Abstract
Spontaneous morbidity primarily affecting female breeders in three independent breeding colonies of NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice prompted an investigation to uncover the cause of disease. Necropsies were performed on 264 (157 female and 107 male) spontaneously sick, experimentally unmanipulated NSG mice. 42 (15.9%) of the mice had acute or chronic renal inflammatory lesions. 12 of the mice with nephritis had concurrent histologic evidence of an ascending urinary tract infection. From 94 kidneys cultured for bacterial organisms, 23 (24.4%) grew Enterococcus species and 19 (20%) grew Klebsiella Oxytoca. Female mice were twice more likely to present with nephritis than males. These findings indicate that bacterial nephritis is a major contributor to morbidity in the NSG strain.
Keywords: IL-2, Immunodeficiency, Mouse, Nephritis, NSG, Urinary tract infection
Introduction
NSG mice carry several mutations affecting the immune system rendering them severely immunodeficient.1,8,9,17,18 Mutant mice combine the features of the NOD/ShiLtJ background, the severe combined immune deficiency mutation (scid) and a IL2 receptor γ chain deficiency. NOD mice lack hemolytic complement because of a two base pair deletion in the C5 structural gene, carry a unique MHC haplotype which leads to defective NK cell function and defects in the immunoregulation of antigen presenting cells.8,15,16 Homozygosity for the scid mutation results in a double stranded DNA repair defect and a defect in the rearrangement of genes that code for antigen-specific receptors on lymphocytes.4,5 As a result, scid mice lack detectable IgM, IgG1, IgG2a, IgG2b, IgG3, or IgA. The IL2 receptor γ chain also known as the common cytokine receptor gamma chain, is shared with receptors for IL-4, IL-7, IL-9, IL-15 and IL-21. The null mutation in the IL2γ receptor leads to deficiencies in cytokine signaling and failure of clonal lymphocyte expansion.1,9 All these mutations are combined together in the NSG strain and as a result, NSG mice lack mature T cells and B cells, and NK cells, lack hemolytic complement, and are deficient in cytokine signaling. Mice of the NSG strain are unable to mount an effective adaptive immune response to foreign organisms or cells and therefore are considered a superior, long-lived model suitable for studies employing xenotransplantation strategies.
In recent years, the NSG strain has become a popular tool in research and has been engrafted with many normal and malignant human cell populations and tissues resulting in “humanized mice” as various types of human cells retain the same tissue functions as in humans. The strain readily supports engraftment of human CD34+ hematopoietic stem cells (HSC), peripheral-blood mononuclear cells (PBMC) and human embryonic stem (ES) cells. 1, 9, 11, 18 Morbidity and mortality of severely immunodeficient mice within breeding colonies occurs in spite of diligent health monitoring programs and in colonies maintained behind barriers known to exclude mouse adapted pathogens. Sporadic morbidity was seen at TJL NSG breeding colonies. In addition, the authors of this manuscript were contacted by two independent academic institutions maintaining NSG breeding colonies. Both institutions reported sporadic morbidity and mortality in their NSG breeders, primarily affecting breeding females over 200 days old. This manuscript describes the pathologic findings of the clinical investigation that ensued.
Materials and Methods
Sick NSG mice were examined from three breeding colonies maintained at The Jackson Laboratory (TJL), either in Bar Harbor or Sacramento. Mice were housed in a positive individually ventilated rack and the bedding used in the mouse boxes was either white pine shavings (Crobb box, Hancock, Maine) in the Bar Harbor colonies, or Beta Chip (Nepco, Warrensburg, NY) in the Sacramento colony. Due to their severe immunodeficiency, production colonies of NSG mice at TJL are housed behind a maximum level barrier. Their health is routinely monitored using immunocompetent sentinel mice by implementing statistically valid sampling procedures and recognized serological and molecular testing protocols. The maximum level barrier excludes a defined list of viral, bacterial, fungal and protozoal organisms to include: Ectromelia virus, Theiler’s mouse encephalomyelitis virus, Hantaan virus, K virus, Lactic dehydrogenase elevating virus, Lymphocytic choriomeningitis virus, Mouse adenovirus, Mouse cytomegalovirus, Mouse hepatitis virus, Mouse minute virus, Mouse norovirus, Mouse parvovirus, Mouse thymic virus, Pneumonia virus of mice, Polyoma virus, Reovirus 3, rotavirus, Sendai virus, Bordetella bronchispetica, CAR bacillus, Citrobacter freundii, Clostridium piliforme, Corynebacterium bovis, Helicobacter spp., K. pneumonia, Mycoplasma pulmonis, Pasteurella pneumotropica, Pneumocystis murina, Pseudomonas spp., salmonella spp., Staphyloccocus aureus, Sterptobacillus moniliformis, Streptoccocus spp., Encephalitozoon caniculi, trichomonads, Giardia, Spironucleus. In addition, all fleas, lice, mites, pinworms, round worms and tapeworms are also excluded. “Sick” mice were submitted to The Jackson Laboratory diagnostic service in Bar Harbor, Maine, or to The Comparative Pathology Laboratory (CPL) at The University of California at Davis for necropsy between June 2007 and May 2009. A total of 226 mice (135 females and 91 males) were submitted, ranging in age from 23 to 537 days. Criteria for submission were clinical assessment of a “sick mouse” which includes any of the following signs: hunched posture, dehydration, ruffled hair coat, weight loss and/or the presence of a grossly visible tumor. From all three breeding colonies at TJL, less than 1% of breeding animals were found to be “sick” and were submitted for pathology evaluation. Complete necropsy was performed on all mice and microbiologic cultures were performed on selected tissues presenting gross lesions. Organ samples were cultured on Columbia agar with 5% defibrinated sheep blood (Northeast labs, Waterville, ME) at TJL or on tryptic Soy Agar with 5% sheep’s blood (Hardy Diagnostics, Santa Maria, CA) at CPL. Additional tissue samples were cultured on MacConkey’s agar (Difco, Sparks, MD) for 24h at 37°C directly or after enrichment in tryptose phosphate at 37°C for up to one week at TJL or on MacConkey’s Agar (Hardy Diagnostics, Santa Maria, CA) and incubated at 37°C for up to 72h at CPL. Plates were processed every 24h using biochemical methods for identification of bacterial colonies. For specimens requiring more extensive identification, Analytical Profile Index testing system was used (BioMerieux, Marcy l’Etoile, France). Tissues collected for histology were fixed in Tellyesniczky/Fekete fixative (100 ml 70% ethanol, 5 ml 37-40% formalin, 5 ml glacial acetic acid) or 10% buffered formalin. Tissues were paraffin embedded and stained with hematoxylin and eosin (H&E), and selected slides were stained with Brown and Brenn (B&B), a tissue gram stain. In addition, fixed tissues or glass slides representing 38 sick NSG mice (22 females and 16 males) from two independent academic institutions maintaining NSG breeding colonies were sent to the authors of this manuscript and were included in this study.
Results
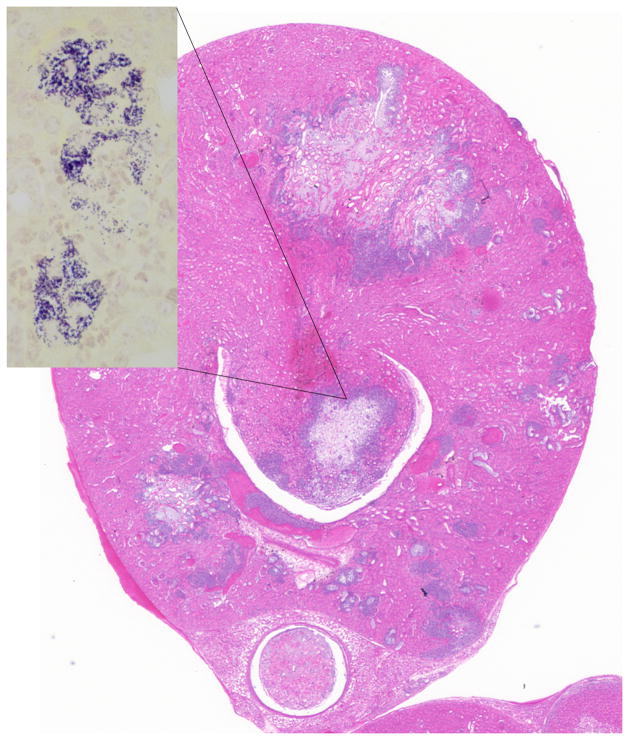
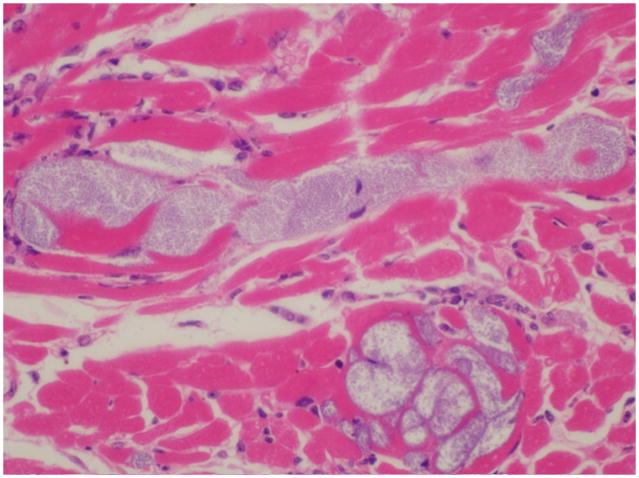
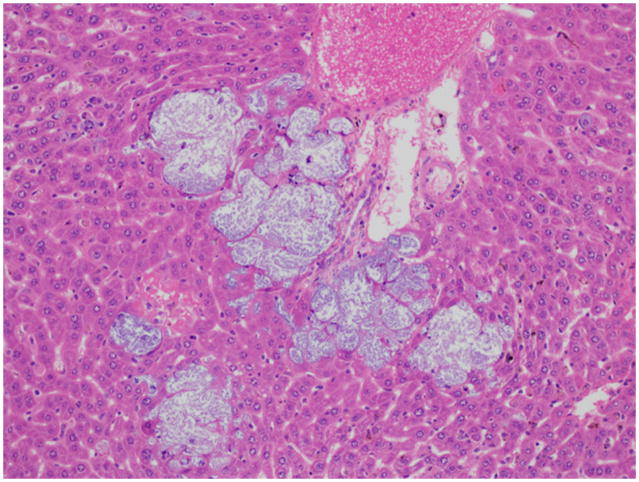
Most sick NSG mice presented with hunched postures and scruffy hair coats but otherwise were alert and responsive. 19 mice appeared “normal” and eight mice were submitted moribund in lateral recumbency. Gross necropsy revealed mottling of the kidneys in 36 (13.6%) mice. 42 (15.9%) of the mice had histologic evidence of acute or chronic renal inflammatory lesions of which 11 (26%) had bilateral renal lesions (Table 1). 34 mice had chronic lesions and 8 had acute lesions. Chronic lesions were characterized by multifocal interstitial fibrosis with tubular degeneration and loss. No inflammatory cells were associated with these areas in any of the mice. In addition, kidneys presenting chronic lesions often had radiating segmental fibrosis suggesting chronic infarcts; however, no thrombi were evident within the vasculature. Acute lesions were characterized by bacterial colonies scattered throughout the renal interstitium and within the distal and proximal tubules and rarely within tubular epithelial cells. Bacterial colonies were accompanied by very small numbers of neutrophils (Fig. 1). No lymphocytic or plasmacytic infiltrates were present in any of the affected kidneys. Twelve mice with renal lesions had also an ascending urinary tract infection with few neutrophils scattered along the lower urinary tract and male accessory reproductive glands. Inflammation was minimal but included cystitis, prostatitis and seminal vesiculitis. Three of these mice had bacteria colonizing their lower urinary tracts, morphologically similar to the bacteria found in their kidneys. All other mice with nephritis had no lesions in the lower urinary tract, as well as no lesions indicating an alternate route of bacterial infection. Seven mice with nephritis had concurrent histologic evidence of bacteremia, with bacterial colonies found in the heart (Fig 2) and/or liver and lungs. In all tissues, bacterial colonies appeared to proliferate undisturbed within the parenchyma and did not seem to provoke an adaptive immune response (Fig. 3). Renal microbiologic cultures were performed on 94 mice, of which 23 (24.4%) and 19 (20%) had positive growth of Enterococcus species and Klebsiella Oxytoca, respectively. All kidneys with positive bacterial cultures had a corresponding histologic renal lesion, and B&B stains performed on selected sections were consistent with bacterial culture results. 48 cultures had negative growth and four had mixed bacterial growth. Female mice were twice more likely to develop nephritis than male mice (19.7% and 10%, respectively). However, the mean age of presentation with nephritis was almost identical for males and females (210 and 217.2 days, respectively). Tumor profiles (unpublished data) included mammary adenocarcinomas, osteosarcomas and lymphomas at frequencies and age of onset compatible with other NOD derived strains.16
Table 1.
Incidence of renal and lower urinary tract bacterial infections in NSG mice and renal culture results
| Both sexes | Males (107 mice) | Females (157 mice) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of cases | Age Range (days) | Mean Age (days) | Number of cases | Age Range (days) | Mean Age (days) | Number of cases | Age Range (days) | Mean Age (days) | |
| Cases with histologic evidence of an ascending urinary tract infection* | 12 | 122 to 180 | 142 | 5 | 122 to 173 | 147 | 7 | 141 to 180 | 162 |
| Cases with histologic evidence of bacteremia | 7 | 168 to 232 | 203 | 2 | 183 to 220 | 201 | 5 | 168 to 232 | 206 |
| Cases with histologic evidence of acute or chronic renal inflammatory lesions | 42 | 146 to 297 | 214 | 11 | 162 to 258 | 210 | 31 | 141 to 297 | 217 |
| Cases with positive cultures of Enterococcus species from the kidney** | 23 | 145 to 297 | 212 | 6 | 145 to 260 | 202 | 17 | 151 to 297 | 223 |
| Cases with positive cultures of Klebsiella Oxytoca from the kidney | 19 | 141 to 282 | 218 | 7 | 166 to 268 | 203 | 12 | 141 to 282 | 234 |
lesions include cystitis and/or prostatitis
microbiologic cultures were performed on 94 mice
Fig. 1.
Kidney; NSG mouse No.1. Acute multifocal parenchymal necrosis. H&E. H&E. Inset: Renal papilla, clusters of cocci bacteria. B&B stain.
Fig. 2.
Heart; NSG mouse No.2. Colonies of cocci bacteria are present within the myocardium.
Fig. 3.
Liver; NSG mouse No.2. Colonies of cocci bacteria are present within the hepatic sinusoids. Note the lack of an inflammatory response. H&E.
Discussion
Lesions were caused by two different bacterial organisms and were most consistent with an ascending urinary tract bacterial infection. Out of 42 mice with nephritis, 12 (28.5%) mice had a concurrent lower urinary tract infection, with three mice having bacterial colonies along their lower urinary tract morphologically similar to the bacteria found in their kidneys. From our experience with the NSG strain, skin wounds and the molar gingival sulcus often serve as port of entry for bacteria which will subsequently disseminate to other organs. In all 42 mice diagnosed with nephritis in this study, no other lesions were noted in any other tissues. These findings support the hypothesis that opportunistic bacteria colonized the lower urinary tract and ascended to the kidneys leading to chronic bacterial nephritis. K. Oxytoca and Enterococcus organisms are not considered primary mouse pathogens and both organisms are often isolated from the gastrointestinal tract of healthy immunocompetent mice.3 Enterococci are facultative anaerobic Gram positive cocci, and are viewed as a commensal organism in the intestines of mice. Enterococci are frequently cultured from the intestines of mice of many inbred strains at all health status levels at TJL. However, there is a single report, in which enterococci were isolated from lung abscesses in a mouse model of x-linked chronic granulomatous disease. 2 The absence of hemolytic complement is likely to play a critical role in the increased susceptibility of NSG mice to Enterococci as neutrophil killing of Enterococci in immunocompetent mice is mediated by hemolytic complement. 8 K. oxytoca is a Gram negative, rod shaped bacteria and although occasionally isolated from mice ceca and feces, the organism is not a permanent resident of the murine intestinal tract, and could be eliminated from a colony by implementing an eradication program as is currently in place in rooms housing colonies of NSG mice at TJL. K. oxytoca is recognized as an opportunistic pathogen of mice that could naturally infect mice and could lead to clinical disease.3 In immunocompetent mice, morbidity caused by K. oxytoca is sporadic and is associated with urinary and genital tract infections, otitis media, pneumonia and abscesses.3 Infections with K. oxytoca leading to clinical disease were reported in immunodeficient strains of mice, such as the C3H/HeJ strain that carries the Tlr4(Lps-d) allele.10 These mice are insensitive to lipopolysaccharide (LPS) signaling by the Toll-like receptor and to the activation of the innate immune system. In addition, the inflammatory mechanisms induced by LPS, and several genetic factors both of the host and the bacteria were found to be essential for resistance to experimental pyelonephritis in mice.10,19,20
NSG mice harbor several mutations that severely impair both their innate and the adaptive immune responses. At TJL, NSG mice are bred behind stringent barriers designed to exclude specific murine pathogens as well as several opportunistic organisms. The elimination of K. oxytoca from NSG breeding colonies previously maintained at TJL was successful and routine culturing of ceca and fecal material has indicates that all breeding colonies at TJL are now free of K. oxytoca (unpublished data). The eradication of K. oxytoca has led to a reduction in the incidence of nephritis and urinary tract infections in this strain, and since the complete exclusion of K. oxytoca from the production colonies, enterococcus is the sole organism cultured from kidneys of sick NSG mice. This finding suggests that the immune deficiency of the NSG strain is so severe, any non pathogenic organism, even an intestinal commensal, could potentially cause clinical disease.
Three breeding colonies of NSG mice were housed in three separate buildings at TJL in Bar Harbor and Sacramento. It is important to note, that morbidity was sporadic and less than 1% of all mice over 200 days old were recognized as “sick” and sent for a diagnostic necropsy. Out of the three NSG colonies, one colony reported significantly lower numbers of sick NSG mice as well as better reproductive performance (LD Shultz, personal communication). This specific colony, as the other two at TJL, practices stringent husbandry procedures and is monitored by the same comprehensive health monitoring program. However, two significant differences were noted between the three colonies. First, entry into this specific colony is restricted and access is limited to two designated and highly trained care takers. Second, change of bedding occurs weekly in this colony, while the other two colonies have a “check-change” (bedding is changed as needed) or a bi weekly cage changing program. As all bedding and cages are autoclaved, this difference might suggest the necessity of a “minimal inoculating dose” of opportunistic bacteria to induce morbidity in NSG mice, and perhaps frequent changes of sterile bedding might reduce infection rates.
In this study, sick individual NSG mice were often part of a breeding unit and were co-housed with unaffected mice. Some of the mice were housed as breeding pairs and others as trio matings. Sick mice submissions were sporadic and often only a single mouse per box showed clinical signs of disease. When their cage mates were examined they were frequently found to be normal. The basis for this disparity remains to be elucidated.
Female mice were twice more likely to develop nephritis. If the route of infection was indeed ascending, this finding is probably associated with anatomic, hormonal and behavioral factors and is compatible with previous studies performed in animals and humans.6,8
In summary, severely immunocompromised strains such as the NSG mouse are susceptible to infections by bacteria considered to be non pathogenic in immuocompetent mice. Both K. oxytoca and enterococcous sp. could cause disease and should be considered pathogens in severely immunodeficient strains. In view of the enhanced susceptibility of these mice to infections by opportunistic organisms, it is essential that NSG mice be housed under optimal conditions, which include eradication of opportunistic bacteria, implementing rigorous management practices and maintaining a stringent barrier system.
Acknowledgments
This work was supported by the following grants (to LDS) National Institutes of Health grants DK072473, CA34196, and AI46629, The Biology of the Beta Cell Consortium, The Juvenile Diabetes Foundation International, and the Helmsley Foundation.
References
- 1.Agliano A, Martin-Padura I, Mancuso P, Marighetti P, Rabascio C, Pruneri G, Shultz LD, Bertolini F. Human acute leukemia cells injected in NOD/LtSz-scid/IL-2Rgamma null mice generate a faster and more efficient disease compared to other NOD/scid-related strains. Int J Cancer. 2008;123:2222–7. doi: 10.1002/ijc.23772. [DOI] [PubMed] [Google Scholar]
- 2.Bingel SA. Pathology of a mouse model of x-linked chronic granulomatous disease. Contemp Top Lab Anim Sci. 2002;41:33–8. [PubMed] [Google Scholar]
- 3.Bleich A, Kirsch P, Sahly H, Fahey J, Smoczek A, Hedrich HJ, Sundberg JP. Klebsiella oxytoca: opportunistic infections in laboratory rodents. Lab Anim. 2008;42:369–75. doi: 10.1258/la.2007.06026e. [DOI] [PubMed] [Google Scholar]
- 4.Blunt T, Finnie NJ, Taccioli GE, Smith GC, Demengeot J, Gottlieb TM, Mizuta R, Varghese AJ, Alt FW, Jeggo PA, Jackson SP. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell. 1995;80:813–23. doi: 10.1016/0092-8674(95)90360-7. [DOI] [PubMed] [Google Scholar]
- 5.Bosma M, Schuler W, Bosma G. The scid mouse mutant. Curr Top Microbiol Immunol. 1988;137:197–202. doi: 10.1007/978-3-642-50059-6_29. [DOI] [PubMed] [Google Scholar]
- 6.Curran EM, Tassell AH, Judy BM, Nowicki B, Montgomery-Rice V, Estes DM, Nowicki S. Estrogen increases menopausal host susceptibility to experimental ascending urinary-tract infection. J Infect Dis. 2007;195:680–3. doi: 10.1086/511275. [DOI] [PubMed] [Google Scholar]
- 7.Harrington RD, Hooton TM. Urinary tract infection risk factors and gender. J Gend Specif Med. 2000;3:27–34. [PubMed] [Google Scholar]
- 8.Harvey BS, Baker CJ, Edwards MS. Contributions of complement and immunoglobulin to neutrophil-mediated killing of enterococci. Infect Immun. 1992;60:3635–40. doi: 10.1128/iai.60.9.3635-3640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–73. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacArthur CJ, Pillers DA, Pang J, Degagne JM, Kempton JB, Trune DR. Gram-negative pathogen Klebsiella oxytoca is associated with spontaneous chronic otitis media in Toll-like receptor 4-deficient C3H/HeJ mice. Acta Otolaryngol. 2008;128:132–8. doi: 10.1080/00016480701387124. [DOI] [PubMed] [Google Scholar]
- 11.Pearson T, Shultz LD, Miller D, King M, Laning J, Fodor W, Cuthbert A, Burzenski L, Gott B, Lyons B, Foreman O, Rossini AA, Greiner DL. Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin(IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment. Clin Exp Immunol. 2008;154:270–84. doi: 10.1111/j.1365-2249.2008.03753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Percy DH, Barta JR. Spontaneous and experimental infections in scid and scid/beige mice. Lab Anim Sci. 1993;43:127–32. [PubMed] [Google Scholar]
- 13.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–8. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ragnarsdóttir B, Fischer H, Godaly G, Grönberg-Hernandez J, Gustafsson M, Karpman D, Lundstedt AC, Lutay N, Rämisch S, Svensson ML, Wullt B, Yadav M, Svanborg C. TLR- and CXCR1-dependent innate immunity: insights into the genetics of urinary tract infections. Eur J Clin Invest. 2008;38 (Suppl 2):12–20. doi: 10.1111/j.1365-2362.2008.02004.x. [DOI] [PubMed] [Google Scholar]
- 15.Serreze DV, Chapman HD, Varnum DS, Gerling I, Leiter EH, Shultz LD. Initiation of autoimmune diabetes in NOD/Lt mice is MHC class I-dependent. J Immunol. 1997;158:3978–86. [PubMed] [Google Scholar]
- 16.Serreze DV, Leiter EH. Genetic and pathogenic basis of autoimmune diabetes in NOD mice. Curr Opin Immunol. 1994;6:900–6. doi: 10.1016/0952-7915(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 17.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–30. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 18.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–89. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 19.Svanborg Edén C, Briles D, Hagberg L, McGhee J, Michalec S. Genetic factors in host resistance to urinary tract infection. Infection. 1985;13 (Suppl 2):S171–6. doi: 10.1007/BF01644425. [DOI] [PubMed] [Google Scholar]
- 20.Svanborg-Edén C, Hagberg L, Hull R, Hull S, Magnusson KE, Ohman L. Bacterial virulence versus host resistance in the urinary tracts of mice. Infect Immun. 1987;55:1224–32. doi: 10.1128/iai.55.5.1224-1232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]