In Arabidopsis, the PHOSPHATE TRANSPORTER1 (PHT1) family encodes the high affinity phosphate transporters. This analysis revealed multiple steps of regulation in various cell compartments modulating the level of PHT1 proteins present in the plasma membrane in response to the level of inorganic phosphate.
Abstract
In Arabidopsis thaliana, the PHOSPHATE TRANSPORTER1 (PHT1) family encodes the high-affinity phosphate transporters. They are transcriptionally induced by phosphate starvation and require PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR (PHF1) to exit the endoplasmic reticulum (ER), indicating intracellular traffic as an additional level of regulation of PHT1 activity. Our study revealed that PHF1 acts on PHT1, upstream of vesicle coat protein COPII formation, and that additional regulatory events occur during PHT1 trafficking and determine its ER exit and plasma membrane stability. Phosphoproteomic and mutagenesis analyses revealed modulation of PHT1;1 ER export by Ser-514 phosphorylation status. Confocal microscopy analysis of root tip cells showed that PHT1;1 is localized to the plasma membrane and is present in intracellular endocytic compartments. More precisely, PHT1;1 was localized to sorting endosomes associated with prevacuolar compartments. Kinetic analysis of PHT1;1 stability and targeting suggested a modulation of PHT1 internalization from the plasma membrane to the endosomes, followed by either subsequent recycling (in low Pi) or vacuolar degradation (in high Pi). For the latter condition, we identified a rapid mechanism that reduces the pool of PHT1 proteins present at the plasma membrane. This mechanism is regulated by the Pi concentration in the medium and appears to be independent of degradation mechanisms potentially regulated by the PHO2 ubiquitin conjugase. We propose a model for differential trafficking of PHT1 to the plasma membrane or vacuole as a function of phosphate concentration.
INTRODUCTION
Phosphorus (P) is an essential mineral nutrient for plant development and metabolism. Previous work revealed that its concentration limits plant development in many soils worldwide (Barber et al., 1963). Mainly absorbed in its oxidized form, phosphate (Pi) is a poorly mobile ion and has a high capacity for association with many cations (Hirsch et al., 2006) or organic compounds (López-Bucio et al., 2000) in the soil. However, only the free Pi form is soluble and can be absorbed by plants. In this form, it is often limiting for plant growth, even in soils where total Pi exceeds the required needs for plant growth. For this reason, around 80% (on average) of the Pi fertilizers applied cannot be exploited by plants.
To cope with this situation, plants have evolved many strategies to optimize their growth in phosphate-limiting conditions (for review, see Raghothama, 1999). One important element of the response is linked to the induction of root high-affinity Pi transporters, which increase the Pi influx capacity by severalfold (Lee, 1993). The molecular identification and analysis of high-affinity phosphate transporter (PHT) genes from many species has revealed that an important part of this regulation takes place at the transcriptional level (Muchhal et al., 1996). In Arabidopsis thaliana, nine sequences of high-affinity transporter homologs have been identified. Most of these transporters exhibit a strong induction of their transcripts within the first 12 h of phosphate starvation conditions (Misson et al., 2005). A second level of regulation has recently been found by a genetic screen that identified PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 (PHF1) (González et al., 2005). This gene encodes a protein located in the endoplasmic reticulum (ER) that is required for the correct targeting of the PHT1;1 transporter to the plasma membrane. The phf1 mutation reduces Pi uptake by 80% in low Pi when compared with a wild-type control (González et al., 2005). In Pi-depleted medium, the effect of the pht1;1 pht1;4 double mutant on the two primary root high-affinity phosphate transporters reduced the uptake by 40% (Shin et al., 2004). This suggests that PHF1 is also required for the proper trafficking of the other members of PHT1 family.
In this study, cellular biology experiments were performed to determine if additional levels of regulation may affect PHT1. We characterized PHT1;1 phosphorylation and intracellular targeting through the different endosomal compartments. Our results identify posttranslational regulatory steps that regulate PHT1;1 accumulation at the plasma membrane and provide greater details for the function of PHF1.
RESULTS
PHF1 Is Required for Efficient PHT1 Targeting
PHF1 has been shown to alter PHT1;1 targeting and is also predicted to affect other PHT1 members (González et al., 2005). To test this prediction, cyan or yellow fluorescent proteins (CFP and YFP) were fused to two other principal PHT1 root transporters: PHT1;2 and PHT1;4. Both fusion proteins gave similar results; thus, we only report data for PHT1;2 here.
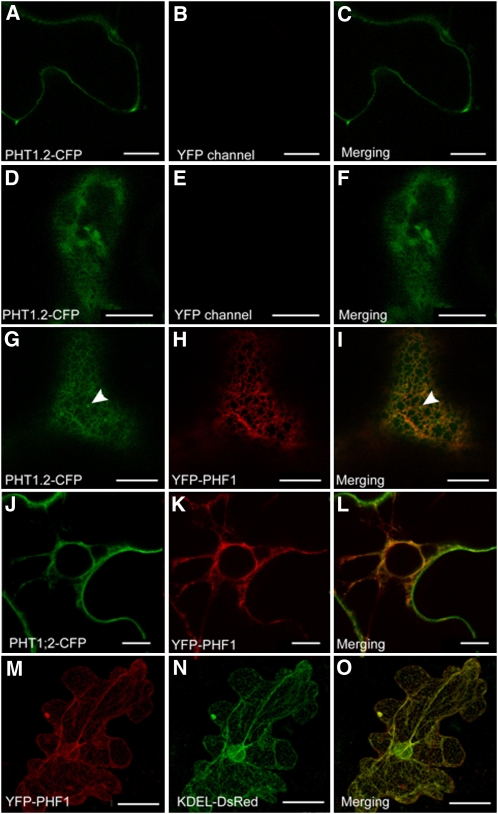
When PHT1;2-CFP is transiently expressed alone in epidermal cells of Nicotiana benthamiana leaves, a weak labeling of plasma membranes is observed (Figure 1A). Strikingly, only a low level of PHT1;2-CFP expression produced this signal. Indeed, a three- to fourfold increase in the amount of Agrobacterium tumefaciens used for transient expression produced a CFP fluorescence signal that was in addition detected in unexpected locations for plasma membrane transporters, such as the cytoplasm (Figures 1D to 1F) or in the nucleus (despite the putative presence of endogenous PHF1 protein). Under these last conditions, correct localization of PHT1;2-CFP is restored when YFP-PHF1 is coexpressed (Figures 1G to 1L). This confirms previous data demonstrating that fusion of green fluorescent protein (GFP) to the PHF1 coding sequence results in a protein that is still functional (González et al., 2005). PHT1;2 is distributed both into reticular structures that colocalize with YFP-PHF1 (Figures 1G to 1L) and into punctate moving structures, such as Golgi stacks that are typical of post-ER compartments (Figures 1G and 1I, arrowheads) where YFP-PHF1 could not been observed. Moreover, PHT1;2-CFP accumulated at the plasma membrane, confirming that targeting is effective (Figures 1J to 1L).
Figure 1.
PHF1 Facilitates PHT1;2-CFP Targeting through the ER Compartment.
Transient protein expression in N. benthamiana epidermal cells analyzed by confocal microscopy 48 h after infiltration. Transient expression of PHT1;2-CFP alone to a low (A) or high level (D) and together with YFP-PHF1 ([G] to [L]). (G) to (I) show colocalization in the ER (arrowheads indicate post-ER compartments). (J) to (L) show PHT1;2-CFP delivery to plasma membrane at cell periphery. (M) to (O) show transient coexpression of YFP-PHF1 (J) with ER marker KDEL-DsRed (K). Bars = 10 mm, except in (J) to (L), where bars = 50 μm.
As previously reported (González et al., 2005), the reticular structures correspond to the ER compartment, as demonstrated by the YFP-PHF1 fluorescence that colocalizes with the ER KDEL-DsRed marker (Figures 1M to 1O).
Immunoblot analysis (see Supplemental Figure 1 online) confirmed that all markers were correctly expressed. Furthermore, it revealed that in the absence of PHF1 overexpression, the pool of accumulated PHT1;2 was strongly reduced. Together, these data suggest that in the absence of an adequate amount of PHF1 protein, only a fraction of the PHT1;2 (or PHT1;4) produced is properly targeted.
PHF1 Is Present Only in the ER Compartment
To investigate if PHF1 is also present in other subcellular compartments, the Golgi-targeted marker ST-mRFP was introgressed into an Arabidopsis PHF1-GFP–expressing line. No colocalization could be detected between the two markers (see Supplemental Figures 2A to 2C online). We tested putative transient PHF1 recycling between ER and Golgi bodies by treating roots that stably coexpress PHF1-GFP and ST-mRFP with the trafficking inhibitor Brefeldin A (BFA). Upon BFA treatment, the trans-Golgi network and early endosomal compartments have been shown to accumulate in the core of big subcellular structures referred to as BFA compartments (Geldner et al., 2001), whereas Golgi markers tend to be found in the periphery (Grebe et al., 2003; Dettmer et al., 2006). After BFA treatment (2 h, 50 μM), PHF1-GFP could not be detected inside BFA compartments or at their periphery (see Supplemental Figures 2D to 2F online). This indicates that the fusion protein was not exported from the ER and did not accumulate in later compartments of the secretory pathway.
PHF1 Is Not Implicated in COPII Recruitment to ER Exit Sites
Sequence comparison and structural analysis indicate that PHF1 is related to the yeast Sec12 protein. Sec12 is an ER resident protein involved in the formation of COPII vesicles, a structure involved in the export of newly synthesized cargo proteins from the ER to the Golgi apparatus in eukaryotic cells (for review, see Russell and Stagg, 2010). This suggested to us that PHF1 could be implicated in the packaging of PHT1 into COPII vesicles to ensure ER export. This was investigated using the N. benthamiana experimental model that is preferred for studies of ER-Golgi transport, as described by daSilva et al. (2004). This approach uses an Agrobacterium-mediated transient expression system in N. benthamiana leaf epidermal cells. This system allowed us to coexpress PHF1 and PHT1 fusion proteins along with various markers involved in COPII trafficking. The conditions selected here promoted targeting of PHF1 and PHT1, identical to those described in Arabidopsis (González et al., 2005), indicating that the use of the N. benthamiana model does not disturb the targeting of these proteins.
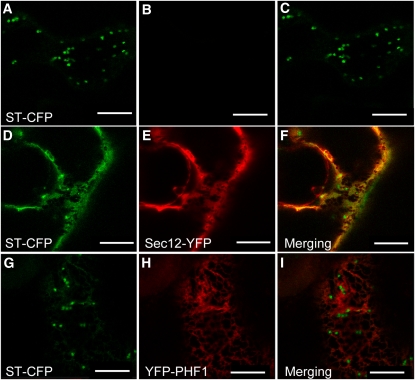
Overexpression of Sec12 protein is known to inhibit the COPII-dependent ER export of proteins (Hanton et al., 2007). We observed this effect here with COPII-exported cargo (such as ST), which displayed abnormal targeting and remained mostly in the ER when coexpressed with Sec12-YFP (cf. Figure 2A with 2D to 2F). Coexpression of Sec12 with PHT1;2-CFP produced similar results (see Supplemental Figure 3 online). In this case, PHT1 targeting to the plasma membrane was impaired (see Supplemental Figures 3D to 3I online), providing evidence that PHT1 proteins are indeed exported from the ER in a COPII-dependent manner. This observation confirms previous work showing that the secretion of proteins exhibiting a diacidic export motif in their C terminus (like PHT1) is predicted to be COPII-dependent (Mikosch et al., 2006; Mikosch and Homann, 2009). However, ST-CFP fluorescence was not modulated by the expression of YFP-PHF1 (cf. Figure 2A with 2G to 2I), even when testing various concentrations of YFP-PHF1 to rule out the possibility of dose limiting effects (all results were similar to Figures 2G to 2I).
Figure 2.
PHF1 Is Not Associated with COPII Component Recruitment to ERES.
Transient protein expression in N. benthamiana epidermal cells analyzed by confocal microscopy 48 h after infiltration. Transient expression of Golgi marker ST-CFP alone ([A] to [C]) or together with Sec12-YFP ([D] to [F]) or YFP-PHF1 ([G] to [I]). Sec12-YFP overexpression induces ST-CFP retention in ER. Bars = 10 μm.
This view is reinforced by additional experiments based on the use of the ER export site (ERES) markers Sar1 and Sec24 fused to YFP. Expression of the YFP-Sec24 marker results in cytosolic fluorescence with additional bright punctate structures (see Supplemental Figure 4B online). These structures correspond to ERES, as revealed by colocalization of the fluorescence signal associated with Sec 24 and ST-CFP (see Supplemental Figures 4D to 4F online). As previously observed, overexpression of COPII-exported cargo, such as ST or ERD2, increased the recruitment of Sec24 to ERES (Hanton et al., 2007). Here, we show that the Sec24 cytosolic fluorescence is reduced and the signal concentrates at the level of the punctate structures (cf. Supplemental Figures 4B and 4E online). Coexpression of PHT1;2-CFP instead of ST-CFP produced similar results, providing additional evidence that PHT1 proteins are indeed exported in a COPII-dependant manner from the ER (see Supplemental Figure 4H online). On the other hand, when YFP-Sec24 and CFP-PHF1 were coexpressed, they did not colocalize, and no modification of YFP fluorescence pattern was observed (see Supplemental Figures 4J to 4L online). Similarly, a fusion of YFP with Sar1, another key component of COPII machinery, was not observed to colocalize with PHF1 (see Supplemental Figures 5A to 5F online); this is in contrast with PHT1;2, which we found to colocalize with Sar1 (see Supplemental Figures 5G to 5L online).
Cumulatively, our results indicate that PHF1, despite its homology with SEC12 proteins, does not appear to be involved in COPII recruitment. However, COPII-dependent vesicles do appear to be used during PHT1 trafficking.
PHF1 Accumulation in Arabidopsis Tissues Is Modulated by the Level of Available Pi
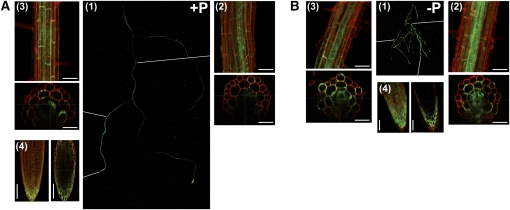
PHF1 expression is induced in phosphate starvation conditions (González et al., 2005). A transgenic Arabidopsis line expressing PHF1-GFP under the control of its own promoter has been used to investigate PHF1 spatial distribution in response to high or low Pi supply. This is a functional construct, as demonstrated by the successful complementation of the phf1 mutant (González et al., 2005). Under a nonlimiting phosphate condition, PHF1-GFP is heterogeneously distributed along the root system (Figure 3A1). The fluorescence of PHF1-GFP is mainly located in the central tissues of primary (Figure 3A2) and secondary roots (Figure 3A3) and is expressed at a very weak level in the outer cell layers (epidermis and cortex). In Pi-starved seedlings, the accumulation of PHF1-GFP is strongly modified. In addition to the central layers, we observed robust fluorescence signal in external cell layers, in particular in root hairs and connected cortex cells (Figures 3B2 and 3B3). The signal also appeared more homogeneously distributed between the various roots of the plantlets (Figure 3B1). Notably, PHF1-GFP accumulated in the root cap (Figures 3A4 and 3B4) in both Pi supply conditions.
Figure 3.
PHF1 Accumulation Is Modulated by Phosphate Supply.
(A) and (B) Seedlings cultivated on medium containing 500 μM (A) or 0 μM (B) Pi.
(A1) and (B1) Whole root system fluorescence observed using a dissecting microscope.
(A2) to (A4) and (B2) to (B4) Cell wall stained with propidium iodide (20 mg/mL) and green (GFP) fluorescence observed by confocal laser scanning microscopy. Sections are derived from Z-stack acquisitions.
(A2) and (B2) Primary root.
(A3) and (B3) Secondary root.
(A4) and (B4) Root tip.
Bars = 50 μm.
Phosphorylation of PHT1 Modulates ER Exit and Targeting to the Plasma Membrane
Phosphoproteomic studies of Arabidopsis plasma membrane proteins (Nühse et al., 2004) indicate that at least two PHT1 transporters (PHT1;1 and PHT1;4) are phosphorylated (for PHT1;1 modification affect Ser-520). Nevertheless, the role of these modifications is not yet understood. We hypothesized that these modifications could be involved in the regulation of PHT1 trafficking or activity.
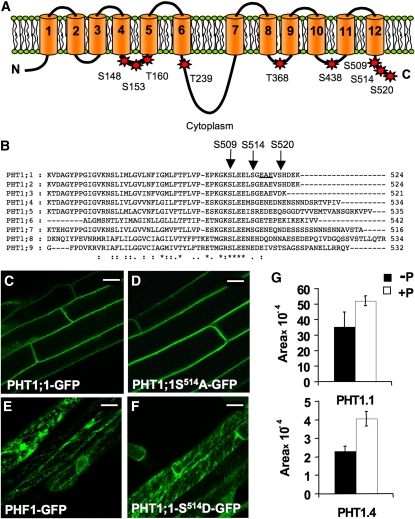
To investigate a putative role of Ser-520 phosphorylation in PHT1;1 subcellular trafficking, this residue was mutated. We also selected eight other putative phosphorylation sites located in the cytoplasmic loops of PHT1;1 (Figure 4A) based on their conservation among the PHT1 transporters and on phosphorylation predictions (Muchhal et al., 1996; Smith et al., 1997). These Ser and Thr residues were changed by site-directed mutagenesis to Ala or Asp, mimicking the unphosphorylated and the phosphorylated forms of the protein respectively. The wild-type and mutated versions of PHT1;1 were fused to GFP at their C termini. In Arabidopsis transformants, the cells expressing PHT1;1-GFP and almost all the mutated versions showed a labeling pattern consistent with a predominant localization of the fusion proteins to the plasma membrane (Figures 4C and 4D). By contrast, the transgenic lines expressing PHT1;1-S514D-GFP presented an intracellular reticular pattern (Figure 4F). The pronounced localization around the nucleus and its fuzzy aspect throughout the cell suggests that PHT1 accumulates in ER, as already shown for PHT1;1-GFP fusion expressed in a phf1 mutant background (González et al., 2005) or for ER PHF1 protein (Figure 4E).
Figure 4.
Targeted Mutagenesis of Putative PHT1 Phosphorylation Sites Prevents Exit from the ER.
(A) Model of PHT1;1 showing potential phosphorylation sites on the cytoplasmic side of the membrane that were mutated to both Ala and Asp. Cylinders represent the 12 predicted transmembrane regions. Ser and Thr residues mutated at potential phosphorylation sites are labeled with their amino acid numbers. C, C terminus; N, N terminus.
(B) Sequence alignment of the C-terminal regions of Arabidopsis PHT1 transporters. Arrows indicate the residues of PHT1;1 that were mutated (at potential phosphorylation sites). The putative ER exit site predicted for PHT1;1 is underlined.
(C) to (F) Representative confocal laser scanning micrographs of the fluorescence emitted by root cells of transgenic plants expressing PHT1;1-GFP (C), PHT1;1-S514A-GFP (D), PHF1-GFP (E), and PHT1;1-S514D-GFP (F).
(G) Quantitative variation of the monophosphorylated C-terminal peptides of PHT1.1 [SLEELSGEAEV(pS)HDEK] and PHT1.4 [SLEEMSGENEDNEN(pS)NNDSR] identified in proteins from root samples Pi-starved (left) or resupplied with phosphate during 2 h. Data show the mean and sd of biological triplicates with technical replicates performed. The P value corresponding to Student’s t test of significant differences between conditions were 0.0326 for PHT1.1 and 0.0006 for PHT1.4.
[See online article for color version of this figure.]
We performed a phosphoproteomic analysis on roots of Arabidopsis and compared plants grown on Pi-deficient soil with plants resupplied with Pi for 2 h. This revealed that Ser phosphorylation events located at the C terminus of PHT1;1 (Ser-520) and PHT1;4 (Ser-524) were significantly less frequent for the plants grown on phosphate-depleted medium. These results suggest a putative regulatory function associated with the phosphorylation of this part of the protein (Figure 4G).
To investigate phosphorylation events on PHT1;1, mass spectrometry analysis was performed on cell suspensions. In these cells, PHT1 is expressed at higher levels, allowing for the detection of rare events. Indeed, an additional PHT1;1 phosphorylation site occurring in vivo on Ser-514 was identified (see Supplemental Figure 6 online). Interestingly, we identified peptides located at the PHT1;1 C-end that exhibited phosphorylation events restricted to either Ser-520 and Ser-514 together or to Ser-520 alone. When pooling all spectra obtained from the different peptide fractions and several independent cell cultures data, the monophosphorylated form appears ~20 times more frequently than the diphosphorylated form. The single phosphorylation event of Ser-514 was never identified.
Altogether, these observations indicate serial phosphorylation events of Ser residues 520 and 514 at the C-end of PHT1;1. The latter very likely prevents PHT1;1 exit from ER and its proper targeting to the plasma membrane.
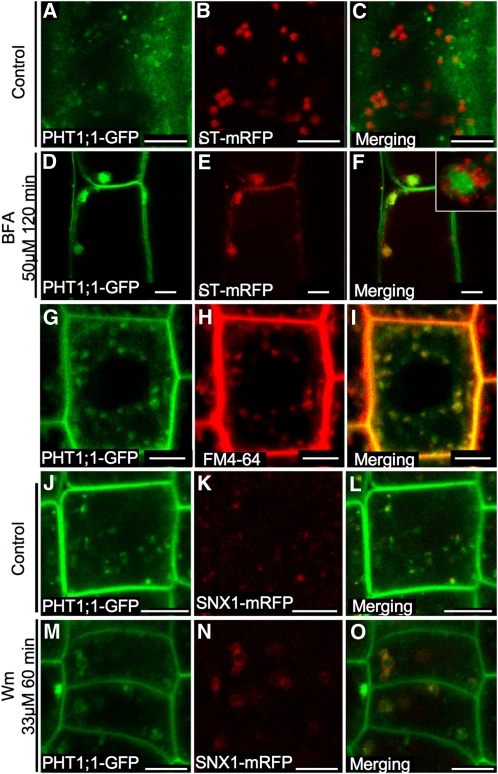
Intracellular Localization of PHT1;1-GFP to Sorting Endosomes
To investigate post-ER targeting of the PHT1;1 transporter, we analyzed an Arabidopsis transgenic line that expresses a PHT1;1-GFP construct under the control of a cauliflower mosaic virus 35S promoter. This allowed overriding of the transcriptional control that affects PHT1 during phosphate starvation. To bypass the limiting PHF1 level, all the observations were performed at the root tip because in this region, PHF1 accumulates independently of Pi supply (Figures 3A4 and 3B4). In p35S:PHT1;1-GFP lines, fluorescence mainly accumulates at the plasma membrane but also in intracellular moving punctate structures, independent of growth conditions (Figures 5A and 5G). In a phf1-1 background, these structures are no longer visible, suggesting they are involved in post-ER trafficking.
Figure 5.
PHT1;1 in Arabidopsis Root Tips Is Localized to Sorting Endosomes.
(A) to (F) Root tip cells coexpressing PHT1;1-GFP and Golgi marker ST-mRFP in control plants ([A] to [C]) or in plants treated with 50 μM BFA for 2 h ([D] to [F]).
(G) to (I) Root tip cells expressing PHT1;1-GFP were stained with the endocytic tracer FM4-64. Observations were made 15 min after incubation.
(J) to (O) Root tip cells coexpressing PHT1;1-GFP and sorting endosomes marker SNX1-mRFP control ([J] to [L]) and 60 min after 33 μM Wm treatment ([M] to [O]).
Bars = 5 μm.
We addressed the intracellular localization of PHT1;1-GFP with transgenic lines combining Golgi marker ST-mRFP and PHT1;1-GFP (Figures 5A to 5C). These observations revealed only partial colocalization of both fusion proteins. However, after BFA treatment (50 μM), both markers rapidly aggregated in BFA compartments (Figures 5D to 5F). A close-up view (Figure 5F) reveals that PHT1;1-GFP was preferentially located in the core of the BFA compartment rather than at the periphery as was ST-mRFP.
Next, we investigated a putative link between PHT1;1-GFP localization and endocytosis processes. We used FM4-64, a specific tracer dye for endosomes (vesicles resulting from endocytosis). GFP and the FM4-64 marker colocalized, confirming the presence of the phosphate transporters in endosomes (Figures 5G to 5I). These compartments form a heterogenous population including the trans-Golgi network, Golgi, and vacuoles. The inhibitor wortmannin (Wm), a covalent inhibitor of phosphoinositide 3-kinases, allows the identification of sorting endosomes, which are a distinct population of endosomes. They are involved in the fine regulation (relocalization and/or degradation) of several membrane proteins and are characterized by the presence of sortin nexin 1 (SNX1) (Geldner et al., 2001; Jaillais et al., 2006). Most of the punctate structures highlighted by PHT1;1-GFP colocalized with SNX1-mRFP (Figures 5J to 5L). Furthermore, Wm treatment (33 μM for 60 min) induced alteration of endosomes containing SNX1-mRFP and PHT1;1-GFP (Figures 5M to 5O). Strikingly, no differences in PHT1;1-GFP localization or Wm sensitivity were observed between control or Pi-starved seedlings (see Supplemental Figure 7 online). A quantitative analysis of the colocalization between PHT1 and SNX1 was performed using the measure of Pearson’s coefficient (Bolte and Cordelières, 2006). This revealed a partial colocalization between the two markers (Pearson’s coefficient ~0.5 for samples with or without Pi; see Supplemental Figure 7N online). We validated this partial colocalization by Coste’s approach (Bolte and Cordelières, 2006). Randomized images gave a Pearson’s coefficient close to 0 with a P value of 100%, indicating a highly probable colocalization. The partial colocalization between PHT1 and SNX1 is not surprising, as PHT1 is present in various endocytic compartments, such as in the Golgi (Figures 5A to 5C).
Analysis of lines expressing PHT1;1-GFP or PHT1;4-GFP under control of their own promoters gave similar results (as illustrated in Figures 5A to 5C), indicating genuine localization of PHT1 transporters to sorting endosomes. Moreover, it suggests that this localization can be extended to other members of PHT1 family.
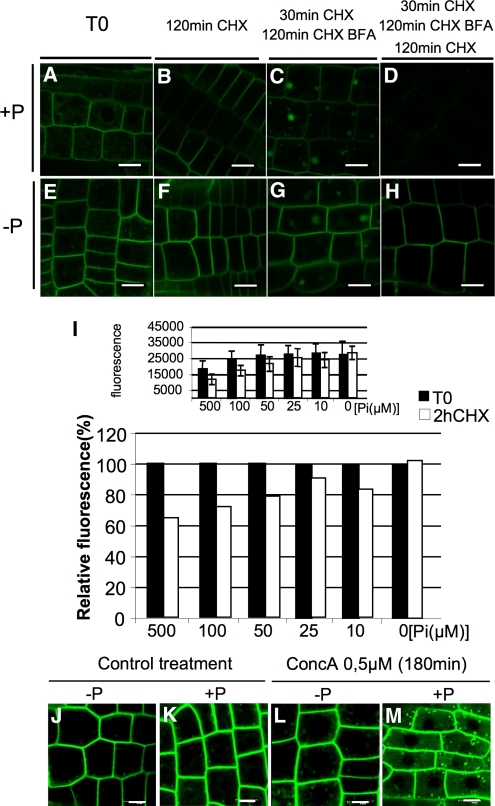
Phosphate Starvation Stabilizes PHT1.1-GFP at the Plasma Membrane
The localization of PHT1;1-GFP to sorting endosomes, independent of external Pi content, suggests either recycling to the plasma membrane or routing of PHT1;1-GFP to lytic vacuoles for degradation. Recycling of membrane proteins between endosomes and the plasma membrane has been demonstrated for PIN1 using a combination of BFA and the protein synthesis inhibitor cycloheximide (CHX) (Geldner et al., 2001, 2004). We investigated whether such a recycling process occurs during PHT1;1-GFP trafficking in seedlings cultivated under Pi-sufficient or limiting conditions (Figures 6A and 6E).
Figure 6.
Phosphate-Induced Degradation of Plasma Membrane PHT1.
(A) to (M) Arabidopsis root tip cells coexpressing PHT1;1-GFP cultivated on medium containing 500 μM ([A] to [D]) or 0 μM ([E] to [H]) Pi. Images represent the following: prior to drug treatment T0 ([A] and [E]), 2 h after CHX (50 μM) treatment ([B] and [F]), 30 min CHX (50 μM) pretreatment and then 2 h CHX (50 μM) and BFA (50 μM) ([C] and [G]), and 30 min CHX (50 μM) pretreatment and then 2 h CHX (50 μM) and BFA (50 μM) followed by 2 h CHX (50 μM) washout ([D] and [H]). Bars = 10 μm.
(I) Top graph shows fluorescence intensity (absolute values in arbitrary units) at T0 and after 2 h of CHX treatment of seedlings cultivated under various Pi concentrations. Bottom graph shows same data in relative level of fluorescence. For each condition, two lateral roots for six plants were observed. The fluorescence from 25 regions of interest for each sample was quantified. The mean and sd from the 300 measurements are indicated here for one of the three independent experiments performed.
(J) to (M) Effect of concA treatment (0.5 μM, 3 h) on root tip cells expressing PHT1;1-GFP grown in −P and +P medium ([L] and [M]) and respective untreated control ([J] and [K]).
[See online article for color version of this figure.]
For control seedlings, a 2-h CHX treatment induced a large decrease in fluorescence signal at the plasma membrane (Figure 6B). The addition of BFA for 2 h resulted in the aggregation of PHT1;1-GFP fluorescence in BFA compartments (Figure 6C). This signal results from endocytosed proteins because de novo synthesis is blocked by CHX addition. Withdrawal of BFA for 2 h induced a dramatic decrease in global cellular fluorescence and no remobilization of PHT1;1-GFP (Figure 6D), indicating no apparent recycling between endosomes and plasma membrane.
Using the same experimental procedure, a PIN1-GFP positive control shows plasma membrane recovery as previously published (Geldner et al., 2001). On Pi-starved seedlings, a 2-h treatment with CHX did not induce a visible decrease in plasma membrane fluorescence (Figure 6F). After BFA treatment, fluorescence accumulated in BFA compartments (Figure 6G). Even if the BFA effect is reversible after washouts, it should be noted that the PHT1;1-GFP fluorescence signal present at the plasma membrane remains strong (Figure 6H). These results suggest a greater stability of the phosphate transporter under the Pi-limiting condition. To test this hypothesis, fluorescence quantitative data were obtained from CHX-treated roots to determine PHT1;1-GFP stability (Figure 6I). Plasma membrane PHT1;1-GFP fluorescence following CHX treatment was measured from 300 regions of interest on roots of seedlings (cultivated in 0, 10, 25, 50, 100, or 500 μM Pi media). For plants growing in Pi-limiting conditions, the level of PHT1;1-GFP fluorescence at the plasma membrane remained constant during the first 2 to 3 h of treatment, whereas this signal rapidly decreased in a dose-dependent manner when phosphate was present in the medium (Figure 6I). Comparison of extreme conditions (0 versus 500 μM Pi) revealed a 50 to 65% drop in fluorescence signal, depending on the experiment (Figure 6I; see Supplemental Figure 8 online). Time-course experiments revealed that the optimum observation of this phenomenon occurs after 2 h of CHX treatments. Longer incubation promoted a slow and continuous decrease of fluorescence signal, which affected plants independently of Pi conditions (cf. 7-h CHX treatments in Supplemental Figure 8 online).
This indicates the occurrence of a rapid degradation mechanism that is specifically active in Pi-sufficient conditions. Putative involvement of the proteasome or PHO2 (a ubiquitin conjugase-related protein involved in the negative regulation of shoot Pi levels; Aung et al., 2006; Bari et al., 2006) in this mechanism was tested here using MG-132 (a proteasome inhibitor) or a pho2-1 mutant. Neither had an impact on this degradation mechanism (see Supplemental Figure 9 online). The mutagenized forms of PHT1;1 described above (mimicking phosphorylation or dephosphorylation) were also subjected to CHX treatment to test the putative role of phosphorylation in these degradation process. These mutants behaved like the native PHT1;1 control (Figures 6B and 6F), suggesting that the regulatory mechanism identified above acts independently of these modifications. We did not test PHT1;1 S514D because it remains in the ER.
It has been recently shown for boron (Takano et al., 2005) and the PIN2 auxin efflux transporter (Kleine-Vehn et al., 2008) that lytic vacuolar sorting and degradation can follow protein endocytosis. This phenomenon was revealed using concanamycin A (concA) or dark treatments (Tamura et al., 2003). The effect of a 3-h treatment with 0.5 μM concA was tested using root tips of 7-d-old seedlings cultivated in Pi-sufficient or -limiting conditions. Accumulation of fluorescence was specifically observed in large vacuolated structures of nonphosphate starved roots (Figures 6L and 6M). Under these conditions, this indicates that PHT1 transporters are targeted to the vacuole for degradation, after removal from the plasma membrane. The various mutagenized forms of PHT1;1 (mimicking phosphorylated or unphosphorylated forms of Ser-509, Ser-514, and Ser-520) were assayed with concA treatment in Pi-sufficient conditions. All lines behaved like the wild-type control (Figure 6M), with the exception of PHT1;1-S514-GFP, which remains trapped in the ER (see Figure 4F). This suggests that the phosphorylation events occurring in vivo and identified in the C terminus do not play any additional role during these degradation processes.
Finally, we compared the internalization kinetic of the endocytic lipid dye FM4-64 from the plasma membrane to the tonoplast, and found revealed no difference between the seedlings cultivated in Pi-sufficient or in Pi-limiting conditions (Supplemental Figure 10 online). This result suggests that there is no general stimulation of endocytosis in the low Pi condition. Consequently, this regulation appears quite specific to PHT1 transporters.
DISCUSSION
Plasma membrane proteins are initially targeted to the ER, after which they require various trafficking steps to reach their final destination. A growing body of evidence indicates that the trafficking of plasma membrane proteins is subject to regulation. In this study, we examined the regulatory events accompanying intracellular trafficking of PHT1 family members. Our results confirm the importance of PHF1 to the ER exit of PHT1 and other members of this family and place its action upstream of COPII vesicle formation. In addition, we found that phosphorylation mimics of PHT1 modulate its exit from the ER and that degradation of plasma membrane PHT1 in the presence of phosphate occurs through endocytosis and its subsequent routing to lytic vacuoles.
PHT1 in the ER: Importance of PHF1
One aspect of the study reported here has been a detailed cell biological analysis of PHF1 localization and function regarding the ER exit of different members of the PHT1 family. In particular, we provide evidence confirming that PHF1 affects not only PHT1;1 exit from the ER as previously shown (González et al., 2005), but also the exit of other PHT1 family members. According to the pPHF1:PHF1-GFP construct, accumulation of PHF1 in the root localizes predominantly to the root tip and the central cylinder (in all Pi conditions) and is induced by Pi starvation in the more external root layers, such as epidermis and cortex. This pattern overlaps with the expression profile of all the various PHT1 transporters expressed in the roots (Karthikeyan et al., 2002; Mudge et al., 2002). In addition, we demonstrate that the other main members of the PHT1 family expressed in the root, such as PHT1;2 (Figure 1), also required PHF1 for efficient targeting to the plasma membrane.
The initial characterization of PHF1 demonstrated its localization to the ER (González et al., 2005). In this study, the putative presence of PHF1 in other compartments of the secretory pathway was investigated in detail, using cellular markers and BFA treatment. Our results indicate that PHF1 is restricted to the ER but is not implicated in recruitment of COPII core components at the ERES. By contrast, PHT1 proteins use these ERES to leave the ER. Therefore, despite the structural similarity with the yeast Sec12p protein, we show that PHF1 has acquired a distinct and specific function in plants, as previously suggested (González et al., 2005), which acts upstream of COPII formation. A role for this could be as a cochaperone, allowing for proper folding of the Pi transporter. Such a mechanism has been identified in yeast, where pho86 mutation leads to ER retention of PHO84 (PHT1 homolog). Previous work has shown that the pho86 mutation induces aggregation of the yeast PHT1 homolog PHO84 (Kota and Ljungdahl, 2005), suggesting that Pho86p acts as a cochaperone essential for proper folding of the transporters. The similarity in phenotypes between the Arabidopsis phf1 and the yeast pho86 mutations suggests that PHF1 might play a similar function. This view is in line with the finding that PHT1-GFP accumulation is reduced in phf1 (González et al., 2005) and by the recent identification (using the split ubiquitin system) of a direct interaction between PHF1 and PHT1 proteins (T.J. Chiou, personal communication).
Phosphorylation Mimics of PHT1 Modulate Its Exit from the ER
Protein phosphorylation is a well-known type of posttranslational modification that is involved in the regulation of numerous cellular processes. Besides modulating protein activity, an important role of protein phosphorylation is to regulate protein targeting. For example, the Arabidopsis nitrate transporter NRT1.1 (Martín et al., 2008) and the aquaporin PIP2;1 (Prak et al., 2008) both require phosphorylation to reach the plasma membrane.
Both this analysis and previous publications (Nühse et al., 2004; Hem et al., 2007) identified phosphorylation in vivo in the C-terminal region of PHT1;1 (Ser-514 and Ser-520) and PHT1;4 (Ser-524). Interestingly, several of these phosphorylations are modulated by phosphate supply, suggesting a conserved regulatory role for this part of the protein. In addition, phosphoproteomics data on cell cultures identified phosphorylations of Ser residues present in the C-terminal region of PHT1;5, PHT1;7, and PHT1;9, (M. Rossignol, personal communication). This provides additional data suggesting that phosphorylation of the C-end of PHT1 is used by plants as a conserved step to regulate plasma membrane targeting.
Our experiments on PHT1.1 identified in vivo phosphorylation of Ser-520 alone or associated with phosphorylation of Ser-514. Another case of multiple phosphorylation has already been identified in the C-terminal tail of Arabidopsis PIP2;1 aquaporins. They are involved in the subcellular trafficking of PIP2;1 toward the plasma membrane (Prak et al., 2008). Phosphorylation modulates the trafficking of PHT1 and PIP2;1, and in both cases only one of the identified phosphorylated Ser residues modulates the targeting (Ser-514 for PHT1; Ser-283 for PIP2;1). However, phosphorylation has opposing effects on these proteins: whereas phosphorylation of the aquaporin is required for its proper allocation, the phosphorylation of PHT1;1 most probably prevents it from reaching its final destination. The reduction of PHT1;1 present at the plasma membrane is correlated with a nonlimiting Pi presence. Thus, it makes sense that a regulatory kinase involved in this process could respond to the level of ion present in the cells. This kinase probably phosphorylates various Ser residues present at the C-end (as proposed for AtPIP2;1 proteins). The regulatory phosphorylation event taking place in vivo on Ser-514 probably occurs just before the degradation of PHT1;1. This could explain why this particular phosphorylation is poorly detected by phosphoproteomic analysis. This is why its identification is facilitated by the use of materials, such as cell suspensions, that can accumulate high levels of PHT1. Interestingly, the PHT1 protein alignment (Figure 4B) revealed the presence of a putative conserved ER export site (D/E-X-D/E) in the C-terminal region of the proteins, close to the phosphorylation sites. This motif in position 516 to 519 is conserved in all PHT1, except for PHT1;8 and PHT1;9 (for which multiple ER export site locations could be predicted due to the presence of more acidic amino acids in the C tails). We hypothesize that the introduction of an additional negative charge adjacent to the ER export site (by mutagenesis or phosphorylation) affects its activity. This could explain the retention of the S514D PHT1;1 protein in the ER. We thereby propose that this regulation could act by altering the recognition process of the ER export motif.
However, since phosphorylation mimics on Ser-514 are all or nothing experiments promoting a retention of the PHT1;1-GFP fusion at the ER, it was not possible to use this approach to investigate whether this phosphorylation could also play a regulatory role at the plasma membrane level.
Specific Degradation of Plasma Membrane PHT1 in the Presence of Phosphate
In this study, we identified a mechanism that regulates PHT1;1 accumulation in the plasma membrane. When phosphate is supplied to a Pi-starved plant, the accumulation of PHT1;1 transporters in the plasma membrane rapidly decreases. This reveals the existence of a degradation process that reduces the pool of PHT1 transporters when phosphate is present in excess (Figure 6). Similar mechanisms have been identified for different types of plasma membrane proteins in yeast, plants, and animals. This involves endocytosis and subsequent degradation in lytic vacuoles and lysosome compartments, as shown here for PHT1. Interestingly, the colocalization of PHT1;1 with SNX1 demonstrates its presence in the sorting endosome. Such compartments, only recently identified in plants (Jaillais et al., 2006), have been associated with various functions of membrane proteins. This includes postendocytic and secretory cargo trafficking (i.e., the recycling of brassinosteroid receptor BRI1 to the plasma membrane or targeting of the boron transporter BOR1 for degradation; Jaillais et al., 2008) or repolarization (auxin transporter PIN1 during secondary root organogenesis; Jaillais et al., 2007). In Arabidopsis root tips, phosphate transporters do not appear to be polarized at the plasma membrane. Therefore, the presence of PHT1;1-GFP in sorting endosomes is most likely associated with recycling to the plasma membrane and/or routing to lytic vacuoles for degradation. This view is confirmed by the accumulation of endocytosed PHT1;1-GFP in BFA-induced compartments in both growing conditions (Figure 5). This suggests a continuous internalization of PHT1 from the plasma membrane to the endosomes for subsequent recycling (in low Pi) or degradation in lytic vacuoles (in high Pi). Additional experiments performed with FM64 dye marker confirmed that endocytocis was not modulated by the level of Pi supply (see Supplemental Figure 10 online). Similar mechanisms have been described for auxin transporters (AUX and PIN), both of which are subjected to continuous recycling between the plasma membrane and endocytic compartments (Geldner et al., 2003). Aside from its recycling, PHT1;1 also accumulated in the vacuole of plants grown in the presence of high Pi (Figure 6M). This has been well characterized in yeast, where rapid internalization and vacuolar degradation of the high-affinity phosphate transporter Pho84, a PHT1 homolog, are observed after addition of Pi (Lagerstedt et al., 2002; Persson et al., 2003). Similar features have been established for the Arabidopsis boron transporter (BOR1), depending on the availability of its own substrate (Takano et al., 2005). Such processes likely aim to avoid the cellular toxicity associated with high internal concentrations of ions such as boron or Pi.
A similar endocytosis mechanism that is followed by vacuolar breakdown has been identified in yeast for the PHT1 homolog PHO84 (Lundh et al., 2009). Interestingly, despite the high conservation between plant and yeast Pi transporters, the sequence identified in the central loop of PHO84 (amino acids 304 to 327), which is required for this regulation, appears to be deleted in all PHT1 members. As in the case of ER exit, which depends on unrelated plant (PHF1) and yeast (PHO86) cofactors, it appears that plant and yeast PHT1 transporters have evolved different mechanisms to regulate degradation of plasma membrane PHT1 in the presence of phosphate. The basis of this mechanism remains to be identified in plants.
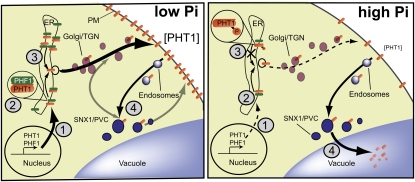
Model of PHT1 Regulations
In Figure 7, we present a model that summarizes the different levels of regulation that affect PHT1. The first regulatory level established by previous analyses is transcriptional control (Figure 7, step 1; Muchhal and Raghothama, 1999; Karthikeyan et al., 2002; Mudge et al., 2002; Misson et al., 2005; Thibaud et al., 2010). It regulates quantitative and spatial distribution of PHT1 in plant tissues in response to environmental modifications. The main known abiotic factor affecting PHT1 expression is the lack of phosphate (Figure 7, step 1). It triggers an important induction in the majority of PHT1 genes within a few hours (Karthikeyan et al., 2002; Misson et al., 2005).
Figure 7.
Multiple Regulatory Events Control PHT1 Trafficking.
Four different levels of control regulate PHT1 accumulation. A first one takes place at the transcriptional level (1), the second one involves PHF1 accumulation in the ER (2), the third one involves specific phosphorylation at the C terminus of PHT1 impairing PHT1 export from the ER (3), and the fourth one involves a recycling/degradation process at the level of plasma membrane (PM; 4). All these steps appear to be modulated by the Pi status of the plant. TGN, trans-Golgi network.
[See online article for color version of this figure.]
All other steps of regulation appear to be acting posttranslationally. Given the very high homologies (45 to 98% amino acid identity; 58 to 99.8% similarity) that exist between PHT1;1 and the eight other members of the PHT1 family (Mudge et al., 2002), we hypothesize that most (if not all) posttranslational regulatory events identified here are conserved. It should be noted that most of the Pi transporters are tightly regulated at the transcriptional level and are therefore present in very limited amount when abundant Pi is present in the medium. Nevertheless, some members of the PHT1 family, such as PHT1;1 (and probably also PHT1;2), remain transcribed at a significant basal level in Pi-sufficient conditions (Muchhal et al., 1996; Karthikeyan et al., 2002). Therefore, the regulations identified in this work may provide a posttranscriptional control particularly relevant to the fine-tuning of these specific PHT1 members.
The first posttranslational regulatory level involves the PHF1 protein (Figure 7, step 2). PHF1 is required to facilitate PHT1 transit through the ER, and we suggest that it acts as a PHT1-specific chaperone. Another level of regulation was also identified in the ER (Figure 7, step 3), which concerns regulatory phosphorylation of particular Ser residues located in the C-end of PHT1. This is illustrated here by the phosphorylation of Ser-514, which prevents ER exit of the PHT1;1 transporters. We propose that phosphorylation of Ser-514 impairs the recognition of the closely linked ER export motif of PHT1;1, thus preventing its exit from the ER when the internal level of Pi is high.
The last level identified in this study (Figure 7, step 4) is the downregulation by Pi levels of PHT1 amount at the plasma membrane. It occurs through endocytosis followed by routing to degradation at lytic vacuoles. A similar regulatory event is observed in yeast but appears to rely on a different mechanism (Lundh et al., 2009).
Such complexity illustrates the capacity for plants to tightly regulate the level of ion transporters (for recent review on other Arabidopsis mineral transporters, see Fuji et al., 2009).
METHODS
Plant Materials and Growth Conditions
Seedlings were grown vertically on Murashige and Skoog medium diluted 10-fold in Petri dishes supplemented with a Pi source of either 500 μM (+P) or 5 μM (−P) NaH2PO4, as previously described (Misson et al., 2004). Nicotiana benthamiana was cultivated in growth chambers as described (Bayle et al., 2008). For all molecular and biochemical analyses, Arabidopsis thaliana plants were grown in a culture chamber under a 16-h-light/8-h-dark regime (24°C/21°C) in vertical plates.
For phosphoproteomics experiments, Arabidopsis (ecotype Columbia-0 [Col-0]) plants and cell suspension were used and grown as described (Hem et al., 2007; Gerbeau et al., 2002, respectively). Eighteen-day-old plants were starved by transfer to a medium depleted of phosphate for 7 d. Phosphate was resupplied by transferring starved plants to the complete medium during 2 h.
Various Arabidopsis transgenic lines expressing different cellular markers were used: SNX1:SNX1-mRFP (Jaillais et al., 2007), 35S:PHT1;1-GFP, pPHF1:PHF1-GFP (González et al., 2005), and ST-mRFP, which was kindly provided by Tomohiro Uemura (University of Tokyo).
pVHK18-en6 ST-CFP (Brandizzi et al., 2002), pVHK18-en6 Sar1-YFP, and pVHK18 YFP-Sec24 (Hanton et al., 2007) plasmids were kindly provided by Federica Brandizzi (Michigan State University).
pVHK18 Sec12-YFP (daSilva et al., 2004) was kindly provided by Jurgen Denecke (University of Leeds)
Constructs and Transient Plant Transformation
Coding sequences for PHF1 and PHT1;2 were amplified from total Col-0 cDNA, cloned into pENTR/D-TOPO (Invitrogen), and recombined into pXCSG-CFP and pXCSG-YFP or pXCS CFP-GW and pXCS YFP-GW (Feys et al., 2005). For cDNA amplification, the following primers were used: PHF1, cDNA forward 5′-CACCATGGAGATTGAAGAAGCGAGTCGTG-3′ and reverse 5′-CCTATAGGTCCAAGTTCCACCTAC-3′ for N-ter fusion or 5′-TAGGTACCAAGTTCCACCTAC-3′ for C-ter fusion; PHT1;2, cDNA forward 5′-CACCATGGCCGAACAACAACTAGGAGTGC-3′ and reverse 5′-TTATTTCTCGTCATGGCTAACCTCAGCC-3′ for N-ter fusion or 5′-TTTCTC GTCATGGCTAACCTCAGCC-3′ for C-ter fusion.
Transient protein expression in N. benthamiana was performed as previously described (Noël et al., 2007).
Site-Directed Mutagenesis and Expression in Transgenic Plants
Mutagenesis of potential PHT1;1 phosphorylation sites was performed by performing PCR on a cDNA of Pht1;1 cloned into a pENTR vector using the pENTR Directional TOPO Cloning kit (Invitrogen). The mutagenic primers used to generate mutations at each selected site are given in Supplemental Table 1 online. PCR-amplified plasmids were treated with DpnI to select the mutated forms and then used to transform Escherichia coli strain DH10B. The presence of each mutation was checked by DNA sequencing (Gexbyweb). The PHT1;1 sequences were placed under the control of a cauliflower mosaic virus 35S promoter and fused to GFP in the C-terminal position by cloning to the pGWB5 vector (Nakagawa et al., 2007) using the Gateway System (Invitrogen). The constructs were then transferred into Agrobacterium tumefaciens strain GV3101 by electroporation. The bacterial strains were used for transformation of Arabidopsis Col-0 by the floral dip method (Harrison et al., 2006). To select transformed plants, seeds were surface sterilized and germinated in a Murashige and Skoog medium complemented with 0.05 g/L hygromycin. At least four independent lines were obtained for each construct.
The roots and shoots of transgenic lines expressing PHT1;1-GFP fusion and the pPHF1:PHF1-GFP line (González et al., 2005) were observed by confocal microscopy. For mutagenesis experiments, two independent lines for each construct were analyzed. Based on the phenotype identified for transformants harboring PHT1;1-S514A-GFP or PHT1;1-S514D-GFP constructs, we extended analysis to four independent lines.
Phosphoproteomic Analysis
Membrane fractions were obtained as previously described (Hem et al., 2007), except for root membranes in which microsome fractions were stripped for soluble proteins before resuspension in Laemmli buffer and subsequent trypsin digestion (Wiśniewski et al., 2009).
Phosphopeptides were purified using a combination of ion exchange (SAX or SCX) and TiO2 microchromatography steps adapted from Hem et al. (2007) and Trinidad et al. (2006), respectively. Peptides were analyzed on a nanoflow liquid chromatography–tandem mass spectrometer (Ion Trap HCT or Q-TOF MaXis; Bruker Daltonics). Mass spectrometry data were identified using Mascot version 2.2.04 (Matrix Science) against an Arabidopsis database (TAIR9, version pep_20090619). For Mascot search, up to one missed cleavage, carbamidomethylation as fixed modification, and phosphorylation (ST) or (Y) as variable modifications were allowed. For quantitative analysis, biological triplicates and technical replicates were performed. Label-free liquid chromatography–mass spectrometry quantification of starved and phosphate-resupplied samples, including statistical analysis (Student’s t test), was made using the ProteinScape version 2.1 software (Bruker Daltonics).
Confocal Imaging
Transient intracellular fluorescence was observed by confocal laser scanning microscopy using a Leica SP2 AOBS inverted confocal microscope (Leica Microsystems) equipped with argon ion (458-, 476-, 488-, 496-, and 514-nm laser lines) and He-Ne (561-nm laser line) lasers. A ×63 HCX PLAN-APO Water immersible objective lens (numerical aperture = 1.2; ref. 506212; Leica, Germany) was used for imaging. GFP, FM4-64, and propidium iodide were excited by the 488-nm argon laser line, CFP using the 458-nm line, and YFP using the 514-nm line. mRFP was excited using a 561-nm He-Ne laser. Fluorescence was detected using photomultiplier tube settings as follows: CFP (463 to 505 nm), GFP (500 to 550 nm), YFP (520 to 560 nm), mRFP (570 to 620 nm), and FM4-64 and propidium iodide (620 to 680 nm). For colocalization experiments, the sequential mode was used to avoid emission spectra overlapping.
For quantification experiments, 5 to 10 plantlets were observed per condition. Fluorescence from 25 regions of interests on two lateral roots per plant was quantified using ImageJ software (http://rsbweb.nih.gov/ij/).
Quantitative Analysis of Colocalization between PHT1-GFP and SNX1-mRFP
Confocal images were analyzed with the ImageJ software using the JACoP plugin and following the Coste’s approach (Bolte and Cordelières, 2006). The Pearson’s coefficient was calculated based on the analysis of 350 root tip cells from 20 plants per condition. To efficiently quantify intracellular colocalization, masks over plasma membrane fluorescence were manually added as previously described (French et al., 2008).
Chemical Treatments
BFA (50 mM; Sigma-Aldrich), Wortmannin (20 mM; Sigma-Aldrich), concA (100 μM; Sigma-Aldrich), and MG-132 (50 mM; Calbiochem) stock solutions were prepared in DMSO and used at 50, 33, 0.5, and 50 μM, respectively, in liquid +P or –P medium according to the experiment. For control experiments, a 0.5% DMSO solution was used. CHX (50 mM; Sigma-Aldrich) stock solution was prepared in water and used at 50 μM for experiments. Various drug effects were assayed on 9-d-old plantlets.
For FM4-64 staining, roots were incubated with 5 μM FM4-64 (Red synaptracer 3.2; Interchim) for 10 min on ice, washed twice with +P or –P liquid medium, and observed at different incubation times. The cell wall was stained with propidium iodide (20 mg/mL).
Immunoblot Analysis
Total protein extract from N. benthamiana leaves were subjected to SDS-PAGE and transferred to nitrocellulose as described (Noël et al., 2007). Protein gel blots were probed with monoclonal anti-GFP antibody (Roche) according to the manufacturer’s instructions.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome initiative under the following accession numbers: PHF1, At3g52190; PHT1;1, At5g43350; PHT1;2, At5g43370; PHT1;3, At5g43360; PHT1;4, At2g38940; PHT1;5, At2g32830; PHT1;6, At5g43340; PHT1;7, At3g54700; PHT1;8, At1g20860; PHT1;9, At1g76430; SNX1, At5g06140; SEC24, At3g07100; and SEC12, At2g01470.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. PHT1;2 Accumulation Is PHF1 Dependent.
Supplemental Figure 2. Strict ER Localization of PHF1.
Supplemental Figure 3. PHT1;2-CFP Export from the ER Is COPII Dependent.
Supplemental Figure 4. PHF1 Is Not Associated with Recruitment of COPII Components to ERES.
Supplemental Figure 5. Coexpression of PHF1 and PHT1;2-CFP Fusion Protein Together with ERES Marker SAR1.
Supplemental Figure 6. MS/MS Fragmentation Spectrum of the Diphosphopeptide SLEEL(pS)GEAEV(pS)HDEK (PHT1.1).
Supplemental Figure 7. Sorting Endosome Localization of PHT1;1 Independently of Pi External Content.
Supplemental Figure 8. Kinetic Analysis of Pi-Induced PHT1 Degradation.
Supplemental Figure 9. Pi-Induced PHT1 Degradation Is Not Affected by MG-132 Treatment or pho2-1 Mutation.
Supplemental Figure 10. Phosphate Starvation Does Not Affect Internalization of Endocytic Tracer FM4-64 in Root Cells.
Supplemental Table 1. Primers Used for Amplification and Site-Directed Mutagenesis of PHT1;1 .
Acknowledgments
This work was supported by a grant from Commissariat à l’Energie Atomique and region Provence-Alpes-Côte d’Azur (to V.B.) and by the Agence Nationale de la Recherche project PhosphoStim (J.V.). We thank T.J. Chiou for sharing unpublished data and E. Dellanoy, M.-C. Thibaud, and E. Javot for the critical reading of the manuscript. We thank J. Denecke for providing the Sec12 markers. We also thank B. Loveall for English editing.
References
- Aung K., Lin S.I., Wu C.C., Huang Y.T., Su C.L., Chiou T.J. (2006). pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol. 141: 1000–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber S.A., Walker J.M., Vasey E.H. (1963). Mechanisms for movement of plant nutrients from soil and fertilizer to plant root. J. Agric. Food Chem. 11: 204–207 [Google Scholar]
- Bari R., Datt Pant B., Stitt M., Scheible W.R. (2006). PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol. 141: 988–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayle V., Nussaume L., Bhat R.A. (2008). Combination of novel GFP mutant TSapphire and DsRed variant mOrange to set up a versatile in planta FRET-FLIM assay. Plant Physiol. 148: 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S., Cordelières F.P. (2006). A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224: 213–232 [DOI] [PubMed] [Google Scholar]
- Brandizzi F., Snapp E.L., Roberts A.G., Lippincott-Schwartz J., Hawes C. (2002). Membrane protein transport between the endoplasmic reticulum and the Golgi in tobacco leaves is energy dependent but cytoskeleton independent: Evidence from selective photobleaching. Plant Cell 14: 1293–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva L.L., Snapp E.L., Denecke J., Lippincott-Schwartz J., Hawes C., Brandizzi F. (2004). Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. Plant Cell 16: 1753–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J., Hong-Hermesdorf A., Stierhof Y.D., Schumacher K. (2006). Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18: 715–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys B.J., Wiermer M., Bhat R.A., Moisan L.J., Medina-Escobar N., Neu C., Cabral A., Parker J.E. (2005). Arabidopsis SENESCENCE-ASSOCIATED GENE101 stabilizes and signals within an ENHANCED DISEASE SUSCEPTIBILITY1 complex in plant innate immunity. Plant Cell 17: 2601–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A.P., Mills S., Swarup R., Bennett M.J., Pridmore T.P. (2008). Colocalization of fluorescent markers in confocal microscope images of plant cells. Nat. Protoc. 3: 619–628 [DOI] [PubMed] [Google Scholar]
- Fuji K., Miwa K., Fujiwara T. (2009). The intracellular transport of transporters: Membrane trafficking of mineral transporters. Curr. Opin. Plant Biol. 12: 699–704 [DOI] [PubMed] [Google Scholar]
- Geldner N., Anders N., Wolters H., Keicher J., Kornberger W., Muller P., Delbarre A., Ueda T., Nakano A., Jürgens G. (2003). The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230 [DOI] [PubMed] [Google Scholar]
- Geldner N., Friml J., Stierhof Y.D., Jürgens G., Palme K. (2001). Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Geldner N., Richter S., Vieten A., Marquardt S., Torres-Ruiz R.A., Mayer U., Jürgens G. (2004). Partial loss-of-function alleles reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis. Development 131: 389–400 [DOI] [PubMed] [Google Scholar]
- Gerbeau P., Amodeo G., Henzier T., Santoni V., Ripoche P., Maurel C. (2002). The water permeability of Arabidopsis plasma membrane is regulated by divalent cations and pH. Plant J. 30: 71–81 [DOI] [PubMed] [Google Scholar]
- González E., Solano R., Rubio V., Leyva A., Paz-Ares J. (2005). PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 is a plant-specific SEC12-related protein that enables the endoplasmic reticulum exit of a high-affinity phosphate transporter in Arabidopsis. Plant Cell 17: 3500–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebe M., Xu J., Möbius W., Ueda T., Nakano A., Geuze H.J., Rook M.B., Scheres B. (2003). Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr. Biol. 13: 1378–1387 [DOI] [PubMed] [Google Scholar]
- Hanton S.L., Chatre L., Renna L., Matheson L.A., Brandizzi F. (2007). De novo formation of plant endoplasmic reticulum export sites is membrane cargo induced and signal mediated. Plant Physiol. 143: 1640–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S.J., Mott E.K., Parsley K., Aspinall S., Gray J.C., Cottage A. (2006). A rapid and robust method of identifying transformed Arabidopsis thaliana seedlings following floral dip transformation. Plant Methods 2: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hem S., Rofidal V., Sommerer N., Rossignol M. (2007). Novel subsets of the Arabidopsis plasmalemma phosphoproteome identify phosphorylation sites in secondary active transporters. Biochem. Biophys. Res. Commun. 363: 375–380 [DOI] [PubMed] [Google Scholar]
- Hirsch J., Marin E., Floriani M., Chiarenza S., Richaud P., Nussaume L., Thibaud M.C. (2006). Phosphate deficiency promotes modification of iron distribution in Arabidopsis plants. Biochimie 88: 1767–1771 [DOI] [PubMed] [Google Scholar]
- Jaillais Y., Fobis-Loisy I., Miège C., Gaude T. (2008). Evidence for a sorting endosome in Arabidopsis root cells. Plant J. 53: 237–247 [DOI] [PubMed] [Google Scholar]
- Jaillais Y., Fobis-Loisy I., Miège C., Rollin C., Gaude T. (2006). AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature 443: 106–109 [DOI] [PubMed] [Google Scholar]
- Jaillais Y., Santambrogio M., Rozier F., Fobis-Loisy I., Miège C., Gaude T. (2007). The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell 130: 1057–1070 [DOI] [PubMed] [Google Scholar]
- Karthikeyan A.S., Varadarajan D.K., Mukatira U.T., D’Urzo M.P., Damsz B., Raghothama K.G. (2002). Regulated expression of Arabidopsis phosphate transporters. Plant Physiol. 130: 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J., Leitner J., Zwiewka M., Sauer M., Abas L., Luschnig C., Friml J. (2008). Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc. Natl. Acad. Sci. USA 105: 17812–17817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota J., Ljungdahl P.O. (2005). Specialized membrane-localized chaperones prevent aggregation of polytopic proteins in the ER. J. Cell Biol. 168: 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerstedt J.O., Zvyagilskaya R., Pratt J.R., Pattison-Granberg J., Kruckeberg A.L., Berden J.A., Persson B.L. (2002). Mutagenic and functional analysis of the C-terminus of Saccharomyces cerevisiae Pho84 phosphate transporter. FEBS Lett. 526: 31–37 [DOI] [PubMed] [Google Scholar]
- Lee R.B. (1993). Control of net uptake of nutrients by regulation of influx in barley plants recovering from nutrient deficiency. Ann. Bot. (Lond.) 72: 223–230 [Google Scholar]
- López-Bucio J., de La Vega O.M., Guevara-García A., Herrera-Estrella L. (2000). Enhanced phosphorus uptake in transgenic tobacco plants that overproduce citrate. Nat. Biotechnol. 18: 450–453 [DOI] [PubMed] [Google Scholar]
- Lundh F., Mouillon J.-M., Samyn D., Stadler K., Popova Y., Lagerstedt J.O., Thevelein J.M., Persson B.L. (2009). Molecular mechanisms controlling phosphate-induced downregulation of the yeast Pho84 phosphate transporter. Biochemistry 48: 4497–4505 [DOI] [PubMed] [Google Scholar]
- Martín Y., Navarro F.J., Siverio J.M. (2008). Functional characterization of the Arabidopsis thaliana nitrate transporter CHL1 in the yeast Hansenula polymorpha. Plant Mol. Biol. 68: 215–224 [DOI] [PubMed] [Google Scholar]
- Mikosch M., Homann U. (2009). How do ER export motifs work on ion channel trafficking? Curr. Opin. Plant Biol. 12: 685–689 [DOI] [PubMed] [Google Scholar]
- Mikosch M., Hurst A.C., Hertel B., Homann U. (2006). Diacidic motif is required for efficient transport of the K+ channel KAT1 to the plasma membrane. Plant Physiol. 142: 923–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misson J., Thibaud M.C., Bechtold N., Raghothama K., Nussaume L. (2004). Transcriptional regulation and functional properties of Arabidopsis Pht1;4, a high affinity transporter contributing greatly to phosphate uptake in phosphate deprived plants. Plant Mol. Biol. 55: 727–741 [DOI] [PubMed] [Google Scholar]
- Misson J., et al. (2005). A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc. Natl. Acad. Sci. USA 102: 11934–11939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchhal U.S., Pardo J.M., Raghothama K.G. (1996). Phosphate transporters from the higher plant Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 93: 10519–10523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchhal U.S., Raghothama K.G. (1999). Transcriptional regulation of plant phosphate transporters. Proc. Natl. Acad. Sci. USA 96: 5868–5872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge S.R., Rae A.L., Diatloff E., Smith F.W. (2002). Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J. 31: 341–353 [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Kurose T., Hino T., Tanaka K., Kawamukai M., Niwa Y., Toyooka K., Matsuoka K., Jinbo T., Kimura T. (2007). Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104: 34–41 [DOI] [PubMed] [Google Scholar]
- Noël L.D., Cagna G., Stuttmann J., Wirthmüller L., Betsuyaku S., Witte C.P., Bhat R., Pochon N., Colby T., Parker J.E. (2007). Interaction between SGT1 and cytosolic/nuclear HSC70 chaperones regulates Arabidopsis immune responses. Plant Cell 19: 4061–4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse T.S., Stensballe A., Jensen O.N., Peck S.C. (2004). Phosphoproteomics of the Arabidopsis plasma membrane and a new phosphorylation site database. Plant Cell 16: 2394–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson B.L., Lagerstedt J.O., Pratt J.R., Pattison-Granberg J., Lundh K., Shokrollahzadeh S., Lundh F. (2003). Regulation of phosphate acquisition in Saccharomyces cerevisiae. Curr. Genet. 43: 225–244 [DOI] [PubMed] [Google Scholar]
- Prak S., Hem S., Boudet J., Viennois G., Sommerer N., Rossignol M., Maurel C., Santoni V. (2008). Multiple phosphorylations in the C-terminal tail of plant plasma membrane aquaporins: Role in subcellular trafficking of AtPIP2;1 in response to salt stress. Mol. Cell. Proteomics 7: 1019–1030 [DOI] [PubMed] [Google Scholar]
- Raghothama K.G. (1999). Phosphate acquisition. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Russell C., Stagg S.M. (2010). New insights into the structural mechanisms of the COPII coat. Traffic 11: 303–310 [DOI] [PubMed] [Google Scholar]
- Shin H., Shin H.S., Dewbre G.R., Harrison M.J. (2004). Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 39: 629–642 [DOI] [PubMed] [Google Scholar]
- Smith F.W., Ealing P.M., Dong B., Delhaize E. (1997). The cloning of two Arabidopsis genes belonging to a phosphate transporter family. Plant J. 11: 83–92 [DOI] [PubMed] [Google Scholar]
- Takano J., Miwa K., Yuan L.X., von Wirén N., Fujiwara T. (2005). Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc. Natl. Acad. Sci. USA 102: 12276–12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Shimada T., Ono E., Tanaka Y., Nagatani A., Higashi S.I., Watanabe M., Nishimura M., Hara-Nishimura I. (2003). Why green fluorescent fusion proteins have not been observed in the vacuoles of higher plants. Plant J. 35: 545–555 [DOI] [PubMed] [Google Scholar]
- Thibaud M.-C., Arrighi J.-F., Bayle V., Chiarenza S., Creff A., Bustos R., Paz-Ares J., Poirier Y., Nussaume L. (2010). Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis. Plant J. 64: 775–789 [DOI] [PubMed] [Google Scholar]
- Trinidad J.C., Specht C.G., Thalhammer A., Schoepfer R., Burlingame A.L. (2006). Comprehensive identification of phosphorylation sites in postsynaptic density preparations. Mol. Cell. Proteomics 5: 914–922 [DOI] [PubMed] [Google Scholar]
- Wiśniewski J.R., Zougman A., Nagaraj N., Mann M. (2009). Universal sample preparation method for proteome analysis. Nat. Methods 6: 359–362 [DOI] [PubMed] [Google Scholar]