Abstract
Daptomycin is a lipopeptide antibiotic with activity against a wide range of gram-positive bacteria. We used the neutropenic murine thigh model to characterize the pharmacodynamics of daptomycin. ICR/Swiss mice were rendered neutropenic with cyclophosphamide; and the thigh muscles of the mice were infected with strains of Staphylococcus aureus, Streptococcus pneumoniae, and Enterococcus faecium. Animals were treated by subcutaneous injection of daptomycin at doses of 0.20 to 400 mg/kg of body weight/day divided into one, two, four, or eight doses over 24 h. Daptomycin exhibited linear pharmacokinetics, with an area under the concentration-time curve (AUC) from time zero to infinity/dose of 9.4 and a half-life of 0.9 to 1.4 h. The level of protein binding was 90%. Free daptomycin exhibited concentration-dependent killing and produced in vivo postantibiotic effects (PAEs) of 4.8 to 10.8 h. Nonlinear regression analysis was used to determine which pharmacokinetic (PK) or pharmacodynamic (PD) parameter was important for efficacy by using free drug concentrations. The peak concentration/MIC (peak/MIC) ratio and 24-h AUC/MIC ratio were the PK and PD parameters that best correlated with in vivo efficacy (R2 = 83 to 87% for peak/MIC and R2 = 86% for the AUC/MIC ratio, whereas R2 = 47 to 50% for the time that the concentration was greater than the MIC) against standard strains of S. aureus and S. pneumoniae. The peak/MIC ratios required for a bacteriostatic effect ranged from 12 to 36 for S. pneumoniae, 59 to 94 for S. aureus, and 0.14 to 0.25 for E. faecium. The AUC/MIC ratios needed for a bacteriostatic effect ranged from 75 to 237 for S. pneumoniae, 388 to 537 for S. aureus, and 0.94 to 1.67 for E. faecium. The free daptomycin concentrations needed to average from one to two times the MIC over 24 h to produce a bacteriostatic effect and two to four times the MIC over 24 h to produce greater than 99% killing. The long PAE and potent bactericidal activity make daptomycin an attractive option for the treatment of infections caused by gram-positive bacteria.
The growing crisis in antibiotic resistance has limited our ability to treat infections caused by resistant pathogens. Vancomycin remains the mainstay of therapy against several resistant gram-positive organisms, but with the 20-fold increase in nosocomial infections caused by vancomycin-resistant enterococci (VRE) (4), there is a growing need for more potent antimicrobials to attack these resistant pathogens. Daptomycin is a lipopeptide antibiotic derived from Streptomyces roseosporus. Among its attributes, daptomycin has potent bactericidal activity against a wide range of gram-positive bacteria (6, 7, 11-14, 16, 18, 21), including methicillin-resistant Staphylococcus aureus (8, 9), VRE (1), and penicillin-resistant Streptococcus pneumoniae (5, 20). Daptomycin is bactericidal against enterococci, including VRE, at concentrations near the MIC (1). Early clinical trials with 2 mg of daptomycin per kg of body weight per day and conventional therapy were suspended by the sponsor in 1992 because of failures of treatment for endocarditis in the daptomycin group (16). The treatment failures were likely due to underdosing, which thus led to a concentration of active drug that was too low and which undermined the potential concentration-dependent bactericidal activity of daptomycin. However, recent phase II and phase III clinical trials with daptomycin have not demonstrated any severe adverse events (F. P. Tally, C. Berman, F. B. Oleson, and M. F. DeBruin, Abstr. 10th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. WeP233, 2000). The potency of daptomycin and the extremely useful role that it could potentially play in the treatment of infections caused by gram-positive organisms merit further evaluation of its pharmacodynamic (PD) activity to determine optimal dosing regimens.
We chose to characterize the PDs of daptomycin in the neutropenic murine thigh infection model to ascertain (i) which pharmacokinetic (PK) or PD parameter best correlates with the efficacy of daptomycin and (ii) whether the magnitudes of these parameters varied for different pathogens. These studies may provide dosing regimen suggestions for the successful administration of daptomycin for the treatment of infections caused by drug-resistant gram-positive bacteria.
(Part of this work was presented at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., September 1999.)
MATERIALS AND METHODS
Organisms.
The organisms studied consisted of nine strains of S. pneumoniae (two strains with intermediate susceptibility to penicillin and seven strains resistant to penicillin), four strains of S. aureus (one strain of methicillin-resistant S. aureus), and two strains of vancomycin-resistant Enterococcus faecium.
Antibiotics.
Daptomycin powder was supplied by Cubist Pharmaceuticals and was stored at −70°C. Solutions were freshly prepared in distilled, deionized sterile water and were diluted to the desired concentrations.
Media.
The in vitro activity of daptomycin has been shown to be dependent on the presence of calcium ions in the medium (2). Hence, Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) was supplemented with calcium (50 mg/liter) and magnesium (25 mg/liter). The media were also supplemented with 3% lysed horse blood for tests with S. pneumoniae.
Sheep blood agar was used for quantitation of S. pneumoniae, and Mueller-Hinton broth was used for S. aureus and E. faecium.
MIC determination.
The MIC of daptomycin for each isolate was determined in duplicate by the standard NCCLS broth microdilution method (3). The broth microdilution wells were read at 20 h after incubation at 35°C.
Animals.
Six-week-old specific-pathogen-free female ICR/Swiss mice (weight, 23 to 25 g; Harlan Sprague-Dawley, Madison, Wis.) were used for all studies. The animals used were maintained in accordance with the criteria of the American Association for Accreditation of Laboratory Animal Care. All studies were approved by the Animal Research Committee of the William S. Middleton Memorial Veterans Affairs Hospital.
Infection model.
The mice were rendered neutropenic (polymorphonuclear cell count, <100/mm3) by injecting cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, Ind.) intraperitoneally 4 days (150 mg/kg) and 1 day (100 mg/kg) before infection (22).
Broth cultures of bacteria were grown to logarithmic phase overnight to an absorbance at 580 nm of 0.3 (Spectronic 88; Bausch & Lomb, Rochester, N.Y.). After dilution 1:10 in fresh Mueller-Hinton broth, the bacterial counts of the inoculum ranged from 106 to 107 CFU/ml. Thigh infections with each of the isolates were produced by injection of 0.1 ml of inoculum into groups of two mice 2 h before therapy with daptomycin. At specified time points the animals were killed by CO2 asphyxiation. After the mice were killed, the thighs were immediately removed and homogenized in 0.9% sterile iced saline. Viable counts were determined by plating duplicate 10-μl aliquots of samples of homogenate serially diluted 10-fold on Mueller-Hinton agar for S. aureus and E. faecium and sheep blood agar for S. pneumoniae.
All datum points represent the mean number of CFU for four thighs (two mice).
PKs.
Plasma samples were obtained by retro-orbital puncture at 0.5, 2, 4, and 6 h from one group of three infected mice and at 1, 3, 5, and 8 h from a second set of three infected mice following the administration of single subcutaneous doses of 10 and 40 mg of daptomycin per kg, respectively. The total volume collected from individual animals was less than 10% of the total blood volume. Concentrations in plasma were determined by microbiologic assay with Micrococcus luteus ATCC 9341 as the test organism (Cubist Pharmaceuticals, unpublished data). The lower limit of detection was 1.5 μg/ml. The intraday variation was less than 10%. Protein binding was determined by ultrafiltration with concentrations in plasma of 50 and 400 μg/ml. Pharmacokinetic parameters were calculated by noncompartmental analysis. The area under the concentration-time curve (AUC) was calculated from the mean concentrations by the trapezoidal rule. Pharmacokinetic constants were interpolated from values obtained in the actual studies for doses for which no kinetics were determined.
In vivo PAE.
Two hours after infection with standard strains of S. aureus (ATCC 25923) and S. pneumoniae (ATCC 10813), single subcutaneous doses of daptomycin at 2.5 and 10 mg/kg were administered to two groups of mice, respectively. Two control mice were killed at 0, 2, 4, 8, and 12 h. Two treated mice were killed at 1, 2, 4, 6, 8, 12, 18, and 24 h. The postantibiotic effect (PAE) was calculated by the following equation:
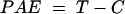 |
(1) |
where C is the time for the growth of 1 log10 CFU/thigh in control animals and T is the time for the growth of 1 log10 CFU/thigh in treated animals after total and free drug levels in plasma had fallen below the MIC.
Dose-response methods.
Neutropenic mice were infected with standard strains of penicillin-susceptible S. pneumoniae or S. aureus. Groups of two mice each were treated for 24 h with multiple daptomycin regimens by using fourfold increasing total doses divided into one, two, four, or eight doses. The total doses of daptomycin ranged from 0.20 to 400 mg/kg. Drug was administered subcutaneously in 0.2-ml volumes. The mice were killed after 24 h of therapy, and the thighs were removed and processed for CFU determination. Untreated control mice were killed just before treatment and after 24 h.
Dose-response studies were performed with 13 additional strains of S. pneumoniae, S. aureus, and E. faecium and dosing with daptomycin every 12 h.
Data analysis.
The results of these studies were analyzed by using the sigmoid dose-effect model. The doses required to produce a net bacteriostatic effect (static dose), 1 log10 killing, and 2 log10 killing were calculated from the following equation, derived from the Hill equation:
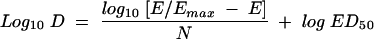 |
(2) |
where D is dose, E is the growth (G; in numbers of CFU per thigh) in untreated controls between 0 and 24 h for the static dose, E is G + 1 log for 1 log killing, and E is G + 2 logs for 2 log killing; Emax is the maximum effect; ED50 is the dose required to achieve 50% of Emax; and N is the slope of the dose-effect curve.
The indices Emax, ED50, and N were estimated by nonlinear least-squares regression. Nonlinear regression analysis with the same Emax dose-response model was used to determine which PK or PD parameter correlated best with efficacy. The coefficient of determination (R2) was used to estimate the percentage of variance in efficacy that could be attributed to regression with each PK or PD parameter.
The results for the different groups are presented as means with standard deviations and 95% confidence intervals. Differences between two groups were determined by the Mann-Whitney test (Sigma Stat; Jandel Scientific Software, San Rafael, Calif.).
RESULTS
The MICs of daptomycin for S. pneumoniae ranged from 0.12 to 0.25 μg/ml, while the MICs for the S. aureus and E. faecium strains were 0.5 and 2.0 μg/ml, respectively.
PKs.
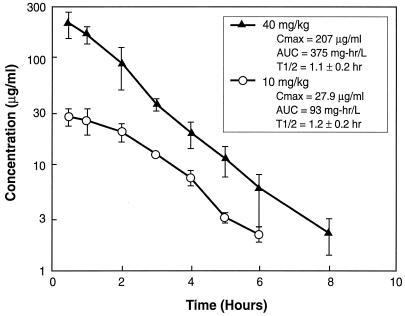
The time course of the mean plasma daptomycin concentrations following the administration of subcutaneous doses of 10 and 40 mg/kg are shown in Fig. 1. At the doses studied, the kinetics of daptomycin were relatively linear, with no change in the elimination half-life with the higher dose. PK analysis revealed peak concentration/dose values of 2.8 and 5.2 for the two doses, respectively, and AUC/dose values of 9.4 for both doses. Since three to four concentrations in plasma were determined for each mouse, individual half-lives were determined and ranged from 0.9 to 1.3 h for the 40-mg/kg dose and 0.9 to 1.4 h for the 10-mg/kg dose. The level of protein binding in mouse plasma ranged from 88.4 to 92.7%, with a mean of 90%.
FIG. 1.
Plasma daptomycin concentrations after administration of single subcutaneous doses of 10 and 40 mg/kg to neutropenic infected mice. Each symbol represents the mean ± standard levels in the plasma of three mice. T1/2, plasma elimination half-life (in hours); Cmax, peak level in plasma.
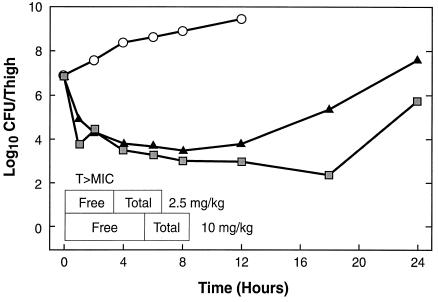
PAE.
The mice had 107.1 and 106.8 CFU of S. pneumoniae and S. aureus per thigh, respectively, when single doses of 2.5 or 10 mg/kg were given. The time course of antimicrobial activity of daptomycin against the standard strain of S. pneumoniae is shown in Fig. 2. Daptomycin reduced the number of bacteria by 3 to 4 log10 CFU/thigh. However, regrowth did not start immediately after total and free drug levels fell below the MIC. The durations of the in vivo PAEs for free daptomycin against both S. pneumoniae and S. aureus are shown in Table 1. Daptomycin exhibited prolonged PAEs against both organisms.
FIG. 2.
In vivo PAE of daptomycin against S. pneumoniae ATCC 10813 after administration of single doses of 2.5 (triangles) and 10 (squares) mg/kg. T > MIC, time that the concentration remains above the MIC.
TABLE 1.
In vivo PAEs of free and total daptomycin concentrations of against S. aureus and S. pneumoniae
| Dose (mg/kg) | Total or free drug | PAE (h) against:
|
|
|---|---|---|---|
| S. aureus | S. pneumoniae | ||
| 2.5 | Total | 0.4 | 2.9 |
| Free | 5.5 | 8.8 | |
| 10 | Total | 5.0 | 6.6 |
| Free | 4.8 | 10.8 | |
Correlation of PK and PD parameters with efficacy.
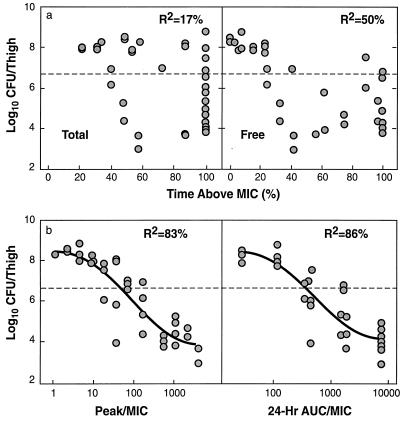
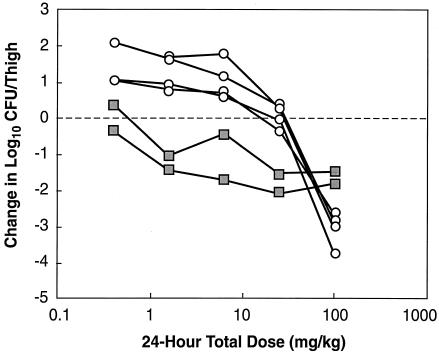
The relationships between the different PK and PD parameters for daptomycin with the number of CFU of S. aureus ATCC 25923 remaining in the thigh after 24 h of treatment are shown in Fig. 3a and b. The results for S. pneumoniae ATCC 10813 were very similar to those shown in Fig. 3. The peak concentration/MIC (peak/MIC) ratio and the 24-h AUC/MIC ratio were the parameters that best correlated with efficacy (R2 = 83 to 87% for the peak/MIC ratio and 86% for the 24-h AUC/MIC ratio, whereas R2 = 8 to 17% for the time that the concentration remains above the MIC for total drug and 47 to 50% for time that the concentration remains above the MIC for free drug). The static doses for the different dosing intervals are shown in Table 2. Values for the 24-h dosing regimen were either similar to or slightly less than those for the more frequent dosing regimens.
FIG. 3.
Relationships between PK and PD parameters and number of organisms remaining in the thighs of neutropenic mice after 24 h of therapy with multiple dosing regimens of daptomycin. (a) The PK and PD parameters are the time that the concentration remains above the MIC for total and free drug; (b) the PK and PD parameters peak/MIC and 24-h AUC/MIC ratios for total drug. The dotted lines represent the log10 CFU per thigh at the start of therapy.
TABLE 2.
Static doses of daptomycin for different dosing intervals
| Organism | Static daptomycin dose (mg/kg over 24 h) with dosing every:
|
|||
|---|---|---|---|---|
| 3 h | 6 h | 12 h | 24 h | |
| S. aureus ATCC 25923 | 53.3 ± 21.3 | 18.5 ± 1.7 | 26.0 ± 2.4 | 17.9 ± 2.4 |
| S. pneumoniae ATCC 10813 | 1.88 ± 0.42 | 1.77 ± 0.24 | 2.04 ± 0.12 | 1.35 ± 0.14 |
Magnitudes of PK and PD parameters determining efficacy against multiple strains.
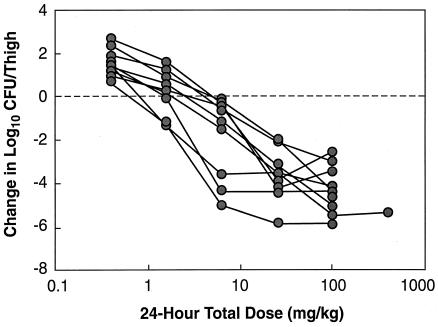
The dose-response curves normalized to the starting inoculum for administration of daptomycin every 12 h for multiple strains of S. pneumoniae and multiple strains of S. aureus and E. faecium are shown in Fig. 4 and 5, respectively. The dose-response curves for various strains of S. pneumoniae were relatively similar. The dose-response curves for the four strains of S. aureus and the two strains of E. faecium were almost identical. The static doses varied and ranged from 0.954 to 5.34 mg/kg/24 h for S. pneumoniae, 20.8 to 28.6 mg/kg/24 h for S. aureus, and 0.203 to 0.360 mg/kg/24 h for E. faecium (Table 3). The low values for E. faecium may reflect the poor growth of the two strains of E. faecium in control mice (0.34 and 0.37 log10 CFU/thigh over 24 h).
FIG. 4.
Dose-response curves for daptomycin against various strains of S. pneumoniae.
FIG. 5.
Dose-response curves for daptomycin against various strains of S. aureus (circles) and E. faecium (squares).
TABLE 3.
MICs, static doses, and magnitudes of 24-h AUC/MIC and peak/MIC ratios required to produce a bacteriostatic effect and killing of 1 and 2 log10 CFU per thigh over 24 h
| Organism | MIC (mg/liter) | Static dose (mg/kg/24 h)a | 24-h AUC/MIC ratio
|
Peak/MIC ratio
|
||||
|---|---|---|---|---|---|---|---|---|
| Static dose | 1 log killing | 2 log killing | Static dose | 1 log killing | 2 log killing | |||
| S. pneumoniae ATCC 10813 | 0.12 | 2.16 | 168 | 390 | 582 | 25.1 | 48.2 | 86.5 |
| S. pneumoniae CDC 145 | 0.12 | 0.954 | 74.7 | 108 | 157 | 11.8 | 16 | 23.4 |
| S. pneumoniae CDC 1199 | 0.12 | 2.62 | 203 | 346 | 594 | 30.5 | 51.5 | 88.1 |
| S. pneumoniae CDC 1396 | 0.12 | 1.5 | 117 | 150 | 190 | 17.4 | 22.3 | 28.3 |
| S. pneumoniae CDC 673 | 0.12 | 3.05 | 237 | 467 | 815 | 35.5 | 69.5 | 121 |
| S. pneumoniae CDC 1325 | 0.25 | 5.34 | 199 | 373 | 673 | 29.8 | 55.5 | 100 |
| S. pneumoniae CDC 49619 | 0.25 | 4.9 | 182 | 337 | 703 | 27.3 | 50 | 104 |
| S. pneumoniae CDC 1020 | 0.25 | 3.39 | 126 | 215 | 395 | 18.9 | 32 | 58.5 |
| Mean ± SD (95% CI) for S. pneumoniae | 160 ± 51 (72-316) | 290 ± 121 (100-660) | 498 ± 131 (117-813) | 24 ± 7.6 (11-44) | 42.1 ± 17.2 (15-98) | 73.9 ± 34.2 (20-204) | ||
| S. aureus ATCC 25923 | 0.5 | 20.8 | 388 | 594 | 896 | 59 | 109 | 197 |
| S. aureus ATCC 33591 | 0.5 | 28.6 | 537 | 733 | 1,099 | 93.6 | 147 | 264 |
| S. aureus ATCC 29213 | 0.5 | 22.5 | 420 | 588 | 788 | 66.2 | 107 | 163 |
| S. aureus ATCC 6538p | 0.5 | 21.9 | 409 | 750 | 1,460 | 63.6 | 152 | 398 |
| Mean ± SD (95% CI) for S. aureus | 438 ± 67 (316-550) | 666 ± 87 (501-832) | 1,061 ± 296 (603-1,738) | 70.6 ± 15.6 (47-102) | 129 ± 24.1 (86-184) | 255 ± 104 (114-507) | ||
| E. faecium VA 20 | 2 | 0.203 | 0.94 | 4.14 | NDb | 0.14 | 0.62 | ND |
| E. faecium VA 21 | 2 | 0.36 | 1.67 | 33.8 | ND | 0.25 | 5.05 | ND |
Calculations are based on total drug levels. Values for free drug are 10% of these values.
ND, not determined.
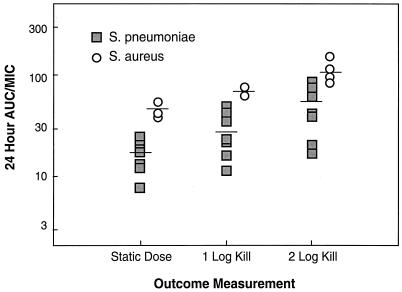
The magnitude of the 24-h AUC/MIC ratios associated with the doses required to produce a static effect or reduce the numbers of CFU by 1 and 2 log10 over 24 h are listed in Table 3 and are shown graphically in Fig. 6. The values in Table 3 are based on total drug, while the data in Fig. 6 are based on free drug (10% of the total drug values). Although the static doses for S. pneumoniae and S. aureus varied 30-fold and ranged from 0.95 to 28.6 mg/kg/day, the 24-h AUC/MIC and peak/MIC ratios for these doses varied 7.1- and 7.9-fold, respectively.
FIG. 6.
AUC over 24 h for free daptomycin associated with the static dose and doses producing killing of 1 and 2 log10 CFU/thigh for multiple strains of S. pneumoniae and S. aureus.
The mean 24-h AUC/MIC and peak/MIC ratios for S. pneumoniae (160 and 24, respectively, for total drug and 16 and 2.4, respectively, for free drug) were significantly lower (P < 0.05) than those for S. aureus (438 and 71, respectively, for total drug and 44 and 7.1, respectively, for free drug). Penicillin and methicillin resistance did not alter the magnitude of the 24-h AUC/MIC and peak/MIC ratios for daptomycin that were required for efficacy. The 24-h AUC/MIC and peak/MIC ratios for E. faecium were much lower than those for the other organisms tested.
DISCUSSION
The burgeoning rates of antibiotic resistance among clinical isolates of gram-positive bacteria and the upsurge in the rates of bacteremia caused by these organisms during recent times are causes for great concern. Daptomycin had potent antimicrobial activity against the multiple strains of S. pneumoniae and S. aureus tested. Unlike vancomycin, daptomycin displays concentration-dependent killing both in vitro (11, 14) and in vivo, as shown in this study. Its long half-life and a prolonged PAE should allow infrequent drug dosing.
In our animal study, exposure of the organisms to daptomycin led to PAEs of 5 to 10 h for S. aureus and S. pneumoniae, respectively. This is similar to the results of in vitro studies, which have also demonstrated prolonged PAEs for daptomycin (7, 13). Hanberger et al. (15), using a bioluminescence assay, showed that the in vitro PAE of daptomycin ranged from 0.6 to 6.7 h against E. fecalis and 1.0 to 6.3 h against S. aureus. Another earlier study also showed an in vitro PAE of up to 2 h against S. aureus (J. Leggett, K. Totsuka, S. Ebert, B. Vogelman, and W. A. Craig, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 154, p. 123, 1987).
Our study demonstrates that the AUC/MIC and peak/MIC ratios are the important PK and PD parameters that determine the in vivo activity of daptomycin against staphylococci and streptococci. Another recent study with a single strain of S. aureus in the neutropenic murine thigh model suggested that the AUC/MIC ratio was the most important PD parameter for daptomycin (17). There are several differences between this study and that reported by Louie et al. (17), even though one of the strains that we studied was the same single isolate that they studied. The static dose in our study was 3.5-fold higher (22.5 versus 6.35 mg/kg/day). Moreover, the starting inoculum in their study was 5.04 log10 CFU/g, while it was 7.38 log10 CFU/thigh (average thigh weight, 1 g) in our study. Finally, the organism had grown 0.07 log10 CFU/g between the time of infection and the time of the start of therapy in their experiment, while the organism had grown 1.49 log10 CFU/thigh in our experiments. The higher inoculum and the growth of the organism in our studies likely account for the differences in the static doses. Nevertheless, the 24-h AUC/MIC ratio required for stasis in their study (43.4 by use of the MIC in 100% mouse serum) is similar to the value that we observed (42.0 [10% of 420]) by using free daptomycin concentrations and the MIC in broth. Thus, the application of these results to the treatment of human infections is based on similar conclusions.
In our study, which used multiple total doses and fractionation down to 3-h dosing regimens, we were not able to differentiate between the peak/MIC ratio and the 24-h AUC/MIC ratio as the more important parameter. The static doses in Table 2 would support the AUC/MIC ratio as the major PK and PD parameter. Furthermore, the magnitudes of the PK and PD parameters predicting a bacteriostatic effect and killing of 1 to 2 log10 CFU/thigh over 24 h were relatively similar among the various strains, despite the presence in some strains of methicillin and penicillin resistance. In terms of free drug concentrations, daptomycin levels need to average from one to two times the MIC over 24 h (i.e., 24-h AUC/MIC ratios of 24 to 48) to produce a bacteriostatic effect and two to four times the MIC over 24 h to produce greater than 99% killing. Peak concentrations of free drug needed to be 2.5 to 7 times the MIC to produce a bacteriostatic effect and 7 to 25 times the MIC to produce greater than 99% killing.
Our studies suggest that the AUCs for total daptomycin that must be obtained in plasma to produce killing of at least 1 log10 CFU/thigh for staphylococci and streptococci and the AUCs for total vancomycin to produce killing of at least 1 log10 CFU/thigh for drug-resistant E. faecium strains in neutropenic mice vary from 294 to 375 μg · h/ml for S. aureus, 13.5 to 93 μg · h/ml for S. pneumoniae, and 8.2 to 67 μg · h/ml for E. faecium. Studies of the PKs of daptomycin with human volunteers have demonstrated mean AUCs from time zero to infinity of 382 and 598 μg · h/ml for doses of 4 and 6 mg/kg, respectively (23). It is appropriate to compare AUCs in humans with those in mice on the basis of total drug concentrations, as the extent of protein binding is identical in both species (10). Thus, doses of 2 to 3 mg/kg twice daily or 4 to 6 mg/kg once daily would surpass the AUC target for efficacy against staphylococci obtained in our study. The use of lower doses should be possible for the successful treatment of pneumococcal infections. Lower doses might also be possible for treatment of infections caused by vancomycin-resistant E. faecium. However, we are concerned about the poor growth of these strains in the thigh model and believe that the activity of the drug against E. faecium may have been overestimated in this study. Additional studies with strains that grow better in the murine thigh are needed before conclusions on optimal dosage regimens for the treatment of infections caused by VRE can be made.
Once-daily administration of daptomycin exhibited efficacy either similar to or slightly better than that of more frequent administration against the standard strains of S. aureus and S. pneumoniae. A recent study with dogs demonstrated that once-daily dosing of daptomycin at 75 mg/kg resulted in less myopathy and greater increases in creatine phosphokinase levels in serum than those obtained with the same total dose administered as 25 mg/kg every 8 h (19). Thus, once-daily dosing may provide a regimen that maintains good efficacy and reduces the risk of muscle-related effects. The mean peak levels obtained in human volunteers treated with 4 and 6 mg/kg were 52 and 82 μg/ml, respectively (23). In our animal study the peak levels required to produce killing of 1 log10 CFU/thigh within 24 h were 30 to 46 μg/ml for S. aureus and 2 to 8 μg/ml for S. pneumoniae.
Our studies with daptomycin suggest that this lipopeptide antibiotic may prove to be valuable for the treatment of infections involving gram-positive bacteria. Its rapid bactericidal activity and long half-life make it a potentially important antibiotic. Once-daily dosing would appear to maintain in vivo antimicrobial activity. Phase III clinical trials that are under way will further define daptomycin's role in the treatment of infections caused by gram-positive bacteria.
REFERENCES
- 1.Amsterdam, D., E. A. Gorzynski, T. R. Beam, and C. Rotstein. 1994. Susceptibility of bacteraemic isolates of gram-positive cocci to daptomycin and other antimicrobial agents. J. Antimicrob. Chemother. 33:1060-1064. [DOI] [PubMed] [Google Scholar]
- 2.Andrew, J. H., M. C. Wale, L. J. Wale, and D. Greenwood. 1987. The effect of cultural conditions on the activity of LY146032 against staphylococci and streptococci. J. Antimicrob. Chemother. 20:213-221. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 1997. Minimum inhibitory concentration interpretive standards, M7-A4. Document 2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 4.Anonymous. 1993. Nosocomial enterococci resistant to vancomycin—United States, 1989-1993. Morb. Mortal. Wkly. Rep. 42:597-599. [PubMed] [Google Scholar]
- 5.Appelbaum, P. C., S. K. Spangler, E. Crotty, and M. R. Jacobs. 1989. Susceptibility of penicillin-sensitive and -resistant strains of Streptococcus pneumoniae to new antimicrobial agents, including daptomycin, teicoplanin, cefpodoxime and quinolones. J. Antimicrob. Chemother. 23:509-516. [DOI] [PubMed] [Google Scholar]
- 6.Barry, A. L., P. C. Fuchs, and S. D. Brown. 2001. In vitro activities of daptomycin against 2,789 clinical isolates from 11 North American medical centers. Antimicrob. Agents Chemother. 45:1919-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartoloni, A., M. G. Colao, A. Orsi, R. Dei, E. Giganti, and F. Parenti. 1990. In-vitro activity of vancomycin, teicoplanin, daptomycin, ramoplanin, MDL 62873 and other agents against staphylococci, enterococci and Clostridium difficile. J. Antimicrob. Chemother. 26:627-633. [DOI] [PubMed] [Google Scholar]
- 8.Bush, L. M., J. A. Boscia, M. Wendeler, P. G. Pitsakis, and D. Kaye. 1989. In vitro postantibiotic effect of daptomycin (LY146032) against Enterococcus faecalis and methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains. Antimicrob. Agents Chemother. 33:1198-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coudron, P. E., J. L. Johnston, and G. L. Archer. 1987. In-vitro activity of LY146032 against Staphylococcus aureus and S. epidermidis. J. Antimicrob. Chemother. 20:505-511. [DOI] [PubMed] [Google Scholar]
- 10.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 11.Debbia, E., A. Pesce, and G. C. Schito. 1988. In vitro activity of LY146032 alone and in combination with other antibiotics against gram-positive bacteria. Antimicrob. Agents Chemother. 32:279-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eliopoulos, G. M., S. Willey, E. Reiszner, P. G. Spitzer, G. Caputo, and R. C. Moellering, Jr. 1986. In vitro and in vivo activity of LY 146032, a new cyclic lipopeptide antibiotic. Antimicrob. Agents Chemother. 30:532-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fass, R. J., and V. L. Helsel. 1986. In vitro activity of LY146032 against staphylococci, streptococci, and enterococci. Antimicrob. Agents Chemother. 30:781-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flandrois, J. P., G. Fardel, and G. Carret. 1988. Early stages of in vitro killing curve of LY146032 and vancomycin for Staphylococcus aureus. Antimicrob. Agents Chemother. 32:454-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanberger, H., L. E. Nilsson, R. Maller, and B. Isaksson. 1991. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob. Agents Chemother. 35:1710-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knapp, C. C., and J. A. Washington II. 1986. Antistaphylococcal activity of a cyclic peptide, LY146032, and vancomycin. Antimicrob. Agents Chemother. 30:938-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louie, A., P. Kaw, W. Liu, N. Jumbe, M. H. Miller, and G. L. Drusano. 2001. Pharmacodynamics of daptomycin in a murine thigh model of Staphylococcus aureus infection. Antimicrob. Agents Chemother. 45:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouton, R. P., and S. L. Mulders. 1987. LY146032: activity and resistance development in vitro. J. Antimicrob. Chemother. 20:513-517. [DOI] [PubMed] [Google Scholar]
- 19.Oleson, F. B., Jr., C. L. Berman, J. B. Kirkpatrick, K. S. Regan, J. J. Lai, and F. P. Tally. 2000. Once-daily dosing in dogs optimizes daptomycin safety. Antimicrob. Agents Chemother. 44:2948-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pankuch, G. A., M. R. Jacobs, and P. C. Appelbaum. 2003. Bactericidal activity of daptomycin against Streptococcus pneumoniae compared with eight other antimicrobials. J. Antimicrob. Chemother. 51:443-446. [DOI] [PubMed] [Google Scholar]
- 21.Rotschafer, J. C., M. W. Garrison, and K. A. Rodvold. 1988. Therapeutic update on glycopeptide and lipopeptide antibiotics. Pharmacotherapy 8:211-219. [DOI] [PubMed] [Google Scholar]
- 22.Vogelman, B., S. Gudmundsson, J. Turnidge, J. Leggett, and W. A. Craig. 1988. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J. Infect. Dis. 157:287-298. [DOI] [PubMed] [Google Scholar]
- 23.Woodworth, J. R., E. H. Nyhart, Jr., G. L. Brier, J. D. Wolny, and H. R. Black. 1992. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy volunteers. Antimicrob. Agents Chemother. 36:318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]