Abstract
This study compared the plasma amprenavir pharmacokinetics of the human immunodeficiency virus (HIV) protease inhibitors amprenavir (Agenerase) 1,200 mg twice daily (BID) and the amprenavir prodrug GW433908, a formulation that substantially reduces the number of tablets per dose compared with amprenavir, at doses of 1,395 mg and 1,860 mg BID, in combination with abacavir 300 mg BID and lamivudine 150 mg BID in patients with HIV infection. Overall, 78 patients received study treatment. Compared with amprenavir 1,200 mg BID, both GW433908 1,395 mg BID and GW433908 1,860 mg BID delivered equivalent steady-state (ss) values for area under the plasma amprenavir concentration-time curve (AUC) at the end of a dosing interval (τ), lower maximum plasma amprenavir concentrations (30% lower), and higher plasma amprenavir concentrations at the end of a dosing interval (28% higher for GW433908 1,395 mg BID and 46% higher for GW433908 1,860 mg BID). Time-variant plasma amprenavir pharmacokinetics were observed with reductions in plasma amprenavir exposure over the first 4 weeks of dosing; the decrease in plasma amprenavir AUCτ,ss versus the AUC from 0 h to ∞ was 27% for GW43308 1,395 mg, 45% for GW433908 1,860 mg, and 23% for amprenavir 1,200 mg. All three regimens reduced plasma HIV-1 RNA (∼2 log10 copies/ml) and increased CD4+ cell counts (∼100 cells/mm3) over the initial 28 days. Adverse event profiles were consistent with those previously reported for amprenavir. Although not statistically tested, the GW433908 groups appeared to have fewer gastrointestinal symptoms. In conclusion, the protease inhibitor GW433908 delivered comparable plasma amprenavir concentrations to those delivered by amprenavir 1,200 mg BID. GW433908, in combination with abacavir and lamivudine, demonstrated potent antiviral activity and was generally well tolerated over a 4-week period.
The importance of human immunodeficiency virus (HIV)-infected patients adhering to their treatment regimens is well recognized. A number of studies have shown that suboptimal adherence is linked with poor treatment outcomes in combination regimens, whether or not they include HIV protease inhibitors (4, 7, 8). There are many factors that affect the level of patient adherence, and one of these is the number of pills in the treatment regimen. For example, a previous meta-analysis showed a negative correlation between the number of pills in a treatment regimen and the proportion of patients achieving plasma HIV type 1 (HIV-1) RNA concentrations of <50 copies/ml after 48 weeks of treatment (J. Bartlett, R. Demasi, J. Quinn, C. Moxham, and F. Rousseau, 7th Conf. Retrovir. Opportunistic Infect., abstr. 519, 2000). Therefore, reducing the number of pills that patients are required to take could have a beneficial effect on antiretroviral treatment outcomes.
GW433908 (908), a prodrug of the HIV-1 protease inhibitor amprenavir (APV; Agenerase), is a calcium phosphate ester that is rapidly hydrolyzed by alkaline phosphatase at the intestinal brush border to form APV and inorganic phosphate. This leads to APV absorption with minimal 908 available systemically (3). Because it has higher water solubility than APV, 908 can be formulated into high-strength film-coated tablets compared with APV, which is formulated as 150-mg soft gelatin capsules. Thus, the number of 908 tablets required per dose is reduced substantially compared with APV (when administered as Agenerase), giving the potential to improve patient adherence.
Previous single-dose studies conducted in healthy adult subjects have shown that 908 1,395 mg delivers an equivalent plasma APV exposure (area under the concentration-time curve from 0 h to infinity [AUC0-∞]) and an approximately 30 to 40% lower maximum plasma APV concentration (Cmax) than APV capsules at a dose of 1,200 mg (3). Prior to the study reported here, the pharmacokinetics of 908 had not been analyzed in HIV-infected individuals.
In this study, HIV-1-infected patients were randomized to receive 908 or APV in combination with the nucleoside reverse transcriptase inhibitors abacavir and lamivudine. Previous studies of some HIV-infected patients suggest that gut levels of alkaline phosphatase may be lower than those found in healthy subjects (1, 13). Consequently, it was considered possible that levels of cleavage of 908 could be lower in the gut of HIV-infected patients than healthy volunteers, resulting in lower blood levels of APV. For this reason, two doses of 908 were used in this study (1,395 mg and 1,860 mg twice daily [BID]) to help determine the appropriate dosing regimen for further study. The primary objectives of this study were to compare the pharmacokinetic parameters of APV in HIV-infected patients after repeat dosing with 908 and APV. Further, the safety, tolerability, and initial antiviral effect of the two doses of 908 BID were assessed when given to HIV-infected patients for 28 days.
(The results of this study were previously presented in part at the Eighth Conference on Retroviruses and Opportunistic Infections, 4 to 8 February 2001, Chicago, Ill.)
MATERIALS AND METHODS
Patients.
Male and nonpregnant female, HIV-1-infected patients were eligible to participate in the study if they were 18 to 65 years of age, were protease inhibitor naïve, and had ≤4 weeks of prior therapy with either nucleoside reverse transcriptase inhibitors or nonnucleoside reverse transcriptase inhibitors. Patients were also to have plasma HIV-1 RNA concentrations of at least 5,000 copies/ml documented in the 28 days before drug administration. Patients were not excluded if they were smokers. All women used adequate contraception during the study. All patients gave written informed consent to participate in the study.
Patients were excluded if they had diabetes, congestive heart failure, cardiomyopathy, or other cardiac dysfunction. Patients were also ineligible for the study if they had a malabsorption syndrome or other gastrointestinal dysfunction that could interfere with drug absorption or a history of clinically relevant pancreatitis or hepatitis within the previous 6 months. Other exclusion criteria included a history of any drug or other allergy felt by the investigator to be clinically significant; current alcohol or illicit drug use; a clinical diagnosis of Centers for Disease Control and Prevention (CDC) AIDS category C; or standard laboratory values outside normal reference ranges. Finally, subjects were excluded if treatment with the following medications occurred within 14 days before starting the study with 908 or if there was an anticipated need during the study: astemizole, bepridil, cisapride, dihydroergotamine, ergotamine, midazolam, terfenadine, triazolam, rifampin, rifabutin, phenobarbital, phenytoin, carbamazepine, erythromycin, clarithromycin, ketoconazole, itraconazole, cimetidine, lovastatin, simvastatin, cerivastatin, atorvastatin, pravastatin, fluvastatin, sildenafil, dexamethasone, troglitazone, primidone, ethosuximide, pimozide, flecainide, propofenone, amiodarone, and quinidine.
Study design.
This was a partially double-blinded (with respect to 908 dose), randomized, repeat-dose, crossover study (protocol number APV20001). The study ran from 15 October 1999 to 11 May 2000 and was conducted in 11 centers in Europe, 8 in the United States, and 1 in South Africa. A national, regional, or investigational center ethics committee or institutional review board approved the study protocol at each site. The study was done in accordance with Good Clinical Practice and the Declaration of Helsinki guidelines applicable at the time. The primary endpoints of the study included a comparison of the following pharmacokinetic parameters after repeat dosing of 908 and APV: AUC of APV during a dosing interval, τ, at steady state (AUCτ,ss), APV concentration at the end of a dosing interval at steady state (trough concentration; Cmin,ss), and APV Cmax at steady state (Cmax,ss). Other endpoints were to assess the safety and tolerability of 908, the change in plasma HIV-1 RNA concentration, and the change in CD4+ cell counts after 28 days of treatment with 908 or APV, in combination with the nucleoside reverse transcriptase inhibitors abacavir and lamivudine.
Patients were screened up to 28 days before the start of study treatment. On day 1 of the study, patients attended the clinical research unit and were randomized to receive one of the following three treatments for 28 days: 908 at 1,395 mg, 908 at 1,860 mg, or APV at 1,200 mg. They received a single dose of their study medication on day 1 and BID dosing from day 2 onwards. At day 28, patients crossed over treatments for an additional 14 days according to the schedule in Table 1. Patients who received either of the 908 doses in treatment period 1 (days 1 to 28) crossed over to APV 1,200 mg in treatment period 2 (day 29 to 42); patients who received APV 1,200 mg in treatment period 1 crossed over to one of the 908 doses, according to the randomization schedule. Investigators and patients were blinded to the dose of 908 by using a double-dummy technique. Patients were randomized using a central randomization procedure (Clinphone, Nottingham, United Kingdom). Study personnel called the 24-h Clinphone service at least 7 days before a patient's anticipated enrollment date and were given a treatment number to assign to the patient.
TABLE 1.
Summary of dosages and dosing
| Group | Treatment period 1 (days 1-28)a | Treatment period 2 (days 29-42)a |
|---|---|---|
| 1 | 908, 1,395 mg BID | APV, 1,200 mg BID |
| 2 | APV, 1,200 mg BID | 908, 1,395 mg BID |
| 3 | 908, 1,860 mg BID | APV, 1,200 mg BID |
| 4 | APV, 1,200 mg BID | 908, 1,860 mg BID |
908 and APV were both administered in combination with abacavir (300 mg BID) and lamivudine (150 mg BID).
The 908 1,395-mg dose was administered as three 465-mg tablets, and the 1,860-mg dose was administered as four 465-mg tablets. Each 465-mg tablet of 908 contained the molar equivalent of 400 mg of APV. To ensure that patients were blinded to which dose of 908 they received, those randomized to 908 1,395 mg also received one matched placebo tablet so that all patients received four tablets for their 908 dose. The selection of the two doses of 908 was based on preliminary data from a phase I study in healthy male volunteers (3). APV (Agenerase; GlaxoSmithKline) was administered as eight 150-mg soft gel capsules BID. All patients received abacavir (Ziagen; GlaxoSmithKline; one 300-mg tablet BID) plus lamivudine (Epivir; GlaxoSmithKline; one 150-mg tablet BID) in combination with their study drug.
The patients fasted for 8 h before pharmacokinetic sampling on days 1, 28, and 42. Fifteen serial whole-blood samples were taken at specified time points for pharmacokinetic analysis over the first 24 h after dosing on day 1. At the end of each dosing period (days 28 and 42), 14 timed whole-blood samples were taken over 12 h. If a cannula were used for collection, it was inserted into an arm vein within sufficient time before dosing. Precautions were taken to prevent dilution of blood samples. In centers where the cannula was kept patent with normal saline or heparin flushes, an initial 0.5- to 1.0-ml volume of blood was discarded before collecting each sample to ensure that samples were not diluted by the saline or heparin flushes. Alternatively, a mandril system was used in the cannula, thus avoiding the use of heparin (Madrin-Intocan Braun Melsugen AG). The cannula was removed after the last blood sample was collected. Blood samples were collected in 2.7-ml sodium citrate-containing (blue-top) Vacutainer tubes, gently inverted after collection, and refrigerated at 4°C. Plasma was collected after refrigerated centrifugation at 2,000 × g for 10 min within 1 h of sample collection. Plasma samples were frozen at −20°C or lower in a non-self-defrosting freezer, where they were stored until shipping for analysis on dry ice.
At screening and at days 1, 7, 14, 21, 28, and 42, blood samples were collected for determination of plasma HIV-1 RNA, CD4+ cell counts, and clinical laboratory tests, and data were collected on HIV-associated conditions and adverse events. Plasma HIV-1 RNA was measured using the Roche Amplicor HIV-1 Ultrasensitive Monitor test (primers 1.0; limit of detection = 50 copies/ml). Samples with plasma HIV-1 RNA levels of >75,000 copies/ml were retested using the Roche Amplicor HIV-1 Monitor test (primers 1.0; standard assay limit of detection = 400 copies/ml). CD4+ cell counts were measured using flow cytometry.
Adverse events and serious adverse events were recorded throughout the study. Adverse events were graded according to their severity using a four-point AIDS Clinical Trials Groups scale, with a score of 4 being the most severe.
At the end of the 42-day pharmacokinetic study, patients could continue in an open-label phase and receive a combination regimen of APV, abacavir, and lamivudine. Results from this phase will be the subject of a separate publication.
Statistical and pharmacokinetic analysis.
A sample size of 24 patients in the combination of treatment groups 1 and 2 and in the combination of treatment groups 3 and 4 (48 patients total; Table 1) was selected as the number of subjects that would provide greater than 90% power at the one-sided 5% level to detect a 20% decrease in AUCτ,ss after administration of 908 compared with APV based on the highest intrasubject standard deviation (0.24) observed in prior APV studies. To obtain additional safety and efficacy data and ensure that 48 evaluable patients completed the study, 84 patients were to be enrolled. The study was not powered to test any hypothesis related to clinical efficacy or safety, and therefore only descriptive statistics are presented.
The pharmacokinetic summary population consisted of patients who had plasma APV pharmacokinetic parameter values on days 1, 28, and 42 of the study. The intent-to-treat (ITT), exposed population consisted of all patients who were randomized into the study with documented evidence of having consumed study drug. For the analysis of safety data, the safety population consisted of all patients randomized in the study with the exception of any patients who did not take any study drug.
Values for the single-dose plasma APV pharmacokinetic parameters were estimated as follows. The Cmax and the first time to reach Cmax (tmax) were the actual observed values. The apparent terminal plasma elimination rate constant (λz) was estimated from log-linear regression analysis of the terminal phase of the plasma concentration-time profile. The associated apparent terminal elimination half-life (t1/2) was calculated as follows: t1/2 = ln2/λz. The AUC to the last sample time (AUClast) and AUC0-∞ were calculated by the log-linear trapezoidal method.
Values for the steady-state plasma APV pharmacokinetic parameters were estimated as follows. The maximum concentration at steady state (Cmax,ss) and the first time to reach Cmax,ss (tmax,ss) were the actual observed values. The concentration at the end of a dosing interval at steady state (Cmin,ss) was calculated as the mean of the predose and 12-h plasma APV concentrations. The AUC during a dosing interval, τ, at steady state (AUCτ,ss) was calculated by the log-linear trapezoidal method.
Plasma APV pharmacokinetic parameters, except tmax,ss, were loge transformed prior to statistical analyses, and the comparisons were expressed as geometric least-squared (GLS) mean ratios (with associated 90% confidence intervals [CI]) on the original scale. Steady-state plasma APV pharmacokinetics following administration of 908 1,395 mg BID or 908 1,860 mg BID were compared within subject to APV 1,200 mg BID in analysis of variance (ANOVA) models. Plasma alpha-1 acid glycoprotein (AAG) concentrations were included as a covariate, treatment was included as a fixed effect, and subject was included as a random effect using the SAS mixed linear models procedure.
Time variance in plasma APV pharmacokinetics was examined by a within-subject comparison of AUCτ,ss on day 28 versus AUC0-∞ on day 1 for each treatment using ANOVA. The ANOVA model included plasma AAG concentrations as a covariate, treatment, day, and the interaction of treatment and day as fixed effects, and subject as a random effect.
Plasma AAG concentrations, age, body weight, sex, race, hepatitis status, baseline CD4+ count, baseline plasma HIV-1 RNA concentration, and treatment were tested as variables correlated with single-dose (day 1) and steady-state (days 28 and 42 separately) plasma APV pharmacokinetic parameters in a correlation analysis using the SAS correlation procedure. The relationship between change in plasma AAG concentrations and change in plasma APV loge-transformed AUCτ,ss values over the first 28 days of the study was examined by stepwise regression analysis using the SAS regression procedure. In addition, the relationship between both plasma AAG concentrations and change in plasma AAG concentrations over the first 28 days of the study and age, body weight, sex, race, baseline CD4+ cell count, baseline plasma HIV-1 RNA concentration, treatment day (tested with plasma AAG concentrations only), and change in plasma HIV-1 RNA average AUC minus baseline (tested with the change in plasma AAG concentrations over the first 28 days only) were examined with stepwise regression.
RESULTS
Patient disposition and baseline characteristics.
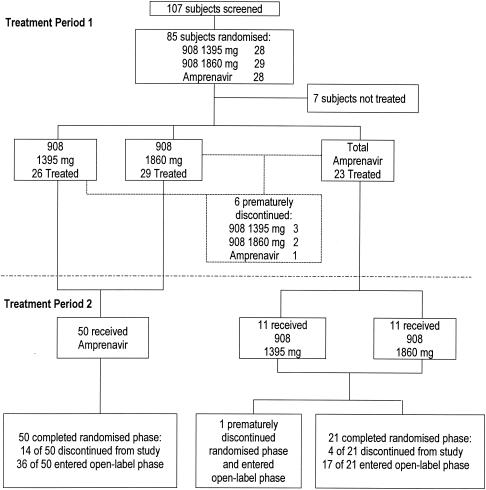
Of the 107 patients screened for this study, 85 patients were randomized and a total of 78 patients received study treatment (Fig. 1). There were six premature discontinuations: three patients withdrew consent (two receiving 908 1,395 mg and one receiving 908 1,860 mg), one patient was lost to follow-up (908 1,395 mg), one patient in the 908 1,860-mg group was unable to tolerate pharmacokinetic sampling, and one patient in the APV 1,200-mg group withdrew because of scheduling conflicts.
FIG. 1.
Summary of patient disposition in treatment periods 1 and 2.
Baseline characteristics were generally similar across the treatment groups, with the possible exception of patients randomized to the APV group, who appeared to have a lower CD4+ cell count at baseline and a higher proportion of patients enrolling with CDC class B HIV infection (Table 2).
TABLE 2.
Summary of baseline characteristics for the ITT (exposed) population
| Characteristic | 908 1,395 mg (n = 26) | 908 1,860 mg (n = 29) | APV 1,200 mg (n = 23) |
|---|---|---|---|
| Age (yrs) median (min, max) | 35 (21, 51) | 36 (22, 54) | 32 (21, 58) |
| No. (%) male | 17 (65) | 20 (69) | 16 (70) |
| Race (n [%]) | |||
| White | 16 (62) | 20 (69) | 11 (48) |
| Black | 8 (31) | 8 (28) | 9 (39) |
| Asian | 0 | 0 | 1 (4) |
| Other | 2 (8) | 1 (3) | 2 (9) |
| Weight (kg) median (min, max) | 70.0 (46, 118) | 72.3 (52, 105) | 65.0 (50, 99) |
| Plasma HIV-1 RNA (log10 copies/ml) median (min, max) | 4.75 (2.71, 6.15) | 4.52 (1.78, 6.37) | 4.58 (3.43, 6.16) |
| CD4+ cell count (cells/mm3) median (min, max) | 245 (16, 1051) | 348 (53, 979) | 177 (4, 677) |
| CDC classification (n [%]) | |||
| A, asymptomatic or lymphadenopathy | 16 (62) | 20 (69) | 7 (30) |
| B, symptomatic, not AIDS | 10 (38) | 9 (31) | 16 (70) |
Pharmacokinetic analyses.
The pharmacokinetic summary population comprised 53 patients: 22 patients received 908 1,395 mg BID, 31 received 908 1,860 mg BID, and all 53 received APV 1,200 mg BID.
APV pharmacokinetics.
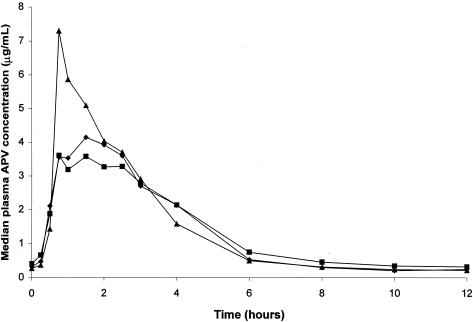
The Cmin,ss was approximately 1.28-fold higher after dosing with 908 1,395 mg than with APV 1,200 mg (GLS mean ratio = 1.28, 90% CI = 1.06 to 1.54; Table 3). Plasma APV Cmin,ss was also higher after dosing with 908 1,860 mg BID than with APV 1,200 mg BID (GLS mean ratio = 1.46, 90% CI = 1.24 to 1.72; Table 3). Both 908 1,395 mg BID and 908 1,860 mg BID delivered near-equivalent plasma APV AUCτ,ss values and lower Cmax,ss values (∼30% lower) compared with APV 1,200 mg BID (Table 3). Median steady-state plasma APV concentration-time profiles at steady state after administration of 908 and APV are shown in Fig. 2.
TABLE 3.
Summary of APV pharmacokinetic parameters
| Parameter | Geometric mean (95% CI), (n)
|
GLS mean ratio (90% CI), (n) for steady-state treatment comparisons with APV 1,200 mgc
|
|||
|---|---|---|---|---|---|
| 908 1,395 mg | 908 1,860 mg | APV 1,200 mg | 908 1,395 mg | 908 1,860 mg | |
| Single dose (day 1) | |||||
| AUC0-∞ (h · μg/ml) | 22.8 (18.7-27.8) (15) | 42.3 (34.1-52.5) (18) | 24.6 (18.9-32.0) (16) | ||
| Cmax (μg/ml) | 4.64 (3.37-6.38) (15) | 7.94 (6.55-9.62) (22) | 7.19 (5.94-8.72) (16) | ||
| tmax (h) | 2.5 (1.5, 4.0)a (15) | 2.0 (0.5, 4.3)a (22) | 1.3 (0.5, 3.0)a (16) | ||
| t1/2 (h) | 7.7 (5.9-10.0) (15) | 7.9 (6.2-10.0) (18) | 9.6 (6.5-14.0) (16) | ||
| Steady state (days 28 and 42) | |||||
| AUCτ,ss (h · μg/ml) | 16.5 (13.8-19.6) (22) | 17.0 (14.9-19.5) (31) | 16.2 (14.3-18.3) (53) | 0.96 (0.85-1.09) (22) | 1.07 (0.96-1.19) (31) |
| Cmax,ss (μg/ml) | 4.82 (4.06-5.72) (22) | 4.78 (4.14-5.53) (31) | 6.80 (5.90-7.84) (53) | 0.70 (0.59-0.82) (22)* | 0.73 (0.63-0.85) (31)* |
| Cmin,ss (μg/ml) | 0.35 (0.27-0.46) (22) | 0.35 (0.27-0.45) (31) | 0.26 (0.21-0.31) (53) | 1.28 (1.06-1.54) (22)* | 1.46 (1.24-1.72) (31)* |
| tmax,ss (μg/ml) | 1.3 (0.8, 4.0)a (22) | 1.5 (0.8, 4.10)a (31) | 1.0 (0.5, 2.5)a (53) | 1.39 (1.08-1.70)b (22)* | 1.75 (1.46-2.03)b (31)* |
Data are presented as median (minimum, maximum).
Data are presented as the least squares mean ration (90% CI).
*, statistically significant difference between 908 and APV 1,200 mg groups.
FIG. 2.
Median steady-state plasma APV concentration-time profiles for the 908 1,395-mg BID (♦; n = 22), 908 1,860-mg BID (▪; n = 31), and APV 1,200-mg (▴; n = 53) groups at the end of treatment period 1 (day 28).
Time variance in plasma APV pharmacokinetics was observed for the three regimens (Table 4). The GLS mean AUCτ,ss/AUC∞ ratio was 0.73 (90% CI, 0.61 to 0.87) for 908 1,395 mg, 0.55 (90% CI, 0.47 to 0.66) for 908 1,860 mg, and 0.77 (90% CI, 0.65 to 0.91) for APV 1,200 mg.
TABLE 4.
Summary of plasma APV pharmacokinetics
| Parameter | Median (interquartile range)
|
||
|---|---|---|---|
| 908 1,395 mg BIDa | 908 1,860 mg BIDb | APV 1,200 mg BIDc | |
| Single dose | |||
| AUC0-∞ (h · μg/ml) | 20.3 (16.4-27.4) | 45.6 (38.1-52.5) | 23.3 (17.4-32.6) |
| Cmax (μg/ml) | 5.29 (3.29-7.42) | 8.61 (6.94-10.66) | 7.37 (5.29-8.46) |
| tmax (h) | 2.5 (1.5-3.0) | 1.9 (1.3-2.5) | 1.3 (1.0-1.8) |
| t1/2 (h) | 6.4 (5.6-11.2) | 7.2 (5.0-9.8) | 6.8 (6.5-12.9) |
| Steady state | |||
| AUCτ,ss (h · μg/mL) | 16.5 (12.6-21.3) | 16.9 (13.9-21.9) | 16.5 (12.0-21.4) |
| Cmax,ss (μg/ml) | 4.67 (3.92-6.52) | 4.82 (3.71-5.8) | 7.01 (4.67-9.27) |
| Cτ,ss (μg/ml) | 0.305 (0.249-0.539) | 0.340 (0.189-0.502) | 0.246 (0.186-0.365) |
| tmax,ss (h) | 1.2 (1.0-2.0) | 1.5 (0.8-2.5) | 1.0 (0.8-1.0) |
n = 15 for single-dose values; n = 22 for steady-state values.
n = 18 for single-dose values, except for Cmax and t1/2, for which n was 22; n = 31 for steady-state values.
n = 16 for single-dose values; n = 53 for steady-state values.
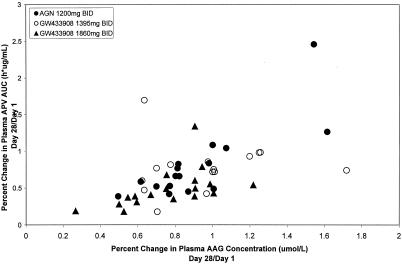
Plasma AAG concentrations were consistently correlated with plasma APV AUCτ,ss and Cmax,ss on days 1 (r2 = 0.337 to 0.589), 28 (r2 = 0.540 to 0.721), and 42 (r2 = 0.626 to 0.768). A linear relationship was seen in the percent change in plasma APV AUC compared to the percent change in AAG from day 28 to day1 (Fig. 3). Age, body weight, sex, race, hepatitis B and/or C status, baseline CD4+ cell count, baseline plasma HIV-1 RNA concentration, and treatment were also assessed but found to have no correlation with plasma APV pharmacokinetic parameters.
FIG. 3.
Percent change in plasma APV AUC versus percent change in AUC (day 28/day1).
908 pharmacokinetics.
908 was rapidly converted to APV with minimal 908 measured systemically. Of the 53 subjects included in the pharmacokinetic summary population, 29 of 37 patients (78%) who received 908 on day 1 had quantifiable 908 concentrations (10 of 15 patients [67%] who received 908 1,395 mg and 19 of 22 patients [86%] who received 908 1,860 mg). Thirty-nine out of 53 patients (74%) who received 908 on days 14 to 28 had quantifiable 908 (14 of 22 patients [64%] who received 908 1,395 mg BID and 25 of 31 patients [81%] who received 908 1,860 mg BID). Plasma 908 concentrations were quantifiable between 0.25 and 4 h postdose. Geometric mean plasma 908 pharmacokinetic parameter values were similar for the two 908 regimens; AUClast,ss was 0.021 h · μg/ml for 908 1,395 mg BID and 0.018 h · μg/ml for 1,860 mg BID, and Cmax,ss was 0.020 μg/ml for 908 1,395 mg BID and 0.021 μg/ml for 1,860 mg BID. In all subjects, plasma 908 AUClast,ss was <0.6% of the corresponding APV AUCτ,ss, and plasma 908 Cmax,ss was <1.6% of the corresponding APV Cmax,ss. 908 was not detected in plasma at the end of the dosing interval on days 14to 28, i.e., Cmin,ss was below the limit of detection.
Efficacy analyses.
The ITT (exposed) population comprised 78 patients: 26 patients received 908 1,395 mg, 29 patients received 908 1,860 mg, and 23 patients received APV 1,200 mg over the first 28 days of the study.
Plasma HIV-1 RNA.
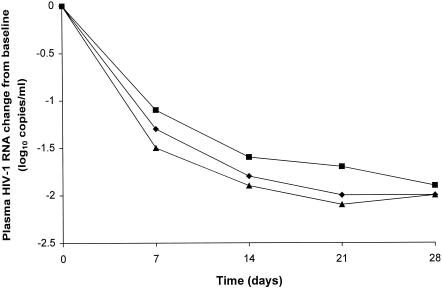
Median (range) baseline plasma HIV-1 RNA concentrations were 4.7 log10 copies/ml (2.7 to 6.2 log10 copies/ml) for the 908 1,395-mg group, 4.5 log10 copies/ml (1.8 to 6.4 log10 copies/ml) for the 908 1,860-mg group, and 4.6 log10 copies/ml (3.4 to 6.2 log10 copies/ml) for the APV 1,200-mg group. Median (range) decreases in plasma HIV-1 RNA concentrations were similar for all three groups during the treatment period: −2.0 log10 copies/ml (−3.0 to +0.49 log10 copies/ml) for the 908 1,395-mg group, −1.9 log10 copies/ml (−2.8 to −0.1 log10 copies/ml) for the 908 1,860-mg group, and −2.0 log10 copies/ml (−3.4 to −0.7 log10 copies/ml) for the APV 1,200-mg group after 28 days of treatment (Fig. 4).
FIG. 4.
Median plasma HIV-1 RNA changes from baseline for the 908 1,395-mg (♦), 908 1,860-mg (▪), and APV 1,200-mg (▴) groups in treatment period 1.
One patient in the 908 1,860-mg group had a plasma HIV-1 RNA level of <400 copies/ml on day 1 of the study. This subject had a screening viral load of >5,000 copies/ml using the Roche Amplicor 1.5 HIV-1 Monitor test and was randomized and treated before it was determined that a non-protocol-defined assay had been used. The subject screening and day 1 HIV RNA sample were reanalyzed using the Roche Amplicor HIV-1 Ultrasensitive Monitor test with HIV-1 RNA results of 733 and 60 copies/ml, respectively. This subject continued in the trial and is included in the efficacy analysis. At day 28, 11 of 26 patients (42%) in the 908 1,395-mg group, 13 of 29 patients (45%) in the 908 1,860-mg group, and 9 of 23 patients (39%) in the APV 1,200-mg group had plasma HIV-1 RNA of <400 copies/ml.
CD4+ cell counts.
Median baseline CD4+ cell counts were 245 cells/mm3 for the 908 1,395-mg group, 301 cells/mm3 for the 908 1,860-mg group, and 183 cells/mm3 for the APV 1,200-mg group. Increases in CD4+ cell counts were similar for all three groups during the treatment period: 111 cells/mm3 for the 908 1,395-mg group, 106 cells/mm3 for the 908 1,860-mg group, and 92 cells/mm3 for the APV 1,200-mg group at day 28.
Safety analyses.
The safety population comprised 78 patients: 26 patients received 908 1,395 mg, 29 patients received 908 1,860 mg, and 23 patients received APV 1,200 mg.
Grade 2 to 4 (moderate to severe) drug-related adverse events were generally evenly distributed between the treatment groups (Table 5), although several gastrointestinal adverse events appeared to be more common in the APV group than the 908 groups (both groups combined). For days 1 to 28, the incidence of nausea was 3 of 55 patients (5%) for 908 and 5 of 23 patients (22%) for APV 1,200 mg. The incidence of abdominal pain was 1 in 55 patients (2%) for 908 and 4 of 23 patients (17%) for APV 1,200 mg. For patients who received 908 from days 1 to 28, diarrhea (five patients, 9%), hypersensitivity reaction to abacavir (four patients, 7%), and sleep disorders (four patients, 7%) were the most frequently reported grade 2 to 4 drug-related adverse events. Nausea (five patients, 22%), abdominal pain, diarrhea (four patients, 17% [both events]), and fatigue (three patients, 13%) were the most frequently reported drug-related adverse events for APV 1,200 mg.
TABLE 5.
Summary of most-frequently reported grade 2 to 4 drug-related adverse events from days 1 to 28
| Adverse eventa | No. (%) reporting the event
|
||
|---|---|---|---|
| 908 1,395 mg (n = 26) | 908 1,860 mg (n = 29) | APV 1,200 mg (n = 23) | |
| Any event | 13 (50) | 11 (38) | 9 (39) |
| Diarrhea | 3 (12) | 2 (7) | 4 (17) |
| Sleep disorder | 3 (12) | 1 (3) | 0 |
| Hypersensitivity reaction to abacavir | 2 (8) | 2 (7) | 1 (4) |
| Vomiting | 2 (8) | 1 (3) | 1 (4) |
| Fever | 2 (8) | 1 (3) | 0 |
| Rash | 2 (8) | 1 (3) | 0 |
| Mood disorder | 2 (8) | 0 | 0 |
| Nausea | 1 (4) | 2 (7) | 5 (22) |
| Headache | 1 (4) | 2 (7) | 0 |
| Fatigue | 1 (4) | 1 (3) | 3 (13) |
| Abdominal pain | 1 (4) | 0 | 4 (17) |
| Decreased white cells | 0 | 3 (10) | 0 |
Events listed were reported in ≥5% of any group (treatment period 1).
After crossing over (days 29 to 42), the incidence of grade 2 to 4 drug-related adverse events was lower than during days 1 to 28: 4 patients (17%) who received 908 and 16 patients (29%) who received APV 1,200 mg reported grade 2 to 4 drug-related adverse events. The most frequently reported grade 2 to 4 drug-related adverse events were diarrhea (one patient, 4%) for patients who received 908 and diarrhea (four patients, 7%) and gaseous symptoms and nausea (three patients each, 5%) for patients who received APV 1,200 mg.
From days 1 to 28, five patients (6%) experienced hypersensitivity reactions to abacavir. One of the patients also experienced an urticarial rash (grade 2) at the same time as the hypersensitivity reaction.
There was a small difference in the median change from baseline in blood cholesterol between the treatment arms at day 28: 0.955 mmol/liter for patients who received 908 versus 0.490 mmol/liter for those who received APV 1,200 mg. However, this difference was not clinically significant. The median change in triglyceride concentrations at day 28 was similar for the two treatment arms: 0.205 mmol/liter for 908 and 0.195 mmol/liter for APV 1,200 mg.
DISCUSSION
In this study, the APV AUCτ,ss was similar for 908 1,395 mg, 908 1,860 mg, and APV 1,200 mg. For both doses of 908, the Cmin,ss was statistically significantly higher than that delivered by APV 1,200 mg under steady-state conditions. The Cmin,ss for APV 1,200 mg was greater than the previously reported APV concentration required to inhibit 50% of viral replication that was determined from clinical isolates of HIV (0.26 μg/ml versus 0.146 μg/ml after adjustment for protein binding) (11). The higher inhibitory quotient of 908 might translate into more favorable antiviral activity and durability. As the Cmax obtained after administering both doses of 908 was significantly lower than that for APV 1,200 mg, this could lead to further increases in tolerability, because a lowered Cmax may decrease the incidence of gastrointestinal adverse drug effects, as was suggested but not conclusively demonstrated by this study.
908 was rapidly converted to APV, with minimal 908 available systemically (<0.6% of corresponding APV AUC and <1.6% of corresponding APV Cmax,ss). This finding is consistent with data observed in a previous single-dose 908 study conducted in healthy adult subjects (3).
It appears that there were disproportionately higher increases in plasma APV AUC0-∞ and Cmax values after administration of a single dose of 908 1,860 mg compared with a single dose of 908 1,395 mg. Although 908 1,860 mg delivered higher plasma APV exposures than 908 1,395 mg following single-dose administration, both doses were equivalent to APV 1,200 mg BID with respect to plasma APV AUCτ,ss upon multiple dose administration for 28 days. Plasma APV AUC values decreased between day 1 and day 28 for all three treatments; however, the proportional decrease for the 908 1,860-mg treatment was the largest (approximately 23% for APV 1,200 mg BID, 26% for 908 1,395 mg, and 46% for 908 1,860 mg BID).
The reason for the differential decrease in plasma APV AUCτ,ss between the two 908 treatments is not fully understood. There are a number of possible reasons, including the following: saturation of absorption, saturation of hydrolysis (conversion of 908 to APV), induction of P-glycoprotein (decreased absorption of APV), induction of cytochrome P450 (CYP) 3A4 (increased metabolism of APV), and/or the disproportionately higher baseline plasma APV exposure for subjects receiving 908 1,860 mg on day 1. If saturation of absorption was involved, the single-dose plasma APV pharmacokinetics would have been similar for the two 908 doses. However, there were obvious differences in plasma APV exposure between single doses of 908 1,395 mg and 1,860 mg. If saturation of hydrolysis was involved, day 28 plasma 908 concentrations would have been higher for 908 1,860 mg than for 908 1,395 mg BID. However, no difference was observed between the doses.
APV induced CYP3A4 and P-glycoprotein in rats that received 139 and 450 mg of APV/kg/day for 14 days (5). Induction effects have not been demonstrated in other animal species but have been seen in humans (2, 6, 12; R. Bertz, C. Foit, D. Burt, A. Hsu, Y.-L. Chiu, T. Chira, R. Wieboldt, L. A. Williams, G. R. Granneman, and E. Sun. Prog. Abstr. 3rd Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 7.6, 2002). The ability to evaluate the metabolic induction potential of APV in HIV-infected patients is confounded by reductions in plasma AAG concentrations as patients are effectively treated. Plasma AAG concentrations were consistently correlated with plasma APV pharmacokinetics on each of days 1, 28, and 42 in this study; this finding is consistent with previous analyses (10). Changes in plasma AAG concentrations and changes in plasma APV AUCτ,ss values over the first 28 days of the study were significantly correlated, confirming the observation of a previous study (11). These data indicate that changes in plasma APV pharmacokinetics over time may be due, in part, to changes in plasma AAG concentrations as well as an inductive effect on either P-glycoprotein or CYP3A4.
In this study, all treatments were generally well tolerated and the adverse event profiles were generally consistent with those previously reported for APV (9): the most frequently reported adverse events were nausea, diarrhea, and abdominal pain for patients on APV and diarrhea and sleep disorders for patients on 908. Notably, the proportion of patients reporting nausea and abdominal pain were lower in the 908 groups than the APV 1,200-mg group. Further large-scale studies are under way to fully define the safety profile of 908.
Across all study regimens, through just 28 days of therapy, plasma HIV-1 RNA concentrations were rapidly reduced by about 2 log10 copies/ml and CD4+ cell counts increased by about 100 cells/mm3. These early potency results are promising, and further studies are ongoing to determine the long-term efficacy of 908.
Providing patients with a protease inhibitor with a low pill count is likely to facilitate adherence and, subsequently, improve treatment outcome (4, 8). Since conducting this study, a 700-mg 908 tablet has become available that is bioequivalent to the 465-mg tablet (GlaxoSmithKline, personal communication) and delivers the equivalent of 600 mg of APV. Therefore, a dosing regimen of four 700-mg tablets per day is likely to result in similar APV levels to the 908 1,395-mg BID dose and, by extension, similar antiretroviral activity.
In conclusion, 908 is a promising protease inhibitor that was administered at doses of 1,395 mg (three tablets) BID and 1,860 mg (four tablets) BID in this study. 908 delivered equivalent APV exposure to APV 1,200 mg and provided Cmin,ss values above the APV 50% inhibitory concentration value previously determined for clinical isolates of HIV. 908 demonstrated potent antiviral activity by reducing plasma HIV-1 RNA concentrations and increasing CD4+ cell counts and was generally well tolerated over the period studied.
Acknowledgments
In addition to some of our authors (K.A., J.E., R.B.P., F.R., H.-J.S., E.T., and R.W.), the following investigators recruited patients for this study: Antonio Diniz, Hospital de Pulido Valente, Lisbon, Portugal; Richard Haubrich, UCSD Treatment Center, San Diego, Calif.; Jean-Michel Livrozet, Hôpital Edoard Herriot, Lyon, France; Didier Sicard, Hôpital Cochin Tarnier, Paris, France; and Christain Trepo, Hôpital Hôtel Dieu, Lyon, France. We thank Justin Cook for editing and writing assistance during preparation of the manuscript.
GlaxoSmithKline Research and Development provided support for this study.
REFERENCES
- 1.Asmuth, D. M., S. M. Hammer, and C. A. Wanke. 1994. Physiological effects of HIV infection on human intestinal epithelial cells: an in vitro model for HIV enteropathy. AIDS 8:205-211. [DOI] [PubMed] [Google Scholar]
- 2.Bart, P. A., P. G. Rizzardi, S. Gallant, K. P. Golay, P. Baumann, G. Pantaleo, and C. B. Eap. 2001. Methadone blood concentrations are decreased by the administration of abacavir plus APV. Ther. Drug Monit. 23:553-555. [DOI] [PubMed] [Google Scholar]
- 3.Falcoz, C., J. M. Jenkins, C. Bye, K. B. Kenney, S. Studenberg, H. Fuder, and W. T. Prince. 2002. Pharmacokinetics of GW433908, a prodrug of APV, in healthy male volunteers. J. Clin. Pharmacol. 42:887-898. [DOI] [PubMed] [Google Scholar]
- 4.Haubrich, R. N., S. J. Little, J. S. Currier, D. N. Forthal, C. A. Kemper, G. N. Beall, D. Johnson, M. P. Dube, J. Y. Hwang, J. A. McCutchan, et al. 1999. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response. AIDS 13:1099-1107. [DOI] [PubMed] [Google Scholar]
- 5.Huang, L., S. A. Wring, J. L. Woolley, K. R. Brouwer, C. Serabjit-Singh, and J. W. Polli. 2001. Induction of P-glycoprotein and cytochrome P450 3A by HIV protease inhibitors. Drug Metab. Dispos. 29:754-760. [PubMed] [Google Scholar]
- 6.Justesen, U. S., N. A. Klitgaard, K. Brosen, and C. Pedersen. 2003. Pharmacokinetic interaction between amprenavir and delavirdine after multiple-dose administration in healthy volunteers. Br. J. Clin. Pharmacol. 55:100-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Moing, V., G. Chene, M. P. Carrieri, A. Alioum, F. Brun-Vezinet, L. Piroth, J. P. Cassuto, J. P. Moatti, F. Raffi, C. Leport, et al. 2002. Predictors of virological rebound in HIV-1-infected patients initiating a protease inhibitor-containing regimen. AIDS 16:21-29. [DOI] [PubMed] [Google Scholar]
- 8.Paterson, D. L., S. Swindells, J. Mohr, M. Brester, E. N. Vergis, C. Squier, M. M. Wagener, and N. Singh. 2000. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann. Int. Med. 133:21-30. [DOI] [PubMed] [Google Scholar]
- 9.Pedneault, L., C. Brothers, G. Pagano, P. Tymkewycz, J. Yeo, J. Millard, and A. Fetter. 2000. Safety profile and tolerability of APV in the treatment of adult and pediatric patients with HIV infection. Clin. Ther. 22:1378-1394. [DOI] [PubMed] [Google Scholar]
- 10.Sadler, B. M., C. Gillotin, Y. Lou, and D. S. Stein. 2001. In vivo effect of α1-acid glycoprotein on pharmacokinetics of APV, a human immunodeficiency virus protease inhibitor. Antimicrob. Agents Chemother. 45:852-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadler, B. M., C. Gillotin, Y. Lou, and D. S. Stein. 2001. Pharmacokinetic and pharmacodynamic study of the human immunodeficiency virus protease inhibitor APV after multiple oral dosing. Antimicrob. Agents Chemother. 45:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran, J. Q., C. Petersen, M. Garrett, M. Schultz-Smith, B. Hee, J. Lillibridge, and B. M. Kerr. 2001. Pharmacokinetic interaction between delavirdine and reduced dose amprenavir in HIV negative adults following multiple dosing. Clin. Infect. Dis. 33:1170. [Google Scholar]
- 13.Ullrich, R., W. Heise, C. Bergs, M. L'age, E. O. Riecken, and M. Zeitz. 1992. Effects of zidovudine treatment on the small intestinal mucosa in patients infected with the human immunodeficiency virus. Gastroenterology 102:1483-1492. [DOI] [PubMed] [Google Scholar]