Abstract
Emtricitabine (FTC; Emtriva), a potent deoxycytidine nucleoside reverse transcriptase inhibitor, has recently been approved by the U.S. Food and Drug Administration for the treatment of human immunodeficiency virus (HIV) infection. In adults, FTC has demonstrated linear kinetics over a wide dose range, and FTC 200 mg once a day (QD) is the recommended therapeutic dose. A phase I open-label trial was conducted in children to identify an FTC dosing regimen that would provide comparable plasma exposure to that observed in adults at 200 mg QD. Two single oral doses of FTC (60 and 120 mg/m2, up to a maximum of 200 mg, in solutions) were evaluated in HIV-infected children aged <18 years old. Children ≥6 years old also received a third dose of ∼120 mg/m2 in capsules. A total of 25 children (two <2 years old, eight 2 to 5 years old, eight 6 to 12 years old, and seven 13 to 17 years old) received at least two doses of FTC. Single escalating oral doses of FTC were well tolerated and produced dose-proportional plasma drug concentrations in children. The FTC pharmacokinetics was comparable between adults and children 22 months to 17 years of age. The capsule formulation provided ∼20% higher plasma FTC exposure than the solution formulation. Using plasma area under the concentration-time curve (AUC) data at the 120-mg/m2 dose, it is projected (based on dose proportionality) that a 6-mg/kg dose (up to a maximum of 200 mg) of FTC would produce plasma AUCs in children comparable to those in adults given a 200-mg dose (i.e., median of ∼10 h·μg/ml). This pediatric FTC dose is being evaluated in long-term phase II therapeutic trials in HIV-infected children.
There has been increasing recognition of the need to develop antiretroviral therapies that combine potency and safety with convenient dosing for the enhancement of adherence. The availability of a once-daily drug is extremely desirable for human immunodeficiency virus (HIV)-infected pediatric patients due to a complex interplay of child and caregiver, daily schedules, developmental obstacles, and challenges with adolescent behavioral patterns. Obstacles to developing a suitable pediatric formulation and determining an appropriate pediatric dosage have limited the broad availability of antiretroviral drugs to pediatric patients. Early attention to and resolution of these issues during antiretroviral drug development are vital to making the therapy as readily available to pediatric patients as to adult patients. We describe here an efficient clinical trial design that led to the early determination of a pediatric dosage for emtricitabine (2′,3′-dideoxy-5-fluoro-3′-thiacytidine [FTC]; Emtriva), a nucleoside reverse transcriptase inhibitor.
Emtricitabine is a potent nucleoside analog with a convenient once-daily dosing schedule that was recently approved for the treatment of HIV infection and is under investigation for the treatment of hepatitis B virus infection. Emtricitabine inhibits HIV reverse transcriptase and hepatitis B virus DNA polymerase and is consistently (4 to 10 times) more potent than lamivudine, another deoxycytidine nucleoside reverse transcriptase inhibitor, against laboratory strains and primary clinical isolates of HIV in vitro (5, 6, 8). In phase I/II trials in HIV-infected adults, emtricitabine up to a dose of 400 mg per day for 14 days has been well tolerated without dose-limiting toxicity (3, 4). In vivo anti-HIV potency of emtricitabine has been demonstrated in these short-term (10- to 14-day) monotherapy trials, where a 1.7 to 1.9 log10 reduction in plasma HIV type 1 (HIV-1) RNA was observed following 100- to 200-mg once-a-day (QD) or twice-a-day doses of emtricitabine. Plasma emtricitabine concentrations increased dose proportionally over the dose range of 100 to 1,200 mg following single oral administration (L. H. Wang, P. Gardner, L. W. Frick, J. E. Noblin, and M. R. Blum, Progr. Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 129, p. 24, 1995) and over the repeated-dose range of 25 to 200 mg administered once or twice daily (3, 4). The pharmacokinetic and pharmacodynamic characteristics of emtricitabine shown in the early phase I/II trials supported the selection of a convenient once-daily dosing schedule (L. H. Wang, J. Begley, J. Feng, J. B. Quinn, and F. Rousseau, Progr. Abstr. XIV Int. AIDS Conf., abstr. TuPeB4546, 2002). The long intracellular half-life of emtricitabine triphosphate (i.e., 39 h) supports the use of emtricitabine as a true once-daily drug (Wang et al., XIV Int. AIDS Conf., 2002). The dose regimen of emtricitabine at 200 mg QD as selected based on optimal anti-HIV activity and pharmacokinetic-pharmacodynamic characteristics has been evaluated in combination therapy in several phase II/III clinical trials in HIV-infected therapy-naïve and -experienced patients for long-term safety and efficacy (M. Saag, P. Cahn, F. Raffi, M. Wolff, D. Pearce, J. M. Molina, J. Hinkle, A. Shaw, J. B. Quinn, and F. Rousseau, Progr. Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 3889/LB-1, 2002; I. Sanne, C. van der Horst, A. Shaw, J. Hinkle, J. B. Quinn, C. Moxham, and F. Rousseau, Progr. Abstr. XIV Int. AIDS Conf., abstr. TuPeB4432, 2002; C. van der Horst, C. Benson, A. Rodriguez, L. Hulett, C. Wakeford, and J. Quinn, Progr. Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. I1932, p. 346, 2001). To facilitate adherence, emtricitabine has also been evaluated in combination with other once-daily agents (i.e., didanosine and efavirenz) to allow for a completely once-daily regimen (1; J. M. Molina, F. Ferchal, V. Journot, A. Maillard, E. Noe, F. Raffi, W. Rozenbaum, D. Sereni, P. Morlat, and G. Chene, Progr. Abstr. 8th Eur. Conf. Clin. Aspects Treat. HIV Infect., p. 221, 2001; Saag et al., 42nd ICAAC, 2002), which would be extremely desirable for pediatric patients.
To expedite the development of emtricitabine in the pediatric population, a single-dose pharmacokinetics study was conducted to identify an emtricitabine dosing regimen in children that will provide comparable plasma emtricitabine exposure to that observed in adults. It is expected that a single-dose pharmacokinetic study of emtricitabine in HIV-infected children concurrently receiving combination antiretroviral therapy would provide a practical assessment of plasma emtricitabine exposure in the clinic without affecting the child's medical treatment or therapy. Several characteristics of emtricitabine have made this approach feasible. The disposition of emtricitabine follows linear kinetics (Wang et al., 35th ICAAC, 1995), and its steady-state pharmacokinetics is predictable based on data from single-dose administration (Wang et al., XIV Int. AIDS Conf., 2002). In addition, emtricitabine has little potential for pharmacokinetic drug-drug interaction, and renal excretion is the primary route of emtricitabine elimination (L. H. Wang, M. R. Blum, J. Hui, L. Hulett, G. Chittick, and F. Rousseau, Progr. Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. A505, p. 18, 2001). The good water solubility of emtricitabine, as is generally the case for nucleosides, has also facilitated the early development of a liquid pediatric formulation. These characteristics have permitted the design of the present single-dose pharmacokinetic study by administering emtricitabine along with the concomitant antiretroviral agents to achieve the goal of establishing a pediatric dosing regimen for emtricitabine early in the clinical development program.
(This work was presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, September 2000, Toronto, Canada.)
MATERIALS AND METHODS
Study design and population.
This was a phase I, open-label, nonrandomized, dose-escalation, multicenter study to evaluate the safety, tolerability, and pharmacokinetics of single doses of emtricitabine at two dose levels in HIV-infected pediatric subjects aged <18 years old as stratified by age into five cohorts, i.e., cohort 1 (birth to <3 months old), cohort 2 (3 months to <2 years old), cohort 3 (2 to <6 years old), cohort 4 (6 to <13 years old), and cohort 5 (13 to <18 years old). After a screening evaluation (≤14 days before the first dose), all eligible subjects received their first dose of emtricitabine at 60 mg/m2. If this dose was well tolerated, a second dose of emtricitabine at 120 mg/m2, up to a maximum of 200 mg, was administered after a washout interval (dose to dose) of ≥4 days to the same patient. Emtricitabine was administered as an oral solution formulation (10 mg of emtricitabine/ml) for these first two doses. To determine relative bioavailability between the solution and the capsule dosage forms, subjects enrolled in cohorts 4 and 5 who tolerated the 120-mg/m2 dose and could swallow solid medications received a second 120-mg/m2 dose (up to a maximum of 200 mg) in capsule formulation (to the nearest 25 mg) after a second washout interval of ≥4 days. A follow-up evaluation was completed 4 to 7 days after administration of the last dose of emtricitabine. The actual dose of emtricitabine administered to an individual subject (i.e., the volume of solution or number of 25-mg or 100-mg capsules) was based on the subject's calculated body surface area (BSA), as determined using the following formula: BSA (in square meters) = [(height or length in centimeters) × (weight in kilograms)][1/2]/3,600.
Although food intake was not expected to significantly affect plasma emtricitabine exposure (Wang et al., 35th ICAAC, 1995), almost all children were withheld food intake for 1 h before and after dose, and 80% of children were withheld food intake until 2 h postdose. Water or other fluids were provided, as needed, to assist the child with swallowing the study drug. This study was approved by the institutional review board at each of the participating centers, and written informed consent was obtained from a parent or guardian of each child prior to the screening evaluation. Where feasible, written informed consent or assent was also obtained from the subject, in accordance with the normal practice of the participating study center. The study was conducted over the period of May 1999 to February 2001.
A total of 26 to 36 HIV-infected children were planned for enrollment (n = 6 to 8 each in cohorts 3 to 5; n = 4 to 6 each in cohorts 1 and 2) at multiple study centers. Eligible subjects were male or female children, weighing ≥2.5 kg, who had a confirmed HIV-1 infection and were clinically stable in the opinion of the responsible physician, based on review of medical history and results of the screening examination. Neonates were to be ≥34 weeks of gestation. Enrollment began in the older age groups (cohorts 3 to 5), with cohorts 1 and 2 opened for enrollment after ≥4 subjects in cohort 3 had completed both dose periods. Eligible subjects could be antiretroviral naïve or experienced. With the exception of concomitant treatment with lamivudine, all children receiving antiretroviral therapy at study enrollment continued their normal antiretroviral drugs without interruption. Children receiving lamivudine were required to withhold lamivudine dosing for 12 h before and until 24 h after each dose of emtricitabine. To avoid interfering with currently effective antiretroviral therapy, subjects taking lamivudine at study enrollment were required to have a plasma HIV-1 RNA level of >400 copies/ml at the screening evaluation. Exclusion criteria included an acute serious opportunistic infection requiring treatment, a history of pancreatitis, unexplained fever, chronic diarrhea or other gastrointestinal disorder within the past 30 days, hemoglobin level of <10 g/dl (>2 years old), absolute neutrophil count of <750 cells/mm3, platelet count of <75,000/mm3, aspartate aminotransferase or alanine aminotransferase levels ≥5 times the upper limit of normal appropriate for the age cohort, creatinine level of >1.1 (<13 years) or >1.8 (≥13 years) mg/dl, amylase level ≥5 times the upper limit of normal for age, bilirubin level ≥2 times the upper limit of normal for age, or history of severe allergic reaction or intolerance to lamivudine or other nucleosides.
Clinical and laboratory monitoring.
The safety and tolerability of emtricitabine were evaluated on the basis of adverse event reports, measurements of vital signs, and results of clinical laboratory tests (serum chemistry, hematology, and urinalysis) and physical examination. All treatment-emergent adverse events were monitored during and between dose periods and at the follow-up evaluation. The onset, duration, severity, and potential relationship to emtricitabine of any adverse events were recorded. Vital signs (blood pressure, pulse rate, respiration rate, and temperature) were measured at predose and at 1, 2, 4, 8, and 12 h following each dose of emtricitabine. Clinical laboratory evaluations were performed predose and at approximately 48 h following each dose of emtricitabine and at the follow-up visit. A complete physical examination was conducted at the screening and follow-up evaluations, and a brief examination was performed prior to each dose administration.
Blood and urine sampling.
Blood samples (up to 1 ml each for subjects in cohorts 1 and 2, and up to 2 ml each for subjects in cohorts 3 to 5) were collected at predose and at 0.5, 1, 2, 4, 8, 12, 24, and 48 h following administration of each dose of emtricitabine. Samples were drawn by individual needlestick or peripheral catheter into precooled VACUTAINER tubes containing dipotassium EDTA and kept at 4°C upon collection. Plasma was separated from blood by centrifugation at 4°C within 2 h of blood collection and stored at −20°C or lower until analysis. Attempts were made to collect urine samples at predose and during the 0-to-6-h and 6-to-12-h postdose intervals and optionally over the 12-to-24-h postdose interval. The total cumulative volume of each urine sample was determined, and an aliquot was stored at −20°C or lower until analysis.
Analytical methods.
Emtricitabine concentrations in plasma and urine were determined by a validated assay using liquid chromatography-tandem mass spectrometry analysis. Briefly, plasma (0.1 ml) was mixed with internal standard (lamivudine) solution and extracted on a solid-phase extraction column (Varian BondElut C18; 1 ml/100 mg). Emtricitabine and the internal standard were then resolved on a high-performance liquid chromatography column (Aquasil C18; 100 by 3 mm; 5 μm; Keystone Scientific) with detection by a triple quadruple mass spectrometer using selected reaction monitoring and atmospheric pressure chemical ionization in the positive ion mode. The resulting chromatograms were constructed using the following precursor ion→product ion transition, i.e., m/z 248 → m/z 130 for emtricitabine and m/z 230 → m/z 112 for lamivudine. Retention times for emtricitabine and lamivudine were approximately 3.8 and 2.1 min, respectively, using an isocratic mobile phase of 0.1% formic acid in water-acetonitrile (93:7 [vol/vol]) at a flow rate of 0.5 ml/min. Quantitation was based on the peak area ratios, using a linear least-squares regression with 1/concentration2 weighting. The range of quantitation was 0.005 to 5 μg/ml. The intra- and interday variabilities were 2.5 to 12.1% and 4.9 to 10.7%, respectively. The intra- and interday biases of the assay ranged from −2.0 to 6.4% and 0 to 5.2%, respectively. Urine samples (10-μl aliquots) were analyzed similarly as plasma samples except that the solid-phase extraction procedure was omitted. The range of quantitation for urine samples was 2.5 to 250 μg/ml. The intra- and interday variabilities of the urine assay were 1.8 to 5.6% and 5.2 to 9.7%, respectively. The intra- and interday biases of the urine assay ranged from −9.1 to 8.0% and −4.0 to 3.3%, respectively.
Pharmacokinetic data analysis.
Plasma emtricitabine concentration-versus-time data (0 to 48 h) following administration of each emtricitabine dose were analyzed by noncompartmental methods using WinNonlin (professional version 3.1; Pharsight Corporation, Mountain View, Calif.) to determine the pharmacokinetics parameter estimates, which included Cmax (maximal or peak plasma concentration), tmax (time at which peak concentration occurred), AUC0-∞ (area under the plasma concentration-time curve from time zero with extrapolation to infinite time), t[1/2] (plasma half-life), and apparent total body clearance (CL/F). The cumulative amount of emtricitabine (urine concentration times volume), the percentage of dose recovered in urine as emtricitabine (amount in urine divided by dose), and renal clearance of emtricitabine (amount in urine divided by AUC0-24) were determined in subjects for whom there were urinary excretion data.
Dose proportionality for Cmax and AUC0-∞ was assessed using a power model: (Cmax or AUC0-∞) = ea × doseb. Following log transformation, this model becomes a linear model: log(Cmax or AUC0-∞) = a + [b × log(dose)]. The population slope (b) and intercept (a) of the regression line were estimated, and the 90% confidence interval (CI) of the slope value (i.e., b) was constructed following linear regression between log(Cmax or AUC0-∞) and log(dose). Dose proportionality was concluded if the 90% CI of the slope was within a prespecified acceptance range (i.e., ±30%) around unity. The 30% range was established based on the wide age range of the population evaluated in this study.
RESULTS
Subject demographics and accountability.
A total of 25 children (from 22 months to 17 years of age) were enrolled in this study at five study centers; two in cohort 2, eight in each of cohorts 3 and 4, and seven in cohort 5. No children were enrolled in cohort 1, notwithstanding that enrollment was open for about 17 months over multiple sites for both cohorts 1 and 2. Patient demographics and baseline characteristics of children enrolled in the study are summarized in Table 1. Except for cohort 2, there was a good range of age distribution among the children enrolled in each age cohort. The distribution of gender or ethnic origin was generally comparable between age cohorts 3, 4, and 5. All 25 children evaluated had confirmed HIV infection, primarily (88%) through vertical transmission. Nearly all subjects (n = 23; 92%) had a history of HIV-related events, 76% with Centers for Disease Control and Prevention (CDC) clinical category A or B events and 16% with an AIDS-defining (CDC category C) event. All subjects were clinically stable in the opinion of the responsible physicians and did not have acute opportunistic infections, diarrhea, or grade 3 clinical or laboratory abnormalities. All subjects were being treated with a combination of at least two antiretroviral drugs, except one subject who was receiving didanosine monotherapy. A total of 15 subjects had lamivudine in their anti-HIV treatment regimens, and lamivudine doses were withheld temporarily during the study drug dosing period, per protocol restriction (see “Study design and population” above). Based on bioanalytical results, lamivudine was either not quantifiable or not detectable in the predose plasma samples from all subjects. All 25 subjects completed the first two dose periods evaluating the oral solution doses (60 and 120 mg/m2) of emtricitabine. Twelve of the 15 subjects enrolled in cohorts 4 and 5 completed the third dose period, receiving a 120-mg/m2 dose of emtricitabine in a capsule(s).
TABLE 1.
Summary of demographics and baseline characteristics of children enrolled
| Demographic or baseline characteristic | Overall (n = 25) | Cohort 2 (n = 2) | Cohort 3 (n = 8) | Cohort 4 (n = 8) | Cohort 5 (n = 7) |
|---|---|---|---|---|---|
| Gender (n [%]) | |||||
| Female | 13 (52) | 0 (0) | 5 (63) | 4 (50) | 4 (57) |
| Male | 12 (48) | 2 (100) | 3 (38) | 4 (50) | 3 (43) |
| Ethnic origin (n [%]) | |||||
| Black | 20 (80) | 2 (100) | 7 (88) | 7 (88) | 4 (57) |
| Caucasian | 1 (4) | 0 (0) | 0 (0) | 1 (13) | 0 (0) |
| Hispanic | 4 (16) | 0 (0) | 1 (13) | 0 (0) | 3 (43) |
| Age (years) | |||||
| Mean ± SD | 8.5 ± 4.9 | 1.9 ± 0.1 | 4.3 ± 1.2 | 8.8 ± 1.6 | 14.9 ± 1.9 |
| Median | 7.6 | 1.9 | 4.1 | 8.7 | 15.4 |
| Min., max. | 1.8, 17.8 | 1.8, 1.9 | 2.3, 5.7 | 6.6, 10.7 | 13.0, 17.8 |
| Body wt (kg) | |||||
| Mean ± SD | 32.5 ± 19.5 | 13.2 ± 1.1 | 16.5 ± 4.8 | 31.2 ± 8.3 | 57.8 ± 14.0 |
| Median | 24.5 | 13.2 | 15.0 | 30.5 | 57.9 |
| Min., max. | 10.2, 76.0 | 12.4, 13.9 | 10.2, 24.5 | 21.5, 43.8 | 35.4, 76.0 |
| Body surface area (m2) | |||||
| Mean ± SD | 1.04 ± 0.42 | 0.56 ± 0.04 | 0.68 ± 0.14 | 1.07 ± 0.19 | 1.57 ± 0.20 |
| Median | 0.92 | 0.56 | 0.65 | 1.06 | 1.58 |
| Min., max. | 0.48, 1.86 | 0.53, 0.59 | 0.48, 0.89 | 0.84, 1.38 | 1.21, 1.86 |
| Estimated CLcr (ml/min) | |||||
| Mean ± SD | 135 ± 84 | 74 ± 21 | 89 ± 39 | 131 ± 53 | 210 ± 110 |
| Median | 120 | 74 | 75 | 126 | 177 |
| Min., max. | 41, 447 | 60, 89 | 41, 165 | 64, 236 | 104, 447 |
| Estimated CLcr (ml/min/1.73 m2) | |||||
| Mean ± SD | 225 ± 92 | 227 ± 47 | 230 ± 103 | 220 ± 106 | 224 ± 88 |
| Median | 199 | 227 | 200 | 199 | 199 |
| Min., max. | 120, 429 | 194, 261 | 145, 392 | 120, 429 | 149, 416 |
| CD4+ cell count (cells/mm3) | |||||
| Mean ± SD | 730 ± 479 | 1636 ± 754 | 886 ± 384 | 571 ± 308 | 475 ± 353 |
| Median | 716 | 1,636 | 904 | 578 | 380 |
| Min., max. | 20, 2,169 | 1,103, 2,169 | 218, 1,479 | 211, 1,153 | 20, 956 |
| Plasma HIV-1 RNA (log10 copies/ml) | |||||
| Mean ± SD | 3.8 ± 1.0 | 3.8 ± 0.7 | 4.0 ± 1.0 | 3.2 ± 0.7 | 4.2 ± 1.1 |
| Median | 3.7 | 3.8 | 4.1 | 2.9 | 4.6 |
| Min., max | <2.6, 5.4 | 3.3, 4.3 | <2.6, 5.3 | <2.6, 4.3 | <2.6, 5.4 |
Safety evaluation.
Emtricitabine doses were well tolerated by all subjects. One serious adverse event was reported in a subject in cohort 5 who experienced moderate cellulitis following an insect bite on his right forearm that required hospitalization for parenteral antibiotic administration. This event was considered by the investigator to be unrelated to study drug. Overall, 29 adverse events, including the above serious adverse event, were reported in 15 of 25 subjects (60%). Thirteen events (45%) reported in seven subjects were considered to be drug related. Twenty-two out of 29 events (76%) were reported during dose period 1, when the lower dose (60 mg/m2) of emtricitabine was administered. There were seven events reported during dose period 2 and none in dose period 3 (120-mg/m2 dose in capsules). The most frequently reported adverse events were vomiting (five subjects), diarrhea (four subjects), abdominal pain (three subjects), and headache (two subjects). Few adverse events were reported on the day of study drug administration. For example, only one event each of diarrhea and abdominal pain reported in one subject commenced on the day of dosing. All episodes of vomiting occurred from about 24 h to 6 days after dosing. In addition, all adverse events were mild (24 of 29; 83%) or moderate (5 of 29; 17%) in intensity, and all drug-related adverse events were mild in intensity.
The number of subjects with laboratory values outside the normal ranges was generally the same or comparable to the number observed at screening or at pretreatment. The most frequently reported abnormal (out of normal range) parameters were in hematology, including low red blood cell count, low hemoglobin, increased mean corpuscular volume, low hematocrit, low absolute neutrophils, and low white blood cell count. The most frequently reported abnormal serum chemistry parameters were increased cholesterol, increased lactate dehydrogenase, and increased total protein. No subject experienced a treatment-emergent laboratory abnormality of ≥grade 3 toxicity, and none of the abnormal test results was considered to be clinically significant. No clinically relevant changes were observed in median vital signs values across time points in any age cohort. Physical examination findings were consistent with preexisting conditions and/or with reported adverse events or HIV-related events.
Pharmacokinetic evaluation.
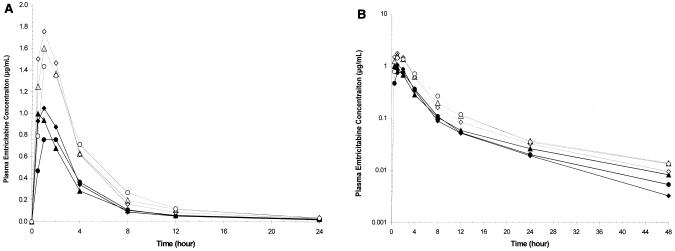
The mean plasma emtricitabine concentration-versus-time profiles by age cohort following administration of oral solution in periods 1 and 2 are depicted in Fig. 1A (linear plots) and B (semi-log plots). Emtricitabine was rapidly and well absorbed in children following administration of the oral solution. Mean plasma concentration-versus-time profiles at each dose level were similar across age cohorts. Plasma emtricitabine concentrations were measurable at the earliest sampling time (0.5 h postdose) and reached a maximum of 0.9 to 1.1 μg/ml for the 60-mg/m2 dose and 1.5 to 2.0 μg/ml for the 120-mg/m2 dose within 2 h following administration of the oral solution in all age cohorts. Peak emtricitabine concentrations and overall plasma exposure to emtricitabine (or plasma emtricitabine concentration-time profiles) were comparable between age cohorts at the 60- and 120-mg/m2 dose levels.
FIG. 1.
Mean plasma emtricitabine concentration-versus-time curves (linear plot [A] and semilog plot [B]) following administration of oral solution by age cohort and dose period. ♦, cohorts 2 and 3, 22 months to 5 years, period 1, n = 10; ▴, cohort 4, 6 to 12 years, period 1, n = 8; •, cohort 5, 13 to 17 years, period 1, n = 7; ⋄, cohorts 2 and 3, 22 months to 5 years, period 2, n = 10; ▵, cohort 4, 6 to 12 years, period 2, n = 8; ○, cohort 5, 13 to 17 years, period 2, n = 7.
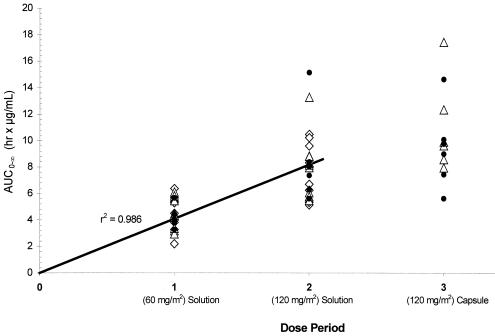
Emtricitabine pharmacokinetics parameter estimates following each dose of emtricitabine are summarized by age cohort in Table 2. In general, tmax values were similar across age cohorts following administration of the solution formulation at each dose level. Cmax and AUC0-∞ values were similar across age cohorts at both dose levels. The mean AUC0-∞ values were 4.2 to 4.8 h·μg/ml for the 60-mg/m2 dose and 7.7 to 8.5 h·μg/ml for the 120-mg/m2 dose (in solution) across all age cohorts. Mean elimination half-life estimates following the 60-mg/m2 dose ranged from 9.3 to 10.4 h in cohorts 3 to 5, but was shorter (mean, 6.4 h) in the two children in cohort 2. At the 120-mg/m2 dose level, mean elimination half-life estimates were more consistent across age cohorts (cohorts 2 to 5), with values ranging from 9.7 to 11.9 h (Table 2; Fig. 1B). These half-life estimates in children across age cohorts are comparable to those observed in adults following 48 h of postdose sampling. There was a linear relationship between emtricitabine AUC0-∞ or Cmax and emtricitabine dose in solution, as characterized by an r2 of 0.986 or 0.960, respectively, by including the origin in the linear regression analysis. The dose proportionality in AUC0-∞ values between the 60- and 120-mg/m2 dose levels is also depicted in Fig. 2. The power model analysis showed that the 90% CI for the slope contained 1.0 for each parameter analyzed, confirming the linearity and dose proportionality in Cmax and AUC0-∞ values of emtricitabine over the dose range studied across all age cohorts.
TABLE 2.
Summary of emtricitabine pharmacokinetic parameter estimates by age cohort following administration of emtricitabine oral solution and capsule formulationsa
| Cohort and age | Dose and formulation | Cmax (μg/ml) | tmax (h) | AUC0-∞ (h · μg/ml) | t1/2 (h) | CL/F (ml/min) | CL/F (ml/min/1.73 m2) |
|---|---|---|---|---|---|---|---|
| 2 (n = 2), 22-23 | 60 mg/m2, solution | 1.02 ± 0.78 | 1.54 ± 0.65 | 4.81 ± 2.19 | 6.43 ± 0.21 | 137 ± 67 | 401 ± 184 |
| mos | 120 mg/m2, solution | 1.51 ± 0.53 | 1.52 ± 0.66 | 8.13 ± 0.25 | 11.93 ± 1.11 | 140 ± 6 | 426 ± 15 |
| 3 (n = 8), 2-5 yrs | 60 mg/m2, solution | 1.13 ± 0.36 | 1.17 ± 0.60 | 4.32 ± 1.14 | 9.51 ± 2.53 | 174 ± 79 | 434 ± 158 |
| 120 mg/m2, solution | 1.99 ± 0.47 | 1.20 ± 0.54 | 7.70 ± 2.17 | 9.65 ± 2.71 | 196 ± 84 | 484 ± 139 | |
| 2 + 3 (n = 10), 22 | 60 mg/m2, solution | 1.11 ± 0.41 | 1.24 ± 0.60 | 4.42 ± 1.26 | 8.89 ± 2.58 | 167 ± 75 | 427 ± 153 |
| mos-5 yrs | 120 mg/m2, solution | 1.90 ± 0.49 | 1.26 ± 0.54 | 7.79 ± 1.92 | 10.11 ± 2.60 | 185 ± 78 | 472 ± 125 |
| 4 (n = 8), 6-12 yrs | 60 mg/m2, solution | 1.06 ± 0.34 | 0.96 ± 0.66 | 4.45 ± 1.23 | 10.37 ± 2.85 | 254 ± 56 | 417 ± 118 |
| 120 mg/m2, solution | 1.91 ± 0.83 | 1.54 ± 1.13 | 8.02 ± 2.51 | 11.70 ± 2.30 | 288 ± 75 | 465 ± 126 | |
| 120 mg/m2, capsuleb | 2.49 ± 0.87 | 1.85 ± 0.42 | 10.93 ± 3.49 | 10.17 ± 3.50 | 213 ± 60 | 338 ± 84 | |
| 5 (n = 7), 13-17 | 60 mg/m2, solution | 0.89 ± 0.26 | 1.61 ± 0.49 | 4.15 ± 0.75 | 9.29 ± 3.53 | 392 ± 90 | 427 ± 69 |
| yrs | 120 mg/m2, solution | 1.55 ± 0.26 | 1.58 ± 0.53 | 8.45 ± 3.42 | 11.08 ± 2.17 | 406 ± 149 | 442 ± 119 |
| 120 mg/m2, capsuleb | 2.15 ± 0.53 | 1.32 ± 0.50 | 9.38 ± 3.03 | 10.77 ± 0.88 | 361 ± 139 | 387 ± 112 |
Values are means ± standard deviations.
n = 6 for the capsule formulation.
FIG. 2.
Plots of emtricitabine AUC0-∞ versus dose period (Treatment) in all subjects. ⋄, cohorts 2 and 3, 22 months to 5 years; ▵, cohort 4, 6 to 12 years; •, cohort 5, 13 to 17 years. The linear regression line is for dose periods 1 and 2.
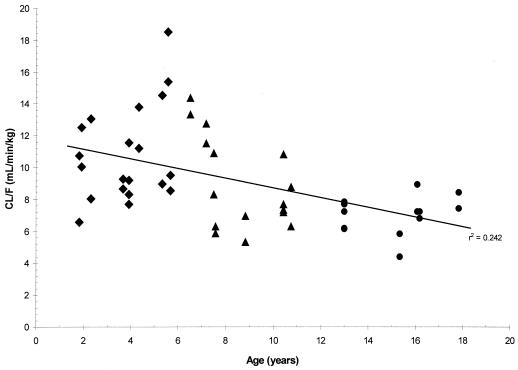
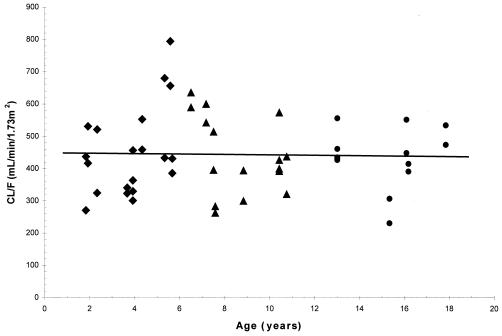
Mean values for CL/F (unadjusted for body weight or BSA) increased with increasing age and ranged from 167 ml/min in cohorts 2 and 3 to 392 ml/min in cohort 5 following the 60-mg/m2 dose, and they ranged from 185 ml/min in cohorts 2 and 3 to 406 ml/min in cohort 5 following the 120-mg/m2 dose. However, the age differences in CL/F values were reduced or diminished once the CL/F values were normalized to body weight or BSA. The mean CL/F values normalized to body weight decreased with increasing age, from 10.2 ml/min/kg in cohorts 2 and 3 to 6.9 ml/min/kg in cohort 5 for the 60-mg/m2 dose level and from 11.4 ml/min/kg in cohorts 2 and 3 to 7.2 ml/min/kg in cohort 5 for the 120-mg/m2 dose level. The trend towards decreased CL/F values normalized to body weight with increasing age for individual children is shown in Fig. 3 for both dose levels. However, the CL/F values normalized to BSA (in the units of milliliters per minute per 1.73 m2) showed almost no difference between age cohorts, with mean values of 401, 434, 417, and 427 ml/min/1.73 m2 for cohorts 2, 3, 4, and 5, respectively, at the 60-mg/m2 dose level and 426, 484, 465, and 442 ml/min/1.73 m2 for cohorts 2, 3, 4, and 5, respectively, at the 120-mg/m2 dose level (Table 2). The lack of age differences in CL/F values normalized to BSA in individual children at both dose levels is shown in Fig. 4 with an almost horizontal trend line.
FIG. 3.
Plots of emtricitabine CL/F normalized to body weight versus age. ♦, cohorts 2 and 3, 22 months to 5 years; ▴, cohort 4, 6 to 12 years; •, cohort 5, 13 to 17 years. Data are from periods 1 and 2; the linear regression line is for all data points.
FIG. 4.
Plots of emtricitabine CL/F normalized to BSA versus age. ♦, cohorts 2 and 3, 22 months to 5 years; ▴, cohort 4, 6 to 12 years; •, cohort 5, 13 to 17 years. Data are from periods 1 and 2; the linear regression line is for all data points.
Emtricitabine was also rapidly absorbed when administered in a capsule formulation in children with comparable mean tmax values observed when a 120-mg/m2 (up to 200-mg) dose was administered in either the solution or capsule formulation (1.54 versus 1.85 h and 1.58 versus 1.32 h for cohorts 4 and 5, respectively). The capsule formulation provided slightly higher plasma exposure to emtricitabine than the solution formulation (Fig. 2). Mean Cmax and AUC0-∞ values were higher following administration of the capsule formulation in both age cohorts (cf. 1.91 versus 2.49 μg/ml and 8.02 versus 10.93 h·μg/ml for cohort 4; 1.55 versus 2.15 μg/ml and 8.45 versus 9.38 h·μg/ml for cohort 5). The relative bioavailability for the capsule formulation (compared to an equal dose in solution) averaged ∼120% over the age range of 6 to 17 years but was variable between subjects. The small sample size and the nonrandomized, pilot study nature precluded a meaningful statistical comparison between the two formulations.
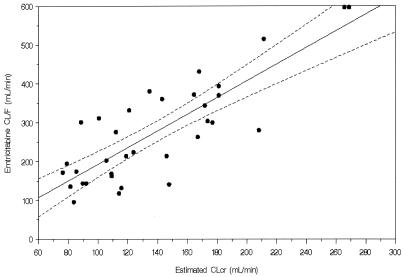
Although the urine collection period was only 24 h or shorter in duration, there was approximately 35 to 60% of the emtricitabine oral dose recovered in urine. In adults, a 48-h postdose urine collection is required to have almost complete urinary recovery of the unchanged emtricitabine in urine (∼60 to 70% of an oral dose). There were no major differences in the percentage of emtricitabine dose recovered in urine between age cohorts following either the solution or capsule administration. Renal clearance values, unadjusted for BSA, increased with increasing age (cf. 70 to 88 ml/min in cohort 3, 114 to 124 ml/min in cohort 4, and 147 to 251 ml/min in cohort 5). The unadjusted renal clearance value in older (cohort 5) children was similar to that reported in adult populations. As expected, once adjusted for BSA, the difference in renal clearance was minimized or normalized between age cohorts (cf. 160 to 214 ml/min/1.73 m2 in cohort 3, 184 to 201 ml/min/1.73 m2 in cohort 4, and 166 to 270 ml/min/1.73 m2 in cohort 5). The urinary excretion data indicated that emtricitabine disposition in children was similar to that in adults, with urinary excretion as the primary route of elimination. There was an almost linear increase in CL/F values as estimated creatinine clearance (a measure of renal function) increased, as described by a statistically significant linear correlation (r2 = 0.681) (Fig. 5) as follows: CL/F (in milliliters per minute) = (−21.92) + (2.14 × CLcr) (in milliliters per minute).
FIG. 5.
Correlation between emtricitabine apparent CL/F (in milliliters per minute) and estimated creatinine clearance (CLcr, in milliliters per minute). CL/F = (−21.92) + 2.14(CLcr); r2 = 0.681.
DISCUSSION
An efficient clinical trial design in a pediatric population has been adopted here to determine the pediatric dosage regimen for emtricitabine early in clinical development. Pediatric dosing parameters were obtained by characterizing the single-dose pharmacokinetics of emtricitabine over a broad age range in children. Plasma emtricitabine profiles were then compared between observed data in children and historical data in adults in order to determine a dosage regimen in children that would provide comparable plasma exposure to that in adults receiving 200 mg of emtricitabine QD. This approach and associated study design are unique to drugs that demonstrate linear pharmacokinetics and have little potential for pharmacokinetic drug interactions with other concomitant medications. Experiences with emtricitabine in adult populations show that emtricitabine possesses both of these characteristics (Wang et al., XIV Int. AIDS Conf., 2002; Wang et al., 41st ICAAC, 2001; Wang et al., 35th ICAAC, 1995). Since the steady-state pharmacokinetics of emtricitabine is predictable based on single-dose data, a single-dose pharmacokinetic study in children will provide relevant data, when comparing to adult data, to support dose recommendation for long-term use of emtricitabine in children. Due to the lack of potential pharmacokinetic drug interaction for emtricitabine, this single-dose pharmacokinetic study can be performed in pediatric patients without interruption of antiretroviral and other treatment medications. To avoid potential interactions, if any, at the absorption site or during the time when plasma exposure was high, there was a 2-h interval separating emtricitabine administration from concomitant drug administration. The only drug that was withheld prior to dosing was lamivudine (for 12 h), primarily due to potential assay interferences. This short duration of temporary interruption of lamivudine could have been avoided if a different internal standard for the plasma assay had been used; nevertheless, it was not expected to have a clinically significant impact on the antiretroviral treatment in patients with plasma HIV1 RNA levels of >400 copies/ml. It would not have been possible to complete study enrollment by excluding those children who were receiving lamivudine in their treatment regimens, due to the wide use of this drug. The minimal risk of temporarily withholding lamivudine is supported by the fact that emtricitabine administered once daily has demonstrated potent anti-HIV activity in phase I/II trials (3, 4) and was being administered in lieu of lamivudine in these patients on the dosing day. The intracellular half-life of lamivudine, i.e., 15 h (2), provided the clinicians an additional comfort level for withholding the evening dose of lamivudine prior to the morning dose of emtricitabine.
Two single, escalating doses of emtricitabine (60 and 120 mg/m2) administered orally to HIV-infected children aged 22 months to 17 years old were safe and generally well tolerated. There were no apparent age-related differences in safety or tolerability over the dose range evaluated. The incidences of adverse events were comparable across age cohorts during each dose period. Most adverse events (22 of 29; 76%) were reported during dose period 1, when subjects received the lower dose of emtricitabine (60 mg/m2), and none were reported during dose period 3, suggesting that most of the events were unrelated to emtricitabine. In addition, the majority of the most frequently reported adverse events, which were vomiting, diarrhea, abdominal pain, and headache, did not occur or were not reported on the day of dosing. At each measurement time point, the number of subjects with abnormal laboratory values was generally similar to the number observed at the screening evaluation and appeared to be related to their underlying disease and not emtricitabine. The good safety profile of emtricitabine is well supported by preclinical toxicology studies and by the early clinical studies in adults (3, 7).
Similar to that observed in adults, emtricitabine was rapidly and well absorbed in children following oral administration of both the liquid and solid dosage forms. When the dose was normalized to BSA (as in this study), the resultant peak concentrations and overall plasma exposures (AUCs) were comparable among age cohorts, i.e., over a broad age range. This finding is supported by the comparable CL/F values normalized to BSA across all age cohorts in children and between adult and pediatric populations. Thus, a pediatric dose administered per BSA in children over a wide age range is desirable when targeting for a plasma AUC value comparable to that in adults. Consistent with what was observed in the adult population (Wang et al., XIV Int. AIDS Conf., 2002; Wang et al., 35th ICAAC, 1995), both the Cmax and AUC values increased dose proportionally over the dose range studied in children. Even though only a twofold dose range was studied, it is expected that dose proportionality in emtricitabine Cmax and AUC values would extend to a greater dose range, based on the similar pharmacokinetic data of emtricitabine observed between adults and children.
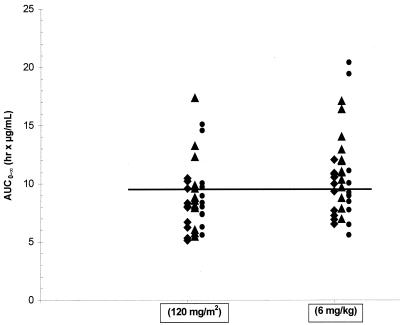
Following the 120-mg/m2 dose in solution, the mean AUC0-∞ values were 7.7 to 8.5 h·μg/ml across all age cohorts of children. These values are similar to or just slightly less than the median AUC0-∞ value or the AUC0-24 value (∼10 h·μg/ml) following a single oral dose of 200 mg of emtricitabine (given as capsules) or following multiple-dose administration of 200 mg of emtricitabine QD, respectively, in adults (3; Wang et al., XIV Int. AIDS Conf., 2002; Wang et al., 35th ICAAC, 1995). Thus, a dose of 120 to 140 mg/m2 could have been chosen as the pediatric dose for emtricitabine that would produce similar plasma exposure of emtricitabine as that in adults given 200 mg once daily. However, the current U.S. Food and Drug Administration recommendation is to use a dose unit of milligrams per kilogram rather than milligrams per square meter when providing a dosing guideline for children in the product labeling. Using the AUC0-∞ values in children of this study following the 120-mg/m2 dose in solution and the body weight data, a dose of 6 mg/kg (up to a maximum of 200 mg) was chosen (based on dose proportionality) as the recommended dose for emtricitabine in children for subsequent studies. As shown in Fig. 6, the projected AUC0-∞ values, based on dose proportionality, in each age cohort of children following the 6-mg/kg dose (up to a maximum of 200 mg) of emtricitabine were similar to or slightly higher than those following the 120-mg/m2 dose observed in this study. Additionally, at least 50% of the children receiving the recommended dose will have emtricitabine AUC0-∞ values above the median AUC value (∼10 h·μg/ml) of emtricitabine in adults receiving a 200-mg dose. This 6-mg/kg dose level is being evaluated in long-term therapeutic trials of emtricitabine in children (R. McKinney, M. Rathore, S. Jankelovich, L. Mofenson, L. Wang, L. Reynolds, M. Hughes, C. Hu, L. Draper, E. Buckley, L. Purdue, A. Ortiz, R. Dickover, A. Weinberg, and J. Rodman, Progr. Abstr. 10th Conf. Retrovir. Opportunistic Infect., abstr. 873, 2003; X. Saez-Llorens, A. Violari, D. Ndiweni, C. Avila-Figueroa, A. Wiznia, M. R. Blum, K. Makhuli, L. H. Wang, G. Shen, and N. Adda, Progr. Abstr. 10th Conf. Retrovir. Opportunistic Infect., abstr. 872, 2003).
FIG. 6.
Plots of emtricitabine AUC0-∞ at 120-mg/m2 and 6-mg/kg doses by age cohort. ♦, cohorts 2 and 3, 22 months to 5 years, data from period 2; ▴, cohort 4, 6 to 12 years, data from periods 2 and 3; •, cohort 5, 13 to 17 years, data from periods 2 and 3. The horizontal line shows the average median AUC in adults (200-mg dose).
The elimination half-life of emtricitabine in children, averaging 11 h, was not age dependent and was similar to the reported values in adults (Wang et al., XIV Int. AIDS Conf., 2002). The available urinary excretion data in children showed that renal clearance values of emtricitabine, adjusted to BSA, in children across the age range of 2 to 17 years were similar to those reported in adults. This indicates that renal function in children ≥2 years old is fully developed as far as the elimination (or urinary excretion) of emtricitabine is concerned. As expected based on observations in adults, the apparent CL/F of emtricitabine in children correlates linearly with the estimated creatinine clearance (a measure of renal function). In addition, renal clearance of emtricitabine is generally greater than the estimated creatinine clearance, suggesting that emtricitabine undergoes secretion in the renal tubules.
The preliminary assessment of the relative bioavailability of the capsule formulation that was performed in this study showed that the emtricitabine capsule formulation was slightly more bioavailable than the solution formulation, averaging ∼120% in both age cohorts. This observation cannot be simply explained by the physicochemical properties of emtricitabine, since emtricitabine is extremely water soluble, is stable under acidic conditions, and its aqueous solubility is not pH dependent. Since plasma concentrations of emtricitabine increased dose proportionally following administration of a wide range of oral doses (from 100 to 1,200 mg) (Wang et al., 35th ICAAC, 1995), it is unlikely that the absorption of a solution dose would be saturated compared to that of a capsule dose. It is likely that the gastrointestinal transit time of the solution formulation is shorter than that of the capsule formulation, thereby reducing the mucosal contact time for emtricitabine to be absorbed. In this case, factors that could prolong gastrointestinal transit time, e.g., food intake, may enhance the bioavailability of the oral solution. Nevertheless, the slightly (∼20%) higher bioavailability of the capsule formulation compared to that of the solution formulation is not expected to result in clinically significant differences in plasma emtricitabine exposure when switching between formulations. Importantly, the pediatric dose of 6 mg of emtricitabine/kg selected herein was primarily based on the direct comparison of AUC data between a solution dose in children and a capsule dose in adults, where the slight difference in bioavailability between the two dosage forms has already been accounted for when determining the pediatric dosage.
In summary, the present study has provided a practical assessment of the pharmacokinetics of emtricitabine over a wide and clinically relevant age range in children, primarily from 22 months to 17 years of age. Single doses of emtricitabine up to 120 mg/m2 are well tolerated in HIV-infected children. There were no clinically significant differences in emtricitabine plasma exposure at each dose level between children over the age range from 22 months to 17 years. The emtricitabine CL/F values normalized to BSA and the overall pharmacokinetic properties of emtricitabine were comparable among children over a wide age range and between children and adults. Using the AUC data in children obtained at the 120-mg/m2 dose, it is projected (based on dose proportionality) that a 6-mg/kg dose (up to a maximum of 200 mg) of emtricitabine will produce plasma AUCs of emtricitabine in children comparable to plasma AUCs of emtricitabine in adults given a 200-mg dose. Since no children younger than 22 months were enrolled in this study, the selected pediatric dosage of emtricitabine requires further evaluation in children much younger than 22 months old. Overall, the results of this study are critical to a rational selection of a dosage regimen for emtricitabine in children, and this dosage is currently being evaluated in long-term therapeutic trials (McKinney et al., 10th Conf. Retrovir. Opportunistic Infect., 2003; Saez-Llorens et al., 10th Conf. Retrovir. Opportunistic Infect., 2003).
Acknowledgments
We thank the study subjects and their guardians for participating in Protocol FTC-105 sponsored by Triangle Pharmaceuticals. Special thanks are also extended to John Begley and John Walsh of Triangle Pharmaceuticals, Inc., for performing the emtricitabine assays in plasma and urine for this study and to Shuching Shaw for performing the statistical analysis for this study.
REFERENCES
- 1.Molina, J. M., F. Ferchal, C. Rancinan, F. Raffi, W. Rozenbaum, D. Sereni, P. Morlat, V. Journot, J. M. Decazes, and G. Chene. 2000. Once-daily combination therapy with emtricitabine, didanosine, and efavirenz in human immunodeficiency virus-infected patients. J. Infect. Dis. 82:599-602. [DOI] [PubMed] [Google Scholar]
- 2.Moore, K. H., J. E. Barrett, S. Shaw, G. E. Pakes, R. Churchus, A. Kapoor, J. Lloyd, M. G. Barry, and D. Back. 1999. The pharmacokinetics of lamivudine phosphorylation in peripheral blood mononuclear cells from patients infected with HIV-1. AIDS 13:2239-2250. [DOI] [PubMed] [Google Scholar]
- 3.Rousseau, F. S., J. O. Kahn, M. Thompson, D. Mildvan, D. Shepp, J.-P. Sommadossi, J. Delehanty, J. N. Simpson, L. H. Wang, J. B. Quinn, C. Wakeford, and C. van der Horst. 2001. Prototype trial design for rapid dose selection of antiretroviral drugs: an example using emtricitabine (Coviracil). J. Antimicrob. Chemother. 48:507-513. [DOI] [PubMed] [Google Scholar]
- 4.Rousseau, F. S., C. Wakeford, H. Mommeja-Marin, I. Sanne, C. Moxham, J. Harris, L. Hulett, L. H. Wang, J. B. Quinn, D. W. Barry, et al. 2003. A prospective randomized trial of emtricitabine versus lamivudine short term monotherapy in HIV-infected patients. J. Infect. Dis. 188:1652-1658. [DOI] [PubMed] [Google Scholar]
- 5.Schinazi, R. F., R. M. Lloyd, M.-H. Nguyen, C. L. Cannon, A. McMillan, N. Ilksoy, C. K. Chu, D. C. Liotta, H. Z. Bazmi, and J. W. Mellors. 1993. Characterization of human immunodeficiency viruses resistant to oxathiolane-cytosine. Antimicrob. Agents Chemother. 37:875-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schinazi, R. F., A. McMillan, D. Cannon, R. Mathis, R. M. Lloyd, A. Peck, J.-P. Sommadossi, M. St. Clair, J. Wilson, P. A. Furman, G. Painter, W.-B. Choi, and D. C. Liotta. 1992. Selective inhibition of human immunodeficiency viruses by racemates and enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob. Agents Chemother. 36:2423-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szczech, G. M., L. H. Wang, J. P. Walsh, and F. S. Rousseau. Favorable reproductive toxicology profile of emtricitabine in mice and rabbits. Reprod. Toxicol. 17:95-108. [DOI] [PubMed]
- 8.Tisdale, M., S. D. Kemp, N. R. Parry, and B. A. Larder. 1993. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc. Natl. Acad. Sci. USA 90:5653-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]