Abstract
Auxin functions as a key morphogen in regulating plant growth and development. Studies on auxin-regulated gene expression and on the mechanism of polar auxin transport and its asymmetric distribution within tissues have provided the basis for realizing the molecular mechanisms underlying auxin function. In eukaryotes, members of the Ras and Rho subfamilies of the Ras superfamily of small GTPases function as molecular switches in many signaling cascades that regulate growth and development. Plants do not have Ras proteins, but they contain Rho-like small G proteins called RACs or ROPs that, like fungal and metazoan Rhos, are regulators of cell polarity and may also undertake some Ras functions. Here, we discuss the advances made over the last decade that implicate RAC/ROPs as mediators for auxin-regulated gene expression, rapid cell surface-located auxin signaling, and directional auxin transport. We also describe experimental data indicating that auxin–RAC/ROP crosstalk may form regulatory feedback loops and theoretical modeling that attempts to connect local auxin gradients with RAC/ROP regulation of cell polarity. We hope that by discussing these experimental and modeling studies, this perspective will stimulate efforts to further refine our understanding of auxin signaling via the RAC/ROP molecular switch.
RAC/ROP GTPases AS MOLECULAR SWITCHES FOR REGULATING CELLULAR FUNCTIONS
Regulation of RAC/ROP Activation and Inactivation
RAC/ROP GTPases (see Supplemental Table 1 online for a list of key genes/proteins/mutants referred to in the text) are a large family of Rho-related molecular switches in plants that together regulate many cellular and developmental processes and responses to the environment (Nibau et al., 2006; Yalovsky et al., 2008; Yang, 2008). Although conserved in their fundamental characteristics as signal transducers, RAC/ROPs belong to a plant-specific clade, related to but clearly distinguishable from their counterparts in animals and fungi (Fowler, 2010). Like other members of the Ras small G protein family, RAC/ROPs bind GTP and GDP with high affinity and hydrolyze GTP inefficiently. RAC/ROPs exist in three conformations: an active GTP-bound state, a short-lived nucleotide free state, and an inactive GDP-bound state. In the active GTP-bound state, RAC/ROPs interact with effector proteins to initiate downstream signaling (Berken and Wittinghofer, 2008). Shuttling between GDP-bound and GTP-bound states is controlled by two major regulators. Guanine nucleotide exchange factors (GEFs) catalyze GDP release, which is exchanged with GTP due the higher concentrations of GTP in cells. GTPase activating proteins (GAPs) enhance GTP hydrolysis, thereby accelerating RAC/ROP inactivation (Berken and Wittinghofer, 2008). The inefficient GTP hydrolysis assures that RAC/ROPs can be maintained in the active conformation for relatively long time periods, as long as they are spatially separated from GAPs. The high affinity binding of GDP requires a GEF function to overcome the energetic barrier of nucleotide release. Hence, RAC/ROPs are maintained in a GDP-bound, inactive state when they are spatially separated from GEFs or when the GEFs are not active. These biochemical properties of the RAC/ROP switch enable the regulation of their function by external signals such as auxin.
RAC/ROPs typically function from the cell membrane, and membrane binding is facilitated by posttranslational lipid modifications. Based on their amino acid sequences, RAC/ROPs have been divided into two subgroups, designated type I and type II (Winge et al., 2000). Type I RAC/ROPs terminate with a canonical CaaL motif and are attached to the membrane following prenylation in the cytoplasm by the protein geranylgeranyltransferase-I (Sorek et al., 2007, 2011) and CaaX processing in the endoplasmic reticulum (ER) (Bracha-Drori et al., 2008). Type II RAC/ROPs terminate with a GC-CG box sequence motif and are attached to the plasma membrane by S-acylation (palmitoylation) (Lavy et al., 2002; Lavy and Yalovsky, 2006).
Modulation of Cell Polarity by Regulation of RAC/ROPs in Time and Space
The mode of RAC/ROP activation/inactivation cycles and their subcellular localization turn them into molecular switches that relay signals in time and space and at specific subcellular locations. Several examples described below exemplify this type of regulation. In the growing pollen tube tip, ROPGEFs (Berken et al., 2005) are localized at or close to the plasma membrane and interact with a tip-localized receptor protein kinase (Kaothien et al., 2005). GAPs were shown to localize either at the shank or proximal to the pollen tube apex, presumably restricting active RAC/ROPs to the growing tip (Klahre and Kost, 2006; Hwang et al., 2008). Hence, localized activation/inactivation of RAC/ROPs by positional signals presumably relays spatial information that modulates cell polarity. The initiation and polar growth of root hairs depends on spatially regulated RAC/ROPs (Molendijk et al., 2001; Jones et al., 2002; Carol et al., 2005; Bloch et al., 2005), possibly in response to a local auxin gradient (Fischer et al., 2006). A ROPGEF-interacting receptor kinase, FERONIA (Escrobar-Restrepo et al., 2007), participates in auxin-regulated root hair development (Duan et al., 2010), thus potentially contributing to mediating auxin signaling from the cell surface.
Besides the family of PRONE domain–containing ROPGEFs (Berken et al., 2005), Arabidopsis thaliana also uses a single DOCK180 family of Rho-GEFs (Rossman et al., 2005), SPIKE1, as a RAC/ROP activator (Qiu et al., 2002; Basu et al., 2008). SPIKE1 has recently been located to ER exit sites, where it colocalizes with RAC/ROPs (Zhang et al., 2010), presumably activating them to initiate signaling from intracellular locations. RAC/ROP activation also promotes their G-domain S-acylation by palmitic and stearic acids and stabilizes interaction with the plasma membrane (Sorek et al., 2010). In the plasma membrane, where they partition into lipid rafts (Sorek et al., 2007, 2010), RAC/ROPs inhibit endocytosis (Bloch et al., 2005), potentially contributing to the auxin-inhibited endocytic process (Paciorek et al., 2005; Robert et al., 2010). Partitioning of small G proteins into nanoclusters is believed to be essential for their function. A model on regulation of mitogen-activated protein kinase cascade suggests that Ras partitioning in membrane microdomains of mammalian cells facilitates its activation in response to local concentrations of epidermal growth factor at a given concentration and postulates Ras functions as an on/off switch, converting the analog signal of the growth factor into a fixed digital output (Tian et al., 2007). In analogy, localized activation of RAC/ROPs and their subsequent partitioning into membrane microdomains may facilitate formation of fixed outputs in response to a local auxin gradient.
Effectors That Mediate RAC/ROP Functions
Activated RAC/ROPs interact with immediate cellular effectors that interact with other cellular components, relaying the signal to the ultimate target systems to effect the corresponding signal-induced responses (Figure 1). RICs (for Rop-interactive CRIB motif-containing proteins) are a family of CRIB (for CDC42/RAC-interactive binding motifs found in fungal and mammalian Rho GTPase effectors) motif-containing proteins (Wu et al., 2001). ICRs (for Interactor of constitutive active ROPs) comprise a family of coiled-coil domain-containing proteins, unique to plants, that likely provide scaffolds to mediate RAC/ROP signaling (Lavy et al., 2007). RICs were shown to control cytoskeletal organization and dynamics in auxin-regulated epidermal pavement cell patterning (Xu et al., 2010), while ICR1 is involved in regulation of polarized secretion, thereby affecting directional auxin transport and asymmetric distribution (Hazak et al., 2010).
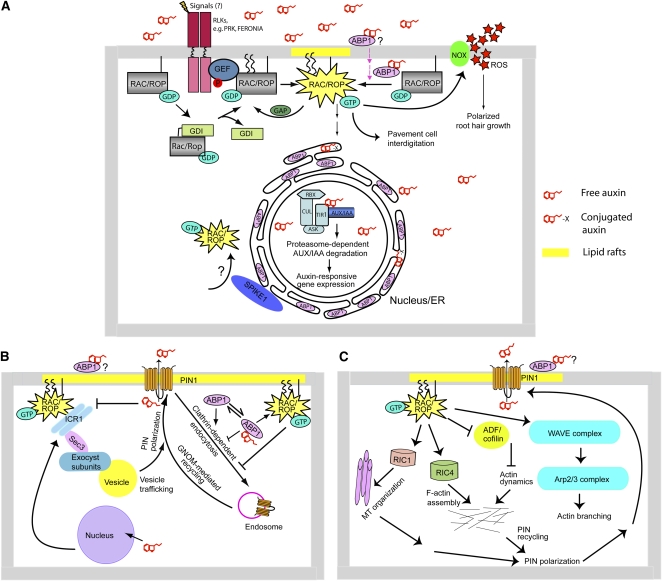
Figure 1.
Regulation of RAC/ROP Signaling and Its Downstream Pathways.
(A) Regulation of RAC/ROP cycling and downstream pathways. Only three cellular target systems are shown: auxin-responsive gene expression, control of polarized root hair growth, and regulation of pavement cell interdigitation. Potential SPIKE1-mediated intracellular RAC/ROP activation remains to be demonstrated. GDI is guanine nucleotide dissociation inhibitor. The straight line connecting RAC/ROP to the membrane represents modification by geranylgeranylation, and the two wavy lines represent S-acylation.
(B) Direct effect of RAC/ROPs on PIN polar localization. The model summarizes the involvement of the RAC/ROP effector ICR1 in recruitments of PIN to polar domain in the plasma membrane and the transcriptional and posttranscriptional regulation of ICR1 expression and stability by auxin. Where RAC/ROP and ABP1 may intersect in their regulation of PIN endocytosis is also depicted.
(C) Indirect effects of RAC/ROPs on polar PIN localization. The model shows how RAC/ROPs may modulate PIN localization indirectly through regulation of actin and microtubule cytoskeleton. MT, microtubule; ADF/cofilin, actin-depolymerizing factor/cofilin; Arp2/3 complex, actin-related protein 2/3 complex, an actin-nucleating protein complex; WAVE complex, a protein complex that activates the Arp2/3 complex.
Auxin Distribution as a Regulator of RAC/ROP Localization and Function
Root hairs develop at the rootward end of trichoblasts, the root hair–forming cells in the root epidermis, as a result of a local auxin gradient that depends on the function of the auxin influx transporter AUX1 (Bennett et al., 1996; Marchant et al., 1999), the ADP Ribosylation Factor GDP/GTP Exchange Factor (ARF-GEF) GNOM (Steinmann et al., 1999), and ER membrane–located Ethylene Insensitive2 (Alonso et al., 1999; Qiao et al., 2009). A local auxin gradient is required for the formation of RAC/ROP patch that precedes the formation of root hairs. This local auxin gradient is abolished in aux1 gnom ein2 triple mutants, resulting in formation of shootward and rootward RAC/ROP patches and root hair formation (Fischer et al., 2006). Furthermore, local production of auxin enhanced rootward positioning of the RAC/ROP patch and root hair formation. Based on these findings, it has been proposed that a local auxin gradient functions upstream of RAC/ROP recruitment to a specific domain in the trichoblast plasma membrane and root hair formation. The activation of RAC/ROPs by auxin (Tao et al., 2002; Xu et al., 2010) and the involvement of the ROPGEF-interacting receptor-like kinase (RLK) FERONIA in root hair development (Duan et al., 2010) are consistent with the notion that a local auxin gradient may indeed activate RAC/ROPs prior to root hair development.
The evidence showing that a RAC/ROP patch forms as a result of a local auxin gradient (Fischer et al., 2006) led to development of a mathematical model that illustrates how RAC/ROPs could function as spatial switches relaying localized auxin signals (Payne and Grierson, 2009). The model shows that an auxin gradient-induced RAC/ROP activation can predict the localization of root hair formation in trichoblasts. The model also properly predicts the root hair phenotypes of various mutants, including those defective in auxin transport (aux1 and gnomeb), auxin signaling (axr1), or ethylene signaling (etr1 and eto1), as well as the phenotypes of plants overexpressing ROP or expressing a constitutively active (CA) ROP and a mutant defective in S-acylation (tip1).
According to the Payne and Grierson (2009) model, a mechanism that depends on root hair length, auxin gradient, low RAC/ROP levels, and low diffusion rate of active RAC/ROPs can explain the formation of RAC/ROP patches, which precede root hair formation. The model assumes that S-acylation of activated RAC/ROPs slows down the RAC/ROP diffusion rate, thus facilitating patch formation (Payne and Grierson, 2009). Measurements of the interaction kinetics of RAC/ROPs with the membrane using fluorescence recovery after photobleaching beam size analysis (Sorek et al., 2010) confirmed some of the predictions that were made in the model. For example, when RAC/ROPs were activated and S-acylated, the only contribution to fluorescence recovery was by lateral diffusion of the RAC/ROPs in the plasma membrane. By contrast, exchange with the cytoplasm had almost the same contribution to fluorescence recovery in RAC/ROP S-acylation mutants. It was further shown that S-acylation is required for RAC/ROP-regulated cell polarity (Sorek et al., 2010). Hence, S-acylation indeed stabilizes interaction of RAC/ROPs with the membrane.
However, the Payne and Grierson model cannot distinguish between the effects of the proposed auxin gradient on RAC/ROP activation or inactivation or that both may exist. RAC/ROPs are presumably bound to the inner leaflet of the plasma membrane and are highly mobile. In line with this assumption, the fluorescence recovery after photobleaching measurements showed that the fluorescence recovery is fast and reaches almost 100% for either S-acylated or non-S-acylated RAC/ROPs (Sorek et al., 2010). Thus, to maintain only a small patch of active RAC/ROPs, they must be quickly inactivated by GAPs, which in turn must be localized in close proximity to the RAC/ROP patch. Similar mechanisms were demonstrated in pollen tubes (Klahre and Kost, 2006; Hwang et al., 2008) and for Cdc42 during bud formation in yeast (Tong et al., 2007). Validating the hypothesis that a local auxin gradient may affect GAP distribution or function of other RAC/ROP regulators awaits experimental proofs.
RAC/ROPs AS MEDIATORS FOR AUXIN SIGNALING
Auxin Activates RAC/ROPs
In 2002, Tao et al. provided the first evidence showing that auxin activates RAC/ROPs. Using a protein pull-down assay that specifically targets activated forms of RAC/ROPs (Lemichez et al., 2001), Tao et al. (2002) showed that addition of exogenous auxin to wild-type tobacco (Nicotiana tabacum) seedlings stimulated activation of endogenous RAC/ROPs in a dose-dependent manner and within a window of time short enough to account for some of the early auxin-inducible gene expression responses (Figure 2). Recently, using similar experimental strategies and auxin-treated protoplasts derived from GFP-ROP2 or GFP-ROP6 transgenic Arabidopsis, Xu et al. (2010) reiterated the observation for auxin-stimulated RAC/ROP activation and showed that it occurred with kinetics rapid enough to account for the earliest cell membrane responses recorded for auxin regulation (Badescu and Napier, 2006). Taken together, these studies that directly monitor RAC/ROP activation provide strong evidence for auxin-signaled activation of these small GTPases.
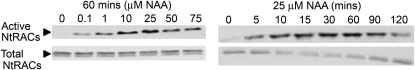
Figure 2.
Auxin Activates RAC/ROPs.
GST-PBD pull-down assays targeting active Nt-RACs in tobacco seedlings treated with increasing concentrations of NAA for 60 min showed RAC/ROP activation with as low as 0. 1 μM NAA (left) or within 5 min with 25 μM NAA (right). The level of total Nt-RACs (active and inactive) was not notably affected. Reproduced from Tao et al. (2002). Figure 2 of Xu et al. (2010) shows similar experiments performed in Arabidopsis protoplasts.
RAC/ROPs Mediate Auxin-Regulated Gene Expression
In exploring the downstream target systems for auxin-activated RAC/ROP signaling, Tao et al. (2002) showed that activated RAC/ROPs mediate auxin-induced gene expression. They showed that expression of wild-type and CA Nt-RAC1 in protoplasts and tobacco seedlings induced the expression of auxin-responsive genes in the absence of exogenous auxin. Moreover, auxin-stimulated expression from auxin-responsive genes was blocked by overexpressing dominant-negative Nt-RAC1 or the negative regulator RhoGDI to downregulate endogenous RAC/ROP signaling, providing evidence that these small GTPases act as signal transducers for auxin-regulated gene expression (Tao et al., 2002). Tao et al. (2005) further showed that auxin and the transcription repressors auxin/indole-3-acetic acid (AUX/IAA) proteins mediate a cellular process that assembles proteolytically active nuclear protein bodies, degrading the repressors therein. Transgene-expressed wild-type and CA Nt-RAC1 mimicked the auxin effect in the absence of exogenous auxin, while dominant-negative Nt-RAC1 blocked auxin-mediated nuclear protein assembly, showing that RAC/ROP signaling is also intermediary to this process. Furthermore, auxin also promotes recruitment of substrates (AUX/IAA proteins) and proteins essential for TIR1/AFB (auxin signaling F-box proteins) mediated proteolysis, including components of the COP9 signalosome, the E3 ligase SCFTIR1, and the 26S proteasome, from the nucleoplasm to these nuclear particles. Therefore, the RAC/ROP-regulated auxin-responsive gene expression pathway clearly integrates with the TIR1/AFBs controlled nuclear pathway of gene derepression via ubiquitin/26S proteasome regulated proteolysis of the repressor AUX/IAA proteins (Dharmasiri et al., 2005; Kepinski and Leyser, 2005; Chapman and Estelle, 2009). However, how auxin-activated RAC/ROP mediates the assembly of proteolytically active protein complexes and leads to derepression of auxin-responsive gene expression in the nucleus remain to be elucidated.
RAC/ROPs AND AUXIN TRANSPORT
Regulation of Auxin Transport and Asymmetric Distribution by RAC/ROPs
Basal localization of auxin efflux transporter PIN proteins requires constitutive vesicle recycling, which is regulated by the ARF-GEF GNOM and the endocytosis regulators clathrin and Rab5 (Kleine-Vehn et al., 2008) (Figure 1B). PIN trafficking is actin dependent (Geldner et al., 2001). Dynamic changes in polarized PIN localization take place by transcytosis and involve phosphorylation/dephosphorylation cycles by PINOID and related AGC3 kinases, PP2A phosphatases, and recruitment to the cell membrane by brefeldin A–insensitive ARF-GEFs (Kleine-Vehn et al., 2008; Dhonukshe et al., 2010). RAC/ROPs have both direct effects on auxin distribution via specific effector proteins and indirect effects as a result of their regulation of the cytoskeleton, vesicle trafficking, and membrane dynamics (Figures 1B and 1C). In the following sections, we review recent advances in understanding these mechanisms.
The Function of the RAC/ROP Effector ICR1 in Polar Auxin Transport and Asymmetric Distribution
Yeast two-hybrid screens using ICR1 as bait showed that it interacts with a specific group of proteins, including the exocyst vesicle tethering complex subunit SEC3A (Lavy et al., 2007). Furthermore, RAC/ROPs can recruit ICR1 and ICR1-SEC3 complexes to the plasma membrane (Lavy et al., 2007) (Figure 1B). Detailed phenotypic analysis showed that root and embryo development is compromised in icr1 mutant plants and that this phenotype is associated with abnormal auxin distribution (Lavy et al., 2007; Hazak et al., 2010). The perturbations in auxin distribution in icr1 plants result from altered PIN recruitment to polar domains in the plasma membrane. It was further shown that ICR1 is required for polarized secretion and likely functions independently of GNOM and Rab5 (Hazak et al., 2010).
Analysis of ICR1 expression indicates that it is a part of an auxin-regulated feedback loop. ICR1 transcription is quickly induced by auxin but it is posttranscriptionally repressed at the site of local auxin maximum formation at the root stem cell niche (Hazak et al., 2010) (Figure 1B). Collectively, the analysis of ICR1 function, mutant phenotype, and expression indicate that it facilitates polar localization of PIN proteins and that its destabilization might enhance the formation of auxin and auxin-response maxima as demonstrated by auxin quantification and auxin-responsive reporters (see Petersson et al., 2009 and references therein).
How RAC/ROP-Regulated Membrane Dynamics May Affect PIN Polarization
Following cell division, PIN localization is initially nonpolar (Boutté et al., 2006; Dhonukshe et al., 2008a; Men et al., 2008). PIN polarization is a gradual process requiring endocytosis (Dhonukshe et al., 2008a; Men et al., 2008) and accumulation in lipid rafts (Men et al., 2008) (Figure 1B). Lipid rafts are sterol- and sphingolipid-rich membrane microdomains that are believed to facilitate specific protein–protein interactions and signaling (Simons and Toomre, 2000; Mongrand et al., 2010; Simons and Gerl, 2010). P-Glycoprotein 19 (PGP19)/ABCB19 have been shown to stabilize PIN1 plasmalemma distribution in lipid rafts (Titapiwatanakun et al., 2009). Hence, signaling processes that affect membrane dynamics and lipid raft formation could affect PIN polarization. In addition, Rho GTPase activation assays showed that ICR1 interacts primarily with lipid raft–localized RAC/ROPs (Sorek et al., 2010). Taken together, it is possible that the lipid raft–located active RAC/ROPs recruit ICR1, which in turn lead to PIN polarization (Figure 1B).
RAC/ROP Regulation of Cytoskeleton Organization and Dynamics and Auxin Transport
F-actin is required for GNOM-positive endosome-dependent PIN recycling to the plasma membrane (Geldner et al., 2001). Some auxin transport inhibitors act as actin stabilizers, further substantiating the link between PIN trafficking and the actin cytoskeleton (Dhonukshe et al., 2008b). A tight link between PIN1 polarization and microtubule arrays was recently demonstrated in the shoot apical meristem (SAM) of Arabidopsis (Heisler et al., 2010). Microtubules are thought to orient the synthesis of cellulose filaments in the cell wall (Somerville, 2006), and an involvement of the cell wall in maintaining PIN polarity was recently demonstrated (Feraru et al., 2011). The effects of RAC/ROPs on actin dynamics and microtubule organization are well documented (reviewed in Nibau et al., 2006; Yalovsky et al., 2008; Yang, 2008 and references therein). For example, it has been shown that RIC4 promotes actin assembly, while RIC1 is a microtubule binding protein and activated RAC/ROPs apparently interfere with RIC1–microtubule interaction. Combined RAC/ROP-regulated RIC1 and RIC4 functions were suggested to underlie auxin-regulated epidermal cell patterning (Fu et al., 2005, 2009; Xu et al., 2010). An ICR family member RIP3 (ROP Interacting Protein)/ICR5 was shown to interact with RAC/ROPs, microtubules, and a kinesin (Mucha et al., 2010). It is not known yet whether RIP3/ICR5 affects auxin transport and whether ICR1 also interacts with microtubules. Hence, it is likely that RAC/ROPs affect auxin transport through their effects on the cytoskeleton (Figure 1C). Taken together, the findings presented above provide a framework for future studies to elucidate the molecular mechanisms by which microtubules affect PIN polarization and how RAC/ROPs regulate microtubule organization, dynamics, and kinesin function.
AUXIN AND RAC/ROP FUNCTIONS IN LEAF DEVELOPMENT
RAC/ROPs Regulate Epidermal Cell Patterning
It is well established that RAC/ROPs control cell polarity, and defects in their signaling lead to cell shape deformation in pollen tubes, root hairs, trichomes, and the jigsaw puzzle pattern of epidermal pavement cells in Arabidopsis (Figure 3) (Nibau et al., 2006; Yalovsky et al., 2008; Yang, 2008). The intercalary growth of neighboring pavement cells arises from differential growth along the cell peripheries, resulting in complementary lobes and indentations along neighboring epidermal cells. Epidermal pavement cells in mutants compromised in RAC/ROP signaling show notable reductions in lobes and indentations (Figure 3) (Qiu et al., 2002; Fu et al., 2005; Lavy et al., 2007; Sorek et al., 2011). RAC/ROPs also affect the polarity of mesophyll cells. For example, ectopic expression of CA At-ROP11 induced formation of short and rounded palisade cells and almost completely eliminated the air spaces between the spongy mesophyll cells (Bloch et al., 2005). This suggests that the structure of the leaf depends on highly specific expression and activation of RAC/ROPs in time and space and thus can be regulated on many levels.
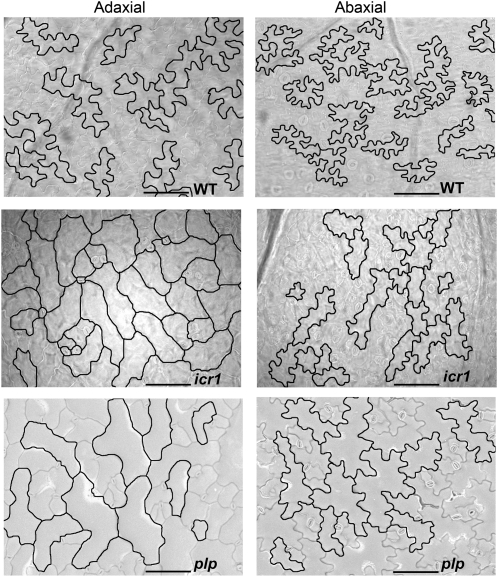
Figure 3.
Epidermal Pavement Cell Structures in Leaves of Wild-Type and RAC/ROP Signaling and Modifying Mutants.
Adaxial and abaxial epidermal pavement cells of leaves of wild-type Columbia-0 (WT), icr1, and plp mutants are shown. Note the differences in cell structures between adaxial and abaxial wild-type pavement cells and the reduced lobe numbers and indentations in the adaxial epidermis of icr1 and plp relative to their abaxial cells. Bars = 100 μm. The plp images were adapted from Figure 7 of Sorek et al. (2011).
Auxin affects leaf development through a number of pathways (Scarpella et al., 2010). As noted above, auxin can lead to RAC/ROP activation (Tao et al., 2002; Xu et al., 2010). However, auxin also affects the function and expression of RAC/ROP-independent transcriptional regulators and microRNAs, which regulate cell division, growth, and differentiation in leaves. Hence, the effects of auxin on cell structure in leaves are likely complex and involve multiple pathways. In the following sections, we discuss how RAC/ROPs may contribute to auxin-regulated leaf development.
ABP1 Mediates Auxin-Activated RAC/ROP Regulation of Pavement Cell Interdigitation
Xu et al. (2010) showed that the pin1 mutant and a quadruple yucca mutant deficient in auxin biosynthesis (Cheng et al., 2006) have significantly reduced numbers of lobes relative to the wild type, similar to that observed in RAC/ROP-deficient rop2RNAi rop4-1 seedlings. However, unlike wild-type and yucca seedlings, exogenous auxin-enhanced pavement cell interdigitation was suppressed in the rop2RNAi rop4-1 background. Together, these observations link RAC/ROP-regulated pavement cell interdigitation to auxin signaling and transport. Xu et al. (2010) further showed that pavement cell interdigitation was reduced in a weak allele of ABP1 (abp1-5), which has a single point mutation in its auxin binding pocket and in ABP1 RNA interference (RNAi)-silenced plants (abp1RNAi) and that auxin failed to induce RAC/ROP activation in abp1-5 protoplasts. Together, these observations suggest an ABP1-dependent auxin-signaled RAC/ROP-mediated pathway to regulate pavement cell patterning. Furthermore, PIN1-GFP localized to the cell membrane in the lobes, but this accumulation was compromised in the abp1-5 and rop2RNAi rop4-1 backgrounds (Xu et al., 2010). Moreover, recruitment of the RAC/ROP effector RIC4 to the plasma membrane was compromised in the pin1 mutant background. Although the mechanisms are far from clear, these observations, together with previously published data (Fu et al., 2005, 2009), led the authors to propose a model stating that local auxin maxima around the lobes in pavement cells leads to ABP1-dependent ROP2 ROP4 activation and ROP6 inactivation (Xu et al., 2010).
Integrating RAC/ROPs into Auxin-Regulated Leaf Development
It is currently not known how the auxin-induced ABP1-dependent activation of RAC/ROPs fits within the framework of leaf development. Early in leaf development, an auxin maximum is formed at the leaf primordium apex and then distributed to the middle of the primordium that would become the central vein. In later developmental stages, additional maxima are formed at specific locations on the leaf primordium margins (Scarpella et al., 2010 and references therein). These lateral maxima are auxin sources for initiation of lateral veins. The local maxima in the leaf primordium likely reflect PIN1-dependent polarized auxin fluxes as well as local auxin biosynthesis. How this highly specific auxin distribution in the developing leaf affects local auxin maxima in individual cells is currently not known.
Specific auxin distribution is required for regulation of gene expression that modulates cell differentiation in leaves. It has been shown that in developing leaves, auxin suppresses expression of the gene encoding the meristem identity KNOX1 transcriptional regulator BREVIPEDICELLUS (BP), and conversely BP expression increases in the pin1 background (Hay et al., 2006; Scarpella et al., 2010). This indicates that reduced auxin levels and disrupted auxin distribution are associated with acquisition of a meristematic differentiation state, which could affect cell division, cell growth, and responses to various stimuli, including RAC/ROP activation.
Auxin is also involved in regulation of transcriptional networks that regulate the adaxial (closer to SAM)/ abaxial (away from the SAM) identity in the leaf (see Efroni et al., 2010; Scarpella et al., 2010 and references therein). Pavement cell structure on the adaxial and abaxial leaf surfaces is different, and they are apparently differentially regulated by RAC/ROPs (Lavy et al., 2007; Sorek et al., 2011) (Figure 3). In icr1 and plp mutants, loss of RAC/ROP signaling capacity strongly affects interdigitaion on the adaxial surface but only to a much lesser extent on the abaxial pavement cells. plp plants have a mutation in the shared farnesyltransferase and geranylgernayltransferase-I α-subunit, lack CaaX box protein prenylation activity (Running et al., 2004), and therefore are compromised in type-I ROP function (Lemichez et al., 2001; Sorek et al., 2007, 2011). Hence, the reduced pavement cell polarity is in line with the proposed function of the type-I ROPs (ROP2, ROP4, and ROP6) in regulation of pavement cell polar growth (Fu et al., 2005, 2009; Xu et al., 2010). However, the differences in epidermal cell structures on the two sides of the leaf in wild-type, icr1, and plp mutant plants indicate that growth of adaxial and abaxial pavement cells is regulated by partially nonoverlapping and type-I ROP-independent mechanisms. The differences between adaxial and abaxial cells should be taken into account in future models of pavement cell interdigitation and its regulation by auxin.
Taken together, it appears that complex feedback mechanisms coordinate auxin distribution, auxin response, and developmental pathways during leaf development. It is likely that disruption of auxin fluxes, biosynthesis, or signaling affect these feedback mechanisms, thereby altering leaf developmental programs. Hence, the changes in pavement cell structure observed in pin1, abp1-5, and quadruple yucca mutants may be associated with multiple pathways, some of which could be RAC/ROP dependent, while others are independent of RAC/ROPs.
POTENTIAL CELL SURFACE REGULATORS FOR AUXIN-STIMULATED RAC/ROP SIGNALING
ABP1 as an Upstream Regulator for Auxin and RAC/ROP-Mediated Epidermal Cell Patterning
Several lines of evidence indicate that ABP1 is an important regulator for auxin-mediated responses, growth, and development. These include the observations that overexpression of ABP1 mediates auxin-dependent cell expansion (Jones et al., 1998), embryo lethality in Arabidopsis abp1 knockout mutants (Chen et al., 2001), compromised auxin transport, and auxin-regulated gene expression and other cellular and developmental defects in abp1/ABP1 heterozygotes (Effendi et al., 2011) and conditionally ABP1-suppressed plants (Braun et al., 2008; Tromas et al., 2009). Immunodetection and quantitative glycan analysis of ABP1 showed that a small portion of this predominantly ER-located protein could be detected on the cell surface (Jones and Herman, 1993; Henderson et al., 1997) and that auxin can induce its clustering on the protoplast surface (Diekmann et al., 1995). In showing that ABP1 acts as a signal mediator for auxin to activate RAC/ROP-regulated epidermal cell patterning, Xu et al. (2010) proposed that it may act as a cell surface regulator for auxin-mediated pavement cell interdigitation. However, it remains unclear how the KDEL-containing ABP1 escapes ER retention and how, without a transmembrane-spanning domain, it associates with the cell surface (Klämbt, 1990; Jones and Herman, 1993; Henderson et al., 1997). A result from an in vitro cross-linking study using a 12–amino acid peptide auxin agonist corresponding to the C terminus of ABP1 and isolated maize (Zea mays) membrane preparations (Shimomura, 2006) was considered as support for how ABP1 may be partially associated with the outer surface of the plasma membrane via binding to a GPI-anchored protein (Xu et al., 2010). Nevertheless, existing evidence is also consistent with intracellular RAC/ROP activation by ABP1. For example, ABP1 is predominantly localized in the ER (Jones and Herman, 1993; Henderson et al., 1997), and the ER is known to have the capacity to regulate auxin homeostasis (see Woodward and Bartel, 2005; Friml and Jones, 2010; Ludwig-Müller, 2011 and reference therein]. Furthermore, localization of PIN5 (Mravec et al., 2009) and of the ROP-GEF SPIKE1 (Zhang et al., 2010) to the ER membrane and at ER exit sites, respectively, was also reported recently. Taken together, it is plausible that the ER-located ABP1 could regulate the ER-located PIN5 (Mravec et al., 2009), affecting intracellular auxin homeostasis, and ABP1-dependent RAC/ROP activation may take place via the ER-located ABP1 and SPIKE1. The compromised pavement cell interdigitation pattern in quadruple yucca seedlings (Xu et al., 2010) and in spike1 mutants (Qiu et al., 2002) are also in line with these alternate considerations of how ABP1 may act. Data showing if and how ABP1 may regulate the function of SPIKE1 or the PRONE ROPGEFs (Berken et al., 2005) will help establish the mechanistic underpinning of ABP1-mediated auxin activation of RAC/ROP signaling.
An RLK as an Upstream Regulator for Auxin and RAC/ROP-Mediated Root Hair Development
In an effort to directly identify upstream regulators for RAC/ROPs and using the Arabidopsis ROPGEF1 as bait in a yeast two-hybrid screen, Duan et al. (2010) identified FERONIA as a ROPGEF-interacting RLK, adding to previous studies that identified pollen-specific RLKs from tomato (Solanum lycopersicum) and Arabidopsis as upstream regulators of ROPGEFs (Kaothien et al., 2005; Zhang and McCormick, 2007). Specifically, Duan et al. showed that FERONIA act as a surface regulator for the well-established RAC/ROP-signaled pathway for NADPH oxidase-dependent ROS-mediated polarized root hair growth and that the feronia mutant had reduced levels of activated RAC/ROPs relative to those observed in wild-type plants. Furthermore, FERONIA, ROPGEFs, and RAC/ROPs appear to be engaged in a transient signaling complex in a guanine nucleotide-dependent manner considerably more favored by GDP-bound RAC/ROPs. Duan et al. also noted that unlike the situation in wild-type seedlings, where auxin inhibits root elongation and stimulates root hair development and NADPH oxidase-dependent ROS accumulation, feronia mutant seedlings are less sensitive to auxin-inhibited root growth; their root hairs remain short and they do not regain ROS production upon auxin treatment. Although these observations hint at an auxin-activated FERONIA RLK-mediated RAC/ROP signaling pathway, definitive studies remain to be performed. In this respect, it would be interesting to test whether FERONIA is engaged in an auxin gradient–regulated RAC/ROP activation postulated to determine the site of root hair initiation (Fischer et al., 2006; Payne and Grierson, 2009).
EMERGING QUESTIONS
The links between auxin transport and responses to RAC/ROP functions have been addressed in only a handful of studies. This is likely due to the redundancy between RAC/ROPs, which has hampered phenotypic analysis of loss-of-function mutants, as well as to the complexity of auxin function. However, these studies provide insights on how auxin may use RAC/ROPs as mediators for plasma membrane–initiated signaling and reveal the function of RAC/ROP effectors in polar auxin transport.
For a hormone with as widespread functional significance as auxin, RAC/ROPs provide enormous versatility for its signal mediation. Depending on their temporal and spatial expression patterns, individual RAC/ROPs have the potential to interact with multiple upstream regulators and downstream effectors. If members of the large receptor kinase family are proven to mediate auxin-activated RAC/ROP signaling, it will provide numerous candidates to mediate the broad array of auxin-regulated responses. Given that the direct activators of RAC/ROPs are ROP-GEFs, the key questions for future studies would be to determine if and how auxin regulates the function of ROP-GEFs, which ROP-GEFs are regulated by auxin, and where in the cell this regulation takes place. The notion that ABP1 may act as a surface receptor mediating auxin activation of RAC/ROPs is intriguing, although the precise understanding for how ABP1 works remains to be defined. For instance, is it indeed the small apoplastic pool of ABP1 that mediates a pathway from auxin to RAC/ROPs to regulate epidermal cell patterning or does the signaling pathway initiate from other cellular locations? Does ABP1 directly activate ROP-GEFs? If so, are the targets of ABP1 members of the cell membrane–associated ROPGEFs or is it the ER exit site–located SPIKE1? Furthermore, given the roles of auxin, ABP1, and RAC/ROPs on PIN polarization, delineating the potentially interdependent pathways that they regulate (Figure 1B) should also provide more precise insight on how ABP1 mediates auxin-signaled RAC/ROP activation. Finally, with the majority of ABP1 being in the ER, knowledge about its biological roles in this intracellular compartment (Woodward and Bartel, 2005; Friml and Jones, 2010; Ludwig-Müller, 2011) is crucial to fully interpret and appreciate its proposed role as a cell surface mediator for RAC/ROP activation and its significance. We believe the questions raised here for RLKs and ABP1 as potential cell surface regulators for auxin signaling are relevant and interesting launching points to advance our understanding of RAC/ROP- mediated auxin signaling.
Downstream of RAC/ROPs, ICR1 appears to play a direct function in PIN recruitment to the plasma membrane (Figure 1B) (Hazak et al., 2010). ICRs are novel Rho GTPase effectors, functioning as adaptors for interactions with other proteins (Lavy et al., 2007; Mucha et al., 2010). In addition to SEC3, several ICR1 interactors have been identified in yeast two-hybrid screens. These interactors must be confirmed by independent assays. It is also not known whether other ICR family members are involved in regulation of auxin trafficking. The ICR family is conserved in higher plants, and it would be interesting to test whether ICR1 homologs have conserved functions in facilitating directional auxin transport. Functional analysis of ICR1 homologs in different plant species together with comparative genomics could show whether the mechanism of polar auxin transport has evolved together with the ICRs. In this respect, further elucidating the links between ICR1 expression and stability and auxin signaling in Arabidopsis and other plant species would be required to establish ICR1 as part of an auxin-modulated feedback loop.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table 1. A List of Key Genes/Proteins/Mutants Referred to in the Text.
Acknowledgments
We thank Sheila McCormick (Plant Gene Expression Center), Jennifer Normanly (University of Massachusetts, Amherst), and Candida Nibau (Aberystwyth University) for helpful comments on the manuscript and apologize to those colleagues whose work we have not cited due to space constraints. We also thank Qiaohong Duan and Daniel Kita (University of Massachusetts, Amherst) for discussions throughout this manuscript project. Research carried out in our laboratories is supported by grants from the USDA (CSREES 2004-35304-14873) and National Science Foundation (IOB0544222) to H.W. and A.Y.C., The ISRAEL Science Foundation (ISF 312/07), the U.S.–Israel Binational Science Foundation (BSF 2009309), and the Deutschland Israel Program (DIP H.3.1) to S.Y.
References
- Alonso J.M., Hirayama T., Roman G., Nourizadeh S., Ecker J.R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Badescu G.O., Napier R.M. (2006). Receptors for auxin: Will it all end in TIRs? Trends Plant Sci. 11: 217–223 [DOI] [PubMed] [Google Scholar]
- Basu D., Le J., Zakharova T., Mallery E.L., Szymanski D.B. (2008). A SPIKE1 signaling complex controls actin-dependent cell morphogenesis through the heteromeric WAVE and ARP2/3 complexes. Proc. Natl. Acad. Sci. USA 105: 4044–4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M.J., Marchant A., Green H.G., May S.T., Ward S.P., Millner P.A., Walker A.R., Schulz B., Feldmann K.A. (1996). Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 273: 948–950 [DOI] [PubMed] [Google Scholar]
- Berken A., Thomas C., Wittinghofer A. (2005). A new family of RhoGEFs activates the Rop molecular switch in plants. Nature 436: 1176–1180 [DOI] [PubMed] [Google Scholar]
- Berken A., Wittinghofer A. (2008). Structure and function of Rho-type molecular switches in plants. Plant Physiol. Biochem. 46: 380–393 [DOI] [PubMed] [Google Scholar]
- Bloch D., Lavy M., Efrat Y., Efroni I., Bracha-Drori K., Abu-Abied M., Sadot E., Yalovsky S. (2005). Ectopic expression of an activated RAC in Arabidopsis disrupts membrane cycling. Mol. Biol. Cell 16: 1913–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutté Y., Crosnier M.T., Carraro N., Traas J., Satiat-Jeunemaitre B. (2006). The plasma membrane recycling pathway and cell polarity in plants: Studies on PIN proteins. J. Cell Sci. 119: 1255–1265 [DOI] [PubMed] [Google Scholar]
- Bracha-Drori K., Shichrur K., Lubetzky T.C., Yalovsky S. (2008). Functional analysis of Arabidopsis postprenylation CaaX processing enzymes and their function in subcellular protein targeting. Plant Physiol. 148: 119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun N., Wyrzykowska J., Muller P., David K., Couch D., Perrot-Rechenmann C., Fleming A.J. (2008). Conditional repression of AUXIN BINDING PROTEIN1 reveals that it coordinates cell division and cell expansion during postembryonic shoot development in Arabidopsis and tobacco. Plant Cell 20: 2746–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol R.J., Takeda S., Linstead P., Durrant M.C., Kakesova H., Derbyshire P., Drea S., Zarsky V., Dolan L. (2005). A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature 438: 1013–1016 [DOI] [PubMed] [Google Scholar]
- Chapman E.J., Estelle M. (2009). Mechanism of auxin-regulated gene expression in plants. Annu. Rev. Genet. 43: 265–285 [DOI] [PubMed] [Google Scholar]
- Chen J.G., Ullah H., Young J.C., Sussman M.R., Jones A.M. (2001). ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev. 15: 902–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Dai X., Zhao Y. (2006). Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 20: 1790–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N., Dharmasiri S., Estelle M. (2005). The F-box protein TIR1 is an auxin receptor. Nature 435: 441–445 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P., Huang F., Galvan-Ampudia C.S., Mähönen A.P., Kleine-Vehn J., Xu J., Quint A., Prasad K., Friml J., Scheres B., Offringa R. (2010). Plasma membrane-bound AGC3 kinases phosphorylate PIN auxin carriers at TPRXS(N/S) motifs to direct apical PIN recycling. Development 137: 3245–3255 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P., et al. (2008a). Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature 456: 962–966 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dhonukshe P., et al. (2008b). Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc. Natl. Acad. Sci. USA 105: 4489–4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann W., Venis M.A., Robinson D.G. (1995). Auxins induce clustering of the auxin-binding protein at the surface of maize coleoptile protoplasts. Proc. Natl. Acad. Sci. USA 92: 3425–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q., Kita D., Li C., Cheung A.Y., Wu H.M. (2010). FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl. Acad. Sci. USA 107: 17821–17826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effendi Y., Rietz S., Fischer U., Scherer G.F. (2011). The heterozygous abp1/ABP1 insertional mutant has defects in functions requiring polar auxin transport and in regulation of early auxin-regulated genes. Plant J. 65: 282–294 [DOI] [PubMed] [Google Scholar]
- Efroni I., Eshed Y., Lifschitz E. (2010). Morphogenesis of simple and compound leaves: A critical review. Plant Cell 22: 1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escrobar-Restrepo J-M., Huck N., Kessler S., Gagliardini V., Gheyselinck J., Yang W-C., Grossniklaus U. (2007). The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science 317: 656–660 [DOI] [PubMed] [Google Scholar]
- Feraru E., Feraru M.I., Kleine-Vehn J., Martinière A., Mouille G., Vanneste S., Vernhettes S., Runions J., Friml J. (2011). PIN polarity maintenance by the cell wall in Arabidopsis. Curr. Biol. 21: 338–343 [DOI] [PubMed] [Google Scholar]
- Fischer U., Ikeda Y., Ljung K., Serralbo O., Singh M., Heidstra R., Palme K., Scheres B., Grebe M. (2006). Vectorial information for Arabidopsis planar polarity is mediated by combined AUX1, EIN2, and GNOM activity. Curr. Biol. 16: 2143–2149 [DOI] [PubMed] [Google Scholar]
- Fowler J.E. (2010). Evolution of the ROP GTPase signaling module. Integrated G Protein Signaling in Plants, Yalovsky S., Baluska F., Jones A., (Berlin: Springer; ), pp. 305–327 [Google Scholar]
- Friml J., Jones A.R. (2010). Endoplasmic reticulum: The rising compartment in auxin biology. Plant Physiol. 154: 458–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Gu Y., Zheng Z., Wasteneys G., Yang Z. (2005). Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120: 687–700 [DOI] [PubMed] [Google Scholar]
- Fu Y., Xu T., Zhu L., Wen M., Yang Z. (2009). A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Curr. Biol. 19: 1827–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N., Friml J., Stierhof Y.D., Jürgens G., Palme K. (2001). Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Hay A., Barkoulas M., Tsiantis M. (2006). ASYMMETRIC LEAVES1 and auxin activities converge to repress BREVIPEDICELLUS expression and promote leaf development in Arabidopsis. Development 133: 3955–3961 [DOI] [PubMed] [Google Scholar]
- Hazak O., Bloch D., Poraty L., Sternberg H., Zhang J., Friml J., Yalovsky S. (2010). A rho scaffold integrates the secretory system with feedback mechanisms in regulation of auxin distribution. PLoS Biol. 8: e1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler M.G., Hamant O., Krupinski P., Uyttewaal M., Ohno C., Jönsson H., Traas J., Meyerowitz E.M. (2010). Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. PLoS Biol. 8: e1000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J., Bauly J.M., Ashford D.A., Oliver S.C., Hawes C.R., Lazarus C.M., Venis M.A., Napier R.M. (1997). Retention of maize auxin-binding protein in the endoplasmic reticulum: Quantifying escape and the role of auxin. Planta 202: 313–323 [DOI] [PubMed] [Google Scholar]
- Hwang J.U., Vernoud V., Szumlanski A., Nielsen E., Yang Z. (2008). A tip-localized RhoGAP controls cell polarity by globally inhibiting Rho GTPase at the cell apex. Curr. Biol. 18: 1907–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.M., Herman E.M. (1993). KDEL-containing auxin-binding protein is secreted to the plasma membrane and cell wall. Plant Physiol. 101: 595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.M., Im K.H., Savka M.A., Wu M.J., DeWitt N.G., Shillito R., Binns A.N. (1998). Auxin-dependent cell expansion mediated by overexpressed auxin-binding protein 1. Science 282: 1114–1117 [DOI] [PubMed] [Google Scholar]
- Jones M.A., Shen J.J., Fu Y., Li H., Yang Z., Grierson C.S. (2002). The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell 14: 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaothien P., Ok S.H., Shuai B., Wengier D., Cotter R., Kelley D., Kiriakopolos S., Muschietti J., McCormick S. (2005). Kinase partner protein interacts with the LePRK1 and LePRK2 receptor kinases and plays a role in polarized pollen tube growth. Plant J. 42: 492–503 [DOI] [PubMed] [Google Scholar]
- Kepinski S., Leyser O. (2005). The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435: 446–451 [DOI] [PubMed] [Google Scholar]
- Klahre U., Kost B. (2006). Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. Plant Cell 18: 3033–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klämbt D. (1990). A view about the function of auxin-binding proteins at plasma membranes. Plant Mol. Biol. 14: 1045–1050 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J., Langowski L., Wisniewska J., Dhonukshe P., Brewer P.B., Friml J. (2008). Cellular and molecular requirements for polar PIN targeting and transcytosis in plants. Mol. Plant 1: 1056–1066 [DOI] [PubMed] [Google Scholar]
- Lavy M., Bloch D., Hazak O., Gutman I., Poraty L., Sorek N., Sternberg H., Yalovsky S. (2007). A novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr. Biol. 17: 947–952 [DOI] [PubMed] [Google Scholar]
- Lavy M., Bracha-Drori K., Sternberg H., Yalovsky S. (2002). A cell-specific, prenylation-independent mechanism regulates targeting of type II RACs. Plant Cell 14: 2431–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy M., Yalovsky S. (2006). Association of Arabidopsis type-II ROPs with the plasma membrane requires a conserved C-terminal sequence motif and a proximal polybasic domain. Plant J. 46: 934–947 [DOI] [PubMed] [Google Scholar]
- Lemichez E., Wu Y., Sanchez J.P., Mettouchi A., Mathur J., Chua N.H. (2001). Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev. 15: 1808–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Müller J. (2011). Auxin conjugates: Their role for plant development and in the evolution of land plants. J. Exp. Bot. 62: 1757–1773 [DOI] [PubMed] [Google Scholar]
- Marchant A., Kargul J., May S.T., Muller P., Delbarre A., Perrot-Rechenmann C., Bennett M.J. (1999). AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 18: 2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men S., Boutté Y., Ikeda Y., Li X., Palme K., Stierhof Y.D., Hartmann M.A., Moritz T., Grebe M. (2008). Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat. Cell Biol. 10: 237–244 [DOI] [PubMed] [Google Scholar]
- Molendijk A.J., Bischoff F., Rajendrakumar C.S., Friml J., Braun M., Gilroy S., Palme K. (2001). Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 20: 2779–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongrand S., Stanislas T., Bayer E.M., Lherminier J., Simon-Plas F. (2010). Membrane rafts in plant cells. Trends Plant Sci. 15: 656–663 [DOI] [PubMed] [Google Scholar]
- Mravec J., et al. (2009). Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 459: 1136–1140 [DOI] [PubMed] [Google Scholar]
- Mucha E., Hoefle C., Hückelhoven R., Berken A. (2010). RIP3 and AtKinesin-13A - a novel interaction linking Rho proteins of plants to microtubules. Eur. J. Cell Biol. 89: 906–916 [DOI] [PubMed] [Google Scholar]
- Nibau C., Wu H.M., Cheung A.Y. (2006). RAC/ROP GTPases: ‘Hubs’ for signal integration and diversification in plants. Trends Plant Sci. 11: 309–315 [DOI] [PubMed] [Google Scholar]
- Paciorek T., Zazímalová E., Ruthardt N., Petrásek J., Stierhof Y.D., Kleine-Vehn J., Morris D.A., Emans N., Jürgens G., Geldner N., Friml J. (2005). Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Payne R.J., Grierson C.S. (2009). A theoretical model for ROP localisation by auxin in Arabidopsis root hair cells. PLoS ONE 4: e8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson S.V., Johansson A.I., Kowalczyk M., Makoveychuk A., Wang J.Y., Moritz T., Grebe M., Benfey P.N., Sandberg G., Ljung K. (2009). An auxin gradient and maximum in the Arabidopsis root apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis. Plant Cell 21: 1659–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H., Chang K.N., Yazaki J., Ecker J.R. (2009). Interplay between ethylene, ETP1/ETP2 F-box proteins, and degradation of EIN2 triggers ethylene responses in Arabidopsis. Genes Dev. 23: 512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J.L., Jilk R., Marks M.D., Szymanski D.B. (2002). The Arabidopsis SPIKE1 gene is required for normal cell shape control and tissue development. Plant Cell 14: 101–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S., et al. (2010). ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell 143: 111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman K.L., Der C.J., Sondek J. (2005). GEF means go: Turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6: 167–180 [DOI] [PubMed] [Google Scholar]
- Running M.P., Lavy M., Sternberg H., Galichet A., Gruissem W., Hake S., Ori N., Yalovsky S. (2004). Enlarged meristems and delayed growth in plp mutants result from lack of CaaX prenyltransferases. Proc. Natl. Acad. Sci. USA 101: 7815–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E., Barkoulas M., Tsiantis M. (2010). Control of leaf and vein development by auxin. Cold Spring Harb. Perspect. Biol. 2: a001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura S. (2006). Identification of a glycosylphosphatidylinositol-anchored plasma membrane protein interacting with the C-terminus of auxin-binding protein 1: a photoaffinity crosslinking study. Plant Mol. Biol. 60: 663–667 [DOI] [PubMed] [Google Scholar]
- Simons K., Gerl M.J. (2010). Revitalizing membrane rafts: New tools and insights. Nat. Rev. Mol. Cell Biol. 11: 688–699 [DOI] [PubMed] [Google Scholar]
- Simons K., Toomre D. (2000). Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1: 31–39 [DOI] [PubMed] [Google Scholar]
- Somerville C. (2006). Cellulose synthesis in higher plants. Annu. Rev. Cell Dev. Biol. 22: 53–78 [DOI] [PubMed] [Google Scholar]
- Sorek N., Gutman O., Bar E., Abu-Abied M., Feng X., Running M.P., Lewinsohn E., Ori N., Sadot E., Henis Y.I., Yalovsky S. (2011). Differential effects of prenylation and s-acylation on type I and II ROPS membrane interaction and function. Plant Physiol. 155: 706–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek N., Poraty L., Sternberg H., Bar E., Lewinsohn E., Yalovsky S. (2007). Activation status-coupled transient S acylation determines membrane partitioning of a plant Rho-related GTPase. Mol. Cell. Biol. 27: 2144–2154 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sorek N., Segev O., Gutman O., Bar E., Richter S., Poraty L., Hirsch J.A., Henis Y.I., Lewinsohn E., Jürgens G., Yalovsky S. (2010). An S-acylation switch of conserved G domain cysteines is required for polarity signaling by ROP GTPases. Curr. Biol. 20: 914–920 [DOI] [PubMed] [Google Scholar]
- Steinmann T., Geldner N., Grebe M., Mangold S., Jackson C.L., Paris S., Gälweiler L., Palme K., Jürgens G. (1999). Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286: 316–318 [DOI] [PubMed] [Google Scholar]
- Tao L.Z., Cheung A.Y., Nibau C., Wu H.M. (2005). RAC GTPases in tobacco and Arabidopsis mediate auxin-induced formation of proteolytically active nuclear protein bodies that contain AUX/IAA proteins. Plant Cell 17: 2369–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L.Z., Cheung A.Y., Wu H.M. (2002). Plant Rac-like GTPases are activated by auxin and mediate auxin-responsive gene expression. Plant Cell 14: 2745–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T., Harding A., Inder K., Plowman S., Parton R.G., Hancock J.F. (2007). Plasma membrane nanoswitches generate high-fidelity Ras signal transduction. Nat. Cell Biol. 9: 905–914 [DOI] [PubMed] [Google Scholar]
- Titapiwatanakun B., et al. (2009). ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. Plant J. 57: 27–44 [DOI] [PubMed] [Google Scholar]
- Tong Z., Gao X.D., Howell A.S., Bose I., Lew D.J., Bi E. (2007). Adjacent positioning of cellular structures enabled by a Cdc42 GTPase-activating protein-mediated zone of inhibition. J. Cell Biol. 179: 1375–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromas A., Braun N., Muller P., Khodus T., Paponov I.A., Palme K., Ljung K., Lee J.Y., Benfey P., Murray J.A., Scheres B., Perrot-Rechenmann C. (2009). The AUXIN BINDING PROTEIN 1 is required for differential auxin responses mediating root growth. PLoS ONE 4: e6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winge P., Brembu T., Kristensen R., Bones A.M. (2000). Genetic structure and evolution of RAC-GTPases in Arabidopsis thaliana. Genetics 156: 1959–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward A.W., Bartel B. (2005). Auxin: Regulation, action, and interaction. Ann. Bot. (Lond.) 95: 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Gu Y., Li S., Yang Z. (2001). A genome-wide analysis of Arabidopsis Rop-interactive CRIB motif-containing proteins that act as Rop GTPase targets. Plant Cell 13: 2841–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Wen M., Nagawa S., Fu Y., Chen J.G., Wu M.J., Perrot-Rechenmann C., Friml J., Jones A.M., Yang Z. (2010). Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 143: 99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S., Bloch D., Sorek N., Kost B. (2008). Regulation of membrane trafficking, cytoskeleton dynamics, and cell polarity by ROP/RAC GTPases. Plant Physiol. 147: 1527–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. (2008). Cell polarity signaling in Arabidopsis. Annu. Rev. Cell Dev. Biol. 24: 551–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Kotchoni S.O., Samuels A.L., Szymanski D.B. (2010). SPIKE1 signals originate from and assemble specialized domains of the endoplasmic reticulum. Curr. Biol. 20: 2144–2149 [DOI] [PubMed] [Google Scholar]
- Zhang Y., McCormick S. (2007). A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 104: 18830–18835 [DOI] [PMC free article] [PubMed] [Google Scholar]