Many plants must reach a certain age before they will flower in response to environmental cues. Perennial Arabis alpina plants are shown not to respond to vernalization until they are 5 weeks old. This effect is found to require the Aa TFL1 gene, which blocks induction of Aa LFY when young plants are exposed to cold.
Abstract
Flowering of many plants is induced by environmental signals, but these responses can depend on the age of the plant. Exposure of Arabidopsis thaliana to vernalization (winter temperatures) at germination induces flowering, whereas a close perennial relative Arabis alpina only responds if exposed when at least 5 weeks old. We show that vernalization of these older A. alpina plants reduces expression of the floral repressor PEP1 and activates the orthologs of the Arabidopsis flowering genes SOC1 (Aa SOC1) and LFY (Aa LFY). By contrast, when younger plants are vernalized, PEP1 and Aa SOC1 mRNA levels change as in older plants, but Aa LFY is not expressed. We demonstrate that A. alpina TFL1 (Aa TFL1) blocks flowering and prevents Aa LFY expression when young plants are exposed to vernalization. In addition, in older plants, Aa TFL1 increases the duration of vernalization required for Aa LFY expression and flowering. Aa TFL1 has similar functions in axillary shoots, thus ensuring that following a flowering episode vegetative branches are maintained to continue the perennial life cycle. We propose that Aa TFL1 blocks flowering of young plants exposed to vernalization by setting a threshold for a flowering pathway that is increased in activity as the shoot ages, thus contributing to several perennial traits.
INTRODUCTION
Flowering is controlled by interactions between regulatory networks that control shoot development and those that mediate responses to the environment. These processes are most thoroughly understood in Arabidopsis thaliana, where six pathways have been described to regulate flowering in response to environmental or developmental cues (Fornara et al., 2010). The photoperiod and vernalization pathways promote flowering in response to seasonal changes in daylength and temperature, respectively (Kobayashi and Weigel, 2007; Turck et al., 2008; Kim et al., 2009), while increases in ambient temperature also promote flowering (Samach and Wigge, 2005). Endogenous pathways include the response to gibberellic acid, the age-dependent pathway that promotes flowering most strongly in older plants, and the autonomous pathway that involves changes in chromatin and RNA metabolism (Farrona et al., 2008; Mutasa-Göttgens and Hedden, 2009; Wang et al., 2009a).
Flowering of many plant species is more sensitive to environmental cues as the plant ages (Hackett, 1985). This increasing sensitivity to the environment is particularly prevalent in perennials. As a typical short-lived annual species, Arabidopsis has a flowering response that is sensitive to environmental cues very early in development, making it a difficult model in which to study the effect of shoot maturation on environmental responses. For example, flowering of Arabidopsis is promoted by daylength (long days [LDs]) within 4 d of seed germination (Mozley and Thomas, 1995) and by vernalization directly from germination (Koornneef et al., 1991). Despite this early response to environmental signals, genes that promote flowering of Arabidopsis have been shown to increase in expression as the plant ages. Several mRNAs encoding members of the SQUAMOSA PROMOTER BINDING-LIKE (SPL) transcription factor family increase in abundance as the plant becomes older (Wu and Poethig, 2006). This increase is due to reduction in levels of miR156/7, which are negative regulators of the abundance of several SPL gene mRNAs. Targets for miR156/7 include the mRNAs of SPL3, SPL5, and SPL9; the SPL3, SPL5, and SPL9 transcription factors directly activate the expression of the flowering genes LEAFY (LFY), CAL, FUL, AGL24, and SOC1 (Wang et al., 2009a; Wu et al., 2009; Yamaguchi et al., 2009). These studies provide a direct link between aging of the plant and flowering, but it remains unclear how the SPL/miR156 system is related to age-dependent sensitivity to environmental signals because even very young Arabidopsis plants flower in response to daylength and vernalization.
Other species of the Brassicaceae, such as Brassica oleracea, grow vegetatively for an extended period of several weeks before they become responsive to exposure to vernalization (Friend, 1985; Lin et al., 2005). In B. oleracea, this delay in responsiveness to vernalization appears to be due to age-dependent regulation of the ortholog of the Arabidopsis gene FLOWERING LOCUS C (FLC). In Arabidopsis, this gene encodes a MADS box transcription factor that represses flowering, but on exposure to vernalization, its mRNA falls progressively, allowing flowering to proceed after vernalization (Michaels and Amasino, 1999; Sheldon et al., 1999). In B. oleracea, this repression of FLC expression in vernalization only occurs in older plants (Lin et al., 2005).
Recently, we used Arabis alpina as a model species to study flowering of perennials (Wang et al., 2009b). It shows classical perennial traits that include surviving for many years and alternating between phases of vegetative growth and flowering. The inbred A. alpina Pajares accession only flowered after exposure to vernalization. The shoot apex and some axillary shoots initiate flowering during vernalization, whereas other axillary shoots remained vegetative. These vegetative axillary shoots were induced to flower when the plant was exposed to vernalization a second time. Such a life cycle that extends over several years and comprises repeated cycles of flowering and vegetative growth is characteristic of perennials. The flowering episode is also restricted to spring or early summer and this seasonal flowering ensures that the plant flowers each year for only a limited period. PERPETUAL FLOWERING1 (PEP1) has a major function in conferring seasonal flowering on A. alpina (Wang et al., 2009b). This gene encodes the ortholog of FLC, and, as in Arabidopsis, blocks flowering of A. alpina until its expression is repressed during vernalization allowing flowering to proceed. However, in contrast with FLC mRNA in Arabidopsis, PEP1 mRNA expression rises after vernalization when PEP1 blocks flowering of any axillary meristems that did not initiate flowering during vernalization.
Here, we study the age-dependent responsiveness to vernalization in A. alpina. We show that these plants must be at least 5 weeks old when exposed to vernalization for flowering to occur, but that in contrast with B. oleraceae, PEP1 mRNA levels fall during vernalization in young and old plants. However, the A. alpina LFY gene is expressed in the shoot apical meristem during vernalization only if the plant is exposed to cold when it is at least 5 weeks old. This observation suggests that an additional repressor of flowering must act after reduction of PEP1 expression but before activation of Aa LFY to prevent flowering of young A. alpina plants during vernalization.
In Arabidopsis, TERMINAL FLOWER1 (TFL1) encodes a floral repressor and is expressed both in the shoot apical meristem and axillary meristems (Shannon and Meeks-Wagner, 1991; Alvarez et al., 1992; Bradley et al., 1997; Conti and Bradley, 2007). TFL1 is a member of the CETS (CEN, TFL1, FT) family, related to phosphatidylethanolamine binding proteins (Bradley et al., 1997; Pnueli et al., 2001). In Arabidopsis and tomato (Solanum lycopersicum), TFL1 has been proposed to repress flowering by antagonizing the activity of the floral promoter FLOWERING LOCUS T (FT) or its tomato ortholog SINGLE FLOWER TRUSS (SFT) (Kardailsky et al., 1999; Kobayashi et al., 1999; Shalit et al., 2009). The FT protein promotes flowering by interacting with the bZIP transcription factor FD, and TFL1 may also bind FD but enhance transcriptional repression rather than activation (Abe et al., 2005; Wigge et al., 2005; Ahn et al., 2006). However, other functions for TFL1 have also been proposed (Sohn et al., 2007). TFL1 mRNA is weakly detected in the lower region of the Arabidopsis shoot apical meristem during vegetative growth and is strongly increased in the shoot apical meristem after the transition to flowering (Bradley et al., 1997). However, TFL1 protein has a broader distribution and is detected throughout the meristem (Conti and Bradley, 2007). TFL1 represses the expression within the meristem of floral meristem identity genes such as LFY and APETALA1 (AP1), which are normally highly expressed only on the flanks of the meristem in developing primordia (Ratcliffe et al., 1999). The role of TFL1 in the vernalization response remains unclear.
Here, we use transgenic A. alpina plants to show that the TFL1 ortholog, Aa TFL1, is required to prevent flowering when young A. alpina plants are exposed to vernalization. In older plants, Aa TFL1 does not prevent the response to vernalization but increases the duration of vernalization required to induce flowering. The effect of vernalization is not age dependent in Arabidopsis, but we show that TFL1 weakly increases the duration of vernalization required for a full flowering response. In addition to repressing flowering of the A. alpina shoot apical meristem during vernalization, Aa TFL1 also contributes to the different flowering behaviors of axillary shoots, contributing to the perennial life cycle of A. alpina. We propose that Aa TFL1 confers an age-dependent vernalization response in A. alpina by setting a threshold for the activity of a flowering pathway whose activity increases with age.
RESULTS
Age-Dependent Flowering Response of A. alpina to Vernalization
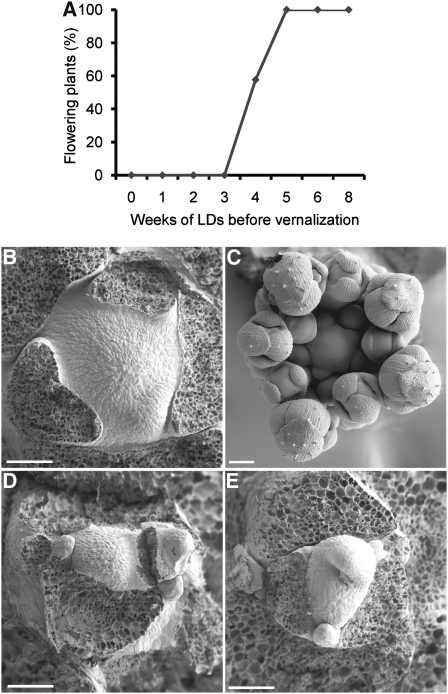
A. alpina accession Pajares plants of different ages were tested for their flowering response to vernalization. This accession does not flower unless it is exposed to vernalization (Wang et al., 2009b). The plants were grown for different lengths of time at 22°C under LDs, exposed to 16 weeks vernalization under short days (SDs), and then returned to 22°C under LDs for 5 weeks before being scored for flowering (Figure 1A; see Methods). This treatment induced flowering of all of the plants that were 5 or more weeks old prior to vernalization. However, plants that were 1, 2, or 3 weeks old before vernalization did not flower. Four-week-old plants showed an intermediate response. Therefore, A. alpina Pajares plants must be at least 5 weeks old to show a full flowering response to vernalization.
Figure 1.
Age-Dependent Effects of Environmental Cues on Flowering of A. alpina.
(A) Effect of age on flowering in response to vernalization in A. alpina Pajares accession. Plants were grown under LDs for different lengths of time, vernalized for 16 weeks, and grown subsequently under LDs.
(B) to (E) Scanning electron microscopy analysis of A. alpina Pajares apices before and after vernalization. Bars = 50 μm. Plants grown for the following lengths of time.
(B) Eight weeks in LDs. No floral primordia are present on the flanks of the shoot apical meristem.
(C) Eight weeks in LDs plus 12 weeks in vernalization. The shoot apical meristem is surrounded by spherical floral primordia.
(D) Two weeks in LDs. No floral primordia are present on the flanks of the shoot apical meristem.
(E) Two weeks in LDs plus 12 weeks in vernalization. No floral primordia are present on the flanks of the shoot apical meristem.
To test the morphology of the apical meristem of plants exposed to vernalization at different ages, apices were compared by scanning electron microscopy before and immediately after vernalization (Figures 1B to 1E). This experiment indicated that 8-week-old plants formed flower buds during cold treatment, whereas the apices of 2-week-old vernalized plants retained a vegetative morphology.
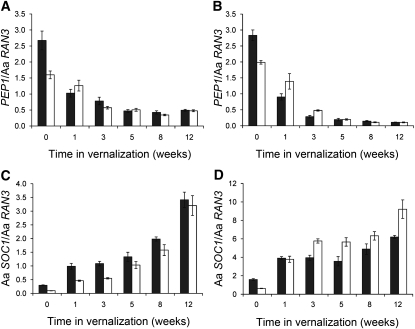
PEP1 and Aa SOC1 mRNA Levels Respond to Vernalization in 2- and 8-Week-Old Plants
PEP1 encodes a floral repressor that prevents flowering of A. alpina Pajares until transcription of the gene is repressed during vernalization (Wang et al., 2009b). Whether the inability of young plants to flower in response to vernalization is due to failure to repress PEP1 was tested. Quantitative RT-PCR was performed on RNA extracted from apices (Figure 2A) and leaves (Figure 2B) of plants of different ages before and during vernalization. PEP1 mRNA levels fell during vernalization at a similar rate in 2- and 8-week-old plants both in apices and leaves. Therefore, young plants detect vernalization and repress PEP1 expression but are still unable to undergo the transition to flowering.
Figure 2.
Effects of Vernalization on PEP1 and Aa SOC1 mRNA Levels in Plants of Different Ages.
(A) PEP1 mRNA levels analyzed by qRT-PCR in apices of old (grown for 8 weeks under LDs before vernalization; black columns) and young (2 weeks under LD before vernalization; white columns) A. alpina Pajares plants exposed to vernalization for different lengths of time.
(B) PEP1 mRNA levels in leaves, otherwise as in (A).
(C) Aa SOC1 mRNA levels analyzed by qRT-PCR in apices of old (age as in [A]; black columns) and young (age as in [A]; white columns) A. alpina Pajares plants exposed to vernalization for different lengths of time.
(D) Aa SOC1 mRNA levels in leaves, otherwise as in (C).
Error bars in indicate se.
In Arabidopsis, SOC1 promotes flowering at the shoot apical meristem and is repressed by FLC (Lee et al., 2000; Samach et al., 2000; Hepworth et al., 2002). The A. alpina SOC1 gene was isolated (see Methods) to test whether its expression is repressed by PEP1 and whether it is expressed in plants of different ages after vernalization. The genes flanking At SOC1 in Arabidopsis also flank the predicted A. alpina SOC1 gene (Aa SOC1), and Aa SOC1 is more similar to At SOC1 than to any other Arabidopsis protein (see Supplemental Figures 1A and 1B and Supplemental Data Set 1 online). This conservation of synteny demonstrates that Aa SOC1 and At SOC1 are orthologs. Also, overexpression of Aa SOC1 in Arabidopsis caused early flowering (see Supplemental Figure 1C online), and Aa SOC1 mRNA levels were much higher in 2-week-old pep1 mutants compared with wild-type plants (see Supplemental Figure 1D online), indicating that Aa SOC1 encodes a promoter of flowering whose expression is repressed by PEP1. In A. alpina, the levels of Aa SOC1 mRNA also increased during vernalization in the apices and leaves of both 2- and 8-week-old plants (Figures 2C and 2D). These results demonstrate that both 2- and 8-week-old plants detect and respond to low temperatures leading to downregulation of PEP1 mRNA and upregulation of Aa SOC1 mRNA, although flowering occurs only in the 8-week-old plants. Therefore, the block on flowering in younger plants must occur downstream of these genes or in a parallel pathway.
During Vernalization, Aa LFY Expression Occurs in the Shoot Apical Meristem of 8-Week-Old but Not 2-Week-Old A. alpina Pajares Plants
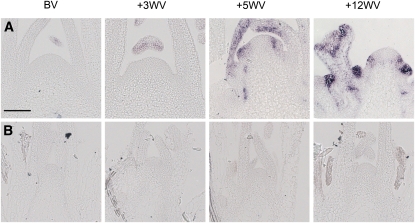
As described above, PEP1 and Aa SOC1 are expressed similarly in 2- and 8-week-old A. alpina Pajares plants during vernalization. In Arabidopsis, LEAFY mRNA acts as a marker for the floral transition (Weigel et al., 1992). Therefore, the A. alpina ortholog of LFY (Aa LFY) was isolated (see Supplemental Figure 2 and Supplemental Data Set 2 online) and its expression tested by in situ hybridization in the shoot apical meristems of 2- and 8-week-old A. alpina Pajares plants during vernalization to determine whether this gene is downstream of the block preventing flowering in the younger plants.
During vernalization of 8-week-old plants, Aa LFY expression was clearly detected in the shoot apical meristem around 5 weeks after transfer to the cold (Figure 3A). By contrast, in 2-week-old A. alpina Pajares plants exposed to vernalization, no expression of Aa LFY was detected throughout vernalization (Figure 3B). These results together with the experiments described above indicate that the block on flowering in response to vernalization in younger plants acts after Aa SOC1 induction but before Aa LFY expression.
Figure 3.
Spatial Pattern of Aa LFY mRNA Accumulation in Apices of A. alpina Pajares Plants of Different Ages during Vernalization.
(A) Aa LFY mRNA in apices of Pajares plants that were grown under LDs for 8 weeks before vernalization.
(B) Aa LFY mRNA in apices of Pajares plants that were grown under LDs for 2 weeks before vernalization.
BV, before vernalization; +3WV, +5WV, and +12WV, plants vernalized for 3, 5, or 12 weeks before harvesting. The same apices were used for each time point in (A) and Figure 7B to analyze the complementary patterns of Aa TFL1 and Aa LFY mRNAs. Bar = 100 μm.
Reduction of Aa TFL1 Expression Does Not Overcome the Obligate Vernalization Response of A. alpina Pajares
In Arabidopsis, TFL1 delays flowering (Shannon and Meeks-Wagner, 1991; Alvarez et al., 1992), but its effect on vernalization response is unclear. Furthermore, At TFL1 has been described to repress At LFY expression (Ratcliffe et al., 1999). To determine whether TFL1 contributes to the repression of flowering required for the obligate vernalization response of A. alpina Pajares, a TFL1 ortholog (Aa TFL1) was isolated (see Methods). Phylogenetic analysis demonstrated that its protein product is closely related to TFL1 of Arabidopsis and the two genes flanking TFL1 in Arabidopsis are present at similar positions in A. alpina (see Supplemental Figures 3A and 3B and Supplemental Data Set 3 online). The conservation of synteny of the regions containing Aa TFL1 and At TFL1 as well as the close sequence relationship between their protein products supports the idea that Aa TFL1 and At TFL1 are orthologs. Overexpression of Aa TFL1 in Arabidopsis delayed flowering, as observed in At TFL1 overexpressing plants (see Supplemental Figures 3C and 3D online) (Ratcliffe et al., 1998), suggesting that Aa TFL1 functions as a floral repressor.
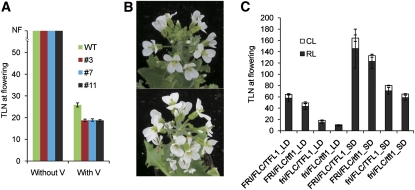
Transgenic A. alpina Pajares plants were then made in which Aa TFL1 expression was reduced using a 35S:Aa TFL1dsRNAi construct. In A. alpina Pajares plants, Aa TFL1 mRNA levels were reduced significantly in the transgenic plants (see Supplemental Figure 4A online), and this effect appears to be gene specific as the mRNA level of a closely related gene, Aa BFT, was unaffected (see Supplemental Figure 4B online). The reduction in Aa TFL1 mRNA did not overcome the obligate vernalization requirement of A. alpina Pajares. In the absence of vernalization, 35S:Aa TFL1dsRNAi A. alpina Pajares plants grew vegetatively indefinitely, producing over 80 leaves before the end of the experiment (Figure 4A). However, after a 16-week vernalization treatment, the 35S:Aa TFL1dsRNAi plants flowered slightly earlier than the A. alpina Pajares progenitor, forming ~18 leaves compared with ~25 leaves for the progenitor (Figure 4A). The inflorescences of 35S:Aa TFL1dsRNAi plants were determinate, ending in a terminal flower, in contrast with the indeterminate development of wild-type A. alpina Pajares (Figure 4B). These results indicated that Aa TFL1 does not contribute to the obligate vernalization requirement of A. alpina Pajares plants but that reducing Aa TFL1 expression causes slightly earlier flowering after vernalization.
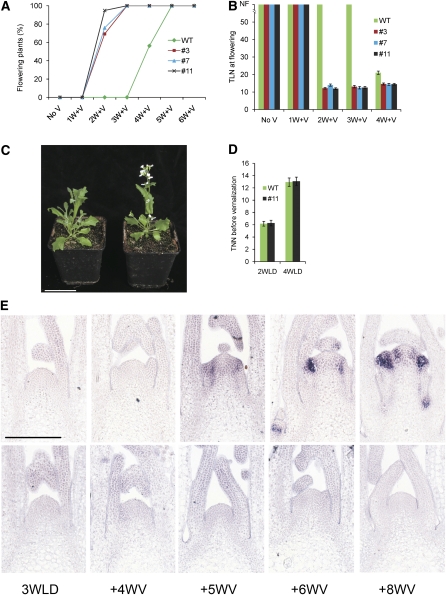
Figure 4.
Effect of Reducing of Aa TFL1 and TFL1 Activity on Vernalization Response of A. alpina and Arabidopsis.
(A) Flowering times of 5-week-old 35S:Aa TFL1 dsRNAi A. alpina Pajares plants exposed to vernalization. Y axis, total leaf number (TLN) at flowering on the main shoot. WT, A. alpina Pajares. #3, #7, and #11, three independent 35S:Aa TFL1 dsRNAi A. alpina Pajares transgenic lines. Plants were exposed to vernalization for 16 weeks. Error bars indicate sd.
(B) Inflorescences of A. alpina Pajares (top) and 35S:Aa TFL1 dsRNAi A. alpina Pajares (bottom) plants. The latter formed terminal flowers.
(C) Flowering times of Arabidopsis Col lines carrying active or inactive alleles at the FRI, FLC, and TFL1 loci. Plants were grown in LDs or SDs. RL, rosette leaves; CL, cauline leaves. Error bars indicate sd.
The effect of tfl1 on flowering of Arabidopsis plants that show a strong vernalization requirement is unknown. Active alleles of FRIGIDA (FRI) and FLC are required to confer a vernalization requirement (Michaels and Amasino, 1999; Sheldon et al., 1999; Johanson et al., 2000). Therefore, FRI FLC tfl1-18 Columbia (Col) plants were constructed and their flowering time scored under LDs and SDs. These plants flowered earlier than FRI FLC plants under both LDs and SDs but still flowered much later than Col plants under both conditions (Figure 4C). Therefore, At TFL1 contributes to the extreme late flowering conferred by FRI FLC, but in the absence of At TFL1 activity, FRI FLC plants were still very late flowering.
These results indicate that Aa TFL1 is not required for the absolute block on flowering before vernalization in A. alpina Pajares, while in Arabidopsis, TFL1 makes a small contribution to the delay in flowering of FLC FRI plants before vernalization.
Reduction in Aa TFL1 Expression Reduces the Age at Which A. alpina Pajares Plants Respond to Vernalization
As shown previously (Figure 1A), A. alpina Pajares plants must be grown for 4 to 5 weeks under LDs for them to flower in response to vernalization. To test whether Aa TFL1 contributes to preventing flowering of young plants after vernalization, A. alpina Pajares plants of different ages carrying 35S:Aa TFL1 dsRNAi were exposed to vernalization from germination or from when they were 1 to 6 weeks old (Figures 5A and 5B). Similar to wild-type plants, 1-week-old 35S:Aa TFL1 dsRNAi A. alpina Pajares plants did not flower when exposed to vernalization. By contrast, 70 to 90% of 2-week-old 35S:Aa TFL1 dsRNAi plants flowered when vernalized, and 100% of 3-week-old plants did so (Figures 5A to 5C). This experiment demonstrated that 35S:Aa TFL1 dsRNAi plants flowered when exposed to cold 2 to 3 weeks younger than progenitor A. alpina Pajares plants. The difference in age at which exposure to cold induced flowering could be due to 35S:Aa TFL1 dsRNAi plants developing at a faster rate; therefore, the number of nodes produced by wild-type and transgenic plants at the age when they started to respond to vernalization (4 and 2 weeks in LDs, respectively) was determined. Whereas wild-type plants did not respond to vernalization until they produced around 13 nodes, 35S:Aa TFL1 dsRNAi plants responded when they had generated only six nodes (Figure 5D). Therefore, Aa TFL1 prevents flowering when young plants are exposed to vernalization, and this conclusion holds whether age is measured in time or number of nodes produced.
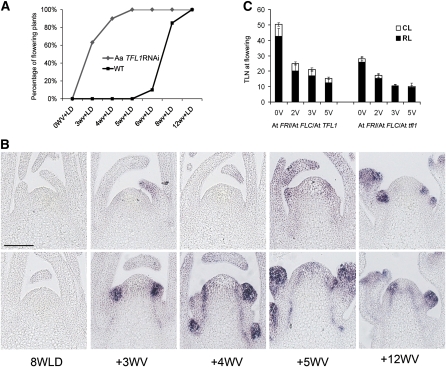
Figure 5.
Reduction of Aa TFL1 mRNA Level Allows Young A. alpina Plants to Flower in Response to Environmental Stimuli.
(A) Flowering behaviors of 35S:Aa TFL1 dsRNAi plants grown under LDs from germination for different lengths of time before 16 weeks of vernalization. Y axis, proportion of plants flowering 5 weeks after the end of the vernalization treatment. NoV, no vernalization; 1W+V, plants grown for 1 week in LDs and then exposed to vernalization. In other treatments, plants were grown for the indicated number of weeks under LDs prior to vernalization treatment. WT, A. alpina Pajares. #3, #7, #11 are three independent lines of 35S:Aa TFL1 dsRNAi A. alpina Pajares plants.
(B) Flowering times of plants treated as in (A). Y axis, total leaf number (TLN) on main shoot at flowering. X axis and genotypes as in (A). For 2W+V transgenic plants and 4W+V wild-type plants, only those that responded to vernalization are included. Error bars indicate sd.
(C) A. alpina Pajares (left) and 35S:Aa TFL1 dsRNAi Pajares (right) plants. Plants were grown under LDs for 2 weeks prior to exposure to vernalization for 16 weeks and then grown under LDs for 3 weeks. Bar = 5 cm.
(D) Number of nodes formed before vernalization by A. alpina Pajares and Aa TFL1 dsRNAi A. alpina Pajares (#11). Total node number (TNN) was counted after 2 weeks in LD (2WLD) or 4 weeks in LD (4WLD). TNN included visible leaves and leaf buds that could be detected by microdissection. Error bars indicate sd.
(E) Spatial patterns of Aa LFY mRNA accumulation in 35S:Aa TFL1 dsRNAi A. alpina Pajares (top row) and A. alpina Pajares (bottom row) plants before and during vernalization. Plants were grown for 3 weeks in LD (3WLD) prior to vernalization and then exposed to vernalization for 4, 5, 6, or 8 weeks. Bar = 100 μm.
To test whether reduction of Aa TFL1 activity allows expression of Aa LFY in the meristem of young plants during vernalization, Aa LFY mRNA expression was tested after 3-week-old 35S:Aa TFL1 dsRNAi A. alpina Pajares plants were exposed to vernalization for different periods of time (Figure 5E). Aa LFY mRNA was clearly detected in the shoot apical meristem and axillary meristems of 3-week-old 35S:Aa TFL1 dsRNAi plants exposed to vernalization for 5 weeks, and floral meristems were visible after 8 weeks of vernalization (Figure 5E, top row). This result contrasts with 3-week-old A. alpina Pajares plants exposed to vernalization, where no Aa LFY mRNA was detected even at the end of the treatment (Figure 5E, bottom row).
Taken together, these data indicate that Aa TFL1 suppresses the flowering response to vernalization in young A. alpina Pajares plants, preventing Aa LFY expression and the transition to flowering.
Aa TFL1 Extends the Duration of Vernalization Required for Floral Induction of Older Plants
Whether Aa TFL1 has an effect on the vernalization response of 5-week-old plants was also tested. Exposure to vernalization induces flowering of both A. alpina Pajares and 35S:Aa TFL1 dsRNAi A. alpina Pajares plants that are at least 5 weeks old, but after vernalization, 35S:Aa TFL1 dsRNAi A. alpina Pajares plants flowered earlier than wild-type plants (Figures 1A and 4A). Earlier flowering of the 35S:Aa TFL1 dsRNAi A. alpina Pajares plants may be due to these plants being induced to flower earlier during the vernalization period than wild-type plants. Therefore, we tested whether shorter vernalization times are sufficient to induce flowering of 8-week-old 35S:Aa TFL1 dsRNAi A. alpina Pajares plants. Whereas no A. alpina Pajares plants flowered after 5 weeks vernalization and at least 9 to 10 weeks vernalization was required for 100% of the treated plants to flower, 60% of 35S:Aa TFL1 dsRNAi A. alpina Pajares plants flowered after 3 weeks of vernalization, and all flowered after 5 weeks of vernalization (Figure 6A). To characterize this effect at the molecular level, the expression of Aa LFY was tested by in situ hybridization in 8-week-old plants exposed to vernalization for different lengths of time (Figure 6B). In A. alpina Pajares plants, Aa LFY mRNA was not detected in the meristem until plants were exposed to vernalization for 5 weeks (Figure 6B, top row). By contrast, in 35S:Aa TFL1 dsRNAi A. alpina Pajares plants, Aa LFY mRNA was strongly detected in floral primordia on the flanks of the apical meristem when plants were exposed to vernalization for only 3 weeks (Figure 6B, bottom row). Therefore, in older 35S:Aa TFL1 dsRNAi A. alpina Pajares plants, Aa LFY mRNA is expressed earlier during vernalization than in younger plants of the same genotype (Figures 5E and 6B). This result suggests that in A. alpina Pajares, Aa TFL1 acts both in young plants to prevent flowering and in older plants to extend the duration of vernalization required to induce flowering by delaying the induction of Aa LFY expression.
Figure 6.
Aa TFL1 Extends the Duration of Vernalization Required to Induce Flowering of 8-Week-Old Plants.
(A) The duration of vernalization required to induce flowering of 8-week-old A. alpina Pajares and 35S:Aa TFL1 dsRNAi A. alpina Pajares. Plants were grown in LD for 8 weeks and then exposed to vernalization for the indicated number of weeks. After vernalization plants were returned to LDs and the proportion of flowering plants measured 5 to 6 weeks later.
(B) Spatial pattern of Aa LFY expression in apices of 8-week old A. alpina Pajares (top) and 35S:Aa TFL1 dsRNAi A. alpina Pajares (bottom) plants exposed to vernalization for different lengths of time. Plants were grown under LDs for 8 weeks (8WLD) and then exposed to vernalization for the indicated number of weeks. Bar = 100 μm.
(C) Flowering time of two Arabidopsis Col lines carrying active alleles at FRI and FLC and active or inactive alleles at TFL1. The two genotypes were grown in the same conditions and compared for flowering time. 0V indicates that the plants were grown directly in LDs at 22°C. 2, 3, and 5V indicate that the seeds were vernalized for 2, 3, or 5 weeks respectively before being shifted to LDs at 22°C. TLN, total leaf number; RL, rosette leaves; CL, cauline leaves. Error bars indicate sd.
To compare this function of Aa TFL1 with TFL1 function during vernalization of Arabidopsis, germinating Arabidopsis seeds of the genotypes FRI FLC TFL1 and FRI FLC tfl1-18 were exposed to vernalization for 2, 3, and 5 weeks and their flowering times measured. Two weeks of vernalization caused a strong acceleration of flowering of both genotypes, so that the number of leaves produced compared with the nonvernalized control was reduced by ~50% (Figure 6C). After 3 weeks of vernalization, the response of FRI FLC tfl1-18 plants was saturated, whereas wild-type plants required at least 5 weeks of vernalization to saturate the response. These experiments indicate that At TFL1 slightly increases the length of cold exposure required for the full flowering response of Arabidopsis to vernalization but has a much weaker effect than observed in A. alpina.
Aa TFL1 Expression Is Remodeled during Vernalization of 8-Week-Old but Not 2-Week-Old A. alpina Pajares Plants
The expression of Aa TFL1 mRNA was then tested by in situ hybridization in the shoot apices of 2- and 8-week-old A. alpina Pajares plants (Figures 7A and 7B, respectively) before and during vernalization. In plants of both ages, Aa TFL1 mRNA showed a similar pattern of expression before vernalization and at early time points in the cold, being present in the shoot apical meristem and in a broader region below the meristem extending down into the upper shoot. This pattern of expression is more extensive and stronger than that described in vegetative apices of Arabidopsis (Bradley et al., 1997; Ratcliffe et al., 1999; Conti and Bradley, 2007). Aa TFL1 mRNA was present in the inner layers of the meristem but was absent from the tip of the meristem, including the epidermis. During vernalization of 8-week-old plants, the expression of Aa TFL1 mRNA in the upper shoot was gradually reduced and at later time points became concentrated in the inflorescence meristem as well as axillary meristems (Figure 7B). After 5 weeks in vernalization, the meristem was domed, resembling an inflorescence meristem, and Aa TFL1 mRNA was still detected in the meristem but was weaker and patchier in the upper stem. The presence of Aa LFY mRNA at this stage in outer layers of the meristem in a complementary pattern to Aa TFL1 mRNA (Figure 3B) is consistent with the conclusion that floral induction has occurred. After 12 weeks in vernalization, Aa TFL1 mRNA was strongly detected in the inflorescence meristem (Figure 7B), which had formed on its flanks floral primordia containing Aa LFY mRNA (Figure 3A), and the pattern of Aa TFL1 mRNA resembled that of At TFL1 in Arabidopsis inflorescences.
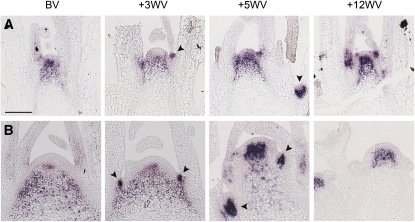
Figure 7.
Spatial Pattern of Aa TFL1 mRNA Accumulation in Apices of A. alpina Pajares Plants of Different Ages during Vernalization.
(A) Aa TFL1 mRNA in apices of Pajares plants that were grown under LDs for 2 weeks before vernalization treatment.
(B) Aa TFL1 mRNA in apices of Pajares plants that were grown under LDs for 8 weeks before vernalization.
BV, before vernalization. +3WV, +5WV and +12WV indicates plants vernalized for 3, 5, or 12 weeks before harvesting. The same apices were used for each time point in (B) and Figure 3A to analyze the complementary patterns of Aa TFL1 and Aa LFY mRNAs. Arrowheads indicate expression in the axillary meristems. Bar = 100 μm.
In contrast with its behavior in 8-week-old plants, the pattern of Aa TFL1 mRNA in the shoot apical meristems of 2-week-old plants remained constant throughout vernalization (Figure 7A). The dynamic expression of Aa TFL1 only in the meristems of older plants during vernalization suggested that Aa TFL1 is regulated by mechanisms that alter with age and that the restriction of its expression may be important in floral induction.
Aa TFL1 and PEP1 Act Additively to Repress Flowering of Axillary Shoots
Axillary shoots of A. alpina show different flowering behaviors (Wang et al., 2009b). After vernalization, an axillary meristem at a particular node shows one of three possible fates: it can form a flowering shoot, form a vegetative shoot, or not develop into a shoot. The different behaviors of these shoots contribute to the perennial life history of A. alpina by ensuring that during a flowering episode some axillary shoots remain vegetative and are available to flower the following year. This ability of a plant to flower multiple times is called polycarpy. At TFL1 and Aa TFL1 are expressed in axillary meristems (Conti and Bradley, 2007) (Figure 7); therefore, whether Aa TFL1 contributes to polycarpic development of A. alpina was tested. Six-week-old plants were vernalized and the flowering behavior of all axillary shoots was scored after vernalization (Figures 8A and 8B). As shown previously, after this treatment, those axillary branches of A. alpina Pajares that are formed by the shoot apical meristem after floral induction (node [N] 17-27) and the oldest axillary shoots at the base of the main shoot axis (N1-2) form flowers (Figure 8A). All of the other axillary shoots that grow out (at N3-16) remain vegetative. A very different pattern was observed for 35S:Aa TFL1 dsRNAi Pajares plants (Figure 8B; see Supplemental Figure 5 online). In these plants, almost all axillary shoots had formed flowers by 5 weeks after vernalization. Only a small proportion (15 to 20%) of axillary shoots at nodes 3 and 4 remained vegetative. At some positions (N5-16), all of the nodes that were scored formed vegetative shoots in Pajares but flowering shoots or even solitary flowers in the transgenic line (Figure 8B; see Supplemental Figure 5 online). Furthermore, even at nodes that produced flowering shoots in Pajares, a higher proportion of flowering shoots was observed in 35S:Aa TFL1 dsRNAi Pajares plants. These data indicate that Aa TFL1 plays an important role in polycarpic development of A. alpina by preventing the formation of flowering shoots at many axillary nodes after vernalization and greatly reducing the proportion of shoots that form flowers at other nodes.
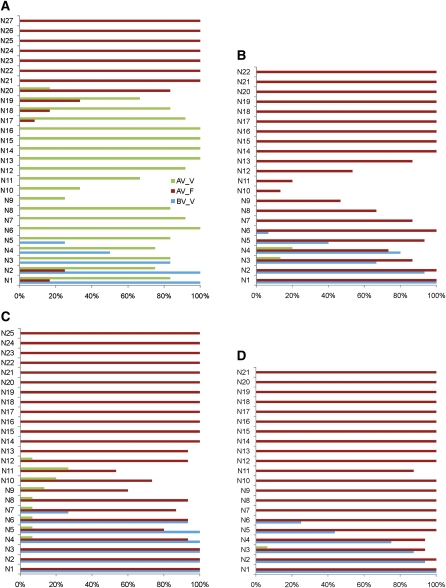
Figure 8.
Behavior of Axillary Shoots in Aa TFL1 dsRNAi A. alpina Pajares and Related Genotypes before and after Vernalization.
(A) Flowering behavior of axillary shoots of A. alpina Pajares before and after vernalization. Y axis, node at which axillary shoot was formed, numbered from base to apex of plant. X axis, proportion of axillary shoots at a particular node exhibiting indicated phenotypes. BV-V, nodes harboring a vegetative axillary shoot before vernalization; AV-F, nodes harboring a flowering axillary shoot after vernalization; AV_V, nodes harboring a vegetative axillary shoot after vernalization. Plants were 6 weeks old prior to vernalization.
(B) Flowering behavior of axillary shoots of 35S:Aa TFL1 dsRNAi A. alpina Pajares plants before and after vernalization. Axes and colors are as in (A).
(C) Flowering behavior of axillary shoots of pep1 mutant plants before and after vernalization. Axes and colors are as in (A).
(D) Flowering behavior of axillary shoots of pep1 mutants carrying 35S:Aa TFL1 dsRNAi before and after vernalization. Axes and colors are as in panel (A).
PEP1 also contributes to polycarpy of A. alpina Pajares by repressing flowering of many axillary shoots after vernalization (Wang et al., 2009b) (Figure 8C). To test whether PEP1 and Aa TFL1 act additively to confer polycarpic development, the pep1 mutant was crossed to 35S:Aa TFL1 dsRNAi Pajares plants. Plants homozygous for both the mutation and the transgene were identified. After vernalization, the flowering behavior of axillary shoots on these plants was scored (Figure 8D). Almost all axillary shoots of pep1 35S:Aa TFL1 dsRNAi plants flowered, only three nodes (N3,4, and 11) produced fewer than 100% flowering shoots and all of these formed over 85%. This genotype formed markedly fewer vegetative shoots than either the pep1 or 35S:Aa TFL1 dsRNAi Pajares progenitors and many fewer than Pajares (Figures 8A to 8C), suggesting that Aa TFL1 and PEP1 act additively to prevent flowering of axillary shoots after vernalization.
An additional feature of A. alpina Parajes development is the arrest of axillary shoot outgrowth at some nodes after vernalization (Wang et al., 2009b). This feature is clearly visible in Pajares and 35S:Aa TFL1 dsRNAi plants where the region between approximately N9 and N12 forms relatively few axillary shoots (Figures 8A and 8B). For example, at N10, fewer than 30% of the nodes that were scored produced axillary shoots in Pajares or 35S:Aa TFL1 dsRNAi. This feature is greatly reduced in pep1 mutants and almost absent in pep1 35S:Aa TFL1 dsRNAi plants. In the latter genotype, only at N4 and N11 do fewer than 100% of nodes form axillary shoots. These data suggest that PEP1 prevents axillary shoot outgrowth at some nodes of A. alpina Pajares and 35S:Aa TFL1 dsRNAi plants.
Taken together, these data demonstrate that the different flowering behaviors of axillary shoots of A. alpina Pajares after vernalization are controlled additively by Aa TFL1 and PEP1.
Patterns of Expression of Aa TFL1 in Axillary Meristems
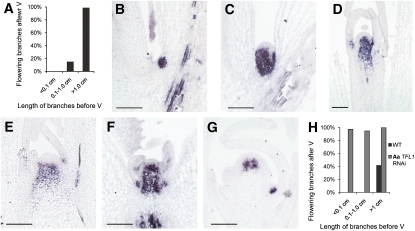
Axillary shoots of A. alpina plants differ in their flowering behavior after vernalization (Figure 8), and this is related to the position on the primary shoot at which the axillary shoot was produced. However, the size of an axillary shoot is also related to its position, suggesting that this may be a factor determining the flowering behavior of an axillary shoot after vernalization. Therefore, the length of individual axillary shoots of 8-week-old A. alpina Pajares plants grown under LDs was measured, the plants were vernalized, and the flowering behavior of each individual axillary shoot was followed after vernalization (see Supplemental Figure 6 online; Figure 9A). Almost all axillary shoots longer than 1 cm before vernalization flowered in response to vernalization, while none of the shoots shorter than 0.1 cm flowered, irrespective of the node at which they were formed (see Supplemental Figure 6B online). Shorter axillary shoots produced from lower nodes, such as N3 and N4 in Supplemental Figure 6B online, which are chronologically older, did not respond to vernalization, while longer shoots from later nodes such as N9 and N10 did flower. This result indicates that the size rather than the position of an axillary shoot correlates with its response to vernalization. In an independent experiment, the relationship between the size of axillary shoots produced before vernalization and their flowering behavior after vernalization was compared in 35S:Aa TFL1 dsRNAi plants and wild-type Pajares plants (Figure 9H). In the transgenic plants, shoots that were shorter than 0.1 cm before vernalization flowered after vernalization (Figure 9H). This result was in contrast with what was observed in wild-type plants and indicates that Aa TFL1 represses flowering in response to vernalization in small axillary shoots.
Figure 9.
Spatial Pattern of Aa TFL1 mRNA Accumulation in A. alpina Pajares Axillary Meristems of Different Sizes before and after Vernalization.
(A) Relationship between flowering of axillary shoots of A. alpina Pajares in response to vernalization and length of the shoot prior to vernalization. Y axis, proportion of flowering axillary shoots 9 weeks after vernalization. Eight-week old plants were vernalized for 12 weeks.
(B) to (G) Spatial pattern of Aa TFL1 mRNA accumulation.
(B) Leaf axil prior to vernalization and before any outgrowth of the axillary shoot is visible.
(C) Prior to vernalization in a young axillary shoot that has not yet formed visible leaf primordia.
(D) An axillary shoot prior to vernalization and after the production of several visible leaf primordia. Shoot was <1 cm in length.
(E) An axillary shoot prior to vernalization. Shoot was longer than 2 cm.
(F) A young axillary side shoot after vernalization. The axillary shoot has formed a few leaves. The plant was harvested after a 12-week vernalization treatment and was not returned to normal growth temperatures.
(G) Apex of an axillary shoot that has undergone the transition to flowering. The shoot was longer than 2 cm, as in (E), before vernalization. Plants were harvested after vernalization, as in (F).
(H) Relationship between flowering of axillary shoots of A. alpina Pajares in respone to vernalization and length of the shoot prior to vernalization in 35S:Aa TFL1 dsRNAi A. alpina Pajares and A. alpina Pajares (WT). Y axis, proportion of flowering axillary shoots 6 weeks after vernalization. Six-week-old plants were vernalized for 16 weeks.
Bars = 100 μm.
Aa TFL1 expression in axillary shoot meristems of different ages was then tested before and after vernalization. Prior to vernalization, strong expression of Aa TFL1 mRNA was detected in leaf axils at the position of the developing axillary meristem (Figure 9B). As young axillary shoots emerged from the leaf axil, before leaf primordia were visible on the growing shoot, strong Aa TFL1 expression was detected throughout the shoot, except for a few cell layers below the epidermis (Figure 9C). This strong Aa TFL1 expression was maintained as the young axillary shoot emerged (Figure 9D). Later in axillary shoot development when several leaves were already present, Aa TFL1 mRNA levels decreased and its expression was restricted to the inner region of the apex resembling that observed in the shoot apical meristem before vernalization (Figure 9E). At this stage, Aa TFL1 mRNA was detected in the axillary meristem and the upper shoot but was absent from the tip of the meristem.
After vernalization, Aa TFL1 mRNA remained strongly and widely expressed at the meristem of vegetative axillary shoots derived from axillary meristems or small side shoots similar to those shown in Figures 9B and 9C (Figure 9F). That Aa TFL1 plays an important role in suppressing flowering of these young axillary shoots is supported by the observation that such shoots flower after vernalization on 35S:Aa TFL1 dsRNAi A. alpina Pajares plants but not on A. alpina Pajares plants (Figure 9H). In axillary shoots that have undergone the transition to flowering, Aa TFL1 expression was remodeled and detected only in the center of the meristem in a pattern similar to that observed in the shoot apical meristem of older plants after vernalization (Figure 9G).
These experiments indicate that Aa TFL1 is expressed strongly in axillary meristems from an early stage in their development and is remodeled only in meristems of long, older axillary shoots exposed to vernalization. This behavior is similar to that observed in older shoot apical meristems, suggesting that Aa TFL1 plays a similar role in preventing flowering of young axillary meristems as it does in the juvenile shoot apical meristem.
DISCUSSION
We showed that in perennial A. alpina Pajares, Aa TFL1 prevents flowering of young plants exposed to vernalization and in older plants markedly increases the duration of vernalization required for flowering. These effects are not observed in Arabidopsis; thus, this gene contributes to species-specific differences in age-dependent sensitivity to vernalization. In young A. alpina plants, vernalization initiates the floral program by reducing PEP1 expression and inducing Aa SOC1, but Aa TFL1 prevents induction of Aa LFY expression and flowering. Aa TFL1 plays a similar repressive role in small axillary shoots. This role of Aa TFL1 is additive with another flowering repressor PEP1, so that when the activity of both genes is reduced almost all axillary shoots flower. These results suggest that Aa TFL1 contributes to the perennial life cycle of A. alpina Pajares by preventing flowering of young plants even when they are exposed to vernalization and by maintaining axillary shoots of flowering plants in the vegetative state so that they are available to flower the following year.
Regulation of Aa TFL1 Expression in A. alpina and Its Role in Flowering
In older plants, the spatial pattern of Aa TFL1 mRNA expression was progressively reduced during vernalization until it was focused only in the center of the inflorescence meristem. This reduction of the spatial pattern of Aa TFL1 expression in older plants might accelerate the floral transition, whereas the strong expression in the center of the inflorescence meristem may be required for indeterminacy. These two roles are conferred by different Aa TFL1 homologs in pea (Pisum sativum; Foucher et al., 2003). In contrast with its dynamic behavior in older plants, the expression pattern of Aa TFL1 mRNA remained constant throughout vernalization in young plants, consistent with these plants remaining in the vegetative phase.
The remodeling of Aa TFL1 expression in older plants during vernalization may be a consequence of increased expression of floral meristem identity genes. In Arabidopsis, TFL1 represses the expression of the floral meristem identity genes LFY and AP1 in the center of the meristem, but at the flanks of the meristem where floral meristems develop, At TFL1 is repressed by AP1 and LFY (Ratcliffe et al., 1999). In support of this idea, in 35S:LFY plants, At TFL1 mRNA was never detected, suggesting that LFY strongly represses At TFL1 transcription (Ratcliffe et al., 1999). Increased expression of floral meristem identity genes in tfl1 mutants may explain how these plants become committed to flower more rapidly than wild-type plants after transient exposures to LDs (Bradley et al., 1997).
We observed that when older A. alpina plants were vernalized for 5 weeks and remodeling of Aa TFL1 expression was first observed, Aa LFY mRNA was also detected and in a largely complementary pattern to that of Aa TFL1. This observation suggests that proteins that confer floral meristem identity such as Aa LFY may play a role during vernalization of older plants in restricting the broader pattern of expression of Aa TFL1 observed during vegetative development. As Aa TFL1 expression is not restricted in young plants, Aa LFY might be activated in older plants by a pathway that is not active or is much less active in young plants. Such a pathway may include SPL transcription factors, which accumulate to a higher level in older plants of several species and in Arabidopsis activate LFY transcription (Wang et al., 2009a; Wu et al., 2009; Yamaguchi et al., 2009). Also, in A. alpina, FT might be involved in activating flowering in older plants and its effect could be blocked by Aa TFL1 in younger plants, as FT/TFL proteins have been proposed to have antagonistic functions in different developmental contexts (Kardailsky et al., 1999; Kobayashi et al., 1999; Shalit et al., 2009). The importance of Aa TFL1 in blocking flowering of young A. alpina plants is evident from young 35S:Aa TFL1 dsRNAi plants, which flower in vernalization and express Aa LFY, presumably by causing premature activation of the adult specific pathway or bypassing its requirement.
Aa TFL1 also plays a role regulating the duration of vernalization treatment required to induce flowering in older plants. Aa LFY mRNA is detected much earlier during vernalization in 35S:Aa TFL1 dsRNAi than in wild-type plants. However, at this stage, Aa LFY mRNA did not appear ectopically in the central region of the meristem, as has been described for tfl1 mutants of Arabidopsis (Liljegren et al., 1999). However, ectopic expression of Aa LFY probably occurs later, after vernalization, as it was only at this stage that 35S:Aa TFL1 dsRNAi plants formed a terminal flower.
In summary, we propose that in older A. alpina plants, as PEP1 expression is repressed at the shoot apical meristem during vernalization, floral meristem identity genes such as Aa LFY are activated. The mechanism by which this activation occurs has not yet been defined but probably involves Aa SOC1, and by analogy with other systems perhaps SPL transcription factors (Wang et al., 2009a; Yamaguchi et al., 2009). The proteins encoded by the floral meristem identity genes in turn repress Aa TFL1 and flowering proceeds. After the transition to flowering occurs, Aa TFL1 is strongly expressed in the inflorescence meristem, where it represses floral meristem gene expression in the central region. An inflorescence is then formed and continues to form flowers indefinitely. Similar processes occur in older axillary shoots. In young plants, PEP1 expression is also repressed during vernalization, but in contrast with older plants, the pathway required for floral meristem identity gene expression is not active. This failure to activate floral meristem identity genes involves Aa TFL1 because in young 35S:Aa TFL1 dsRNAi plants, floral meristem identity genes are expressed early during vernalization. One possibility is that Aa TFL1 antagonizes the age-specific pathway setting a threshold of activity that it must reach to cause expression of floral meristem identity genes. Such a function would explain how the antagonistic role of Aa TFL1 is eventually overcome as the plant ages. In young wild-type plants, Aa TFL1 remains highly expressed throughout vernalization and blocks flowering as the age-dependent pathway never reaches the threshold of activity required to activate floral meristem identity gene expression.
The Roles of Aa TFL1 in Reproductive Competence
A. alpina does not flower in response to cold exposure until it is 4 to 5 weeks old. Several terms have been used to define such a phase of insensitivity to environmental stimuli. These include a maturation phase or a juvenile phase (Hackett, 1985). This period has also been defined in developmental terms as the meristem becoming competent to respond to floral signals. To avoid confusion with the juvenile phase of vegetative development, we term this period of A. alpina growth as maturation or acquisition of competence.
Extensive physiological studies were performed on various species to study the age-dependent responsiveness to environmental stimuli in the induction of flowering. These experiments indicated that this maturation process is controlled by a mechanism intrinsic to the shoot apex (Robinson and Wareing, 1969; Hackett, 1985).
The role of TFL1-like genes in the meristems of several species and their roles in floral repression suggest that they could have roles in maturation. We demonstrated the importance of Aa TFL1 in determining the length of the maturation phase of A. alpina, as measured by sensitivity to vernalization.
Outside the Brassicaceae, the significance of TFL1 homologs in regulating flowering time varies between species. The role of TFL1 in reproductive competence has been studied in perennial poplar (Populus spp) and apple (Malus domestica). Decreased expression of TFL1 homologs accelerated first flowering by several years and was proposed to play a role in conferring juvenility (Kotoda et al., 2006; Mohamed et al., 2010). However, the roles of apple and poplar TFL1 homologs were not tested in response to specific environmental cues. Our work extends these data by explicitly demonstrating the contribution of Aa TFL1 homologs to determining the age at which plants become sensitive to an environmental stimulus. Also in perennial ryegrass (Lolium perenne), Lp TFL1 expression was induced after vernalization and was proposed, based on its expression pattern in heterologous Arabidopsis plants, to have a role in axillary meristem identity (Jensen et al., 2001). In Antirrhinum majus, CEN1 has a similar role to At TFL1 in conferring indeterminacy on the inflorescence meristem but has no effect on flowering time (Bradley et al., 1996; Ratcliffe et al., 1998). In pea, these functions are divided between two TFL1 homologs, so that one, DETERMINATE, controls determinacy of the shoot apical meristem and the other, LATE FLOWERING, delays flowering (Foucher et al., 2003). In tomato, the TFL1 homolog SELF-PRUNING increases the number of nodes formed between inflorescences, effectively delaying inflorescence production, and antagonizes the effect of the FT homolog SFT in several tissues (Pnueli et al., 1998; Shalit et al., 2009).
The Function of Aa TFL1 in the Perennial Life Cycle
The perennial life cycle of A. alpina has been described in detail (Wang et al., 2009b). This species lives for many years and flowers repeatedly. The first flowering episode follows vernalization and involves the shoot apical meristem and some axillary meristems; subsequent episodes are induced by later exposures to vernalization and involve a further subset of axillary meristems. Thus, at the end of a flowering episode, some vegetative axillary shoots always survive seed set, remain vegetative, and will flower the following year. We propose that in wild-type plants, Aa TFL1 blocks flowering of the axillary meristems of young shoots during vernalization when PEP1 mRNA levels are reduced and that after vernalization, PEP1 expression rises ensuring that these shoots do not flower as they become older. Reduction in Aa TFL1 expression in the 35S:Aa TFL1 dsRNAi line causes young shoots to flower during vernalization so that rising PEP1 expression after vernalization can no longer block flowering. Such an interaction would explain how Aa TFL1 and PEP1 act additively to enhance polycarpy.
Aa TFL1 therefore contributes in two major ways to the perennial life cycle. In the first year after germination, it blocks flowering at the shoot apical meristem if the plant is exposed to vernalization while young. Thus, older plants that flower in response to vernalization will have formed more nodes producing axillary shoots, some of which will be available to flower the following year. Similarly, Aa TFL1 blocks flowering of young axillary shoots, ensuring that these will not undergo flowering and will be available to flower the following year. Perennial and annual plants appear in most angiosperm families, indicating that this distinction has arisen independently many times. How conserved the contributions of TFL1 homologs are to the life cycles of different perennial species will require detailed functional analyses in phylogenetically diverse species.
METHODS
Oligonucleotide Primers
Sequences of the oligonucleotide primers used are shown in Supplemental Table 1 online.
Plant Material and Growth Conditions
All vernalization treatments were performed at 4°C as described previously (Wang et al., 2009b).
The Arabis alpina Pajares accession and pep1 mutant were described previously (Wang et al., 2009b). The tfl1-18 (fri/FLC/tfl1) mutant was a kind gift from Desmond Bradley (John Innes Centre, Norwich, UK). Arabidopsis thaliana Col accession with functional FRI introgressed from sf-2 accession (FRIsf-2-Col; FRI/FLC/TFL1) was kindly provided by Richard M Amasino (University of Wisconsin, Madison, WI). The tfl1-18 mutant was crossed to FRIsf-2-Col, and homozygous FRI/FLC/tfl1 plants were created through selfing. The pep1 mutant described previously (Wang et al., 2009b) was crossed to 35S:Aa TFL1 dsRNAi A. alpina Pajares plants, and homozygous pep1 35S:Aa TFL1 dsRNAi plants were created through selfing.
Flowering time was measured by recording the date at the appearance of the first visible floral bud. At this time, the total primary leaf number on the stem was counted to provide the leaf number at flowering. To count the total leaves produced, including in the inflorescence, the leaf number was counted again after the inflorescence had elongated. All flowering time experiments were performed with at least 15 plants.
BAC Analysis and Sequencing
The BAC library made from A. alpina Pajares was described previously (Wang et al., 2009b). Positive BAC clones were identified by screening the library using gene specific probes from At SOC1 (generated with primers RH94 and RH95 in Supplemental Table 1 online), At TFL1 (generated with primers RH78 and RH79 in Supplemental Table 1 online), or At LFY (generated with primers W125 and W126 in Supplemental Table 1 online). BAC DNA from the positive colonies was sequenced using the Sanger method and assembled with SeqMan in the DNAStar software package (http://www.dnastar.com) and annotated manually according to the BLAST results. BAC sequences were aligned by BLAST algorithm to their putatively homologous region from the Arabidopsis genome downloaded from The Arabidopsis Information Resource using the program GATA (Nix and Eisen, 2005). The sequence of Arabidopsis was set as reference, while that of A. alpina was used as comparative sequence. The minimum bits value was set to 24.7. GATA color codes the score obtained for the subalignments. The gray shades represent sequences having the same orientation, while red shades are used to show inversions; the intensity of the color represents their degree of conservation: the stronger the more conserved. An option for obtaining a color legend was added to the source code of the program to obtain more intuitive figures.
Phylogenetic Analysis
Protein-protein BLAST was performed using the deduced amino acid sequences of Aa SOC1, Aa TFL1, and Aa LFY, respectively. MEGA4 (Tamura et al., 2007) was used to create the multiple alignments and generate phylogenic trees using the neighbor-joining method. Bootstrap analysis was performed to evaluate the node support on the basis of 10,000 replicates. The accession numbers of the protein sequences used for phylogenetic analysis are listed in Supplemental Table 2 online.
Plasmid Constructs
Overexpression constructs 35S:Aa SOC1 and 35S:Aa TFL1 were generated by introducing the full-length coding sequences of Aa SOC1 and Aa TFL1 (see accession numbers below), respectively, into a GATEWAY-compatible binary vector pLEELA through site-directed recombination. Similarly, to make the 35S:Aa TFL1 dsRNAi construct, a gene-specific cDNA fragment of Aa TFL1 (for sequence details, see Supplemental Table 1 online) was amplified with RT-PCR and recombined to both side of an intron in a GATEWAY-compatible binary vector pJawohl-8-RNAi (GenBank accession number AF408413) to generate an intron-spliced hairpin construct.
Plant Transformation
Transgenic Arabidopsis and A. alpina plants were generated by the floral dip method (Clough and Bent, 1998).
Gene Expression Analysis
Total plant RNA was extracted using the RNeasy plant mini kit from Qiagen, and an on-column DNase treatment was performed to reduce any DNA contamination. Total RNA (2.5 μg) was used to synthesize cDNA through reverse transcription with oligo(dT15) as primer. The cDNA was diluted into a final volume of 180 μL, and 3.5 μL was used for each PCR. The quantitative PCR was performed using a Bio-Rad iQ5 apparatus and SYBR Green I detection. The error bars shown are based on three technical replicates. Biological replicates were performed for most experiments and produced similar results.
In Situ Hybridization
In situ hybridization was performed on the apices of main shoots and axillary shoots using the method described previously (Wang et al., 2009b).
Scanning Electron Microscopy
The detailed method for performing scanning electronic microscopy on A. alpina is the same as described previously (Wang et al., 2009b).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: BAC sequence containing Aa TFL1 (JF436962), coding sequence of Aa TFL1 (JF436953), genomic contig containing Aa LFY (JF436959), coding sequence of Aa LFY (JF436956), genomic region containing Aa SOC1 (JF436960), coding sequence of Aa SOC1 (JF436957), Aa CEN1 genomic sequence (JF436961), Aa CEN1 predicted coding sequence (JF436955), Aa BFT genomic sequence (JF436958), and Aa BFT coding sequence (JF436954).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Relationship between Aa SOC1 and At SOC1.
Supplemental Figure 2. Relationship between Aa LFY and At LFY.
Supplemental Figure 3. Relationship between Aa TFL1 and At TFL1.
Supplemental Figure 4. Gene Expression in TFL1 dsRNAi Pajares Plants.
Supplemental Figure 5. Comparison of A. alpina Pajares and 35S:TFL1 dsRNAi A. alpina Pajares Illustrating the Role of Aa TFL1 in Polycarpy.
Supplemental Figure 6. Relationship between the Length of Axillary Branches and Flowering.
Supplemental Table 1. Sequences of the Oligonucleotide Primers Used in This Study.
Supplemental Table 2. Accession Numbers of the Protein Sequences Used for Phylogenetic Analysis.
Supplemental Data Set 1. Text File of Alignment Corresponding to Phylogenetic Tree in Supplemental Figure 1.
Supplemental Data Set 2. Text File of Alignment Corresponding to Phylogenetic Tree in Supplemental Figure 2.
Supplemental Data Set 3. Text File of Alignment Corresponding to Phylogenetic Tree in Supplemental Figure 3.
Acknowledgments
This work was partly funded by the Deutsche Forschungsgemeinshaft through SFB680 to G.C., the Bundesministerium für Bildung und Forschung through the TRANSNET program to G.C., the International Max Planck Research School (R.W.), and the EC Training Program ADOPT (S.B.). The laboratory of G.C. also received a core grant from the Max Planck Society.
References
- Abe M., Kobayashi Y., Yamamoto S., Daimon Y., Yamaguchi A., Ikeda Y., Ichinoki H., Notaguchi M., Goto K., Araki T. (2005). FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056 [DOI] [PubMed] [Google Scholar]
- Ahn J.H., Miller D., Winter V.J., Banfield M.J., Lee J.H., Yoo S.Y., Henz S.R., Brady R.L., Weigel D. (2006). A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J. 25: 605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J., Guli C.L., Yu X.H., Smyth D.R. (1992). terminal flower: A gene affecting inflorescence development in Arabidopsis thaliana. Plant J. 2: 103–116 [Google Scholar]
- Bradley D., Carpenter R., Copsey L., Vincent C., Rothstein S., Coen E. (1996). Control of inflorescence architecture in Antirrhinum. Nature 379: 791–797 [DOI] [PubMed] [Google Scholar]
- Bradley D., Ratcliffe O., Vincent C., Carpenter R., Coen E. (1997). Inflorescence commitment and architecture in Arabidopsis. Science 275: 80–83 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Conti L., Bradley D. (2007). TERMINAL FLOWER1 is a mobile signal controlling Arabidopsis architecture. Plant Cell 19: 767–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrona S., Coupland G., Turck F. (2008). The impact of chromatin regulation on the floral transition. Semin. Cell Dev. Biol. 19: 560–573 [DOI] [PubMed] [Google Scholar]
- Fornara F., de Montaigu A., Coupland G. (2010). SnapShot: Control of flowering in Arabidopsis. Cell 141: 550. [DOI] [PubMed] [Google Scholar]
- Foucher F., Morin J., Courtiade J., Cadioux S., Ellis N., Banfield M.J., Rameau C. (2003). DETERMINATE and LATE FLOWERING are two TERMINAL FLOWER1/CENTRORADIALIS homologs that control two distinct phases of flowering initiation and development in pea. Plant Cell 15: 2742–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend D.J.C. (1985). Brassica. In Handbook of Flowering, Vol. II, Halvey A.H., ed (Boca Raton, FL: CRC Press; ), pp. 48–77 [Google Scholar]
- Hackett W.P. (1985). Juvenility, maturation, and rejuvenation in woody plants. Hortic. Rev. (Am. Soc. Hortic. Sci.) 7: 109–155 [Google Scholar]
- Hepworth S.R., Valverde F., Ravenscroft D., Mouradov A., Coupland G. (2002). Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J. 21: 4327–4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen C.S., Salchert K., Nielsen K.K. (2001). A TERMINAL FLOWER1-like gene from perennial ryegrass involved in floral transition and axillary meristem identity. Plant Physiol. 125: 1517–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson U., West J., Lister C., Michaels S., Amasino R., Dean C. (2000). Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290: 344–347 [DOI] [PubMed] [Google Scholar]
- Kardailsky I., Shukla V.K., Ahn J.H., Dagenais N., Christensen S.K., Nguyen J.T., Chory J., Harrison M.J., Weigel D. (1999). Activation tagging of the floral inducer FT. Science 286: 1962–1965 [DOI] [PubMed] [Google Scholar]
- Kim D.H., Doyle M.R., Sung S., Amasino R.M. (2009). Vernalization: Winter and the timing of flowering in plants. Annu. Rev. Cell Dev. Biol. 25: 277–299 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Kaya H., Goto K., Iwabuchi M., Araki T. (1999). A pair of related genes with antagonistic roles in mediating flowering signals. Science 286: 1960–1962 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Weigel D. (2007). Move on up, it’s time for change—Mobile signals controlling photoperiod-dependent flowering. Genes Dev. 21: 2371–2384 [DOI] [PubMed] [Google Scholar]
- Koornneef M., Hanhart C.J., van der Veen J.H. (1991). A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol. Gen. Genet. 229: 57–66 [DOI] [PubMed] [Google Scholar]
- Kotoda N., Iwanami H., Takahashi S., Abe K. (2006). Antisense expression of MdTFL1, a TFL1-like gene, reduces the juvenile phase in apple. J. Am. Soc. Hortic. Sci. 131: 74–81 [Google Scholar]
- Lee H., Suh S.S., Park E., Cho E., Ahn J.H., Kim S.G., Lee J.S., Kwon Y.M., Lee I. (2000). The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev. 14: 2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljegren S.J., Gustafson-Brown C., Pinyopich A., Ditta G.S., Yanofsky M.F. (1999). Interactions among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. Plant Cell 11: 1007–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.I., Wang J.G., Poon S.Y., Su C.L., Wang S.S., Chiou T.J. (2005). Differential regulation of expression by vernalization FLOWERING LOCUS C in cabbage and Arabidopsis. Plant Physiol. 137: 1037–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels S.D., Amasino R.M. (1999). FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11: 949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed R., Wang C.T., Ma C., Shevchenko O., Dye S.J., Puzey J.R., Etherington E., Sheng X.Y., Meilan R., Strauss S.H., Brunner A.M. (2010). Populus CEN/TFL1 regulates first onset of flowering, axillary meristem identity and dormancy release in Populus. Plant J. 62: 674–688 [DOI] [PubMed] [Google Scholar]
- Mozley D., Thomas B. (1995). Developmental and photobiological factors affecting photoperiodic induction in Arabidopsis thaliana Heynh Landsberg erecta. J. Exp. Bot. 46: 173–179 [Google Scholar]
- Mutasa-Göttgens E., Hedden P. (2009). Gibberellin as a factor in floral regulatory networks. J. Exp. Bot. 60: 1979–1989 [DOI] [PubMed] [Google Scholar]
- Nix D.A., Eisen M.B. (2005). GATA: A graphic alignment tool for comparative sequence analysis. BMC Bioinformatics 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnueli L., Carmel-Goren L., Hareven D., Gutfinger T., Alvarez J., Ganal M., Zamir D., Lifschitz E. (1998). The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development 125: 1979–1989 [DOI] [PubMed] [Google Scholar]
- Pnueli L., Gutfinger T., Hareven D., Ben-Naim O., Ron N., Adir N., Lifschitz E. (2001). Tomato SP-interacting proteins define a conserved signaling system that regulates shoot architecture and flowering. Plant Cell 13: 2687–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe O.J., Amaya I., Vincent C.A., Rothstein S., Carpenter R., Coen E.S., Bradley D.J. (1998). A common mechanism controls the life cycle and architecture of plants. Development 125: 1609–1615 [DOI] [PubMed] [Google Scholar]
- Ratcliffe O.J., Bradley D.J., Coen E.S. (1999). Separation of shoot and floral identity in Arabidopsis. Development 126: 1109–1120 [DOI] [PubMed] [Google Scholar]
- Robinson L.W., Wareing P.F. (1969). Experiments on juvenile-adult phase change in some woody species. New Phytol. 68: 67–78 [Google Scholar]
- Samach A., Onouchi H., Gold S.E., Ditta G.S., Schwarz-Sommer Z., Yanofsky M.F., Coupland G. (2000). Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288: 1613–1616 [DOI] [PubMed] [Google Scholar]
- Samach A., Wigge P.A. (2005). Ambient temperature perception in plants. Curr. Opin. Plant Biol. 8: 483–486 [DOI] [PubMed] [Google Scholar]
- Shalit A., Rozman A., Goldshmidt A., Alvarez J.P., Bowman J.L., Eshed Y., Lifschitz E. (2009). The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proc. Natl. Acad. Sci. USA 106: 8392–8397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon S., Meeks-Wagner D.R. (1991). A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell 3: 877–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon C.C., Burn J.E., Perez P.P., Metzger J., Edwards J.A., Peacock W.J., Dennis E.S. (1999). The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11: 445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn E.J., Rojas-Pierce M., Pan S., Carter C., Serrano-Mislata A., Madueño F., Rojo E., Surpin M., Raikhel N.V. (2007). The shoot meristem identity gene TFL1 is involved in flower development and trafficking to the protein storage vacuole. Proc. Natl. Acad. Sci. USA 104: 18801–18806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. (2007). MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Turck F., Fornara F., Coupland G. (2008). Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu. Rev. Plant Biol. 59: 573–594 [DOI] [PubMed] [Google Scholar]
- Wang J.W., Czech B., Weigel D. (2009a). miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138: 738–749 [DOI] [PubMed] [Google Scholar]
- Wang R.H., Farrona S., Vincent C., Joecker A., Schoof H., Turck F., Alonso-Blanco C., Coupland G., Albani M.C. (2009b). PEP1 regulates perennial flowering in Arabis alpina. Nature 459: 423–427 [DOI] [PubMed] [Google Scholar]
- Weigel D., Alvarez J., Smyth D.R., Yanofsky M.F., Meyerowitz E.M. (1992). LEAFY controls floral meristem identity in Arabidopsis. Cell 69: 843–859 [DOI] [PubMed] [Google Scholar]
- Wigge P.A., Kim M.C., Jaeger K.E., Busch W., Schmid M., Lohmann J.U., Weigel D. (2005). Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309: 1056–1059 [DOI] [PubMed] [Google Scholar]
- Wu G., Park M.Y., Conway S.R., Wang J.W., Weigel D., Poethig R.S. (2009). The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138: 750–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Poethig R.S. (2006). Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 133: 3539–3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A., Wu M.F., Yang L., Wu G., Poethig R.S., Wagner D. (2009). The microRNA-regulated SBP-Box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Dev. Cell 17: 268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]