In this study, the authors find that the expansion of the xylem in Arabidopsis hypocotyls observed upon flowering is directly triggered by signaling through the plant hormone gibberellin. The authors also demonstrate that this involves mobile gibberellin, which therefore can act as a long-distance signal.
Abstract
Secondary growth of the vasculature results in the thickening of plant structures and continuously produces xylem tissue, the major biological carbon sink. Little is known about the developmental control of this quantitative trait, which displays two distinct phases in Arabidopsis thaliana hypocotyls. The later phase of accelerated xylem expansion resembles the secondary growth of trees and is triggered upon flowering by an unknown, shoot-derived signal. We found that flowering-dependent hypocotyl xylem expansion is a general feature of herbaceous plants with a rosette growth habit. Flowering induction is sufficient to trigger xylem expansion in Arabidopsis. By contrast, neither flower formation nor elongation of the main inflorescence is required. Xylem expansion also does not depend on any particular flowering time pathway or absolute age. Through analyses of natural genetic variation, we found that ERECTA acts locally to restrict xylem expansion downstream of the gibberellin (GA) pathway. Investigations of mutant and transgenic plants indicate that GA and its signaling pathway are both necessary and sufficient to directly trigger enhanced xylogenesis. Impaired GA signaling did not affect xylem expansion systemically, suggesting that it acts downstream of the mobile cue. By contrast, the GA effect was graft transmissible, suggesting that GA itself is the mobile shoot-derived signal.
INTRODUCTION
In higher land plants, any excess sugar provided by photosynthesis and not needed for maintenance of the general metabolism is invested into growth and transported from photosynthetic source organs toward sink organs (Ye, 2002; Thompson, 2006). This transport occurs in the vascular phloem, a highly specialized tissue comprised of various cell types, such as the sieve elements, which perform the actual transport of the phloem sap; companion cells, which are responsible for loading and unloading of phloem sap cargo; phloem parenchyma cells, which transfer metabolites to and from companion cells; and phloem fibers, cells with thick secondary cell walls that provide structural support. The vasculature also comprises xylem tissue, which transports soil water and minerals to the leaves. Xylem is a sink tissue, which incorporates sugar into novel cell wall material and represents the principal site of biomass accumulation in perennial dicotyledons (Demura and Ye, 2010). Three general cell types are found in the xylem of nearly all vascular plants: xylem vessels, which are the actual conducts for water and solutes; xylem fibers, which possess thick secondary cell walls to provide structural support; and xylem parenchyma cells, which can differentiate into fibers.
The extent of xylem tissue is an important factor for plant growth because water and solute transport capacity limits shoot growth as plants become bigger. The major group of extant plants, the dicotyledons, have solved this developmental problem by continuously expanding their vascular tissues throughout their life cycle, resulting in the radial expansion of stems and roots. This so-called secondary growth also offers the advantage that nonfunctional xylem vessels can be replaced, as they do not forever resist the strain of the negative pressure created by the transpiration stream. In perennial plants, for instance, this permits the replacement of water transport capacity lost through cavitation of xylem vessels. Moreover, because negative pressure and thus mechanical strain increase with height, secondary growth permits plants to grow taller and thus represents the key invention that permitted the evolution of trees (Spicer and Groover, 2010).
Secondary growth is driven by the vascular cambium, a secondary meristem that is located between phloem and xylem within a vascular bundle (Ye, 2002; Elo et al., 2009). The cambial stem cells produce daughter cells that will acquire phloem or xylem fate, depending on the given polarity. Studies in Arabidopsis thaliana have identified various genes that are critical for the proper differentiation of vascular tissue types (e.g., Bonke et al., 2003; Kubo et al., 2005; Mitsuda et al., 2007), and several among them implicate plant hormone pathways in vascular development. For instance, polar auxin transport and auxin signaling are required for de novo vascular specification and patterning (Scarpella et al., 2006; Nilsson et al., 2008), while cytokinin signaling has a crucial role in the determination of xylem versus phloem identity (Mähönen et al., 2000; Carlsbecker and Helariutta, 2005; Mähönen et al., 2006).
In various species, the gibberellin (GA) pathway has been implicated in promoting xylogenesis in combination with the auxin pathway, as well as in the induction of fiber differentiation (Digby and Wareing, 1966; Eriksson et al., 2000; Biemelt et al., 2004; Mauriat and Moritz, 2009; Dayan et al., 2010). However, in these studies, GA also exerted a general growth-promoting effect, suggesting that elevated GA signaling might merely enhance xylem production along with the overall growth increase in height. For instance, while Populus trees overexpressing a GA biosynthetic gene produced more total fibers than the control trees, relative abundance of fibers remained approximately constant (Eriksson et al., 2000). In summary, it remains unclear to what degree the effects of GA on xylem production and fiber development are specific.
Other hormone pathways that impinge on vascular development in Arabidopsis have been shown to play an equally important role in Populus (Schrader et al., 2003; Dettmer et al., 2009; Nieminen et al., 2008; Nilsson et al., 2008). Moreover, despite being an annual, herbaceous plant, Arabidopsis displays significant secondary growth, including lignification of cells walls, suggesting that Arabidopsis can serve as a model to investigate this trait (Chaffey et al., 2002; Nieminen et al., 2004). This potential is underpinned by a recent study that found that manipulation of flowering time regulators can turn Arabidopsis into a quasiperennial plant that displays strong secondary growth and bush-like morphology (Melzer et al., 2008). Already in wild-type Arabidopsis lines, secondary growth is observed in the hypocotyl, the seedling stem (Chaffey et al., 2002; Sibout et al., 2008). Moreover, in the hypocotyl, elongation growth and thickening are uncoupled; therefore, unlike in stems, secondary growth at any position along the hypocotyl is not obscured by parallel ongoing elongation growth and formation of novel vasculature at the apex (Sibout et al., 2008). Finally, secondary growth in Arabidopsis hypocotyls proceeds in two distinct phases, an early phase of proportional secondary growth of all tissues followed by a later phase of xylem expansion and fiber differentiation, which is highly reminiscent of the mode of secondary growth in trees (Chaffey et al., 2002; Nieminen et al., 2004). Collectively, these characteristics render the Arabidopsis hypocotyl a particularly suitable model for the investigation of basic mechanisms of secondary growth.
The transition between the early and late stages of hypocotyl secondary growth is triggered by flowering (Sibout et al., 2008). Transient transgenic expression of flowering inducers, such as CONSTANS (CO), can therefore be used to accelerate xylem production at will (Sibout et al., 2008). A combination of micrografting and gene expression analyses has suggested that this requires signaling from the shoot via a mobile shoot-derived signal (Sibout et al., 2008). In this study, we set out to identify the factors that limit and induce hypocotyl xylem expansion in response to flowering. Guided by the analysis of natural genetic variation in this trait, we identified local determinants as well as a systemic determinant, which appears to be identical with the mobile signal.
RESULTS
Flowering as a General Condition for Hypocotyl Xylem Expansion in Rosette Plants
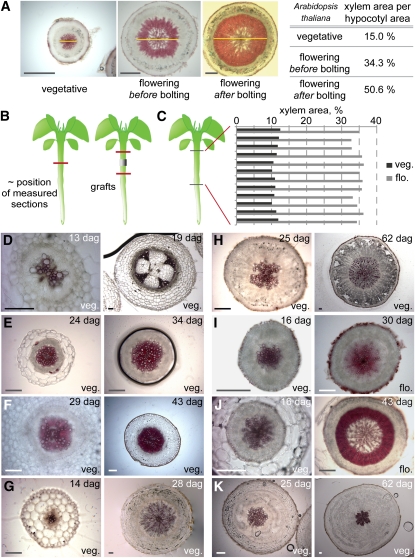
The aerial part of plants consists of repeating units called phytomeres, which each comprise a stem internode, one or several organs, and an axillary meristem. The internodes of vegetative Arabidopsis phytomeres do not elongate significantly before bolting, and Arabidopsis is thus classified as a plant with rosette habit. The switch from vegetative to reproductive growth (flowering) reprograms the shoot apical meristem to produce inflorescence rather than leaf primordia and coincides with the appearance of elongated internodes bearing inflorescences (bolting). Since flowering also triggers xylem expansion along Arabidopsis hypocotyls (Figures 1A to 1C) (Sibout et al., 2008), we investigated whether this phenomenon is conserved in other species and whether it correlates with the rosette growth habit. To this end, we selected species within the Brassicacae and Astereacae (rosette plants mostly belong to these two families) that do or do not display a rosette growth habit. In addition, we analyzed two species from the Solanaceae, which do not encompass rosette plants. For all species, hypocotyl cross sections were obtained at different developmental stages and stained with phloroglucinol to highlight xylem tissue. In all nonrosette plants analyzed (Arabis alpina, Aster alpinus, Nicotiana benthamiana, and Solanum lycopersicum), xylem started to expand during the vegetative phase (Figures 1D to 1G), as indicated by the appearance of fibers, whereas in rosette plants, (Arabidopsis, Cardamine hirsuta, Barberea verna, and Taraxacum officinalis), no xylem expansion was observed before flowering (Figures 1H to 1K). Thus, flowering appears to be a general condition for xylem expansion in the hypocotyl of rosette plants.
Figure 1.
Flowering as a Condition for Hypocotyl Xylem Expansion in Plants with Rosette Growth Habit.
(A) Progression of xylem expansion in Arabidopsis illustrated by transverse sections, stained for lignin with phloroglucinol to highlight xylem vessels and fibers (yellow line indicates xylem diameter). Xylem area compared with total hypocotyl area increases slowly during the vegetative phase but accelerates considerably once flowering occurs.
(B) Schematic illustration of the approximate position of sections (red lines) sampled to measure the relative xylem area in the genotypes analyzed in this study. Sections were taken from the hypocotyl center, except in the case of grafting experiments. For the latter, scion and stock sections were taken about halfway between the apical and basal ends of the hypocotyls, respectively, and the silicon tubing collar of the grafts.
(C) Relative xylem area as determined from serial sections along individual Arabidopsis hypocotyls before and after flowering.
(D) to (K) Hypocotyl cross sections of species with nonrosette growth habit (i.e., the stem internodes elongate already during vegetative growth) ([D] to [G]) and with rosette growth habit (i.e., the stem internodes elongate only once flowering has been induced) ([H] to [K]), demonstrating that in nonrosette plants, xylem expansion already starts during vegetative growth. S. lycopersicum (D), A. alpina (E), Aster alpinus (F), N. benthamiana (G), B. verna (H), Arabidopsis (I), C. hirsute (J), T. officinalis (K). dag, days after germination; veg., vegetative growth phase; flo., flowering, at appearance of inflorescence meristem. Bars = 200 μm.
Flower Specification and Bolting Are Not Required for Hypocotyl Xylem Expansion
An open question that we also aimed to answer was whether hypocotyl xylem expansion depends on the actual formation of flowers? To this end, we analyzed mutants in the FLOWERING LOCUS T (FT) gene, which produces a mobile protein that moves from the leaf vasculature to induce flowering in the shoot apical meristem in long-day conditions (Corbesier et al., 2007). In addition, FT acts as a floral pathway integrator (a central regulator of flowering) in the inflorescence meristem. ft-1 mutants flower late compared with the wild type; thus, xylem expansion was determined at the same developmental stage (i.e., at or shortly after the appearance of the inflorescence, as throughout this article unless indicated otherwise) rather than the same age to allow meaningful comparison of secondary growth vigor (Sibout et al., 2008). In these assays, ft-1 mutants displayed wild-type levels of xylem expansion (see Supplemental Figures 1A and 1B online), also suggesting that FT is not the elusive mobile signal for hypocotyl expansion. LEAFY (LFY) is another flowering pathway integrator and also controls flower and inflorescence identity (Weigel et al., 1992). lfy ft double mutants bolt very late and produce leaf-like structures instead of flowers (Kardailsky et al., 1999). Still, the double mutants displayed wild-type xylem expansion (see Supplemental Figure 1C online), suggesting that floral identity is not a prerequisite for this process.
Somewhat opposite to lfy ft double mutants, terminal flower1 (tfl1) mutants are tiny plants, which flower early after producing only few leaves and terminate their inflorescence prematurely (Kobayashi et al., 1999). Again, as in the wild type, xylem expansion in tfl1 occurred at flowering (see Supplemental Figure 1D online), corroborating that neither inflorescence weight nor bolting are the triggering factor (Sibout et al., 2008). Additional evidence that bolting is not required for xylem expansion was obtained from investigation of pennywise pound-foolish (pny pnf) double mutants. PNY and PNF are two homeodomain transcription factors that promote flowering. Their combined loss of function results in plants that never flower and continue to produce leaves instead. pny pnf mutants respond to flowering stimuli but do not complete floral induction and fail to express the majority of floral meristem identity genes (Smith et al., 2004; Kanrar et al., 2008). Surprisingly, we observed some xylem expansion in pny pnf plants, although with a long delay compared with the wild type (see Supplemental Figures 1E to 1G online). In summary, these analyses of key flowering regulators suggest that neither floral specification nor bolting is required for hypocotyl xylem expansion.
Hypocotyl Xylem Expansion Is Not an Age-Related Trait
The result from the analysis of the pny pnf double mutants suggested that age might be a factor in triggering hypocotyl xylem expansion. Indeed, a novel, age-regulated flowering induction pathway that acts in parallel to FT and might still be functional in pny pnf has been identified; this pathway is based on the interplay of two microRNAs, miR156 and miR172, which control the general transition from the juvenile to the adult phase (Wu et al., 2009). The direct targets of miR156 are transcription factors of the SQUAMOSA PROMOTER BINDING PROTEIN-LIKE family (Cardon et al., 1999), whereas the targets of miR172 are APETALA2-like transcription factors (Aukerman and Sakai, 2003). To determine whether these miRNAs could trigger xylem expansion, we analyzed both overexpressor lines of miR156 and miR172 as well as so-called mimic lines, in which the activity of the respective miRNA is reduced by expression of a complementary, artificial miRNA (Franco-Zorrilla et al., 2007; Todesco et al., 2010). Both overexpressor and mimic lines did not show any xylem phenotype during development, whether assayed before or at flowering (see Supplemental Figures 1H to 1M online). Moreover, loss of function in miR156 targets also displayed wild-type xylem expansion (see Supplemental Figure 2 online). Collectively, these data suggest that the miR156/miR172 pathway does not control xylem expansion and that xylem expansion is in sync with the transition to reproductive development irrespective of absolute age.
Exploring Natural Variation to Identify Novel Rate-Limiting Factors for Xylem Expansion
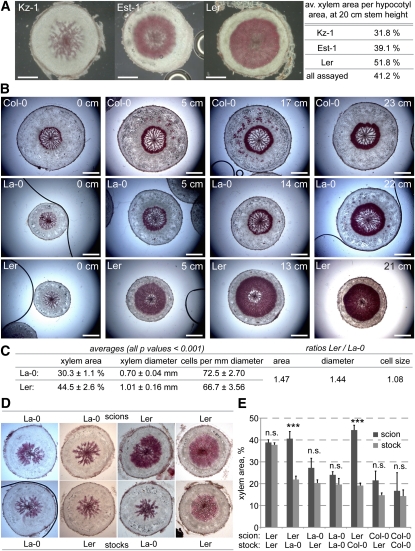
In summary, the investigation of mutant and transgenic lines affected in various flowering pathways, as well as variation in relevant environmental conditions, such as photoperiod, did not yield any candidate regulators of xylem expansion. Since the same was true for genes involved in bolting and flower specification, it appears that xylem expansion is a consequence of the incipient reprogramming of the shoot apical meristem upon flowering. To find relevant regulators, we turned to the analysis of natural genetic variation, which enabled us previously to isolate a major QTL for xylem expansion, the central flowering time regulator FLOWERING LOCUS C (Sibout et al., 2008). As suggested by our earlier study, we revisited this trait to assay Arabidopsis accessions at their individual time of flowering rather than the same age to select against identification of flowering time genes and reveal natural variation in genuine regulators of hypocotyl secondary growth (Sibout et al., 2008). Indeed, natural variation in our indicator trait for xylem expansion, the ratio of xylem area to total hypocotyl area in transverse sections, was observed. Among the few dozen investigated, two accessions stood out because of their atypical secondary growth phenotypes: whereas xylem expansion was strongly enhanced in the ommonly used Landsberg erecta (Ler) accession, xylem expansion was significantly delayed in Kazakhstan-1 (Kz-1) (Figure 2A).
Figure 2.
ER Is a Negative Regulator of Hypocotyl Xylem Expansion.
(A) Natural quantitative variation in hypocotyl xylem expansion between Arabidopsis accessions, exemplified as xylem area per total transverse hypocotyl area in plants with 20-cm-tall main inflorescence stems. Est-1, Estland-1.
(B) Progression of hypocotyl xylem expansion in the Col-0 reference strain and the er mutant (Ler) in La-0 background. Plants with roughly equal stem height are shown for comparison; 0-cm stem height represents flowering (i.e., appearance of inflorescence meristem).
(C) Quantification of Ler and La-0 xylem expansion traits determined 8 d after flowering. Standard errors are indicated. All differences are significant with P values < 0.001.
(D) Examples of scion and stock hypocotyl sections from micrograftings of indicated genotypes, sampled at 8 d after flowering.
(E) Xylem area quantification of scion and stock hypocotyls obtained from indicated micrograftings, sampled at 8 d after flowering. Error bars are standard error. n.s., not significant; *P < 0.05; **P < 0.01; ***P < 0.001. Bars = 200 μm.
ERECTA Is a Locally Acting Negative Regulator of Xylem Expansion
Detailed analysis of the Ler accession revealed that the onset of xylem expansion is normal at the time of bolting but proceeds at an enhanced rate afterwards compared with other accessions, such as the Arabidopsis reference accession Columbia (Col-0) (Figure 2B). Ler is a mutant derived from the original Landsberg-0 (La-0) accession and carries a loss-of-function mutation in the ERECTA (ER) gene (Redei, 1962), which encodes a Leu-rich receptor-like Ser/Thr kinase (Torii et al., 1996). The La-0 accession displayed normal, less prominent xylem expansion, comparable to Col-0 (Figures 2B and 2C), suggesting that the Ler phenotype is due to the er loss of function. This idea fits with the fact that ER acts as a major general regulator of growth (van Zanten et al., 2009). However, while ER is a positive regulator of elongation growth, it appears to be a negative regulator of secondary growth. Notably, although xylem cells in Ler are somewhat bigger, the increased xylem area in Ler was only to a minor part due to cell size (Figure 2C), corroborating that the effect of the er mutation manifests primarily at the level of enhanced xylogenesis rather than cellular anisotropy.
To determine whether ER could have a role in the mobile signal, we took advantage of the micrografting technique (Turnbull et al., 2002). To facilitate this approach, we germinated seedlings at 25°C to enhance hypocotyl elongation before performing reciprocal grafts between hypocotyls of the different genotypes. Xylem expansion was then measured in the upper hypocotyl, derived from the scion, and the lower hypocotyl, derived from the stock, at 8 d after appearance of the inflorescence meristem. These experiments revealed that the stimulation of xylem expansion observed in Ler scions was not graft transmissible and that this stimulation was suppressed in Ler stocks if the scion was derived from La-0 or Col-0 (Figures 2D and 2E). This might mean that the extent of secondary growth in the scion and, thus, ER limits the secondary growth of the stock, possibly by limiting transmission of the mobile signal. In summary, it appears that ER is not involved in generating or suppressing the mobile signal and that ER acts locally, downstream of the mobile signal.
Analysis of the Kz-1 Accession Suggests a Role of the GA Pathway in Hypocotyl Xylem Expansion
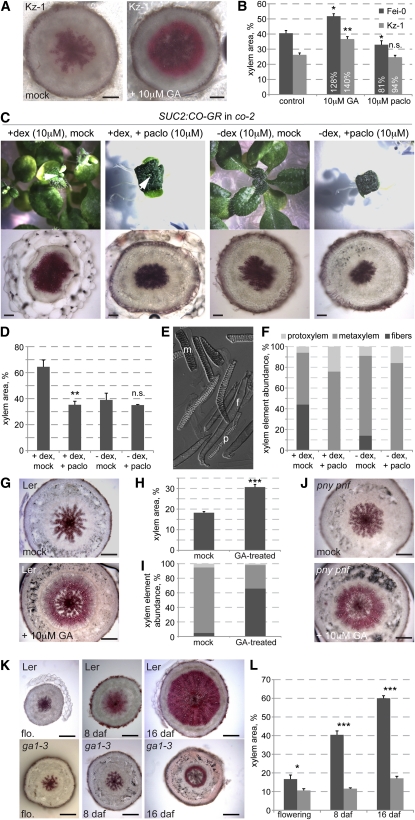
Opposite to Ler, xylem expansion in the Kz-1 accession was strongly delayed and did not occur even well after flowering and bolting (Figure 2A). Interestingly, in an independent survey of hormone response of Arabidopsis accessions, Kz-1 displayed outlier phenotypes in response to application of the GA biosynthesis inhibitor, paclobutrazol (M. Blazquez, personal communication). This suggested that Kz-1 might to some degree be altered in GA biosynthesis, perception, or signaling. Indeed, xylem expansion in Kz-1 could be restored by GA application (Figure 3A). Moreover, GA treatment of other genotypes also resulted in a significant increase in the xylem area to total area ratio (Figures 3B and 3G).
Figure 3.
GA Is Necessary for Hypocotyl Xylem Expansion.
(A) Rescue of the reduced xylem expansion phenotype of the Kz-1 accession (assayed at 26 d after germination) by treatment with 10 μM GA.
(B) Quantification of xylem expansion in Kz-1 (assayed at 29 d after germination) and an average accession, Fei-0 (St. Maria d. Feiria) (assayed at 34 d after germination) with or without GA treatment.
(C) Suppression of hypocotyl xylem expansion in SUC2:CO-GR transgenic plants (co-2 mutant background) upon interference with GA biosynthesis by treatment with the inhibitor paclobutrazol (paclo). Plants were first treated with dexamethasone (dex) from 0 to 5 d after germination to induce flowering and thereby xylem expansion. This was followed by a 14-d treatment with paclobutrazol, inducing a GA-deficient phenotype (top). Induced plants flowered at 19 d after germination, and hypocotyls were sampled and sectioned at that point (bottom). Arrowheads point out the inflorescence meristem in dexamethasone-induced plants.
(D) Quantification of xylem area in the experiment illustrated by examples in (C). Error bars are se.
(E) Example of xylem elements released from a hypocotyl sample by maceration. Elements can be distinguished by their morphology and counted to determine relative abundance. f, fiber; m, metaxylem; p, protoxylem.
(F) Quantification of xylem element abundance in the experiment illustrated by examples in (C).
(G) Stimulation of xylem expansion in the Ler accession by GA treatment, sampled at vegetative state.
(H) Quantification of xylem area in the experiment illustrated by examples in (G). Error bars are se.
(I) Quantification of xylem element abundance in the experiment illustrated by examples in (G). See (F) for xylem element coding.
(J) Stimulation of xylem expansion in the pny pnf double mutant by GA treatment, sampled at vegetative state.
(K) Reduction of xylem expansion in the GA-biosynthetic mutant ga1-3 compared with its background, Ler.
(L) Quantification of xylem area in the experiment illustrated by examples in (K). Error bars are se. daf, days after flowering; n.s., not significant; *P < 0.05; **P < 0.01; ***P < 0.001. Bars = 200 μm.
To further investigate a role of the GA pathway in xylem expansion, we took advantage of transgenic lines that allow dexamethasone-induced nuclear import of a CO-glucocorticoid receptor fusion protein (CO-GR) expressed under control of the SUCROSE TRANSPORTER2 promoter in a co-2 null mutant background (SUC2:CO-GR). Induction enables the CO-GR fusion protein to trigger the expression of FT and, thus, flowering and consequently hypocotyl xylem expansion (Sibout et al., 2008). Combination of this induction with paclobutrazol treatment prevented xylem expansion as well as fiber formation (Figures 3C and 3D). Maceration of the hypocotyls allowed us to score the abundance of the different xylem elements (Muñiz et al., 2008), confirming the absence of fibers and an increased amount of vessels in response to paclobutrazol treatment (Figures 3E and 3F). We also conducted the inverse experiment and investigated whether GA application could promote xylem expansion before flowering. To this end, plants were grown in short-day conditions to prevent flowering and sprayed with GA at 28 d. Hypocotyls were then sectioned and macerated once the inflorescence meristem became visible (at 35 d for GA-treated plants and at 41 d for the control plants). The GA-treated plants displayed a significant increase in the xylem area to total area ratio and in the proportion of fibers (Figures 3G to 3I). This finding was corroborated by GA treatment of the pny pnf double mutant, which did not restore flowering, but again induced xylem expansion compared with mock-treated plants (Figure 3J).
We confirmed our results by analysis of the prototypical GA-deficient mutant ga1-3 (Sun and Kamiya, 1994). ga1-3 plants are dwarfs with small, dark-green leaves and severely stunted inflorescences. They also flower very late, as is typical for GA-deficient plants (Willige et al., 2007) (i.e., in our conditions, ga1-3 flowered 50 d later than the Ler control). Hypocotyl sections of both genotypes were again taken at the same stage after flowering. Consistent with our earlier observations, ga1-3 mutants displayed severely reduced xylem expansion, although fibers were still present (Figures 3K and 3L).
GA Signaling Is Necessary to Permit Hypocotyl Xylem Expansion
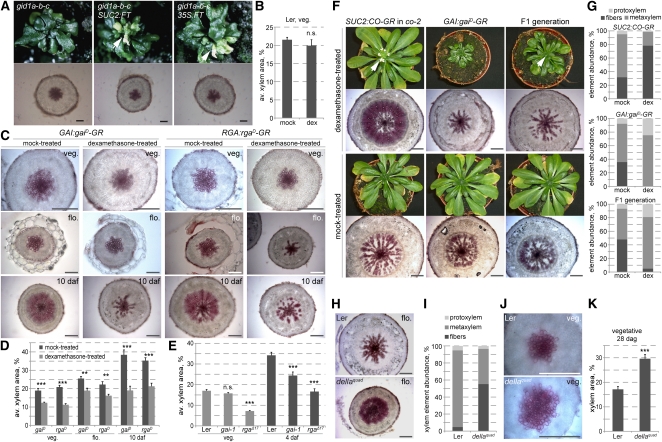
To investigate whether the GA effect on xylem expansion is mediated by the described GA signaling pathway, we also assayed GA signaling mutants. First, we sought to analyze mutants in the GA receptor, which is encoded by three redundantly acting genes, GA INSENSITIVE DWARF (GID) 1a, GID1b, and GID1c (Griffiths et al., 2006; Nakajima et al., 2006; Willige et al., 2007). gid1a-b-c triple null mutants (as described in Willige et al., 2007) largely resemble ga1-3 both in their phenotypic and molecular characteristics; however, their phenotype is even more severe in that they never flower (Willige et al., 2007). This is unlike another gid1a-b-c triple mutant (Griffiths et al., 2006), which was constructed with a GID1C mutant allele (gid1c-1) that carries an intronic T-DNA insertion. Although it has been reported that wild-type transcript was not found in this allele and it should be considered null, the flowering phenotype suggests that it might be leaky. By contrast, the gid1a-b-c triple used in our study has been constructed using the gid1c-2 allele, an exon insertion (Willige et al., 2007). This line never flowered. Therefore, we introduced either a SUC2:FT or a 35S:FT transgene into segregating lines to obtain gid1a-b-c triple mutants that constitutively express the FT florigen. In these lines, we eventually observed flower-like structures, indicating that reprogramming of the shoot apical meristem by the transgene was successful (Figure 4A). However, no hypocotyl xylem expansion was observed in these plants, suggesting that the GA receptors are required for this process.
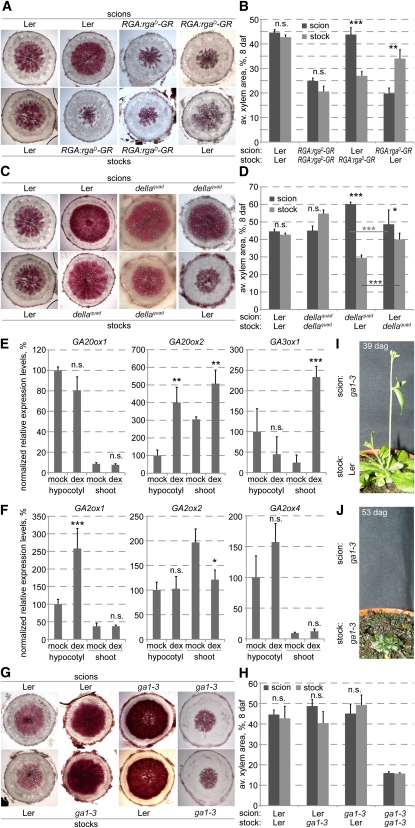
Figure 4.
Control of Hypocotyl Xylem Expansion by GA Signaling.
(A) gid1a-b-c triple mutants that fail to flower (top) unless transgenes driving constitutive expression of the florigen, FT, are introduced. Transverse hypocotyl sections (bottom) were taken at 14 days after flowering of the transgenic lines for all lines. Arrowheads point out the inflorescence meristem and flower-like structures in transgenic plants.
(B) Control experiment demonstrating the absence of dexamethasone (dex) effects on xylem expansion in the Ler control background.
(C) Suppression of xylem expansion by dex activation of dominant-negative versions of transgenic GAI and RGA genes (gaiD and rgaD) fused with the glucocorticoid receptor domain (GR).
(D) Quantification of xylem area in the experiments illustrated in (C). Error bars are se.
(E) Reduced xylem expansion in the rgaΔ17 and the gai-1 mutants compared with their Ler background at vegetative state and after flowering. Error bars are se.
(F) Suppression of xylem expansion by dex activation of dominant-negative transgenic GAI and simultaneous dex induction of flowering and, thus, xylem expansion through the SUC2:CO-GR transgene. Arrowheads point out the inflorescence meristems.
(G) Quantification of xylem element abundance in the hypocotyls sampled in the experiment illustrated in (F).
(H) Enhanced xylem expansion at flowering in the rga-24 gai-t6 rgl1-1 rgl2-1 quadruple loss-of-function mutant (dellaquad) compared with its Ler background (dellaquad at 36 d after germination; Ler at 42 d after germination).
(I) Quantification of xylem element abundance in the hypocotyls sampled in the experiment illustrated in (H).
(J) Xylem expansion in hypocotyls of Ler and dellaquad plants at same age vegetative state (28 d after germination).
(K) Quantification of xylem area in the experiment illustrated by examples in (J). Error bars are se. daf, days after flowering; n.s., not significant; *P < 0.05; **P < 0.01; ***P < 0.001. Bars = 200 μm.
The targets of the GA receptors are transcriptional regulators of the so-called DELLA class (Silverstone et al., 2001), which repress growth and are targeted for degradation upon GA binding to the GID1 receptors. In Arabidopsis, the DELLA family comprises five members, GA-INSENSITIVE (GAI), REPRESSOR OF ga1-3 (RGA), and RGA-LIKE (RGL) 1-3 (Davière et al., 2008), which were identified by mutant analysis (Peng et al., 1997; Dill et al., 2001). Dominant gai and rga mutants encode stabilized variants of the proteins that are insensitive to GA-triggered degradation. Thus, the mutant proteins continue to suppress elongation growth even in the presence of GA, leading to a stunted dwarf phenotype. We obtained transgenic plants that express functional fusions of these dominant versions with the GR domain under control of the respective endogenous promoters (GAI:gaiD-GR and RGA:rgaD-GR), enabling their dexamethasone-inducible activation as described above. Dexamethasone treatment as such had no effect on the xylem expansion phenotype of the wild-type background (Figure 4B). However, dexamethasone activation of the transgenic proteins not only resulted in a dwarf phenotype similar to the gai-1 and rgaΔ17 mutants but also in a large reduction in xylem expansion from the vegetative stage onwards compared with controls (Figures 4C and 4D). This effect was confirmed by analysis of the original dominant gai-1 and rgaΔ17 mutants; however, at the vegetative stage, only the rgaΔ17 mutants were significantly affected at earlier stages. Reduced xylem expansion also became obvious in gai-1 later, but the phenotype remained quantitatively milder than the one observed in rgaΔ17, suggesting that RGA has a genuinely more prominent role in xylem expansion than GAI (Figure 4E).
One shortcoming of the experiments described above is that GA itself has an effect on flowering time (Willige et al., 2007), for instance, flowering was suppressed in paclobutrazol-treated plants. We thus sought to corroborate our results by uncoupling flowering and GA signaling. To this end, we crossed the SUC2:CO-GR and the GAI:gaiD-GR lines and investigated the resulting F1 generation that carried both transgenes. In these plants, dexamethasone-treatment thus triggered flowering (which occurred simultaneously in the SUC2:CO-GR parents and the F1 plants) and at the same time interfered with GA signaling. Again, this resulted in the suppression of xylem expansion (Figure 4F). Quantification of xylem elements by the maceration assay confirmed that this coincided with a shift toward preferentially early cell types (Figure 4G). In summary, our results thus suggest that both GA biosynthesis and signaling are necessary for xylem expansion to occur.
GA Signaling Is Sufficient to Induce Xylem Expansion in the Absence of Flowering
Opposite to the dominant della mutants is a della quadruple mutant that combines loss-of-function alleles (rga-24 gai-t6 rgl1-1 rgl2-1) (Achard et al., 2006), largely abolishing the redundancy between the DELLA proteins and resulting in constitutively elevated GA signaling even in the absence of the hormone (Cao et al., 2005). In this della quadruple mutant, xylem expansion was increased as compared with the wild type at flowering (Figure 4H), concomitant with a higher abundance of fibers (Figure 4I). This quadruple mutant also enabled us to determine whether GA signaling is sufficient to trigger xylem expansion, as implied by the stimulatory effect of GA treatment described above. To this end, we analyzed the mutants during the early, proportional phase of hypocotyl secondary growth, prior to flowering. To increase the trait resolution, we conducted these experiments in short-day conditions, which promoted overall secondary growth but not xylem expansion, and collected samples at 26 to 28 d after germination, well before any signs of flowering as determined by parallel grown control groups. In the hypocotyls of della quadruple mutants, the area occupied by xylem vessels and parenchyma cells was increased relative to the total area compared with the Ler control plants (Figures 4J and 4K). Thus, GA signaling appears to be sufficient to stimulate xylem production, even in the absence of flowering. Notably, in this experiment, no xylem fibers were observed, suggesting that GA does not exclusively promote fiber differentiation and, further, that fiber differentiation might be a secondary effect of GA-triggered xylem expansion.
GA-Mediated Stimulation of Xylem Expansion Does Not Require the Auxin Pathway or Polar Auxin Transport
The GA pathway has been shown to interact with the auxin pathway in the regulation of diverse developmental processes (e.g., Fu and Harberd, 2003; Frigerio et al., 2006), including the regulation of cambial activity (Björklund et al., 2007). We thus examined whether the stimulatory effect of GA on xylem expansion might also be mediated through or in conjunction with auxin signaling or polar auxin transport. To this end, we again employed the inducible secondary growth system based on the SUC2:CO-GR line. Treatment with the polar auxin transport inhibitor N-1-naphthylphthalamic acid did not affect xylem expansion, neither in control plants nor when xylem expansion was induced (see Supplemental Figure 3A online). Likewise, compared with Col-0 controls, xylem expansion was still observed in null mutants of the prototypical auxin efflux carrier, PIN-FORMED1, in which polar auxin transport is impaired (Gälweiler et al., 1998). Thus, our data suggest that polar auxin transport is not a limiting factor for xylem expansion.
Next, we analyzed xylem expansion in diverse auxin-related signaling mutants that display a hypocotyl phenotype, corroborating that the respective genes are active in our tissue of interest. None of the genotypes with constitutively reduced (non-phototropic hypocotyl4 and transport inhibitor response1) auxin signaling examined displayed altered xylem expansion or fiber formation, suggesting that auxin signaling is not limiting this process (see Supplemental Figure 3B online).
GA Signaling Acts Locally to Promote Xylem Expansion
An open question that remained was whether GA signaling stimulates xylem expansion locally or whether it functions at the shoot apex to launch the production of the elusive mobile signal. To decide this issue, we performed reciprocal micrografting as described above between RGA:rgaD-GR or della quadruple plants and their Ler control background. In self-graftings of either genotype, xylem expansion proceeded at a similar rate in both scion and stock and was suppressed in the RGA:rgaD-GR background as expected if dexamethasone was applied (Figures 5A to 5D). By contrast, in dexamethasone-treated Ler onto RGA:rgaD-GR or RGA:rgaD-GR onto Ler grafts, the Ler scions or stocks displayed prominent xylem expansion, whereas the RGA:rgaD-GR scions or stocks did not (Figures 5A and 5B). The grafting between the della quadruple mutant and Ler confirmed the local action of GA signaling. In both Ler onto della and della onto Ler grafts, the della scions or stocks displayed more prominent xylem expansion than their Ler counterparts (Figure 5D). A possible explanation for the observation that expansion in the Ler stock was smallest when combined with a della quadruple mutant scion is that this scion may have very low GA levels due to the feedback regulation of GA homeostasis, thereby limiting the GA supply to the Ler stock (see below). In the respective self-grafts, xylem expansion proceeded at a similar rate in both scion and stock (Figures 5C and 5D). Collectively, our results thus suggest that the effects of altered GA signaling on xylem expansion are not graft transmissible. Therefore, GA signaling is not required for the production of the elusive mobile signal and acts locally to promote xylem expansion, downstream of this signal.
Figure 5.
Evidence for GA as the Mobile Shoot-Derived Signal That Triggers Hypocotyl Xylem Expansion upon Flowering.
(A) Examples of scion and stock hypocotyl sections from micrograftings of indicated genotypes, sampled at 8 d after flowering.
(B) Xylem area quantification of scion and stock hypocotyls obtained from indicated micrograftings, sampled at 8 d after flowering. Error bars are se.
(C) Examples of scion and stock hypocotyl sections from micrograftings of indicated genotypes, sampled at 8 d after flowering.
(D) Xylem area quantification of scion and stock hypocotyls obtained from indicated micrograftings, sampled at 8 d after flowering. Error bars are se.
(E) Expression level quantifications of GA biosynthetic genes in SUC2:CO-GR transgenic plants (co-2 mutant background), comparing hypocotyls and shoots of 9-d-old mock-treated samples and samples treated with dexamethasone (dex) from 6 to 8 d after germination to induce flowering and thereby xylem expansion. Averaged relative expression levels normalized with respect to the EF1 housekeeping gene are indicated. Expression was quantified for all genes in each of three replicates; measurements represent the average of the relative expression with respect to EF1 from each sample. Error bars are se.
(F) Same experiment as (E), for GA catabolic genes. Error bars are se.
(G) Examples of scion and stock hypocotyl sections from micrograftings of indicated genotypes, sampled at 8 d after flowering.
(H) Xylem area quantification of scion and stock hypocotyls obtained from indicated micrograftings, sampled at 8 d after flowering. Error bars are se.
(I) Development of a ga1-3 shoot scion grafted onto a Ler root stock.
(J) Development of a ga1-3 shoot scion grafted onto a ga1-3 root stock. n.s., not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
GA Is the Mobile Shoot-Derived Signal That Induces Hypocotyl Xylem Expansion
The local role of GA signaling in xylem expansion together with the ga1-3 secondary growth phenotype supports the idea that GA itself must also act locally to induce xylem expansion. Moreover, the documented induction of GA biosynthesis in the shoot upon flowering (Eriksson et al., 2006) suggested that GA might also act systemically. We corroborated this result by analysis of the transcript levels of those GA biosynthetic and catabolic enzymes (Hedden and Phillips, 2000) for which we could detect expression in the hypocotyl or shoot. Comparison of the expression of three biosynthetic enzymes in the hypocotyl or shoot (i.e., shoot apical meristem plus cotyledons and the first true leaves) of dexamethasone- versus mock-treated SUC2:CO-GR plants revealed a significant increase in GA20ox2 levels in both hypocotyl and shoot and of GA3ox1 in the shoot (Figure 5E). Thus, expression of GA biosynthetic enzymes increased in both hypocotyl and shoot (Figure 5E) upon flowering induction. By contrast, of the three catabolic enzyme genes assayed, only GA2ox1 displayed increased expression in the hypocotyl upon flowering (Figure 5F).
Interestingly, although GA biosynthesis genes were generally induced in both hypocotyl and shoot upon flowering (Figure 5E), increased expression of the gene encoding the last enzymatic step leading to the formation of bioactive GA, GA3ox1, was only observed in the shoot. This severalfold increase confirmed that upon flowering induction, bioactive GA mostly accumulates in the shoot (Eriksson et al., 2006). We thus wondered whether GA could be the mobile shoot-derived signal that triggers hypocotyl xylem expansion. To determine whether endogenous GA is mobile, we performed micrografting experiments between the ga1-3 mutant and the Ler background. Technically, these grafts were more demanding because of the severe phenotype of ga1-3. For instance, ga1-3 does not germinate unless GA is supplied externally, and the mutants are stunted and accordingly have short hypocotyls. Since we did not want to interfere with our experiment even by low transient levels of GA, we germinated ga1-3 seeds by mechanically opening the seed coat (as we did in all experiments using this mutant). We then cut the shoot off at the very top of the hypocotyl to graft a Ler scion on top of it. Strikingly, in these plants, the Ler scions restored xylem expansion in the ga1-3 stocks (Figures 5G and 5H). Interestingly, in the reciprocal graftings, the Ler stock was also able to gradually rescue the GA-deficient ga1-3 shoot phenotype, although with a strong delay. That is, the rosette leaves turned from dark to light green, they expanded in size and the inflorescence stem was able to elongate (Figure 5I). No such rescue was observed in self-grafts of the ga1-3 mutant (Figure 5J). In summary, our results therefore suggest that in the wild type, GA can move through the plant and is the mobile shoot-derived signal that triggers xylem expansion in the hypocotyl.
DISCUSSION
In our study, we aimed to determine the cause for the acceleration of secondary growth and the shift toward xylogenesis observed in Arabidopsis hypocotyls. A previous study had established that the transition toward this so-called xylem expansion is triggered by flowering and involves an elusive, shoot-derived mobile signal (Sibout et al., 2008). Our results suggest that this mobile signal is GA and that GA signaling and ER act locally and downstream of GA to regulate xylem expansion.
Among the accessions examined for their secondary growth vigor corrected for differences in flowering time, Ler stood out as the strain with strongest xylem expansion. This does not reflect a head start in this trait, as the onset of xylem expansion in Ler still depends on flowering. However, once triggered, it proceeds at an enhanced rate. Ler carries a loss-of-function mutation in the ER gene and was derived from the original La-0 accession by mutagenesis (Redei, 1962; Torii et al., 1996). As La-0 displays much weaker xylem expansion, comparable to the Col-0 reference accession, the er mutation appears to be responsible for the enhanced xylem expansion. ER is known to function in the regulation of aerial organ shape and size (van Zanten et al., 2009) and is a commonly used genetic background for Arabidopsis research due to its practical, compact growth habit. Thus, while ER has been shown to be a positive regulator of elongation growth, it appears to be a negative regulator of xylem expansion. ER has also been implicated in modulating various hormone pathways, including auxin, brassinosteroid, ethylene, and GA (van Zanten et al., 2009). Thus, the er mutation possibly enhances the xylem expansion phenotype through modification of cambial hormonal signaling, including the GA pathway.
Previous studies have painted a somewhat complex picture of the role of GA signaling in xylem development. Application of GA to decapitated, auxin-depleted Populus stems was shown to stimulate cell divisions in the cambial zone (Björklund et al., 2007). However, the identity of cells formed through GA-induced divisions was reported to be relatively obscure, as they appeared more spherical than the flat, thin-walled cells of the untreated cambium. Furthermore, they failed to differentiate into xylem cells and instead preserved their parenchymatic phenotype. When GA was applied together with auxin, both cambial cell divisions and xylem differentiation was stimulated, leading to the conclusion that GA signaling has a role in inducing cambial cell divisions but functions together with auxin in promoting xylem differentiation. This idea fits with a report of GA concentrations in Populus stems, which found only trace amounts in dividing cells of the active cambium, whereas the highest levels were detected in differentiating xylem cells (Israelsson et al., 2005). Consistently, the study also found low expression of GA biosynthetic enzymes and GA signaling genes in the dividing cambial cells and higher expression in both differentiating phloem and xylem cells. By contrast, a recent study indicates that the GA receptors are expressed the highest in the phloem and dividing cambial cells (Mauriat and Moritz, 2009). Thus, it appears that a comprehensive expression analysis of GA biosynthesis and signaling genes across the cambial zone is still missing, and it remains to be determined when and where exactly GA signaling is active in secondary growth. The observed synergism between GA and auxin application in Populus was accompanied by an increased auxin concentration in stem tissues. Combined with the finding that GA treatment induced expression of a cambial auxin efflux carrier gene, these results indicated that GA action might promote auxin transport (Björklund et al., 2007). Reciprocally, auxin treatment stimulated expression of GA biosynthesis genes and inhibited expression of genes encoding GA degrading enzymes (Björklund et al., 2007), suggesting a feed forward loop that would promote cambial activity.
In Arabidopsis, the GA pathway has been shown to interact with the auxin pathway in the regulation of diverse developmental processes. For instance, auxin signaling in roots has been shown to induce degradation of RGA (Fu and Harberd, 2003), thereby promoting root growth, and it has also been suggested that auxin can induce GA biosynthesis (Frigerio et al., 2006). However, the relationship between GA and auxin also appears to be complex and context specific. In the root meristem for instance, while auxin induces RGA degradation, GA signaling in turn dampens auxin signaling and transport through a multistep process involving signaling components of another plant hormone, cytokinin (Moubayidin et al., 2010). The direct stimulatory effect of GA observed in our studies does not appear to depend on auxin transport or signaling as suggested by analysis of respective mutants. We would like to clarify, however, that this should be seen in the context of the quantitative xylem expansion trait and should not be extended to the qualitative level. That is to say that the auxin pathway is clearly indispensable for vascular development (Berleth et al., 2000) but does not appear to be limiting for the observed quantitative acceleration in xylogenesis.
On the one hand, our result that GA stimulates xylogenesis directly could reflect a fundamental difference between Arabidopsis and Populus. On the other hand, it could reflect the fact that unlike in the hypocotyl, analyses of secondary growth in stems are intrinsically complicated by parallel elongation growth (Sibout et al., 2008). For example, this issue is illustrated by another secondary growth trait that has been attributed to GA, that is, fiber formation. Analyses of transgenic trees overproducing GA and external GA application experiments have shown that GA can stimulate xylem fiber differentiation and elongation (Digby and Wareing, 1966; Eriksson et al., 2000). For instance, Populus trees overexpressing a GA biosynthetic gene produced more total fibers than the control trees. However, this was accompanied by a general growth-promoting effect, including an increase in both stem height and diameter. Thus, while these Populus produced more total fibers than the control trees, their relative abundance remained approximately constant (Eriksson et al., 2000). In Arabidopsis hypocotyls, fiber differentiation occurs coincident with flowering, similar to xylem expansion, however, with a delay compared with the latter. In the della quadruple mutants investigated, the constitutive GA signaling led to xylem expansion well before flowering, concomitant with fiber differentiation. Moreover, in the gid1 a-b-c genotypes, fibers were absent but could still be detected in the ga1-3 mutant. This suggests that GA primarily promotes the proliferation of xylem, while the occurrence of fibers appears to be a delayed secondary effect.
Previous studies, which have relied on external application of labeled GA, have suggested that GA can move inside the plant. For instance, applied GA was transported downwards in Populus stems and from leaves to the shoot apex in Arabidopsis (Eriksson et al., 2006; Björklund et al., 2007), whereas it moved upwards in pea (Pisum sativum) stems (Proebsting et al., 1992). The latter study also demonstrated cross-complementation between scions and stocks of one GA biosynthesis mutant and another in pea. The results from our ga1-3 versus Ler micrografting experiments confirm that GA is mobile. They also confirm that endogenous GA is able to move inside the plant in both acro- and basipetal directions because GA production in either the shoot or root was sufficient to compensate for impaired GA biosynthesis in the grafted scion or stock. This finding is underlined by the basically complete phenotypic rescue of ga1-3 shoot scions by Ler root stocks, albeit with a strong temporal delay of over 2 weeks with respect to other grafts. This might mean that the drain of GA by the GA-deficient shoot leads to decreased feedback inhibition of GA biosynthesis in the root, thereby elevating its GA export capacity. However, an alternative explanation would be that the observed rescue results from GA export from the root system that increases with its growth. This would explain the considerable delay in the rescue of the ga1-3 scions, as well as the eventual, albeit rather weak, xylem expansion observed in pny pnf mutants.
Beyond genetically proving the mobility of GA, our grafting experiments also suggest that in the wild-type situation, GA itself is the mobile shoot-derived signal that is transported to the cambial zone of the hypocotyl upon flowering initiation to trigger xylem expansion. This idea is consistent with the described feed-forward upregulation of GA levels at the shoot apex upon flowering induction (Eriksson et al., 2006). In particular, the concentration of GA was shown to increase dramatically already before floral initiation and to stay at constantly high levels thereafter (Eriksson et al., 2006). Combined with the observed upregulation of the last step GA biosynthetic enzyme GA3ox1 in the shoot, this suggests that it is likely GA that, at least initially, triggers xylem expansion. In an ontogenetic context, this sequence of events would make sense, as it would increase the water and solute transport capacity of the hypocotyl in anticipation of the substantial height increase associated with inflorescence formation and bolting.
METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana and other species were grown either in standard tissue culture conditions or in soil as described, in continuous light or in short-day conditions (16-h-dark/8-h-light cycle) depending on the experiment as indicated in the text and figure legends. The spl3, spl4, spl9, ft-1, lfy-1, lfy ft double, tfl1, pin1, tir1, nph4, hy5, hy5 hyh double, pny pnf double, ga1-3, rga-24 gai-t6 rgl1-1 rgl2-1 quadruple, gai-1, and rgaΔ17 mutants, as well as the 35S:MiM156, 35S:MiR156, 35S:MiM172, 35S:MiM172, RGA:rgaD-GR, GAI:gaiD-GR, and SUC2:CO-GR transgenic lines used in this study have been described previously (Kardailsky et al., 1999; Kobayashi et al., 1999; Smith et al., 2004; Sibout et al., 2006; Corbesier et al., 2007; Franco-Zorrilla et al., 2007; Kanrar et al., 2008; Sibout et al., 2008; Todesco et al., 2010). The gid1a-b-c triple mutants and their transgenic derivatives containing either a SUC2:FT or a 35S:FT transgene were generated by crossing and selection of the triple mutant in segregating lines by genotype (Willige et al., 2007). Arabis alpina, Aster alpinus, Nicotiana benthamiana, Solanum lycopersicum, Cardamine hirsuta, Barberea verna, and Taraxacum officinalis seeds were either lab stocks or obtained from colleagues or garden centers. Natural Arabidopsis accessions correspond to the lines distributed by the Nottingham Arabidopsis Stock Centre (http://nasc.nott.ac.uk). Absence of flowering was defined as absence of an inflorescence meristem. Where applicable, flowering time was determined in parallel grown control plants.
GA, Paclobutrazol, N-1-Naphthylphthalamic Acid, and Dexamethasone Treatment
For tissue culture experiments to analyze the effects of paclobutrazol and N-1-naphthylphthalamic acid, SUC2:CO-GR plants were grown for 5 d on half-strength Murashige and Skoog media in the presence or absence of 10 μM dexamethasone (Duchefa) to induce flowering before being transferred on plates containing either 10 μM paclobutrazol (Duchefa) or 5 μM N-1-naphthylphthalamic acid (Duchefa) or mock solution. After 14 d, floral meristems appeared in all dexamethasone-induced plants, and hypocotyls were sampled for phenotypic analyses. Induction of soil-grown SUC2:CO-GR, RGA:rgaD-GR, or GAI:gaiD-GR plants was achieved by watering with a 10 μM dexamethasone solution three times per week. For GA treatments, plants were sprayed with a 1 mM GA3 (Sigma-Aldrich) or mock solution at similar frequency (King et al., 2008).
Micrografting
Grafting between hypocotyls of scions and stocks using 1-mm pieces of silicon tubing was performed in tissue culture as described (Turnbull et al., 2002; Sibout et al., 2008) using young, 6-d-old seedlings grown on plates at 25°C in 16-h light days. Successful grafts were transferred into soil 7 d after grafting and grown under continuous light conditions.
Phenotypic Analyses of Hypocotyl Sections
Maceration of hypocotyls was performed as described (Muñiz et al., 2008), and xylem element abundance was scored by light microscopy, typically classifying at least 200 cells. To determine xylem area, hypocotyls were embedded in 6% agarose (Sigma-Aldrich) immediately after harvest and sectioned (85 μm thick) using a Leica-VT 1000S vibratom. Sections were stained for lignin with phloroglucinol (Prolabo VWR 26337) to highlight xylem vessels and fibers. Sections were photographed using a Diaplan 3 microscope (Leica) and analyzed using ImageJ software as described (Sibout et al., 2008). All images were taken at high resolution, and measurements were performed at zoom levels that allowed unequivocal identification of the xylem border.
Molecular Biology
Molecular biology procedures were performed according to standard procedures as described (Sibout et al., 2008). For quantitative PCR analyses, hypocotyls and shoots were dissected and quantitative PCR was performed using a Stratagene MX3000P instrument as described (Sibout et al., 2006). Expression levels were normalized with respect to the elongation initiation factor 1 gene (EF1). All quantitative PCR experiments were repeated in independent triplicates. The following oligonucleotides were used: EF1 (AT5G60390), 5′-GGTCACCAAGGCTGCAGTGAAGAA-3′ and 5′-GCTCAAACGCCATCAAAGTTTTAAGAA-3′; GA20ox1 (AT4G25420), 5′-GGGTATCTTCTTGATGTGATGCTGTCCAAA-3′ and 5′-GGTGAACAGCGAGAGCGAGAGGAAA-3′; GA20ox2 (AT5G51810), 5′-CAAGGAACATAGACCAAGTGAAGTCAGGGTA-3′ and 5′-ACGGGATATTCAAGAGCTGTTTGCATAGA-3′; GA3ox1 (AT1G15550), 5′-ACCAGAACAATACCGCCGGTCTACAA-3′ and 5′-ATCAGATTGCGGACCCCAAAGGAA-3′; GA2ox1 (AT1G78440), 5′-TTGGTGACTCTCTCCAGGTGATGACAAAG-3′ and 5′-GTCAACGGAGCGATTCTCTGAGTCAAT-3′; GA2ox2 (AT1G30040), 5′-AGGAAGAGGCGAGAAGATGGTGAA-3′ and 5′-GGGACCTGAAGAGCATCTCCAACATTAA-3′; GA2ox4 (AT1G47990), 5′-TACGAAACATGTCTAAACGGCTATCCTCAA-3′ and 5′-GGCATAGAGCATTGACCTACGGAGAAGAAA-3′.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: EF1 (AT5G60390), GA20ox1 (AT4G25420), GA20ox2 (AT5G51810), GA3ox1 (AT1G15550), GA2ox1 (AT1G78440), GA2ox2 (AT1G30040), GA2ox4 (AT1G47990), GAI (AT1G14920), RGA (AT2G01570), and ER (AT2G26330).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Xylem Expansion in Various Inflorescence Specification and Flowering Time Mutants.
Supplemental Figure 2. Xylem Expansion in Mutants of Genes Targeted by the Age-Related Flowering Pathway.
Supplemental Figure 3. Xylem Expansion in Hypocotyls with Impaired Auxin Transport or Signaling.
Acknowledgments
We thank our colleagues D. Alabadi, M. Blazquez, G. Coupland, M. Schmid, H. Smith, M. Tsiantis, and D. Weigel for providing various plant materials and transgenic lines. This work was supported by Swiss National Science Foundation Grant 31003A_129783, a long-term postdoctoral fellowship awarded by the Finnish Academy of Sciences to K.N., and an EMBO long-term postdoctoral fellowship awarded to L.R.
References
- Achard P., Cheng H., De Grauwe L., Decat J., Schoutteten H., Moritz T., Van Der Straeten D., Peng J., Harberd N.P. (2006). Integration of plant responses to environmentally activated phytohormonal signals. Science 311: 91–94 [DOI] [PubMed] [Google Scholar]
- Aukerman M.J., Sakai H. (2003). Regulation of flowering time and floral organ identity by a MicroRNA and its APETALA2-like target genes. Plant Cell 15: 2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berleth T., Mattsson J., Hardtke C.S. (2000). Vascular continuity and auxin signals. Trends Plant Sci. 5: 387–393 [DOI] [PubMed] [Google Scholar]
- Biemelt S., Tschiersch H., Sonnewald U. (2004). Impact of altered gibberellin metabolism on biomass accumulation, lignin biosynthesis, and photosynthesis in transgenic tobacco plants. Plant Physiol. 135: 254–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund S., Antti H., Uddestrand I., Moritz T., Sundberg B. (2007). Cross-talk between gibberellin and auxin in development of Populus wood: Gibberellin stimulates polar auxin transport and has a common transcriptome with auxin. Plant J. 52: 499–511 [DOI] [PubMed] [Google Scholar]
- Bonke M., Thitamadee S., Mähönen A.P., Hauser M.T., Helariutta Y. (2003). APL regulates vascular tissue identity in Arabidopsis. Nature 426: 181–186 [DOI] [PubMed] [Google Scholar]
- Cao D., Hussain A., Cheng H., Peng J. (2005). Loss of function of four DELLA genes leads to light- and gibberellin-independent seed germination in Arabidopsis. Planta 223: 105–113 [DOI] [PubMed] [Google Scholar]
- Cardon G., Höhmann S., Klein J., Nettesheim K., Saedler H., Huijser P. (1999). Molecular characterisation of the Arabidopsis SBP-box genes. Gene 237: 91–104 [DOI] [PubMed] [Google Scholar]
- Carlsbecker A., Helariutta Y. (2005). Phloem and xylem specification: pieces of the puzzle emerge. Curr. Opin. Plant Biol. 8: 512–517 [DOI] [PubMed] [Google Scholar]
- Chaffey N., Cholewa E., Regan S., Sundberg B. (2002). Secondary xylem development in Arabidopsis: a model for wood formation. Physiol. Plant. 114: 594–600 [DOI] [PubMed] [Google Scholar]
- Corbesier L., Vincent C., Jang S., Fornara F., Fan Q., Searle I., Giakountis A., Farrona S., Gissot L., Turnbull C., Coupland G. (2007). FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316: 1030–1033 [DOI] [PubMed] [Google Scholar]
- Davière J.M., de Lucas M., Prat S. (2008). Transcriptional factor interaction: a central step in DELLA function. Curr. Opin. Genet. Dev. 18: 295–303 [DOI] [PubMed] [Google Scholar]
- Dayan J., Schwarzkopf M., Avni A., Aloni R. (2010). Enhancing plant growth and fiber production by silencing GA 2-oxidase. Plant Biotechnol. J. 8: 425–435 [DOI] [PubMed] [Google Scholar]
- Demura T., Ye Z.H. (2010). Regulation of plant biomass production. Curr. Opin. Plant Biol. 13: 299–304 [DOI] [PubMed] [Google Scholar]
- Dettmer J., Elo A., Helariutta Y. (2009). Hormone interactions during vascular development. Plant Mol. Biol. 69: 347–360 [DOI] [PubMed] [Google Scholar]
- Digby J., Wareing P.F. (1966). The effect of applied growth hormones on cambial division and the differentiation of the cambial derivatives. Ann. Bot. (Lond.) 30: 539–548 [Google Scholar]
- Dill A., Jung H.S., Sun T.P. (2001). The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc. Natl. Acad. Sci. USA 98: 14162–14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo A., Immanen J., Nieminen K., Helariutta Y. (2009). Stem cell function during plant vascular development. Semin. Cell Dev. Biol. 20: 1097–1106 [DOI] [PubMed] [Google Scholar]
- Eriksson M.E., Israelsson M., Olsson O., Moritz T. (2000). Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat. Biotechnol. 18: 784–788 [DOI] [PubMed] [Google Scholar]
- Eriksson S., Böhlenius H., Moritz T., Nilsson O. (2006). GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. Plant Cell 18: 2172–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Zorrilla J.M., Valli A., Todesco M., Mateos I., Puga M.I., Rubio-Somoza I., Leyva A., Weigel D., García J.A., Paz-Ares J. (2007). Target mimicry provides a new mechanism for regulation of microRNA activity. Nat. Genet. 39: 1033–1037 [DOI] [PubMed] [Google Scholar]
- Frigerio M., Alabadí D., Pérez-Gómez J., García-Cárcel L., Phillips A.L., Hedden P., Blázquez M.A. (2006). Transcriptional regulation of gibberellin metabolism genes by auxin signaling in Arabidopsis. Plant Physiol. 142: 553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X., Harberd N.P. (2003). Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421: 740–743 [DOI] [PubMed] [Google Scholar]
- Gälweiler L., Guan C., Müller A., Wisman E., Mendgen K., Yephremov A., Palme K. (1998). Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Griffiths J., Murase K., Rieu I., Zentella R., Zhang Z.L., Powers S.J., Gong F., Phillips A.L., Hedden P., Sun T.P., Thomas S.G. (2006). Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18: 3399–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P., Phillips A.L. (2000). Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 5: 523–530 [DOI] [PubMed] [Google Scholar]
- Israelsson M., Sundberg B., Moritz T. (2005). Tissue-specific localization of gibberellins and expression of gibberellin-biosynthetic and signaling genes in wood-forming tissues in aspen. Plant J. 44: 494–504 [DOI] [PubMed] [Google Scholar]
- Kanrar S., Bhattacharya M., Arthur B., Courtier J., Smith H.M.S. (2008). Regulatory networks that function to specify flower meristems require the function of homeobox genes PENNYWISE and POUND-FOOLISH in Arabidopsis. Plant J. 54: 924–937 [DOI] [PubMed] [Google Scholar]
- Kardailsky I., Shukla V.K., Ahn J.H., Dagenais N., Christensen S.K., Nguyen J.T., Chory J., Harrison M.J., Weigel D. (1999). Activation tagging of the floral inducer FT. Science 286: 1962–1965 [DOI] [PubMed] [Google Scholar]
- King R.W., Hisamatsu T., Goldschmidt E.E., Blundell C. (2008). The nature of floral signals in Arabidopsis. I. Photosynthesis and a far-red photoresponse independently regulate flowering by increasing expression of FLOWERING LOCUS T (FT). J. Exp. Bot. 59: 3811–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Kaya H., Goto K., Iwabuchi M., Araki T. (1999). A pair of related genes with antagonistic roles in mediating flowering signals. Science 286: 1960–1962 [DOI] [PubMed] [Google Scholar]
- Kubo M., Udagawa M., Nishikubo N., Horiguchi G., Yamaguchi M., Ito J., Mimura T., Fukuda H., Demura T. (2005). Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev. 19: 1855–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mähönen A.P., Bishopp A., Higuchi M., Nieminen K.M., Kinoshita K., Törmäkangas K., Ikeda Y., Oka A., Kakimoto T., Helariutta Y. (2006). Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science 311: 94–98 [DOI] [PubMed] [Google Scholar]
- Mähönen A.P., Bonke M., Kauppinen L., Riikonen M., Benfey P.N., Helariutta Y. (2000). A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes Dev. 14: 2938–2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauriat M., Moritz T. (2009). Analyses of GA20ox- and GID1-over-expressing aspen suggest that gibberellins play two distinct roles in wood formation. Plant J. 58: 989–1003 [DOI] [PubMed] [Google Scholar]
- Melzer S., Lens F., Gennen J., Vanneste S., Rohde A., Beeckman T. (2008). Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat. Genet. 40: 1489–1492 [DOI] [PubMed] [Google Scholar]
- Mitsuda N., Iwase A., Yamamoto H., Yoshida M., Seki M., Shinozaki K., Ohme-Takagi M. (2007). NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell 19: 270–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moubayidin L., Perilli S., Dello Ioio R., Di Mambro R., Costantino P., Sabatini S. (2010). The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Curr. Biol. 20: 1138–1143 [DOI] [PubMed] [Google Scholar]
- Muñiz L., Minguet E.G., Singh S.K., Pesquet E., Vera-Sirera F., Moreau-Courtois C.L., Carbonell J., Blázquez M.A., Tuominen H. (2008). ACAULIS5 controls Arabidopsis xylem specification through the prevention of premature cell death. Development 135: 2573–2582 [DOI] [PubMed] [Google Scholar]
- Nakajima M., et al. (2006). Identification and characterization of Arabidopsis gibberellin receptors. Plant J. 46: 880–889 [DOI] [PubMed] [Google Scholar]
- Nieminen K., et al. (2008). Cytokinin signaling regulates cambial development in poplar. Proc. Natl. Acad. Sci. USA 105: 20032–20037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen K.M., Kauppinen L., Helariutta Y. (2004). A weed for wood? Arabidopsis as a genetic model for xylem development. Plant Physiol. 135: 653–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J., Karlberg A., Antti H., Lopez-Vernaza M., Mellerowicz E., Perrot-Rechenmann C., Sandberg G., Bhalerao R.P. (2008). Dissecting the molecular basis of the regulation of wood formation by auxin in hybrid aspen. Plant Cell 20: 843–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Carol P., Richards D.E., King K.E., Cowling R.J., Murphy G.P., Harberd N.P. (1997). The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev. 11: 3194–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proebsting W.M., Hedden P., Lewis M.J., Croker S.J., Proebsting L.N. (1992). Gibberellin concentration and transport in genetic lines of pea: Effects of grafting. Plant Physiol. 100: 1354–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redei G.P. (1962). Single locus heterosis. Z. Vererbungsl. 93: 164–170 [Google Scholar]
- Scarpella E., Marcos D., Friml J., Berleth T. (2006). Control of leaf vascular patterning by polar auxin transport. Genes Dev. 20: 1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J., Baba K., May S.T., Palme K., Bennett M., Bhalerao R.P., Sandberg G. (2003). Polar auxin transport in the wood-forming tissues of hybrid aspen is under simultaneous control of developmental and environmental signals. Proc. Natl. Acad. Sci. USA 100: 10096–10101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibout R., Plantegenet S., Hardtke C.S. (2008). Flowering as a condition for xylem expansion in Arabidopsis hypocotyl and root. Curr. Biol. 18: 458–463 [DOI] [PubMed] [Google Scholar]
- Sibout R., Sukumar P., Hettiarachchi C., Holm M., Muday G.K., Hardtke C.S. (2006). Opposite root growth phenotypes of hy5 versus hy5 hyh mutants correlate with increased constitutive auxin signaling. PLoS Genet. 2: e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone A.L., Jung H.S., Dill A., Kawaide H., Kamiya Y., Sun T.P. (2001). Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell 13: 1555–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H.M., Campbell B.C., Hake S. (2004). Competence to respond to floral inductive signals requires the homeobox genes PENNYWISE and POUND-FOOLISH. Curr. Biol. 14: 812–817 [DOI] [PubMed] [Google Scholar]
- Spicer R., Groover A. (2010). Evolution of development of vascular cambia and secondary growth. New Phytol. 186: 577–592 [DOI] [PubMed] [Google Scholar]
- Sun T.P., Kamiya Y. (1994). The Arabidopsis GA1 locus encodes the cyclase ent-kaurene synthetase A of gibberellin biosynthesis. Plant Cell 6: 1509–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M.V. (2006). Phloem: The long and the short of it. Trends Plant Sci. 11: 26–32 [DOI] [PubMed] [Google Scholar]
- Todesco M., Rubio-Somoza I., Paz-Ares J., Weigel D. (2010). A collection of target mimics for comprehensive analysis of microRNA function in Arabidopsis thaliana. PLoS Genet. 6: e1001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii K.U., Mitsukawa N., Oosumi T., Matsuura Y., Yokoyama R., Whittier R.F., Komeda Y. (1996). The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell 8: 735–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull C.G., Booker J.P., Leyser H.M. (2002). Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J. 32: 255–262 [DOI] [PubMed] [Google Scholar]
- van Zanten M., Snoek L.B., Proveniers M.C., Peeters A.J. (2009). The many functions of ERECTA. Trends Plant Sci. 14: 214–218 [DOI] [PubMed] [Google Scholar]
- Weigel D., Alvarez J., Smyth D.R., Yanofsky M.F., Meyerowitz E.M. (1992). LEAFY controls floral meristem identity in Arabidopsis. Cell 69: 843–859 [DOI] [PubMed] [Google Scholar]
- Willige B.C., Ghosh S., Nill C., Zourelidou M., Dohmann E.M., Maier A., Schwechheimer C. (2007). The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell 19: 1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Park M.Y., Conway S.R., Wang J.-W., Weigel D., Poethig R.S. (2009). The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138: 750–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z.H. (2002). Vascular tissue differentiation and pattern formation in plants. Annu. Rev. Plant Biol. 53: 183–202 [DOI] [PubMed] [Google Scholar]