lower plasma lamivudine trough concentrations (by 56% versus 53% in the present study) and higher Cmax values (by 67% versus 66%) with the 300-mg once-daily regimen in the absence of significant differences in plasma lamivudine Cave, AUC, and tmax. In addition, Bruno noted that the elimination half-life of lamivudine (not evaluated in the present study) following 300 mg once daily did not differ from that observed following 150 mg twice daily.
The AUC24,ss, Cave,ss, and Cmax,ss results of this study indicate that the intracellular lamivudine-TP pharmacokinetics following lamivudine at 300 mg once daily are essentially equivalent to those following 150 mg twice daily. Pharmacokinetic differences between the two lamivudine regimens were less striking intracellularly than in plasma. Thus, in contrast to what occurred in plasma, the intracellular lamivudine-TP Cmax,ss did not differ between the regimens, and trough concentrations, reflected in the C24,ss and C0,ss values, were only 18 to 24% lower with the once-daily regimen. The latter difference is of unknown clinical significance in view of the high interpatient variability in intracellular pharmacokinetics that was seen in this study and the observation that median lamivudine-TP C24,ss and C0,ss concentrations (1.90 and 1.61 pmol/106 cells, respectively) remained within the range reported in lamivudine responders (1.01 to 4.32 pmol/106 cells) (Robbins et al., 40th ICAAC, abstr. 1168). The smaller difference between the 300-mg once-daily and 150-mg twice-daily regimens for intracellular lamivudine-TP trough concentrations compared with plasma lamivudine trough concentrations is probably due to the saturable enzymatic step from intracellular lamivudine-DP to lamivudine-TP and the effect of pooling of intracellular lamivudine-DP. These factors allow continued production of lamivudine-TP despite lower plasma lamivudine concentrations in the 12- to 24-h postdose period for the 300-mg once-daily regimen compared to the 150-mg twice-daily regimen.
The plasma lamivudine and intracellular lamivudine-TP pharmacokinetics observed in our study population of healthy volunteers were comparable to those previously described in HIV-infected patients (7, 10). We felt that a study population consisting of healthy subjects was appropriate, because earlier studies showed no differences in lamivudine pharmacokinetic parameters in healthy males and females from those reported in patients with HIV infection (11, 21). Furthermore, because a population pharmacokinetics study showed that lamivudine pharmacokinetics are unaffected by surrogate markers of HIV disease (CD4+ counts, HIV-1 RNA PCR, and Centers for Disease Control and Prevention Classification), it is unlikely that lamivudine biodispositions would differ between healthy and HIV-infected populations (12).
Our study showed a high degree of intersubject variability in intracellular lamivudine-TP pharmacokinetics (CV, 48 to 124%). This magnitude of variability is consistent with the CVs generally exceeding 50% that have been found in other studies of intracellular lamivudine-TP pharmacokinetics (10; Moore et al., Abstr. 7th Conf. Retrovir. Opportunistic Infect., abstr. 96; B. L. Robbins, T. T. Tran, F. H. Pinkerton, Jr., et al., Program Abstr. 5th Conf. Retrovir. Opportunistic Infect., abstr. 361, p. 224, 1998). Variability in nucleoside phosphorylation is likely due to a combination of patient-related factors (immune status, cellular function, phenotype, genetic variation), pharmacokinetic/pharmacodynamic factors (endogenous enzyme activity, competition for enzymes by endogenous substrates, and concomitantly administered nucleoside antiretrovirals), and assay-related factors (e.g., bioanalytical limitations) (1, 2, 15, 16).
In this short-term study, the absence of differences in the relative safety profiles of lamivudine at 300 mg once daily and 150 mg twice daily was expected. Two longer-term phase I to phase II studies previously confirmed that the safety profile of lamivudine did not differ over the dose range of 0.5 to 12 mg/kg/day (∼37.5 to 900 mg/day), administered for 24 weeks or longer (14, 20). Moreover, in the NUCA3001 study, which compared lamivudine at 300 mg twice daily (twice the daily dose administered in this study) with 150 mg twice daily in combination with zidovudine over 24 weeks, no differences in type or incidence of adverse events were noted (5).
In conclusion, for key AUC-related parameters, lamivudine at 300 mg once daily is pharmacokinetically equivalent to lamivudine at 150 mg twice daily and is equally well tolerated.
Acknowledgments
We thank Gary Pakes for assistance with the writing of the manuscript.
REFERENCES
- 1.Barry, M., M. Wild, G. Veal, D. Back, A. Breckenridge, R. Fox, N. Beeching, F. Nye, P. Carey, and D. Timmins. 1994. Zidovudine phosphorylation in HIV-infected patients and seronegative volunteers. AIDS 8:F1-F5. [DOI] [PubMed] [Google Scholar]
- 2.Barry, M. G., S. H. Khoo, G. J. Veal, P. G. Hoggard, S. E. Gibbons, E. G. Wilkins, O. Williams, A. M. Breckenridge, and D. J. Back. 1996. The effect of zidovudine dose on the formation of intracellular phosphorylated metabolites. AIDS 10:1361-1367. [DOI] [PubMed] [Google Scholar]
- 3.Cammack, N., P. Rouse, C. L. Marr, P. J. Reid, R. E. Boehme, J. A. Coates, C. R. Penn, and J. M. Cameron. 1992. Cellular metabolism of (−) enantiomeric 2′-deoxy-3′-thiacytidine. Biochem. Pharmacol. 43:2059-2064. [DOI] [PubMed] [Google Scholar]
- 4.Coates, J. A., N. Cammack, H. J. Jenkinson, I. M. Mutton, B. A. Pearson, R. Storer, J. M. Cameron, and C. R. Penn. 1992. The separated enantiomers of 2′-deoxy-3′-thiacytidine (BCH 189) both inhibit human immunodeficiency virus replication in vitro. Antimicrob. Agents Chemother. 36:202-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eron, J. J., S. L. Benoit, J. Jemsek, R. D. MacArthur, J. Santana, J. B. Quinn, D. R. Kuritzkes, M. A. Fallon, and M. Rubin. 1995. Treatment with lamivudine, zidovudine, or both in HIV-positive patients with 200 to 500 CD4+ cells per cubic millimeter. N. Engl. J. Med. 333:1662-1669. [DOI] [PubMed] [Google Scholar]
- 6.Gray, N. M., C. L. P. Marr, C. R. Penn, J. M. Cameron, and R. C. Bethell. 1995. The intracellular phosphorylation of (−) 2′-deoxy-3′-thiacytidine (3TC) and the incorporation of 3TC 5′-monophosphate into DNA by HIV-1 reverse transcriptase and human DNA polymerase γ. Biochem. Pharmacol. 50:1043-1051. [DOI] [PubMed] [Google Scholar]
- 7.Johnson, M. A., K. H. P. Moore, G. J. Yuen, A. Bye, and G. E. Pakes. 1999. Clinical pharmacokinetics of lamivudine. Clin. Pharmacokinet. 36:41-66. [DOI] [PubMed] [Google Scholar]
- 8.Kewn, S., G. J. Veal, P. G. Hoggard, M. G. Barry, and D. J. Back. 1997. Lamivudine (3TC) phosphorylation and drug interactions in vitro. Biochem. Pharmacol. 54:589-595. [DOI] [PubMed] [Google Scholar]
- 9.Moore, J. D., G. Valette, A. Darque, X. J. Zhou, and J. P. Sommadossi. 2000. Simultaneous quantitation of the 5′-triphosphate metabolites of zidovudine, lamivudine, and stavudine in peripheral mononuclear blood cells of HIV-infected patients by high-performance liquid chromatography tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 11:1134-1143. [DOI] [PubMed] [Google Scholar]
- 10.Moore, K. H. P., J. E. Barrett, S. Shaw, G. E. Pakes, R. Churchus, A. Kapoor, J. Lloyd, M. G. Barry, and D. Back. 1999. The pharmacokinetics of lamivudine phosphorylation in peripheral blood mononuclear cells from patients infected with HIV-1. AIDS 13:2239-2250. [DOI] [PubMed] [Google Scholar]
- 11.Moore, K. H. P., S. Shaw, A. L. Laurent, P. Lloyd, B. Duncan, D. M. Morris, M. J. O'Mara, and G. E. Pakes. 1999. Lamivudine/zidovudine as a combined formulation tablet: bioequivalence compared with lamivudine and zidovudine administered concurrently and the effect of food on absorption. J. Clin. Pharmacol. 39:593-605. [DOI] [PubMed] [Google Scholar]
- 12.Moore, K. H. P., G. J. Yuen, E. K. Hussey, G. E. Pakes, J. J. Eron, Jr., and J. A. Bartlett. 1999. Population pharmacokinetics of lamivudine in adult human immunodeficiency virus-infected patients enrolled in two phase III clinical trials. Antimicrob. Agents Chemother. 43:3025-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry, C. M., and D. Faulds. 1997. Lamivudine. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in the management of HIV infection. Drugs 53:657-680. [DOI] [PubMed] [Google Scholar]
- 14.Pluda, J. M., T. P. Cooley, J. S. G. Montaner, L. E. Shay, N. E. Reinholter, S. N. Warthan, J. Ruedy, H. M. Hirst, C. A. Vicar, J. B. Quinn et al. 1995. A phase I/II study of 2′-deoxy-3′-thiacytidine (lamivudine) in patients with advanced human immunodeficiency virus infection. J. Infect. Dis. 171:1438-1447. [DOI] [PubMed] [Google Scholar]
- 15.Robbins, B. L., J. Rodman, C. McDonald, R. V. Srinivas, P. M. Flynn, and A. Fridland. 1994. Enzymatic assay for measurement of zidovudine triphosphate in peripheral blood mononuclear cells. Antimicrob. Agents Chemother. 38:115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodman, J. H., B. Robbins, P. M. Flynn, and A. Fridland. 1996. A systemic and cellular model for zidovudine plasma concentrations and intracellular phosphorylation in patients. J. Infect. Dis. 174:490-499. [DOI] [PubMed] [Google Scholar]
- 17.Soudeyns, H., X. J. Yao, Q. Gao, B. Belleau, J.-L. Kraus, N. Nguyen-Ba, B. Spira, and M. A. Wainberg. 1991. Anti-human immunodeficiency virus type 1 activity and in vitro toxicity of 2′-deoxy-3′thiacytidine (BCH-189), a novel heterocyclic nucleoside analog. Antimicrob. Agents Chemother. 35:1386-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staszewski, S., A. Haberl, P. Gute, G. Nisius, V. Miller, and A. Carlebach.1998. Nevirapine/didanosine/lamivudine once daily in HIV-1-infected intravenous drug users. Antiviral Ther. 3(Suppl. 4):55-56. [PubMed] [Google Scholar]
- 19.Staszewski, S., P. Keiser, J. Montaner, F. Raffi, J. Gathe, V. Brotas, C. Hicks, S. M. Hammer, D. Cooper, M. Johnson, S. Tortell, A. Cutrell, D. Thorborn, R. Isaacs, S. Hetherington, H. Steel, W. Spreen et al. 2001. Abacavir-lamivudine-zidovudine vs. indinavir-lamivudine-zidovudine in antiretroviral-naïve HIV-infected adults. A randomized equivalence trial. JAMA 285:1155-1163. [DOI] [PubMed] [Google Scholar]
- 20.van Leeuwen, R., C. Katlama, V. Kitchen, C. A. Boucher, R. Tubiana, M. McBride, D. Ingrand, J. Weber, A. Hill, H. McDade et al. 1995. Evaluation of safety and efficacy of 3TC (lamivudine) in patients with asymptomatic or mildly symptomatic human immunodeficiency virus infection: a phase I/II study. J. Infect. Dis. 171:1166-1171. [DOI] [PubMed] [Google Scholar]
- 21.Yuen, G. J., Y. Lou, N. F. Thompson, V. R. Otto, T. L. Allsup, W. B. Mahony, and H. W. Hutman. 2001. Abacavir/lamivudine/zidovudine as a combined formulation tablet: bioequivalence compared with each component administered concurrently and the effect of food on absorption. J. Clin. Pharmacol. 41:277-288. [DOI] [PubMed] [Google Scholar]