The desiccation-tolerant Sporobolus stapfianus and desiccation-sensitive Sporobolus pyramidalis form a sister group contrast to investigate adaptive metabolic responses to dehydration using untargeted global metabolomic analysis. The metabolic profiles obtained reveal a state of preparedness and a cascade of biochemical regulation strategies critical to the survival of S. stapfianus under desiccation.
Abstract
Understanding how plants tolerate dehydration is a prerequisite for developing novel strategies for improving drought tolerance. The desiccation-tolerant (DT) Sporobolus stapfianus and the desiccation-sensitive (DS) Sporobolus pyramidalis formed a sister group contrast to reveal adaptive metabolic responses to dehydration using untargeted global metabolomic analysis. Young leaves from both grasses at full hydration or at 60% relative water content (RWC) and from S. stapfianus at lower RWCs were analyzed using liquid and gas chromatography linked to mass spectrometry or tandem mass spectrometry. Comparison of the two species in the fully hydrated state revealed intrinsic differences between the two metabolomes. S. stapfianus had higher concentrations of osmolytes, lower concentrations of metabolites associated with energy metabolism, and higher concentrations of nitrogen metabolites, suggesting that it is primed metabolically for dehydration stress. Further reduction of the leaf RWC to 60% instigated a metabolic shift in S. stapfianus toward the production of protective compounds, whereas S. pyramidalis responded differently. The metabolomes of S. stapfianus leaves below 40% RWC were strongly directed toward antioxidant production, nitrogen remobilization, ammonia detoxification, and soluble sugar production. Collectively, the metabolic profiles obtained uncovered a cascade of biochemical regulation strategies critical to the survival of S. stapfianus under desiccation.
INTRODUCTION
The impact of drought on crop production is of continuous and growing concern as the world struggles to meet food production targets for an increasing global population. The predicted and emerging changes in global climate patterns generally forecast an increase in the number and severity of drought events that will negatively impact the production and stability of food supplies (Schmidhuber and Tubiello, 2007). Drought usually implies a composite stress condition that includes soil water deficits, increased daytime temperatures, and reduced nutrient availability, but, occasionally, also increased salinity in the soil. However, the most important factor limiting growth and impairing plant productivity is the drop in water availability to the plant. For example, Arabidopsis thaliana seedlings exposed to mild water deficits cease shoot and root growth at water potentials of only −1 and −0.6 megapascal (MPa), respectively, in controlled conditions (van der Weele et al., 2000). More severe water deficits that send leaf osmotic potentials to −2.68 ± 0.46 MPa result in a 95% lethality rate for mature Arabidopsis plants (Columbia ecotype; Yang et al. 2005). In general, most crop species are very sensitive to soil water potential and only rarely survive soil water deficits that drive leaf water potentials to −4 MPa (Proctor and Pence, 2002). Thus, Arabidopsis and other models are not appropriate for studies aimed at elucidating mechanisms of dehydration tolerance.
Cellular responses to water deficits include growth inhibition, stomatal closure, limited transpiration, and reduced photosynthesis, and those responses that enhance cellular dehydration tolerance (Mullet and Whitsitt, 1996). Understanding which responses are critical and adaptive for maintaining plant growth and productivity is essential for developing strategies that improve drought tolerance of all major crops. Work with the major archetypal species, both crop (e.g., maize) and noncrop (e.g., Arabidopsis), has established a wealth of information and a substantial catalog of cellular responses to water deficits. However, most studies of water deficit responses in plants do not attempt to reach water potentials that would generate significant cellular dehydration, and many remain in the range where osmotic adjustment can prevent significant dehydration of the cellular environment. This is because most present-day angiosperms cannot survive dehydration of their vegetative tissues to 20 to 30% of full turgor (RWC), which translates to between −5 and −10 MPa (Proctor and Pence, 2002). The lowest reported water potential reached for an angiosperm that is not DT is −12.1 MPa for Larrea divaricata, a desert shrub (Cunningham and Burk, 1973). Despite the array of data characterizing water deficit responses that may relate to dehydration tolerance, there is still little understanding as to which responses, whether at the gene or cellular level, are actually adaptive in nature and truly critical for or central to tolerance (Bray, 2002).
Much of what we know of dehydration tolerance in vegetative tissues of plants comes from studies involving the so-called DT or resurrection plants that can tolerate water deficits so severe that the plants loose all available free water from their tissues. The increased interest in drought tolerance and the accelerating search for novel genetic components and strategies for improving or maintaining crop production under soil water deficits has renewed interest in DT plants (Moore et al., 2009; Oliver et al., 2010). Several species have been studied in depth with a view toward understanding the genes that control the underlying mechanisms and processes involved in desiccation tolerance. These include a bryophyte Tortula ruralis; the clubmosses Selaginella lepidophylla and Selaginella tamariscina; the dicots Craterostigma plantagineum, Craterostigma wilmsii, Boea hygrometrica, and Myrothamnus flabellifolia; and the monocots Xerophyta viscosa, Xerophyta humalis, and S. stapfianus (reviewed in Ingram and Bartels, 1996; Alpert and Oliver, 2002; Moore et al., 2009; Cushman and Oliver, 2010; Oliver et al., 2010). Many studies using resurrection species are now focusing on gene discovery, large-scale transcriptome profiling of dehydration responses (Rodriguez et al., 2010), signaling pathways, and functional roles of individual genes in the desiccation response (reviewed in Cushman and Oliver, 2010; Oliver et al., 2010). Proteomic-level investigations have also been undertaken, and novel insights into the dehydration response of several DT species have enriched our understanding of the cellular protection mechanisms that underpin desiccation tolerance (Alamillo and Bartels, 2001; Georgieva et al., 2009; reviewed in Cushman and Oliver, 2010).
Perhaps some of the more revealing insights come from small-scale but in-depth analyses of metabolic processes that emerge or respond during the systemic loss of water from resurrection plants (reviewed in Cushman and Oliver, 2010). Soluble sugars accumulate, and associated enzyme activities increase, in all DT tissues studied to date, sometimes in combination with oligosaccharides (Vertucci and Farrant, 1995; Whittaker et al., 2001; Phillips et al., 2002; Illing et al., 2005; Farrant, 2007; Peters et al., 2007; Iturriaga et al., 2009). The disaccharide Suc is the most common soluble sugar associated with desiccation tolerance in resurrection plants and accumulates during drying (Smirnoff, 1992; Ghasempour et al., 1998). Amino acids such as Arg and Asn, perhaps derived from the breakdown of damaged proteins, accumulate in large amounts during the later stages of dehydration in some resurrection species, as observed in S. stapfianus (Whittaker et al., 2007), and may, in addition to their roles as osmolytes, provide nitrogen and carbon for the return of growth and metabolism upon rehydration (Martinelli et al., 2007a).
In addition to metabolites associated with cellular protection, other metabolites are thought to play important roles in protecting cellular constituents from reactive oxygen species (ROS) that build up during dehydration and in the desiccated state, particularly under high light conditions. Anthocyanins, which may act as photoprotectants by masking photosynthetic pigments and by quenching free radicals, typically increase during dehydration in resurrection angiosperms (Sherwin and Farrant, 1998; Hoekstra et al., 2001). Antioxidants and their attending enzymes also appear central to the desiccation response in DT plants and tissues (Sherwin and Farrant, 1998; Illing et al., 2005; Kranner and Birtic, 2005; Berjak, 2006; Farrant, 2007). For example, ascorbate-glutathione cycle metabolites are often elevated during drying to combat ROS activity in resurrection plants (Navari-Izzo et al., 1997; Jiang et al., 2007). In fact, the length of time a resurrection plant can survive the dried state has been correlated to the level of antioxidants in its tissue (Kranner et al., 2002). Lastly, polyphenol oxidase, which catalyzes the oxidation of mono- and o-diphenols to o-diquinones, showed increased protein abundance and enzyme activity in the dehydrating leaves of several resurrection species (Jiang et al., 2007; Veljovic-Jovanovic et al., 2008). Polyphenols are powerful detoxifiers of toxic ROS and may function as antioxidants during the first few hours of rehydration (Veljovic-Jovanovic et al., 2008). Although we begin to grasp how resurrection plants respond to desiccation or rehydration, without a relevant comparison between the responses of sensitive and tolerant tissues, any insight into the adaptive processes remains simply speculative. Martinelli et al. (2007a) compared the metabolic response of older DS leaves of S. stapfianus to that of younger DT leaves. Although there are significant and seemingly important differences in the desiccation response of these two leaf types, it remains unclear whether they result only from a change in leaf maturity or if they do indeed relate to a loss in DT. What might cause more mature leaves (cellular or otherwise) to lose their desiccation tolerance remains unknown. Another metabolic comparison performed by Farrant et al. (2009) in the fern Mohria caffrorum, which is DT during the dry season and DS during the wet season, led to clear differences between the DT and DS fronds in terms of response to dehydration, but was limited to only a few metabolites.
In this study, we have taken advantage of a natural experiment, comparing the metabolomic responses of closely related species (sister group contrast) that differ in their sensitivity or tolerance to desiccation. We compared the metabolome of young leaves from the DT grass S. stapfianus with that of young leaves from the DS species S. pyramidalis. By contrasting two closely related species that differ primarily in their abilities to tolerate dehydration of their vegetative tissues, we hope to better infer not only which processes or components relate directly to desiccation tolerance in these angiosperms, but also the metabolic mechanisms by which desiccation tolerance is acquired. We compared 167 metabolites in S. stapfianus and S. pyramidalis at full hydration and at various RWC levels during dehydration, and, in the case of S. stapfianus, all the way to the desiccated state. The data demonstrate that S. stapfianus is metabolically primed for a desiccation event and responds quickly as water is lost from the plant. In contrast, S. pyramidalis fails to respond in a measurable way to dehydration to 60% RWC and is metabolically focused on energy metabolism, presumably for growth. The data also point to the strong involvement of the glutathione biosynthesis pathway, other antioxidant processes, and sugars in the desiccation tolerance phenotype of S. stapfianus. A novel plant compound also appears to be associated with the response of S. stapfianus to desiccation: ophthalmate, which is also linked to the glutathione biosynthesis pathway. These findings not only enable us to better understand how plants withstand desiccation of their vegetative tissues, but also to make inferences as to the processes and genetic components of adaptive value to the evolution of this important plant phenotype.
RESULTS
Phenotypic Responses to Dehydration
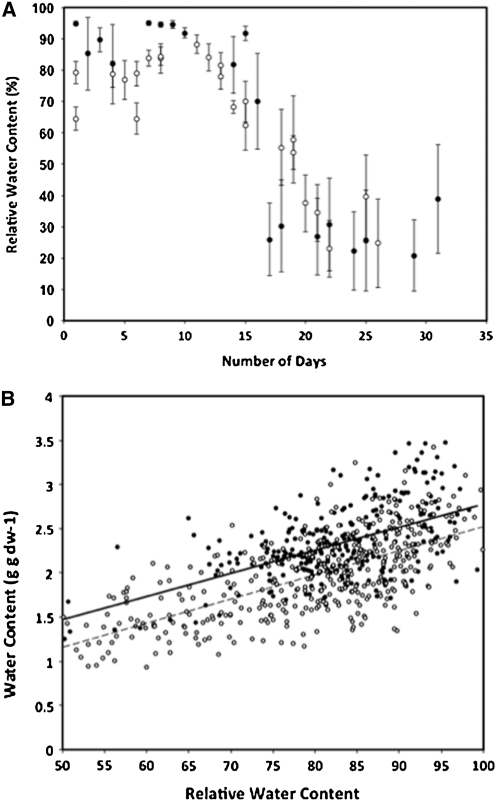
The two species exhibited similar drying curves (Figure 1a), but S. stapfianus displayed an initially lower absolute water content and throughout the drying process (Figure 1b). Both species initially lose water at a rate that is consistent with field observations for S. stapfianus (Gaff et al., 2009). S. pyramidalis loses water faster once the overall water contents decline around d 16 or 17. The leaves of S. stapfianus curl as dehydration progresses after the cessation of irrigation, and, under our conditions, appear to curl to particular degrees at specific RWCs; leaves are halfway curled at 68% RWC and fully curled (leaf margins touching) at 44% RWC, as described previously (Gaff et al., 2009). Leaves of S. pyramidalis also begin to curl, but only when RWCs are between 80 and 60%. At 60% RWC, the leaves of S. pyramidalis are fully wilted and curled, but have not yet begun to visibly lose chlorophyll. At RWCs below 60%, the leaves of S. pyramidalis start to yellow, and between 40 and 30% RWC, they start to senesce and turn brown. Under our drying regimen, if the plants are allowed to dry beyond 40%, all of the extant leaves loose viability. Based on this phenological data, we chose 60% as our dehydration level for comparisons between the two species—a level at which S. pyramidalis is severely stressed (beyond the wilting point, but not to the point where chlorophyll is lost or senescence is initiated). A RWC of 60% for S. pyramidalis is the equivalent to a leaf water potential of −3 to −3.5 MPa, a water potential that results in leaf senescence in maize (Boyer, 1976).
Figure 1.
Drying Curve and the Relationship of RWC to Water Content on a Gram Dry Weight Basis for Both S. stapfianus (s) and S. pyramidalis (•).
Each point in the drying curve (A) is an average of a minimum of four samples from individual plants; the vertical lines through each point represents the standard deviation from the mean. Each point in the RWC to water content plot (B) is an individual sample taken from individual plants from multiple drying experiments (solid line, S. pyramidalis; dashed line, S. stapfianus).
Metabolomic Profiles and Statistical Analysis
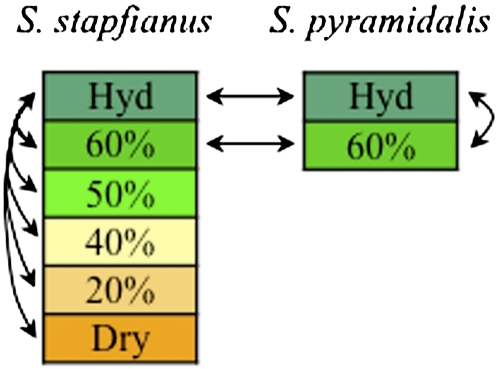
The metabolomic profiling approach used in this study was a nonbiased, global analysis technology based on ultrahigh-performance liquid chromatography/tandem mass spectrometry (UHLC/MS/MS2) and gas chromatography/mass spectrometry (GC/MS). In short, the leaf samples were extracted, analyzed on the three MS platforms, ion peaks were matched to standards in a reference library, and their relative levels were quantified. A total of 167 metabolites that matched biochemicals with known structures was detected in the samples (see Supplemental Data Set 1 online). The metabolites were mapped onto general biochemical pathways, as illustrated in the Kyoto Encyclopedia of Genes and Genomes (http://www.genome.jp/kegg/) and the Plant Metabolic Network (http://www.plantcyc.org/). As illustrated in Figure 2, interspecies comparisons were performed at each dehydration level, where possible. Within each species, the metabolomic data for each dehydration level were compared with its fully hydrated control. The full statistical table generated from this analysis is presented in Supplemental Data Set 2 online.
Figure 2.
Statistical Comparison Design.
Statistical comparisons of samples from each species, indicated by arrows, were conducted between hydrated (Hyd), 60, 50, 40, 20, and ~5% RWC (Dry) within S. stapfianus, Hyd and 60% RWC within S. pyramidalis, and Hyd and 60% RWC between species.
[See online article for color version of this figure.]
Metabolomic Differences between S. stapfianus and S. pyramidalis under Fully Hydrated Conditions
From 167 metabolites detected, a number of them had missing values (not detected) in certain experimental samples. This was likely due to biological variations. To build a robust model using partial least squares-discriminant analysis (PLS-DA), we excluded metabolites with missing values in either species and metabolites lacking more than 66% of sample replicates (four or more of six replicates missing), resulting in n = 105 for the hydration state comparisons within S. stapfianus, and for the comparisons between S. stapfianus and S. pyramidalis at 100 and 60% RWC. PLS-DA with three components produced discrete clustering of 100 and 60% RWC treatments for both species (Figure 3; R2 = 0.65, Q2 = 0.61). Different metabolites are clearly responsible for the differences observed in the model, suggesting an obvious metabolic predisposition to water deficit stress at 100% RWC that also persists at 60% RWC. PLS-DA was also performed on S. stapfianus at all hydration states tested, and also revealed clear differentiation among the treatments (see Supplemental Figure 1 online). The supervised classification method produced a slightly less robust model, with three components separating the treatments (R2 = 0.68, Q2 = 0.31).
Figure 3.
Global Metabolite Comparison at 100% Hydrated and at 60% RWC between S. stapfianus and S. pyramidalis.
PLS-DA model is constructed from 105 variables (i.e., metabolites) generating a three-PLS-DA component model with R2 = 65.2, and Q2 = 61.0. S. pyramidalis: squares, 100% hydrated; crosses, 60% RWC. S. stapfianus: asterisks, 100% hydrated; upside down triangles, 60% RWC.
[See online article for color version of this figure.]
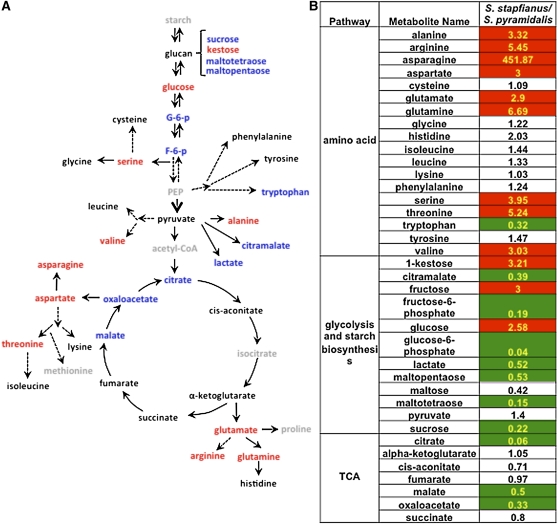
In the comparison of the metabolomic profiles for fully hydrated S. stapfianus and S. pyramidalis, a total of 70 metabolites with significantly altered concentrations (P < 0.05) was identified; 36 had higher concentrations in S. stapfianus and 34 had higher concentrations in S. pyramidalis. From these significant differences, two clear clusters of metabolism emerged that involve 34 of the 70 metabolites: amino acid biosynthesis and energy production (Figure 4).
Figure 4.
Differences of the Metabolites in Glycolysis/TCA Cycle and Amino Acids between S. stapfianus and S. pyramidalis.
(A) Amino acid biosynthetic pathway and glycolysis/TCA cycle. The metabolites in red indicate higher levels in S. stapfianus. The metabolites in blue indicate lower levels in S. stapfianus. The metabolites in black indicate that there were no significant differences between S. stapfianus and S. pyramidalis. The metabolites in gray indicate that they are below detection level (not detected).
(B) Heat map showing the ratio of the metabolite levels between S. stapfianus and S. pyramidalis and their statistical significance of the differences. Cells shaded with red indicate higher levels in S. stapfianus with P < 0.05. Cells shaded with green indicate lower levels in S. stapfianus with P < 0.05. Cells not shaded indicate that the difference between S. stapfianus and S. pyramidalis are not statistically significant (P > 0.05). The number in each cell indicates the fold change between S. stapfianus and S. pyramidalis.
Amino acid biosynthesis in the hydrated state is remarkably different between the two species (Figure 4; see Supplemental Data Set 2 online). To some degree, with the exception of Trp, all amino acids had accumulated to higher concentrations in S. stapfianus. Among the 18 amino acids detected, nine (Ala, Arg, Asn, Asp, Glu, Gln, Ser, Thr, and Val) exhibited significantly higher concentrations (from three- to 451-fold increase) in S. stapfianus than in S. pyramidalis.
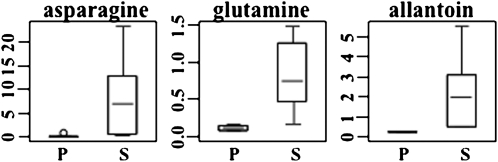
In fully hydrated plants, Asn exhibited the largest difference in concentration between the two species, a 451-fold higher level in S. stapfianus than in S. pyramidalis (Figure 4). In many plants, Asn is the major metabolite for nitrogen storage and transportation, along with allantoin and Gln (Schubert, 1986), which are also in greater abundance, eight- and sixfold, respectively, in S. stapfianus than in S. pyramidalis (Figure 5; see Supplemental Data Set 2).
Figure 5.
The Relative Amounts of Asn, Gln, and Allantoin between S. pyramidalis and S. stapfianus under Fully Hydrated Condition by Box Plots.
The box represents the middle 50% of the distribution and upper and lower whiskers represent the entire spread of the data. The hyphen refers to the median. The outlier determined by the statistical program R, if any, is represented by a circle. The y axis is the median scaled value (relative level). The P values for all comparisons are referenced in the Supplemental Data online. P, S. pyramidalis; S, S. stapfianus.
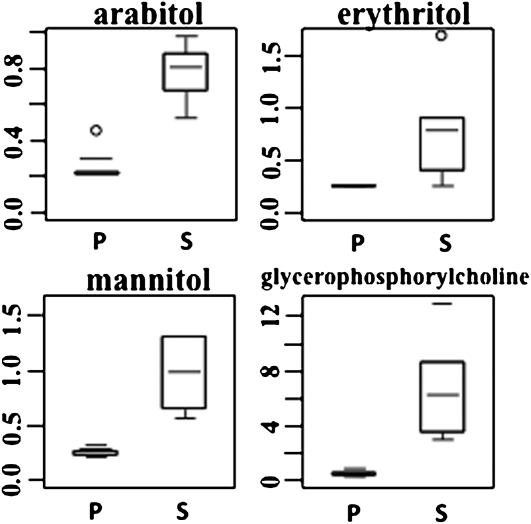
Amino acids are often associated with osmoregulation (Morgan, 1984), and in concordance with this possible role, other osmolytic metabolites also exhibit relatively higher concentrations in S. stapfianus than S. pyramidalis, including a threefold difference in arabitol, erythritol, and mannitol concentrations, although the concentration of galactinol was much lower in S. stapfianus (Figure 6; see Supplemental Data Set 2 online). The concentration of glycerophosphorylcholine, a well-recognized osmolyte in mammalian cells (Neuhofer and Beck, 2005) only sparsely described in plants, also markedly differed between the two species, with an 11-fold greater accumulation in S. stapfianus than in S. pyramidalis (Figure 6).
Figure 6.
The Relative Amounts of Several Osmolytes between S. pyramidalis and S. stapfianus under Fully Hydrated Conditions by Box Plots.
The P values for all comparisons are referenced in the Supplemental Data online. P, S. pyramidalis; S, S. stapfianus.
In energy metabolism, differences in the glycolytic pathway between the two species were observed. Both fructose-6-P and glucose-6-P levels were significantly lower in S. stapfianus, as were the concentrations of Suc, maltotetraose, and maltopentaose, which are key metabolites in starch synthesis. By contrast, the concentrations of Glc, the primary substrate for glycolysis and starch biosynthesis, and kestose, another storage carbohydrate, were higher in S. stapfianus. Downstream of glycolysis, the difference between the two species in energy metabolism is clear. Levels of lactate and citramalate, both derived from pyruvate, were lower in S. stapfianus than in S. pyramidalis. In addition, concentrations of the tricarboxylic acid (TCA) cycle metabolites citrate, malate, and oxaloacetate were significantly lower in S. stapfianus. In summary, the metabolomes of S. stapfianus and S. pyramidalis under fully hydrated conditions were significantly different. S. stapfianus had higher concentrations of osmolytes, lower concentrations of compounds that would indicate a lower apparent rate of energy metabolism, and higher concentrations of nitrogen storage compounds.
Metabolic Regulation during Early Stages of Dehydration
In the initial phase of dehydration, both species were sampled at 60% RWC, a dehydration level that, while nonlethal to both species, did result in a visible dehydration phenotype: leaf-curling in S. stapfianus and wilting and leaf curl in S. pyramidalis. The leaf metabolism of each species responded differently to the imposition to this level of dehydration. Compared with its fully hydrated state, leaves of S. stapfianus displayed an increased abundance of many metabolites, chiefly, putative cellular osmolytes, including amino acids and antioxidants (Figure 7; see Supplemental Data Set 2 online). The amino acids Gly, Ile, Leu, Pro, Trp, Tyr, and Val all increased between two- and fivefold. Sugars such as Fru, Gal, Glc, maltose, raffinose, sophorose, and Suc increased between 1.8-fold and more than 18-fold. The sugar alcohols arabitol and mannitol were elevated 2- and 1.6-fold, respectively, which had not been reported for S. stapfianus in previous studies (Gaff et al., 2009). In addition, β-tocopherol, a strong cellular antioxidant, increased more than threefold, also novel information for this species. By contrast, S. pyramidalis did not appear to respond to dehydration by instigating a significant metabolic shift toward the production of these potentially protective antioxidants and osmolytes, of which there was no apparent statistically significant accumulation. Only the amounts of Pro, Glc, and Fru exhibited substantial elevations, but as none of these reached the threshold of significance set for the analysis, their response to dehydration in S. pyramidalis remains to be validated. The only significant changes in metabolites during the response of S. pyramidalis to dehydration were decreases in various metabolites, most likely due to the suppression of their biosynthesis by dehydration stress (Figure 7; see Supplemental Data Set 2 online).
Figure 7.
Heat Map of Metabolic Responses of S. stapfianus and S. pyramidalis to the Dehydration Stress Reducing RWC from Fully Hydrated to 60%.
Cells shaded with red indicate higher levels in 60% RWC conditions with P < 0.05. Cells shaded with green indicate lower levels in 60% RWC conditions with P < 0.05. Cells not shaded indicate that the difference between 60% and fully hydrated are not statistically significant (P > 0.05). The number in each cell indicates the fold change between 60% and fully hydrated.
Metabolic Regulation during the Late Stages of Dehydration toward Desiccation
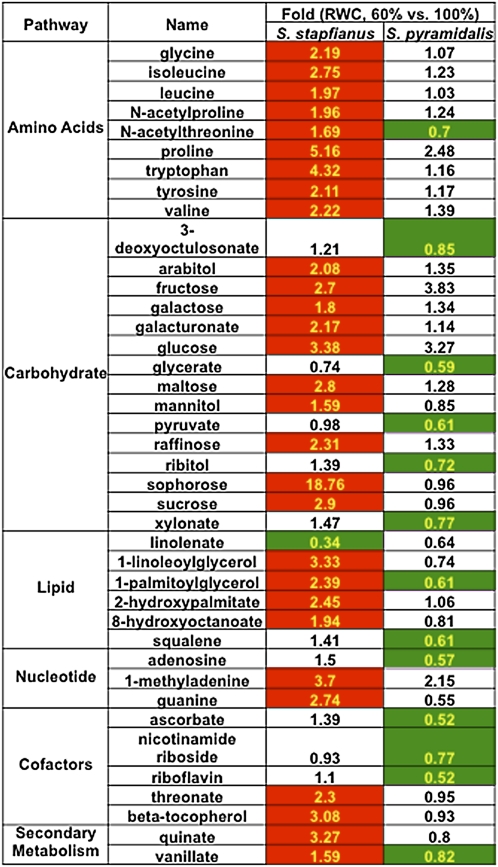
Reduction of leaf RWC below 60% rapidly and severely damages S. pyramidalis leaves, resulting in leaf senescence. If leaves dehydrate to ~40% RWC, then extant leaves of S. pyramidalis do not survive desiccation. Therefore, only the DT S. stapfianus was employed to study the regulation of metabolism during stages of increasing dehydration leading to the fully desiccated state. Samples from S. stapfianus at four additional dehydration states were collected for analysis: 50, 40, and 20% RWC and dry (~5%). Compared with the fully hydrated control state, the total number of metabolites with altered relative abundance increased gradually with decreasing RWC. At the dry stage, over one-half (89 of 167 metabolites) of the metabolome had changed significantly (see Supplemental Data Set 2 online) on a dry weight basis. Mapping the altered metabolites to their respective biochemical pathways clearly indicated that the major responses to severe dehydration were directed toward antioxidant production, continued amino acid production, and an accumulation of several carbohydrates.
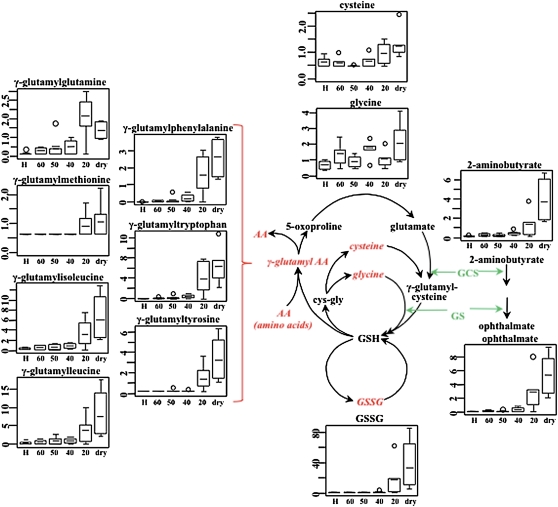
Further dehydration of S. stapfianus leaves beyond 60% RWC resulted in a significant increase in glutathione, predominantly in the oxidized form, primarily late in the drying regimen, to an ~100-fold greater amount in desiccated versus hydrated control tissue (Figure 8; see Supplemental Data Set 2 online). Coincident with the increase in glutathione, its precursors Gly (to threefold) and Cys (to twofold) also accumulated as drying continued.
Figure 8.
Elevation of Glutathione Biosynthesis in S. stapfianus in Response to Desiccation.
The enzymes γ-glutamylcysteine synthetase (GCS) and glutathione synthetase (GS) are shared between glutathione biosynthesis and ophthalmate biosynthesis. The metabolites in red italics indicate higher levels during the dehydration process. The relative levels of the significantly altered metabolites were displayed using box plots. The P values for all comparisons are referenced in the Supplemental Data online. The x axis for each plot is RWC as a percentage. H, fully hydrated sample.
[See online article for color version of this figure.]
The most dramatic increases associated with the glutathione pathway were observed for a number of γ-glutamyl dipeptides that play a role in glutathione recycling via the γ-glutamyl cycle (Grzam et al., 2007). These included, on a dry weight basis: γ-glutamylphenylalanine (up to 1873-fold), γ-glutamyltryptophan (up to 955-fold), and γ-glutamylisoleucine (up to 21-fold). Additional glutathione conjugates with increased relative abundance included γ-glutamyl-Glu, Met, Ile, and Leu (Figure 8).
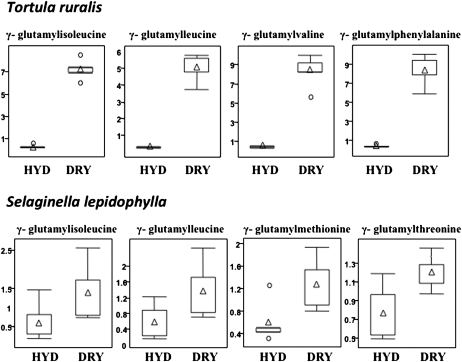
The increase in γ-glutamyl dipeptides during dehydration is a novel discovery and, given the magnitude of the response in S. stapfianus during dehydration, it appeared possible that this might be an important aspect of the mechanism of desiccation tolerance in this plant and perhaps for vegetative desiccation tolerance in general. To explore the latter possibility, we extended the metabolite profile to investigate whether or not γ-glutamyl dipeptides accumulated in response to dehydration in two other species that have long served as models for vegetative desiccation tolerance: the bryophyte T. ruralis (moss) and the lycophyte (spike moss) S. lepidophylla. The results of this analysis are presented in Figure 9. In T. ruralis, the four most dehydration responsive γ-glutamyl dipeptides, as evidenced by a statistically significant increase in accumulation in the dried state, are γ-glutamyl-isoleucine (26-fold), -leucine (19-fold), -valine (20-fold), and -phenylalanine (22-fold). For S. lepidophylla, the accumulation levels are less but still significant for γ-glutamyl-isoleucine (twofold), -leucine (twofold), Met (twofold), and -Thr (1.6-fold).
Figure 9.
The Relative Amounts of γ-Glutamyl Amino Acids in Response to Severe Desiccation in T. Ruralis and Selaginella Lepidophylla By Box Plots.
The P-values for all comparisons are referenced in the Supplemental Data online. The x axis for each plot is RWC as a percentage. HYD, fully hydrated sample; DRY, dried sample.
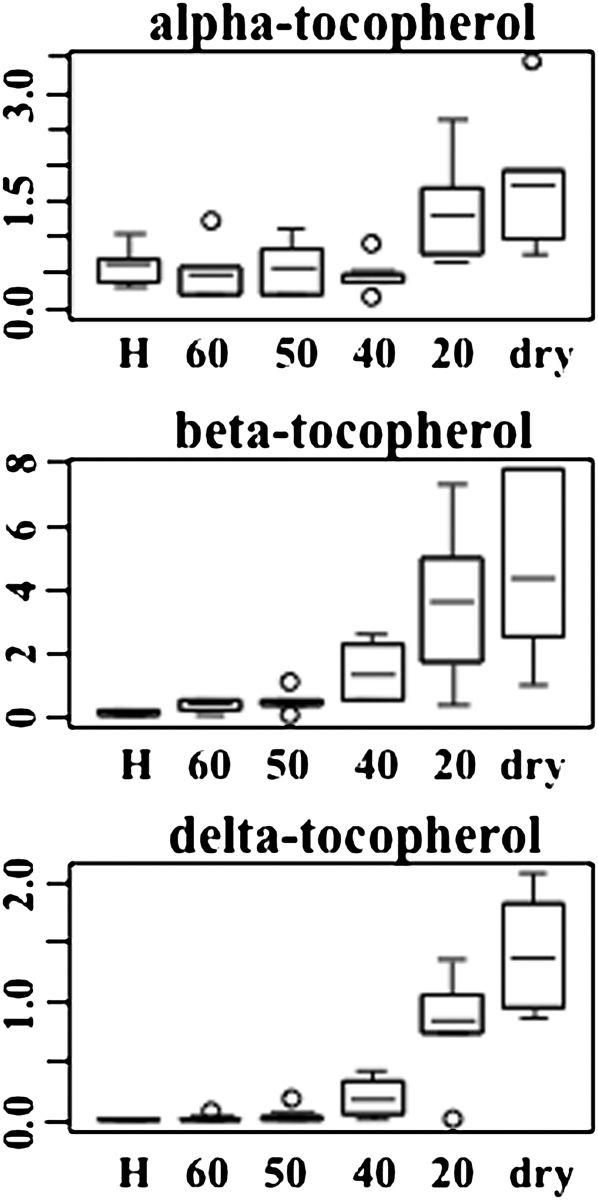
Along with the increased abundance of glutathione pathway metabolite, the concentrations of related compounds ophthalmate and 2-aminobutyrate (Figure 8; see Supplemental Data Set 2 online) also increased during dehydration. Ophthalmate (glu-2-aminobutyrate-gly) is an analog of glutathione that has not been previously reported in plants and has not been demonstrated to have antioxidant properties. Other antioxidants that accumulated in dehydrating S. stapfianus leaves included α-, β-, and δ-tocopherols (2.8-, 35-, and 89-fold, respectively; Figure 10), and the polyamine putrescine (up to twofold) and its precursor agmatine (up to 4.7-fold), which are known to have antioxidant activities that prevent lipid peroxidation. Ascorbate does not appear to be an important antioxidant in S. stapfianus, as its concentrations did not display significant alterations in response to dehydration stress.
Figure 10.
The Relative Amounts of Tocopherols in Response to Severe Desiccation in S. stapfianus by Box Plots.
The P values for all comparisons are referenced in the Supplemental Data online. The x axis for each plot is RWC as a percentage. H, fully hydrated sample.
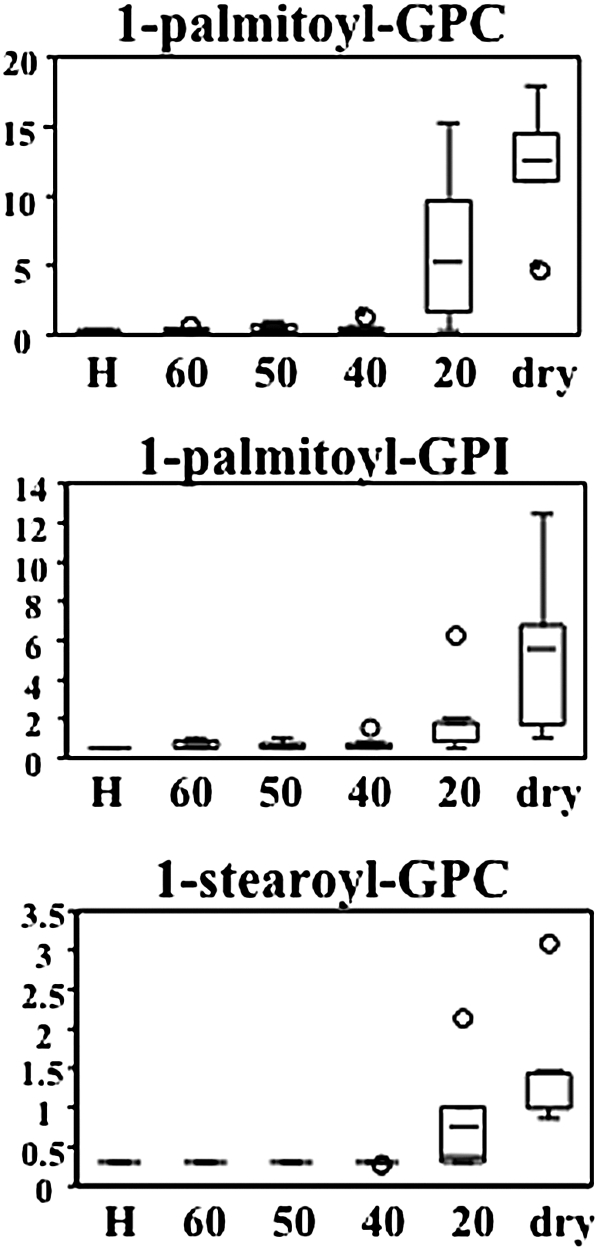
Lysolipids accumulated in the latter stages of dehydration when the RWC of leaves reaches 20% or lower (Figure 11; see Supplemental Data Set 2 online). Of the seven lysolipids identified, 1-palmitoylglycerophospocholine appeared to increase to the greatest degree, a 45-fold elevation, but several others increased between two- and 10-fold above control concentrations. The accumulation of these compounds might have significant ramifications for membrane properties in desiccating tissues.
Figure 11.
The Relative Amounts of Lysolipids in Response to Severe Desiccation in S. stapfianus by Box Plots.
The P values for all comparisons are referenced in the Supplemental Data online. The x axis for each plot is RWC as a percentage. H, fully hydrated sample; GPC, glycerophosphocholine; GPI, glycerophosphoinositol.
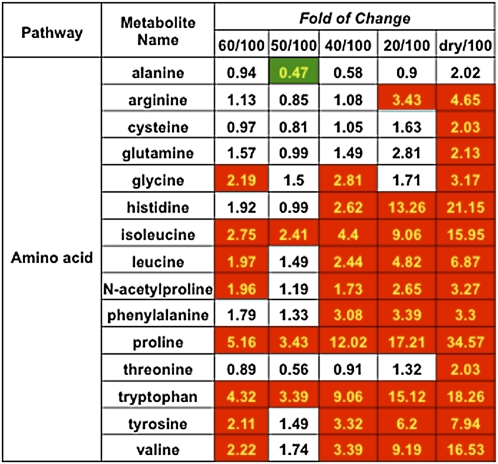
A steady accumulation of many amino acids was observed during the progression of desiccation in S. stapfianus (Figure 12; see Supplemental Data Set 2 online). These included the branched chain amino acids (Ile [up to 16-fold], Leu [up to 6.9-fold], and Val [up to 16.5-fold]), and the aromatic amino acids (Phe [up to 3.3-fold], Trp [up to 18-fold], and Tyr [up to eightfold]). The most prominent amino acid accumulations were for Pro (up to 34.5-fold) and His (up to 21-fold).
Figure 12.
Heat Map of the Amino Acid Changes in S. stapfianus in Response to the Dehydration.
There were five dehydration stages to reduce RWC to 60, 50, 40, and 20% and dry. Cells shaded with red indicate higher levels in comparison to 100% RWC conditions, with P < 0.05. Cells shaded with green indicate lower levels in comparison to 100% RWC conditions, with P < 0.05. Cells not shaded indicate that the differences were not statistically significant (P > 0.05). The number in each cell indicates the fold change.
The nitrogen storage and ammonia capture metabolites Asn (which was in greater abundance in S. stapfianus than in S. pyramidalis), allantoin, and Gln also exhibited a positive response to dehydration (Figure 13). Changes in these metabolites were not readily identified by the statistical analysis, possibly obscured by the wide dynamic range of these metabolites in the fully hydrated group. Asn decreased rapidly during initial dehydration to 60% RWC. During later stages of dehydration, Asn accumulated dramatically, with allantoin and Gln exhibiting a similar pattern.
Figure 13.
The Relative Amounts of Nitrogen Storage Metabolites in Response to Severe Desiccation in S. stapfianus by Box Plots.
The P values for all comparisons are referenced in the Supplemental Data online. The x axis for each plot is RWC as a percentage. H, fully hydrated sample.
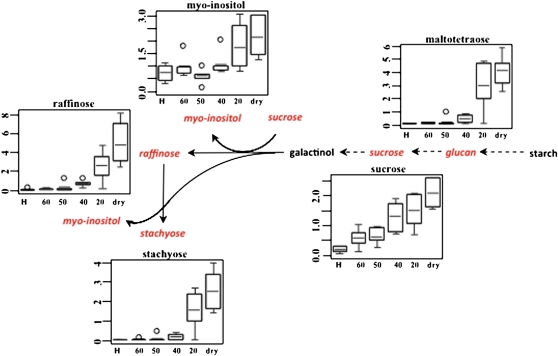
Carbohydrates are another major class of metabolites that accumulate in leaf tissues in response to dehydration in all vegetative DT tracheophytes studied to date (Alpert and Oliver, 2002). In S. stapfianus, as reported earlier (Whittaker et al., 2004), Suc accumulated steadily during dehydration to a maximum elevation of 10.5-fold above control concentrations in the desiccated state (Figure 14, see Supplemental Data Set 2 online). Raffinose and stachyose also gradually increased over the course of dehydration, and reached 74- and 62-fold increases in concentration, respectively, above the hydrated control. Significant increases were also observed for maltotetraose (up to 46-fold) and myo-inositol (up to threefold), which are likely synthesized from glucose-6-phosphate, which might store phosphate during the dehydration process.
Figure 14.
Elevation of Raffinose Pathways in S. stapfianus in Response to Severe Desiccation.
The metabolites in red indicate higher amounts during the dehydration process of reducing RWC. The relative abundance of the significantly altered metabolites was displayed using box plots. The P values for all comparisons are referenced in the Supplemental Data online. The x axis for each plot is RWC as a percentage. H, fully hydrated sample.
[See online article for color version of this figure.]
DISCUSSION
The Hydrated State
The contrast between the responses of two closely related grass species to dehydration, one DT and the other DS, forms a natural experiment in comparative stress biology. This natural experiment highlights the adaptive significance of particular processes within the fundamental trait of desiccation tolerance, as evolved in the monocotyledonous resurrection plant, S. stapfianus, a C4 grass. Sporobolus is one of the few plant genera containing resurrection species that offers a close sister-group contrast, and as such, presents a unique opportunity to study the essential aspects of this complex trait.
Our large-scale metabolite profiles of the young leaves of these two species in their respective fully hydrated state demonstrate that the metabolic disposition for each species is very distinct. S. pyramidalis accumulates several metabolites of the glycolytic pathway and the TCA cycle in greater abundance than does S. stapfianus, along with a greater accumulation of metabolites involved in starch biosynthesis (Figure 4). The accumulation of these metabolites indicates a greater emphasis on energy metabolism and growth compared with S. stapfianus. This is reflected in the relative growth rates of the two species: S. pyramidalis grows at a faster rate and generally produces greater biomass than S. stapfianus at all stages of development (Wood and Gaff, 1989; J.C. Cushman and M.J. Oliver, unpublished data). By contrast, S. stapfianus focuses its metabolism on amino acid biosynthesis and on maintaining a certain level of sugar alcohols and other osmolytes, such as the methylamine glycerophosphorylcholine (Figures 4 and 5). This flow of nitrogen into amino acid metabolism and a lesser emphasis on energy metabolism might account for the lower growth rate of S. stapfianus compared with its DS sister species. S. stapfianus might also have a lower baseline metabolic rate, with less emphasis on energy metabolism and a lower growth rate than S. pyramidalis. This tendency might play a part in its overall desiccation tolerance strategy; the lower the baseline metabolic rate, the less disruption of metabolism that can occur during dehydration, a hypothesis requiring more study.
The apparent focus on amino acid metabolism and the greater accumulation of sugar alcohols and other compatible solutes in S. stapfianus compared with S. pyramidalis strongly suggest that this species is primed to resist dehydration by accumulating osmolytes in its leaves to inhibit loss of water from the plant as the soil dries. The observation that S. stapfianus leaves, in general, have lower water contents on a gram to gram dry weight basis (Figure 1b), and lower leaf water potential (data not shown), than S. pyramidalis under fully hydrated conditions supports this suggestion. DT angiosperms are known to require time to establish desiccation tolerance within their leaves, and thus must dry slowly in order to survive (Alpert and Oliver, 2002). The higher concentrations of amino acids, sugar alcohols, and other compatible solutes in S. stapfianus are thought to reduce the rate at which water is lost from the plant. This inference is supported by the more rapid initiation and more precipitous rate of water loss following soil depletion observed for S. pyramidalis compared with S. stapfianus (Figure 1a). A greater emphasis on compatible solute metabolism in the fully hydrated state, where the plant is primed to delay water loss during dehydration, could be an important component of desiccation tolerance in this species. A comparison between old leaves (DS) and young leaves (DT) of S. stapfianus demonstrated that amino acid concentrations were identical in the fully hydrated state (Martinelli et al., 2007a), indicating that the loss of desiccation tolerance as leaves mature is probably not because they lose their ability to slow water loss, but could be due to other factors. High Asn concentrations were also seen in both young and old leaves of hydrated S. stapfianus, as noted in our study, which is indicative of a large capacity for nitrogen transport within the plant, presumably from the soil. The comparison with S. pyramidalis suggests that S. stapfianus is better able to extract nitrogen from the soil and/or move it around the plant.
The emphasis on amino acid metabolism, perhaps indicating the storage of these metabolites during the hydrated state in S. stapfianus, implies not only that this grass is primed to resist dehydration and associated damage, but also that it possesses a growth strategy with major implications for the ecological adaptation and evolution of this species. DT Sporobolus species are typically found growing in shallow depressions on rocky outcrops known as inselbergs (Porembski and Barthlott, 2000), which are generally low in organic matter and consequently nitrogen poor (Burke, 2002). The ability to emphasize nitrogen metabolism, when fully hydrated, may simply reflect the superior ability of S. stapfianus, inferred from our studies where nitrogen was not limiting, to make use of available nitrogen, and thus better survive in the ecological niche in which it evolved. The ability to store nitrogen under nitrogen-limiting conditions also suggests an adaptive storage pattern for DT species, perhaps as a strategy for recovery from catastrophe, as described by Chapin et al. (1990). Moreover, the higher concentrations of these nitrogenous compounds might provide a natural advantage for the metabolic regulation required to protect against desiccation. As discussed below, many dehydration responses, such as the synthesis of amino acids and the antioxidant glutathione, require intensive nitrogen supply. By having an elevated, intrinsic nitrogen storage pool, S. stapfianus can produce these cellular protective compounds quickly and efficiently.
Initial Response to Dehydration
As can be seen in Figure 1, and as discussed above, these two species differ in their dehydration kinetics following the cessation of watering, although the variability between plants grown in pots in a greenhouse might mask those differences. S. stapfianus appeared to delay the crash in water content and slowed its progression to equilibrium.
S. pyramidalis exhibited little in the way of a metabolic response to dehydration stress, despite experiencing a level of water loss that causes a visible phenotype (leaf wilt and curling). The increase in Pro, Fru, and Glc concentrations, although significant at P of 0.05 or less, does suggest an attempt to osmoregulate, maintain turgor, and slow water loss (Figure 7). Pro accumulation as a means of osmoregulation is one of a number of mechanisms by which plants limit water loss when subjected to a water deficit that occurs either by soil dehydration, or salt or cold stress (Bray, 1997). This is a response common among many species within the Sporobolus genus, including S. stapfianus (Tymms and Gaff, 1979; Martinelli et al., 2007a), S. pyramidalis, S. elongatus (Ghasempour and Kianian, 2007), and S. virginicus (Naidoo and Mundree, 1993). Pro accumulation can also help in removing hydroxyl radicals (Akashi et al., 2001).
The accumulation of soluble sugars in response to water deficit is also associated with osmoregulation (Morgan, 1984). The increase in Fru and Glc observed here is reminiscent of the response of soybean hypocotyls to dehydration stress, in which 70% of the solutes accumulating in response to water deficit were Fru, Glc, and free amino acids (Meyer and Boyer, 1981). Accumulation of Fru has also been associated with the protection of membranes from oxidative stress in response to chilling temperatures, a dehydrating stress (Bogdanović et al., 2008). As shown in this study, S. pyramidalis clearly responds to dehydration metabolically in a manner common to dehydration-sensitive species, slowing and limiting water loss through osmoregulation and perhaps limiting oxidative damage. That the metabolic response of S. pyramidalis to dehydration is not strong, at a RWC of 60%, might account for its relative sensitivity.
By contrast, S. stapfianus quickly responds metabolically to dehydration by increasing concentrations of several metabolites above those seen in the hydrated control (Figure 7). A large part of this initial response involves an increase in several free amino acids, including Pro, and an array of carbohydrates including mono-, di-, and trisaccharides and sugar alcohols, a novel observation for S. stapfianus, compared with S. pyramidalis. Similar increases in amino acids and carbohydrates in response to dehydration have been noted for S. stapfianus, particularly in detailed studies by Martinelli et al. (2007a) and Whittaker et al. (2007). These authors concluded that during the early response to dehydration, while RWC is high, metabolism is focused on osmoregulation and the need to limit or reduce the rate at which water is lost from the plant. Many free amino acids might serve as compatible solutes for osmoregulation, as do soluble sugars (Morgan, 1984, Rhodes et al., 1986, Cushman, 2001). By studying the effect of dehydration on the carbon-to-nitrogen balance, Whittaker et al. (2007) suggested that when the RWC is within the 100 to 70% range, photosynthesis and starch breakdown provide the carbon needed for the buildup of these metabolites. Our data, along with similar data reported by Martinelli et al. (2007a), indicate that the initial buildup in amino acids is fueled by the high concentrations of Asn found within fully hydrated leaves, a significant mobile nitrogen source that is not present in S. pyramidalis. Maintenance of elevated concentrations of Asn in hydrated leaves as a feedstock for the increase in nitrogenous osmolytes might allow this and other resurrection species to slow water loss sufficiently enough to allow the adaptive cellular protection aspects of desiccation tolerance to be established. However, Martinelli et al. (2007a) argued that the initial loss of Asn from the leaves during the early dehydration phases was the result of bulk transport of nitrogen to the root as a metabolic response to stress. This might be the case, but we contend that the high level of leaf Asn could allow for, at least in part, the generation of an osmotic response in the shoot.
Although there was not a major antioxidant response associated with the initial dehydration step to 60% RWC in S. stapfianus, there was an early, significant increase in the lipophilic antioxidant β-tocopherol (an approximate threefold increase; Figure 10). This response was not evident in S. pyramidalis, and this too might account for its inability to survive severe dehydration. For S. stapfianus, the increase in tocopherol might indicate a need to protect membranes from oxidative damage, but might also signal the initiation of the programmed metabolic response that prepares the plant for desiccation, given what we observed when dehydration drops below 40% RWC. Clearly, when the two species are exposed to a significant dehydration event that results in a loss of 40% of bulk water (60% RWC), they generated a very different metabolic response in their leaf tissues. S. pyramidalis, for which this is a relatively severe stress, responded with a weak metabolic attempt to osmoregulate, similar to what is seen in many drought-sensitive plants. S. stapfianus, already primed to modulate the rate at which water is lost from the plant, responded strongly by increasing its osmoregulatory capabilities and further slowing water loss. Given what occurs as dehydration continues, this early response to dehydration appears to be the start of a metabolic program that prepares the plant for desiccation. Thus, at least within this genus, desiccation tolerance appears not to be simply an elaboration of the type of response to water deficit so well described for sensitive species (Hanson and Hitz, 1982; Bray 1997), but rather represents a novel metabolic regulatory strategy.
Preparation for Desiccation
The metabolic responses of leaves of DT S. stapfianus have received a great deal of attention, more so than any other resurrection species studied to this point (Gaff et al., 2009). Much of this work has been accomplished without the advantage of modern metabolomic techniques as reported here, thus, this study builds upon a solid base of work and offers some novel insights into a relatively well-understood process.
In our study, 40% RWC appears to be an important dehydration threshold where the majority of the metabolites that respond to dehydration start to accumulate at an increasing rate, peaking at 20% RWC. Leading up to this point, the cells are thought to initiate the major cellular protection programs, as water loss reaches a state where irreversible cellular damage becomes imminent and metabolism shifts to focus significant resources to protect cells from the stress and survive.
For most resurrection species, dehydration toward desiccation results in a substantial increase in compatible solutes and a major metabolic focus on strategies to prevent or nullify oxidative damage (Vicré et al., 2004; Farrant et al., 2007; França et al., 2007), and S. stapfianus is no exception (Sgheiri et al., 1997; Whittaker et al., 2001, 2004, 2007; Martinelli et al., 2007a, 2007b; Martinelli, 2008). Our data demonstrate that accumulation of amino acids to concentrations far exceeding those seen in hydrated controls begins in the early dehydration phases, and continues to increase as leaves reach the desiccated state. The majority of amino acids accumulate in dehydrating young leaves (Martinelli et al., 2007a). While the general pattern of accumulation tends to be similar for each amino acid, Asn concentrations in our study increased, although not significantly below 40% RWC. However, all of the other amino acids continued to accumulate in comparison to controls at RWC below 40%. This observation differs from that of the Martinelli study (Martinelli et al. 2007a) wherein Asn concentrations recovered during the last stages of dehydration and the level of most other amino acids declined at RWC below 30%. The former observation is a significant distinction, as Martinelli et al. (2007a) concluded that at late stages, between 35 and 6% RWC, accumulation of Asn and Arg is important and serves as nitrogen and carbon reservoirs for protein synthesis and growth upon rehydration. This difference might simply reflect natural variation among genotypes or drying conditions. As will be discussed, there appears to be an ample source of nitrogen for growth upon recovery.
The accumulation of amino acids in the stages of dehydration leading up to the desiccated state cannot be attributed to preexisting pools of transportable Asn because these have been depleted during the initial accumulation of amino acids in the early drying stage, as discussed earlier. Martinelli et al. (2007a) suggested that the buildup of amino acids during dehydration is fueled by nitrogen released from protein breakdown, largely from chloroplastic proteins, including ribulose-1,5-bisphosphate carboxylase/oxygenase in old leaves, which are not DT and subsequently senesce. Young, DT leaves also lose some protein during drying, but older leaves lose up to 50% of their soluble proteins during dehydration (Martinelli et al., 2007b). This protein mobilization is thought to occur via a series of transamination reactions that utilize the proteolysis-derived amino acids to generate an increase in the glutamate pool, which in turn serves as substrate for glutamate dehydrogenase (GDH), generating ammonia and 2-oxoglutarate. The released ammonia is used as a substrate by Gln synthetase 1 to produce Gln for transport (Martinelli et al., 2007a: Masclaux-Daubresse et al., 2010). However, in this study, we do not see a significant accumulation of Glu or Gln until dehydration is almost complete at 20% RWC and below. In the Martinelli et al. (2007a) study, Glu did increase in the initial stages of dehydration, but declined steadily as drying continued; Gln concentrations were so low that they were not reported. Martinelli et al. (2007a) argued that Gln is channeled into Asn and Arg in young leaves, and they reported a modest increase in these amino acids late in the dehydration process. We observed no significant change in Asn, and only modest, but significant increases in Arg, Gln, and Glu in the latter stages of drying. These data support the suggestion that Gln is channeled into other amino acids for nitrogen reassimilation in Sporobolus, but other mechanisms for mobilization could also exist.
If GDH is involved in the reassimilation of nitrogen from proteolysis, it is possible that ammonia concentrations could rise in leaf cells as water is lost (Skopelitis et al., 2006). GDH is relatively stable in S. stapfianus during desiccation (Martinelli et al., 2007a), and ammonia generation was observed to increase substantially at RWC below 30%. Photorespiration is another possible source of ammonia and is active throughout much of the dehydration phase leading to desiccation, but more so early in the dehydration process (Martinelli et al., 2007b). This stress-induced increase in ammonia is potentially toxic to the leaf cells (Lutts et al., 1999: Skopelitis et al., 2006). S. stapfianus apparently responds to intracellular hyperammonia accumulation by a slight elevation in the synthesis of Asn and Gln, and by a corresponding depression in allantoin biosynthesis, a pathway that generates ammonia.
Protein degradation likely involves, in part, the proteosome pathway and ubiquitination, as polyubiquitin transcripts rapidly accumulate during dehydration in S. stapfianus leaves (O’Mahony and Oliver, 1999). The remobilization of nitrogen from senescent leaves is a common occurrence in plants (Masclaux-Daubresse et al., 2010) and serves to conserve valuable resources and energy. In S. stapfianus, and perhaps other DT plants, the sacrifice of older leaves by initiation of senescence upon drying and tight control of nitrogen metabolism might be integral to the overall strategy of survival in environments where dehydration extremes are relatively commonplace and nitrogen is a limited resource.
Our results show clearly that nitrogen metabolism and protection from oxidative stress are tightly linked in S. stapfianus as it dries beyond 40% RWC (Figure 9). Dehydration- or desiccation-induced accumulation of ROS has been well documented (Mittler, 2002; França et al., 2007). Protection against ROS activity, which can lead to phospholipid, protein, and nucleic acid damage (França et al., 2007), appears to be delivered principally by the glutathione biosynthesis pathway in S. stapfianus (this study; Navari-Izzo et al., 1997). The role of the glutathione biosynthetic pathway in protecting cells from the activity of ROS is a common finding for resurrection species (Farrant, 2007). Interestingly, we report the occurrence of the glutathione analog, ophthalmate, in S. stapfianus. The existence and biosynthesis of ophthalmate were recently established in mammalian systems (Soga et al., 2006) and although its biological function is uncertain, it is synthesized from 2-aminobutyrate by γ-glutamylcysteine synthetase and glutathione synthetase, two enzymes also responsible for glutathione production (Figure 9). In mammalian systems, ophthalmate is proposed as a marker for glutathione production and oxidative stress. Our result indicates that the ophthalmate pathway exists in plants and, as in mammalian systems, ophthalmate production mirrors glutathione biosynthesis, the significance of which, as stated previously, is as yet unclear.
Apart from an increased accumulation of oxidized glutathione, presumably the product of oxidative damage repair, and the intermediates Gly and Cys, there was a major increase in the concentrations of γ-glutamyl-amino acids during dehydration, a phenomenon not recorded until now (Figure 9; see Supplemental Table 1 online). The γ-glutamyl amino acids are derived from the transfer of γ-glutamine from glutathione (GSH) to an amino acid molecule via the activity of the enzyme γ-glutamyl transpeptidase (GGT), the initial step in the glutathione recycling pathway. γ-Glutamyl cyclotransferase (GGC) converts γ-glutamyl amino acids into 5-oxoproline (5OP), which cycles to form GSH, and releases the free amino acid. In Arabidopsis, this is a minor pathway for the recycling of GSH (Ohkama-Ohtsu et al., 2008), and the majority of GSH is recycled in the cytoplasm by conversion to 5OP via a combination of GGC and 5-oxoprolinase. In animal systems, GSH recycling via synthesis of γ-glutamyl-amino acids occurs in the extracellular spaces between cells utilizing a plasma membrane-bound GGT. In this manner, animal cells are able to utilize amino acids that are outside of the cell to form γ-glutamyl-amino acids that are then transported into the cells and then converted to 5OP by GGC in the cytoplasm, releasing the amino acid into the cell. In this way, the GSH recycling pathway is thought to be an important route for amino acid reuptake into animal cells (Meister and Larsson, 1995). The dramatic increase in γ-glutamyl-amino acids (up to 1800-fold relative to control concentrations in the case of γ-glutamylphenylalanine) would tend to suggest that S. stapfianus does not follow the Arabidopsis model, and recycles GSH in a mechanism not unlike that described for animal systems. Interestingly, GGT transcripts increase significantly during dehydration of S. stapfianus leaves (M.J. Oliver, unpublished data). The accumulated γ-glutamyl-amino acids could play a dual role in recycling of GSH for protection from the effects of ROS and the storage of nitrogen in young leaves, in a manner similar to that suggested for seeds (Higgins and Payne, 1982).
The increase in the accumulation of γ-glutamyl-amino acids during dehydration, as a trait, might be a common and ancient feature of vegetative responses to desiccation in DT species. This is suggested by their accumulation in dried T. ruralis (Figure 8), a bryophyte that exhibits a mechanism of desiccation that is long thought to reflect the desiccation tolerance mechanisms that were present in the first plants that transitioned from water to land, and S. lepidophylla (Figure 8), a DT plant from the first clade that evolved vegetative DT after it was lost from land plants following the establishment of the tracheophytes (Oliver et al., 2000, 2005). The accumulation of γ-glutamyl-amino acids in vegetative DT plants might not be directly related to desiccation tolerance per se, but it might reflect a close relationship between cellular protection strategies that are integral to desiccation tolerance mechanisms and nitrogen storage and remobilization pathways that are essential to reinitiate growth following a dehydration event.
Along with the emphasis on glutathione biosynthesis to counteract oxidative stress, S. stapfianus also accumulates tocopherols, as mentioned above. The accumulation of tocopherols increased significantly as dehydration exceeded 40% RWC, coincident with the need to protect membrane integrity during drying (Figure 10). Tocopherols are lipid-soluble antioxidants that prevent the proliferation of lipid peroxidation in membranes (Munné-Bosch and Alegre, 2002) that is critical for membrane maintenance in the dried state. Protection of membrane integrity during desiccation has long been discussed as an important aspect of desiccation tolerance in plants (Hoekstra et al., 1997; Walters et al., 2002). Nevertheless, it is also clear that desiccation resulted in an elevation in lysolipid content compared with hydrated controls (Figure 11), suggesting that some degree of membrane damage might occur. In animal systems, lysolipids are formed via the activity of phospholipases that target lipids that are damaged by peroxidation (Atkinson et al., 2008), and in plants, they have been associated with stress and cell ageing (van Bilsen and Hoekstra, 1993; Welti et al., 2002). The presence of lysolipids in biological membranes alters the biophysical properties of the lipid bilayers such as fluidity, permeability, and bending properties, and in sufficient concentrations, they are toxic to the membrane (Atkinson et al., 2008; Howland and Parikh, 2010). Tocopherols associate with lysolipids in the membranes, and counteract their effects, as well as prevent oxidative attack on the target phospholipids (Atkinson et al., 2008).
Last, our results fully support the importance of soluble sugars in the preparation of S. stapfianus leaves to withstand and survive the desiccated state. As reported by others (Ghasempour et al., 1998; Whittaker et al., 2001, 2004, 2007), we observed a general increase in Suc above that found in the hydrated controls, together with a late reduction in Fru and Glc levels (Figures 4 and 14). Whittaker et al. (2007) determined that the early increase in carbohydrates is derived from photosynthesis and starch reserves, but that the later accumulation of Suc is derived from phosphorylation of hexose, driven by an increase in Suc phosphate synthase activity and protein. Suc is thought to be derived from photosynthate (starch) stored when plants are hydrated and active, but some DT species, including C. plantagineum, C. wilmsii, and Lindernia brevidens, store 2-octulose when hydrated, which is converted to Suc during dehydration (Bianchi et al., 1991; Cooper and Farrant, 2002; Phillips et al., 2008). Increases in raffinose and stachyose have also been reported for S. stapfianus (Ghasempour et al., 1998) and have often been linked with protection mechanisms in desiccating plant tissues (Farrant, 2007). In our experiments, we also saw an elevation in myo-inositol, a precursor for raffinose and stachyose and an effective free radical scavenger (Smirnoff, 1993), and maltotetraose, a starch breakdown product (Figure 14). This observed buildup of maltotetraose might simply be a product of an inhibition of starch breakdown as dehydration deepens; however, the buildup of a reducing sugar is generally considered detrimental to desiccating tissues, as these compounds enhance ROS activity (Obendorf, 1997).
In most resurrection plants, Suc concentrations correlate strongly with the ability to survive desiccation, whereas other oligosaccharides apparently play only a minor role. Sugars are widely believed to protect cellular structures from mechanical and metabolic stresses during dehydration. Their protective attributes can be explained by the osmotic and volumetric properties of the sugars, acting as osmotic spacers that prevent membrane fusion and reduce compressive stresses that cause damage (Bryant et al., 2001; Koster and Bryant, 2006). Sugars and complex polysaccharides can also scavenge ROS (Smirnoff, 1993; Van den Ende and Valluru, 2009) that are often associated with metabolic imbalances induced by dehydration.
Conclusions
The strategy of using a sister-group contrast for metabolite profiling has revealed some important features of the mechanism by which S. stapfianus achieves desiccation tolerance of its vegetative tissues. The comparison between the metabolomes of fully hydrated young leaf tissue from the two sister species uncovered the metabolic predisposition for dehydration tolerance and perhaps a greater capacity to resist water loss in the DT S. stapfianus. As dehydration stress is imposed on the two species, the young leaves of S. stapfianus respond quickly to produce more osmolytes and lipid-soluble antioxidants to further slow the loss of water and to protect the cells from membrane damage resulting from lipid peroxidation. The DS S. pyramidalis has only a minimal capacity to respond to the dehydration event and likely succumbs to the stress as a result. These findings lead to the hypothesis that S. stapfianus, presumably because of selection pressure in the form of long periods of dryness in its natural habitat, has evolved a mechanism of desiccation tolerance that involves a metabolic readiness for a drying event coupled with the ability to rapidly respond to dehydration when it occurs. This response, at least in part, might slow the rate of water loss from the plant to enable the initiation of a metabolic program designed to prepare the plant for desiccation.
The metabolic profiles of S. stapfianus leaves as they dry indicated that the metabolic program that leads to desiccation tolerance is a complex one that is intertwined with an apparent need to retain and remobilize important nutrients, particularly nitrogen, from senescing older leaves that are sensitive to desiccation, for tissue revival and growth when the plants rehydrate. One novel aspect of the metabolome of dehydrating S. stapfianus is the substantial increase in abundance of γ-glutamyl peptide conjugates, indicating that, at least in this grass, GSH recycling involves transpeptidases and amino acid uptake into cells in a manner similar to that found in animal systems. This pathway, in contrast to the GSH recycling pathway in Arabidopsis, provides a substantive link between nitrogen remobilization and antioxidant synthesis (via glutamate), which appears to be an integral part of the desiccation tolerance mechanism of S. stapfianus. The metabolic regulation that occurs during desiccation involves a significant investment in protection from oxidative stress (ROS) via glutathione and lipid-soluble antioxidants. In addition, the metabolome of desiccating S. stapfianus also revealed a need for detoxification of ammonia, presumably generated by nitrogen mobilization and GDH activity. The attainment of desiccation tolerance in S. stapfianus also appears to require a large investment of carbon, in the form of soluble sugars, to establish a cellular milieu that protects cellular integrity and infrastructure as the cells dry. Although this metabolic feature, along with establishing a cellular environment to resist oxidative damage, is common to all resurrection plants (Cushman and Oliver, 2010; Oliver et al., 2010), S. stapfianus exhibits some metabolic idiosyncrasies that might be unique to this resurrection plant, such as the increase in abundance of ophthalmate, a metabolite that has not been reported in other plant species to date.
METHODS
Plant Material and Stress Treatments
Sporobolus stapfianus (original provenance: Verena, Transvaal, South Africa) and Sporobolus pyramidalis (also known as Sporobolus indicus var pyramidalis), accession number PI310012 (National Plant Germplasm System: Germplasm Resource Information Network) were grown, maintained, and seed stock increased as described by O’Mahony and Oliver (1999) in one-gallon pots under greenhouse conditions (16-h light and day/night temperatures of 28°C/19°C). The PI310012 accession of S. pyramidalis was chosen from a number of accessions for its germination rate and its definitive sensitivity to desiccation (all plants die when desiccated). Three-month-old plants were subjected to a drying event by withholding water. Young leaf tissue was collected at regular intervals from individual plants and flash frozen in liquid nitrogen and stored at −80°C. Six replicate samples, from individual plants, were collected for each dehydration step representing fully hydrated tissue, 60% RWC for both species, 50, 40, and 20% RWC, and desiccated. The samples were ground in liquid nitrogen using a ceramic mortar and pestle under liquid nitrogen with the addition of a known weight of silica sand. The samples were lyophilized, which allowed us to normalize the samples on a dry weight basis, and were again stored frozen at −80°C prior to extraction.
Duplicate samples were taken to allow for the determination of the RWC of the plant at time of sampling. RWC was calculated as the formula (Fwt − Dwt) (FTwt − Dwt), where Fwt was the fresh sample weight, FTwt was the full turgor weight of the same sample after submersion in deionized water overnight in the dark, and Dwt was the weight of the sample after being dried at 65°C for 4 h.
Tortula ruralis was dried by placing fresh gametophytes of known weights on a nylon mesh in a closed chamber over a stirred saturated solution of sodium nitrite at 20°C (relative humidity of 66%) for 24 h. Using this regime, the air-dried weight (~20% of original fresh weight) was obtained within 6 h (Oliver, 1991).
Selaginella lepidophylla fully hydrated plants were air-dried in an atmosphere of 37% relative humidity, 27°C, and 175 μM M−2 S−1 for 24 h by which time the plants had reached a constant weight.
Metabolomic Profiling Platform
The global unbiased metabolic profiling platform was based on a combination of three independent platforms: UHLC/MS/MS2 optimized for basic species, UHLC/MS/MS2 optimized for acidic species, and GC/MS. This platform was described in detail in a previous publication (Evans et al., 2009). The samples were extracted, analyzed with the three instruments, and their ion features were matched against a chemical library for identification. The major components of the process are summarized below.
For sample extraction, 20 mg of each leaf sample was thawed on ice and extracted using an automated MicroLab STAR system (Hamilton Company) in 400 μL of methanol containing recovery standards.
UPLC/MS was performed using a Waters Acquity UHPLC (Waters Corporation) coupled to an LTQ mass spectrometer (Thermo Fisher Scientific Inc.) equipped with an electrospray ionization source. Two separate UHPLC/MS injections were performed on each sample: one optimized for positive ions and one for negative ions. Derivatized samples for GC/MS were analyzed on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole MS operated at unit mass resolving power. Chromatographic separation, followed by full-scan mass spectra, was performed to record retention time, molecular weight (m/z), and MS/MS2 of all detectable ions present in the samples.
Metabolites were identified by automated comparison of the ion features in the experimental samples to a reference library of chemical standard entries that included retention time, molecular weight (m/z), preferred adducts, and in-source fragments, as well as their associated MS/MS2 spectra. This library allowed the rapid identification of metabolites in the experimental samples with high confidence. Comparison of experimental samples to process blanks (water only) and solvent blanks allowed the removal of artifactual peaks.
Data Imputation and Statistical Analysis
The samples were analyzed over the course of 2 d. After the data were corrected for minor variations resulting from instrument interday tuning differences (Evans et al., 2009), the missing values for a given metabolite were assigned the observed minimum detection value, based on the assumption that the missing values were below the limits of detection. For the convenience of data visualization, the raw area counts for each biochemical were rescaled by dividing each sample value by the median value for the specific biochemical.
Statistical analysis of the data was performed using JMP (SAS, http://www.jmp.com), a commercial software package, and R (http://cran.r-project.org/), a freely available open-source software package. A log transformation was applied to the observed relative concentrations for each biochemical because the variance generally increased as a function of each biochemical’s average response. Welch’s t tests, a variation of Student’s t test for samples with unequal variances, were performed to compare data obtained between experimental groups. The false-positive rate associated with multiple comparisons was calculated using the false discovery rate (FDR) method of Storey and Tibshirani; q values for all tests are included in Supplemental Data Set 2 online. The q values for the vast majority of significant tests (P < 0.05) fell well below the 10% FDR (q < 0.10), the greatest being q = 0.37. In the biochemical pathway analysis, all tests with significance of P < 0.05 were considered without restriction by q value.
Metabolites identified were then imported into to the chemometrics software Solo (Eigenvector Research, Inc.) for PLS-DA to determine classification of the treatments. PLS-DA is an extension of principal component analysis used to model group classification. R2 and Q2 are used as measures for the robustness of a PLS-DA model, where R2 is the fraction of variance explained by a model. Cross-validation of R2 estimates Q2, which explains the fraction of the total variation predicted by the model. Thus, both of these values indicate how well the overall model classifies group membership in a data set. A robust model has an R2 value of 0.50 and a Q2 value of 0.40.
To visualize the entire data set, we generated a heat map to show fold change for each compound identified from GC-MS and LC-MS analyses of the tissue samples (see Supplemental Figure 1 online). Fold change was calculated as the means ratios of each treatment compared with the fully hydrated state. In addition, fold changes in metabolite concentrations were determined for the two [[DRY]] treatments and two partially hydrated treatments. Welch’s two-sample t tests were then used to determine whether each metabolite was significantly increased or decreased in abundance. For five treatments, there was a total of 10 different two-sample t test combinations. We used the FDR to correct for multiple Welch’s two-sample t test comparisons for the hundreds of compounds detected. Metabolite expression studies are very similar to gene array studies with a very large number of statistical comparisons. For gene array studies, the FDR is more commonly used than family-wise error rate adjustments, such as the Bonferroni or Tukey corrections. The FDR for a given set of metabolites is estimated by the q value (Storey, 2002). Box plots were generated for those compounds that showed a significant increase or decrease in both using the Welch two-sample t test and FDR (i.e., P < 0.05 and q < 0.10) significance values.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Global Metabolite Comparison of 100% Hydrated with Five States of Increasing Dehydration in S. stapfianus.
Supplemental Data Set 1. Metabolites Detected in This Research.
Supplemental Data Set 2. Metabolomic Profiles for S. stapfianus and S. pyramidalis, within and between Species Comparisons.
Acknowledgments
We would like to acknowledge the expert technical assistance of Jim Elder in the preparation and growth of the plant material used in this study and the meticulous water content measurements of the tissues. This work was partially supported by a grant from the USDA National Institute of Food and Agriculture to M.J.O. and J.C.C. (Grant 2007-55100-18374). We would also like to thank Abou Yobi for Selaginella samples, Karen Koster and Dan Bush for their contributions to the discussions that helped interpret the meaning of some of the metabolic changes observed in this study, and Mary Ann Cushman for her helpful and clarifying comments on the manuscript. The mention of a trademark or proprietary product does not constitute a guarantee or warranty of the product by the USDA and does not imply its approval to the exclusion of other products that may also be suitable.
References
- Akashi K., Miyake C., Yokota A. (2001). Citrulline, a novel compatible solute in drought-tolerant wild watermelon leaves, is an efficient hydroxyl radical scavenger. FEBS Lett. 508: 438–442 [DOI] [PubMed] [Google Scholar]
- Alamillo J.M., Bartels D. (2001). Effects of desiccation on photosynthesis pigments and the ELIP-like dsp 22 protein complexes in the resurrection plant Craterostigma plantagineum. Plant Sci. 160: 1161–1170 [DOI] [PubMed] [Google Scholar]
- Alpert P., Oliver M.J. (2002). Drying without dying. In Desiccation and Survival in Plants: Drying without Dying. Black M., Pritchard H., eds (Oxford: CABI Publishing; ), pp. 3–43 [Google Scholar]
- Atkinson J., Epand R.F., Epand R.M. (2008). Tocopherols and tocotrienols in membranes: A critical review. Free Radic. Biol. Med. 44: 739–764 [DOI] [PubMed] [Google Scholar]
- Berjak P. (2006). Unifying perspectives of some mechanisms basic to desiccation tolerance across life forms. Seed Sci. Res. 16: 1–15 [Google Scholar]
- Bianchi G., Gamba A., Murelli C., Salamini F., Bartels D. (1991). Novel carbohydrate metabolism in the resurrection plant Craterostigma plantagineum. Plant J. 1: 355–359 [DOI] [PubMed] [Google Scholar]
- Bogdanović J., Mojović M., Milosavić N., Mitrović A., Vucinić Z., Spasojević I. (2008). Role of fructose in the adaptation of plants to cold-induced oxidative stress. Eur. Biophys. J. 37: 1241–1246 [DOI] [PubMed] [Google Scholar]
- Boyer J.S. (1976). Photosynthesis at low water potentials. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 273: 501–512 [Google Scholar]
- Bray E.A. (1997). Plant responses to water stress. Trends Plant Sci. 2: 48–54 [Google Scholar]
- Bray E.A. (2002). Classification of genes differentially expressed during water-deficit stress in Arabidopsis thaliana: An analysis using microarray and differential expression data. Ann. Bot. (Lond.) 89 (Spec No): 803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant G., Koster K.L., Wolfe J. (2001). Membrane behavior in seeds and other systems at low water content: The various effects of solutes. Seed Sci. Res. 11: 17–25 [Google Scholar]
- Burke A. (2002). Properties of soil pockets on arid Nama Karoo inselbergs: The effect of geology and derived landforms. J. Arid Environ. 50: 219–234 [Google Scholar]
- Chapin F.S., III, Schulze E., Mooney H.A. (1990). The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 21: 423–447 [Google Scholar]
- Cooper K., Farrant J.M. (2002). Recovery of the resurrection plant Craterostigma wilmsii from desiccation: Protection versus repair. J. Exp. Bot. 53: 1805–1813 [DOI] [PubMed] [Google Scholar]
- Cunningham G.L., Burk J.H. (1973). The effect of carbonate deposition layers (“Caliche”) on the water status of Larrea divaricata. Am. Midl. Nat. 90: 474–480 [Google Scholar]
- Cushman J.C. (2001). Osmoregulation in plants: Implications for Agriculture. Am. Zool. 41: 758–769 [Google Scholar]
- Cushman J.C., Oliver M.J. (2011). Understanding vegetative desiccation tolerance using integrated functional genomics approaches within a comparative evolutionary framework. In Ecological Studies: Desiccation Tolerance in Plants, Luttge U., Beck E., Bartels D., eds (Heidelberg: Springer-Verlag), in press [Google Scholar]
- Evans A.M., Dehaven C.D., Barrett T., Mitchell M., Milgram E. (2009). Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 81: 6656–6667 [DOI] [PubMed] [Google Scholar]
- Farrant J.M. (2007). Mechanisms of desiccation tolerance in angiosperm resurrection plants. In Plant Desiccation Tolerance, Jenks M.A., Wood A.J., eds (Ames, IA: Blackwell Publishing; ), pp. 51–90 [Google Scholar]
- Farrant J.M., Brandt W., Lindsey G.C. (2007). An overview of mechanisms of desiccation tolerance in selected angiosperm resurrection plants. Plant Stress 1: 72–84 [Google Scholar]
- Farrant J.M., Lehner A., Cooper K., Wiswedel S. (2009). Desiccation tolerance in the vegetative tissues of the fern Mohria caffrorum is seasonally regulated. Plant J. 57: 65–79 [DOI] [PubMed] [Google Scholar]
- França M.B., Panek A.D., Eleutherio E.C.A. (2007). Oxidative stress and its effects during dehydration. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 146: 621–631 [DOI] [PubMed] [Google Scholar]
- Gaff G.F., Blomstedt C.K., Neale A.D., Le T.N., Hamill J.D., Ghasempour H.R. (2009). Sporobolus stapfianus, a model desiccation-tolerant grass. Funct. Plant Biol. 36: 589–599 [DOI] [PubMed] [Google Scholar]
- Georgieva K., Röding A., Büchel C. (2009). Changes in some thylakoid membrane proteins and pigments upon desiccation of the resurrection plant Haberlea rhodopensis. J. Plant Physiol. 166: 1520–1528 [DOI] [PubMed] [Google Scholar]
- Ghasempour H., Gaff D., Williams R.B., Gianellow R. (1998). Contents of sugars in leaves of drying desiccation tolerant flowering plants, particularly grasses. Plant Growth Regul. 24: 185–191 [Google Scholar]
- Ghasempour H.R., Kianian J. (2007). The study of desiccation-tolerance in drying leaves of the desiccation-tolerant grass Sporobolus elongatus and the desiccation-sensitive grass Sporobolus pyramidalis. Pak. J. Biol. Sci. 10: 797–801 [DOI] [PubMed] [Google Scholar]
- Grzam A., Martin M.N., Hell R., Meyer A.J. (2007). gamma-Glutamyl transpeptidase GGT4 initiates vacuolar degradation of glutathione S-conjugates in Arabidopsis. FEBS Lett. 581: 3131–3138 [DOI] [PubMed] [Google Scholar]
- Hanson H.D., Hitz W.D. (1982). Metabolic responses of mesophytes to plant water deficits. Annu. Rev. Plant Physiol. 33: 163–203 [Google Scholar]
- Higgins C.F., Payne J.W. (1982). Plant peptides. In Encyclopedia of Plant Physiology 14A, Boulder D., Parthier B., eds (Berlin: Springer-Verlag; ), pp. 438–458 [Google Scholar]
- Hoekstra F.A., Golovina E.A., Buitink J. (2001). Mechanisms of plant desiccation tolerance. Trends Plant Sci. 6: 431–438 [DOI] [PubMed] [Google Scholar]
- Hoekstra F.A., Wolkers W.F., Buitink J., Golovina E.A., Crowe J.H., Crowe L.M. (1997). Membrane stabilization in the dry state. Comp. Biochem. Physiol. 117A: 335–341 [Google Scholar]
- Howland M.C., Parikh A.N. (2010). Model studies of membrane disruption by photogenerated oxidative assault. J. Phys. Chem. B 114: 6377–6385 [DOI] [PubMed] [Google Scholar]
- Illing N., Denby K., Collett H., Shen A., Farrant J. (2005). The signature of seeds in resurrection plants: A molecular and physiological comparison of desiccation tolerance in seeds and vegetative tissues. Integr. Comp. Biol. 45: 771–787 [DOI] [PubMed] [Google Scholar]
- Ingram J., Bartels D. (1996). The molecular basis of dehydration tolerance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47: 377–403 [DOI] [PubMed] [Google Scholar]
- Iturriaga G., Suárez R., Nova-Franco B. (2009). Trehalose metabolism: From osmoprotection to signaling. Int. J. Mol. Sci. 10: 3793–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G., Wang Z., Shang H., Yang W., Hu Z., Phillips J., Deng X. (2007). Proteome analysis of leaves from the resurrection plant Boea hygrometrica in response to dehydration and rehydration. Planta 225: 1405–1420 [DOI] [PubMed] [Google Scholar]
- Koster K.L., Bryant G. (2006). Dehydration in model membranes and protoplasts: Contrasting effects at low, intermediate and high hydrations. In Cold Hardiness in Plants, Chen T.H.H., Uemura M., Fujikawa S., eds (Wallingford, UK: CABI: ). pp. 219–234 [Google Scholar]
- Kranner I., Birtic S. (2005). A modulating role for antioxidants in desiccation tolerance. Integr. Comp. Biol. 45: 734–740 [DOI] [PubMed] [Google Scholar]
- Kranner I., Beckett R.P., Wornik S., Zorn M., Pfeifhofer H.W. (2002). Revival of a resurrection plant correlates with its antioxidant status. Plant J. 31: 13–24 [DOI] [PubMed] [Google Scholar]
- Lutts S., Majerus V., Kinet J.M. (1999). NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol. Plant. 105: 450–458 [Google Scholar]
- Martinelli T. (2008). In situ localization of glucose and sucrose in dehydrating leaves of Sporobolus stapfianus. J. Plant Physiol. 165: 580–587 [DOI] [PubMed] [Google Scholar]
- Martinelli T., Whittaker A., Bochicchio A., Vazzana C., Suzuki A., Masclaux-Daubresse C. (2007a). Amino acid pattern and glutamate metabolism during dehydration stress in the ‘resurrection’ plant Sporobolus stapfianus: A comparison between desiccation-sensitive and desiccation-tolerant leaves. J. Exp. Bot. 58: 3037–3046 [DOI] [PubMed] [Google Scholar]
- Martinelli T., Whittaker A., Masclaux-Daubresse C., Farrant J.M., Brilli F., Loreto F., Vazzana C. (2007b). Evidence for the presence of photorespiration in desiccation-sensitive leaves of the C4 ‘resurrection’ plant Sporobolus stapfianus during dehydration stress. J. Exp. Bot. 58: 3929–3939 [DOI] [PubMed] [Google Scholar]
- Masclaux-Daubresse C., Daniel-Vedele F., Dechorgnat J., Chardon F., Gaufichon L., Suzuki A. (2010). Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. (Lond.) 105: 1141–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., Larsson A. (1995). Glutathione synthetase deficiency and other disorders of the γ-glutamyl cycle. In The Metabolic and Molecular Bases of Inherited Disease, Vol. 1, Scriver C.R., Beaudet A.L., Sly W.S., Valle D., eds (New York: McGraw-Hill: ) pp. 1461–1495 [Google Scholar]
- Meyer R.F., Boyer J.S. (1981). Osmoregulation, solute distribution, and growth in soybean seedlings having low water potentials. Planta 151: 482–489 [DOI] [PubMed] [Google Scholar]
- Mittler R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Moore J.P., Le N.T., Brandt W.F., Driouich A., Farrant J.M. (2009). Towards a systems-based understanding of plant desiccation tolerance. Trends Plant Sci. 14: 110–117 [DOI] [PubMed] [Google Scholar]
- Morgan J.M. (1984). Osmoregulation and water stress in higher plants. Annu. Rev. Plant Physiol. 35: 299–319 [Google Scholar]
- Mullet J.E., Whitsitt M.S. (1996). Plant cellular responses to water deficit. Plant Growth Regul. 20: 119–124 [Google Scholar]
- Munné-Bosch S., Alegre L. (2002). The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 21: 31–57 [Google Scholar]
- Naidoo G., Mundree S.G. (1993). Relationship between morphological and physiological responses to waterlogging and salinity in Sporobolus virginicus (L.) Kunth. Oecologia 93: 360–366 [DOI] [PubMed] [Google Scholar]
- Navari-Izzo F., Meneguzzo S., Loggini B., Vazzana C., Sgherri C. (1997). The role of the glutathione system during dehydration of Boea hygroscopica. Physiol. Plant. 99: 23–30 [Google Scholar]
- Neuhofer W., Beck F.-X. (2005). Cell survival in the hostile environment of the renal medulla. Annu. Rev. Physiol. 67: 531–555 [DOI] [PubMed] [Google Scholar]
- Obendorf R.L. (1997). Oligosaccharides and galactosyl cyclitols in seed desiccation tolerance. Seed Sci. Res. 7: 63–74 [Google Scholar]
- Ohkama-Ohtsu N., Oikawa A., Zhao P., Xiang C., Saito K., Oliver D.J. (2008). A γ-glutamyl transpeptidase-independent pathway of glutathione catabolism to glutamate via 5-oxoproline in Arabidopsis. Plant Physiol. 148: 1603–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver M.J. (1991). Influence of protoplasmic water loss on the control of protein synthesis in the desiccation-tolerant moss Tortula ruralis: Ramifications for a repair-based mechanism of desiccation tolerance. Plant Physiol. 97: 1501–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver M.J., Koster K.L., Cushman J.C. (2010). Dehydration tolerance in plants. In Plant Stress Tolerance: Methods in Molecular Biology, Vol. 639, Part 1, Sunkar R., ed (New York: Humana Press; ) pp. 3–24 [DOI] [PubMed] [Google Scholar]
- Oliver M.J., Tuba Z., Mishler B.D. (2000). Evolution of desiccation tolerance in land plants. Plant Ecol. Divers. 151: 85–100 [Google Scholar]
- Oliver M.J., Velten J., Mishler B.D. (2005). Desiccation tolerance in bryophytes: A reflection of the primitive strategy for plant survival in dehydrating habitats? Integr. Comp. Biol. 45: 788–799 [DOI] [PubMed] [Google Scholar]
- O’Mahony P.J., Oliver M.J. (1999). The involvement of ubiquitin in vegetative desiccation tolerance. Plant Mol. Biol. 41: 657–667 [DOI] [PubMed] [Google Scholar]
- Peters S., Mundree S.G., Thomson J.A., Farrant J.M., Keller F. (2007). Protection mechanisms in the resurrection plant Xerophyta viscosa (Baker): Both sucrose and raffinose family oligosaccharides (RFOs) accumulate in leaves in response to water deficit. J. Exp. Bot. 58: 1947–1956 [DOI] [PubMed] [Google Scholar]
- Phillips J.R., Fischer E., Baron M., van den Dries N., Facchinelli F., Kutzer M., Rahmanzadeh R., Remus D., Bartels D. (2008). Lindernia brevidens: A novel desiccation-tolerant vascular plant, endemic to ancient tropical rainforests. Plant J. 54: 938–948 [DOI] [PubMed] [Google Scholar]
- Phillips J.R., Hilbricht T., Salamini F., Bartels D. (2002). A novel abscisic acid- and dehydration-responsive gene family from the resurrection plant Craterostigma plantagineum encodes a plastid-targeted protein with DNA-binding activity. Planta 215: 258–266 [DOI] [PubMed] [Google Scholar]
- Porembski S., Barthlott W. (2000). Granitic and gneissic outcrops (inselbergs) as center of diversity for desiccation-tolerant vascular plants. Plant Ecol. Divers. 151: 19–28 [Google Scholar]
- Proctor M., Pence V. (2002). Vegetative tissues: Bryophytes, vascular resurrection plants and vegetative propagules. In Desiccation and Survival in Plants: Drying without Dying, Black M., Pritchard H., eds (Oxford: CABI Publishing; ), pp. 207–237 [Google Scholar]
- Rhodes D., Handa S., Bressan R.A. (1986). Metabolic changes associated with adaptation of plant cells to water stress. Plant Physiol. 82: 890–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M.C., Edsgärd D., Hussain S.S., Alquezar D., Rasmussen M., Gilbert T., Nielsen B.H., Bartels D., Mundy J. (2010). Transcriptomes of the desiccation-tolerant resurrection plant Craterostigma plantagineum. Plant J. 63: 212–228 [DOI] [PubMed] [Google Scholar]
- Schmidhuber J., Tubiello F.N. (2007). Global food security under climate change. Proc. Natl. Acad. Sci. USA 104: 19703–19708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K.R. (1986). Products of biological nitrogen fixation in higher plants: Synthesis, transport, and metabolism. Annu. Rev. Plant Physiol. 37: 539–574 [Google Scholar]
- Sgheiri C.L.M., Loggini B., Puliga S., Navari-Izzo F. (1997). Antioxidant system in Sporobolus stapfianus: Changes in response to desiccation and rehydration. Phytochemistry 35: 561–565 [Google Scholar]
- Sherwin H., Farrant J. (1998). Protection mechanisms against excess light in the resurrection plants Craterostigma wilmsii and Xerophyta viscosa. Plant Growth Regul. 24: 203–210 [Google Scholar]
- Skopelitis D.S., Paranychianakis N.V., Paschalidis K.A., Pliakonis E.D., Delis I.D., Yakoumakis D.I., Kouvarakis A., Papadakis A.K., Stephanou E.G., Roubelakis-Angelakis K.A. (2006). Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 18: 2767–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. (1992). The carbohydrates of bryophytes in relation to desiccation tolerance. J. Bryol. 17: 185–191 [Google Scholar]
- Smirnoff N. (1993). Role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 125: 27–58 [DOI] [PubMed] [Google Scholar]
- Soga T., Baran R., Suematsu M., Ueno Y., Ikeda S., Sakurakawa T., Kakazu Y., Ishikawa T., Robert M., Nishioka T., Tomita M. (2006). Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J. Biol. Chem. 281: 16768–16776 [DOI] [PubMed] [Google Scholar]
- Storey J.D. (2002). A direct approach to false discovery rates. J. Royal Statist. Soc. Ser. B. 64: 479–498 [Google Scholar]
- Tymms M.J., Gaff D.F. (1979). Proline accumulation during water stress in resurrection plants. J. Exp. Bot. 30: 165–168 [Google Scholar]
- van Bilsen D.G.J.L., Hoekstra F.A. (1993). Decreased membrane integrity in aging Typha latifolia L. pollen (accumulation of lysolipids and free fatty acids). Plant Physiol. 101: 675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ende W., Valluru R. (2009). Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging? J. Exp. Bot. 60: 9–18 [DOI] [PubMed] [Google Scholar]
- van der Weele C.M., Spollen W.G., Sharp R.E., Baskin T.I. (2000). Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J. Exp. Bot. 51: 1555–1562 [DOI] [PubMed] [Google Scholar]
- Veljovic-Jovanovic S., Kukavica B., Navari-Izzo F. (2008). Characterization of polyphenol oxidase changes induced by desiccation of Ramonda serbica leaves. Physiol. Plant. 132: 407–416 [DOI] [PubMed] [Google Scholar]
- Vertucci C.W., Farrant J.M. (1995). Acquisition and loss of desiccation-tolerance. In Seed Development and Germination, Kigel J., Galili G., eds (New York: Marcel Dekker; ), pp. 237–271 [Google Scholar]
- Vicré M., Lerouxel O., Farrant J., Lerouge P., Driouich A. (2004). Composition and desiccation-induced alterations of the cell wall in the resurrection plant Craterostigma wilmsii. Physiol. Plant. 120: 229–239 [DOI] [PubMed] [Google Scholar]
- Walters C., Farrant J.M., Pammenter N.W., Berjak P. (2002). Desiccation stress and damage. In Desiccation and Survival in Plants: Drying without Dying, Black M., Pritchard H., eds (Oxford: CABI Publishing; ), pp. 263–291 [Google Scholar]
- Welti R., Li W., Li M., Sang Y., Biesiada H., Zhou H.-E., Rajashekar C.B., Williams T.D., Wang X. (2002). Profiling membrane lipids in plant stress responses. Role of phospholipase D α in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 277: 31994–32002 [DOI] [PubMed] [Google Scholar]
- Whittaker A., Bochicchio A., Vazzana C., Lindsey G., Farrant J.M. (2001). Changes in leaf hexokinase activity and metabolite levels in response to drying in the desiccation-tolerant species Sporobolus stapfianus and Xerophyta viscosa. J. Exp. Bot. 52: 961–969 [DOI] [PubMed] [Google Scholar]
- Whittaker A., Martinelli T., Bochicchio A., Vazzana C., Farrant J.M. (2004). Comparison of sucrose metabolism during the rehydration of desiccation-tolerant and desiccation-sensitive leaf material of Sporobolus stapfianus. Physiol. Plant. 122: 11–20 [Google Scholar]
- Whittaker A., Martinelli T., Farrant J.M., Bochicchio A., Vazzana C. (2007). Sucrose phosphate synthase activity and the co-ordination of carbon partitioning during sucrose and amino acid accumulation in desiccation-tolerant leaf material of the C4 resurrection plant Sporobolus stapfianus during dehydration. J. Exp. Bot. 58: 3775–3787 [DOI] [PubMed] [Google Scholar]
- Wood J.N., Gaff D.F. (1989). Salinity studies with drought resistant Sporobolus species. Oecologia 78: 559–564 [DOI] [PubMed] [Google Scholar]
- Yang C.Y., Chen Y.C., Jauh G.Y., Wang C.S. (2005). A Lily ASR protein involves abscisic acid signaling and confers drought and salt resistance in Arabidopsis. Plant Physiol. 139: 836–846 [DOI] [PMC free article] [PubMed] [Google Scholar]