Abstract
Viral replication, as well as an immunopathological component, is assumed to be involved in coxsackie B virus-induced myocarditis. We evaluated the efficacy of the interferon inducer Ampligen on coxsackie B3 virus-induced myocarditis in C3H/HeNHsd mice. The efficacy of Ampligen was compared with that of the interferon inducer poly(inosinic acid)-poly(cytidylic acid) [poly(IC)], alpha interferon 2b (INTRON A), and pegylated alpha interferon 2b (PEG-INTRON-α-2b). Ampligen at 20 mg/kg of body weight/day was able to reduce the severity of virus-induced myocarditis, as assessed by morphometric analysis, by 98% (P = 3.0 × 10−8). When poly(IC) was administered at 15 mg/kg/day, it reduced the severity of virus-induced myocarditis by 93% (P = 5.6 × 10−5). Alpha interferon 2b (1 × 105 U/day) and pegylated alpha interferon 2b (5 × 105 U/day) were less effective and reduced the severity of virus-induced myocarditis by 66% (P = 0.0009) and 78% (P = 0.0002), respectively. The observed efficacies of Ampligen and poly(IC) were corroborated by the observation that the drugs also markedly reduced the virus titers in the heart, as detected by (i) quantitative real-time reverse transcription-PCR and (ii) titration for infectious virus content. Whereas the electrocardiograms for untreated mice with myocarditis were severely disturbed, the electrocardiographic parameters were normalized in Ampligen- and poly(IC)-treated mice. Even when start of treatment with Ampligen was delayed until day 2 postinfection, a time at which lesions had already appeared in untreated control animals, a marked protective effect on the development of viral myocarditis (as assessed at day 6 postinfection) was still noted.
Myocarditis is a common cause of idiopathic dilated cardiomyopathy, one of the single most important reasons for heart transplantation (13). Coxsackie B viruses (CBVs) are considered the principal etiological agents of viral myocarditis (1, 2). The management of viral myocarditis is complicated by (i) the fact that the majority of the patients remain asymptomatic and (ii) the absence of rapid and reliable diagnostic tools. The clinical manifestations may differ among patients who are symptomatic. They may show abnormalities in left ventricular systolic and diastolic functions and electrical activation, leading to arrhythmias and conduction disorders (16). Abnormalities in electrocardiographic (ECG) findings are common in patients with acute myocarditis, and ECG is recognized as a useful tool in the diagnosis of this heart disease. Patients with acute viral myocarditis usually develop arrhythmias such as atrioventricular block, which are also important risk factors for morbidity and mortality (15). Total QRS amplitudes during the acute stage of the disease are significantly decreased in both patients (23) and experimentally infected animals (17, 30).
The protective effect of human interferon against coxsackie B3 virus (CBV3) replication in vitro (8, 9, 14, 25) and CBV-induced myocarditis in murine models has been well documented (20, 21, 22). Pegylation (“peg” refers to polyethylene glycol) of interferon has been shown to increase the relatively short half-life of the molecule and to result in more constant levels in blood (32). Pegylated alpha interferon 2b (PEG-IFN), either alone or in combination with ribavirin, has been approved for use for the treatment of chronic hepatitis C virus infection in patients with compensated liver disease (27, 29); and PEG-IFN is being evaluated in phase III clinical trials for the treatment of chronic myelogenous leukemia and malignant melanoma (4, 26). Poly(inosinic acid)-poly(cytidylic acid) [poly(IC)] is a highly effective inducer of interferon both in vitro and in vivo (5). This molecule has been shown to offer protection against a variety of experimental viral infections in mice (3, 6, 18). Ampligen [poly(I)-poly(C12U)] is an interferon inducer that consists of poly(IC) with a U mismatch at every 12th base of the C strand. The major effect of Ampligen is on the Th1 arm of the immune system. This has been demonstrated in delayed-type hypersensitivity reactions and in current clinical studies with human immunodeficiency virus-infected patients. Other cells targeted are NK cells, cytotoxic CD8 cells, and LAK-NK cells. Ampligen likely acts through binding to the Toll-like receptor 3 and entry into the cell (i.e., endocytosis of the receptor-ligand complex), although this has not been directly demonstrated. We wanted to study the effect of Ampligen on the development of viral myocarditis because no single compound in clinical use (with the exception of pleconaril, which is given on a compassionate-use basis) can be used for the treatment of viral myocarditis. Because Ampligen has already been extensively studied for the treatment of a variety of conditions in patients (such as myalgic encephalomyelitis-chronic fatigue syndrome and human immunodeficiency virus disease [31]), it was of interest to explore the efficacy of Ampligen in a murine model of CBV3-induced myocarditis. The activity of this drug was compared with the activities of poly(IC), alpha interferon 2b (IFN), and PEG-IFN.
MATERIALS AND METHODS
Cells and viruses.
CBV3 (Nancy strain) was obtained from the American Type Culture Collection (VR-30). African green monkey kidney (Vero) cells were propagated in minimal essential medium (Gibco, Life Technologies, Rockville, Md.) supplemented with 10% fetal calf serum (Integro, Zaandam, The Netherlands), 1% l-glutamine (Gibco, Life Technologies), and 0.3% sodium bicarbonate (Gibco, Life Technologies). Confluent cultures of Vero cells were infected and incubated at 37°C until an extensive cytopathic effect was observed (generally 5 to 7 days postinfection). At that time, the culture medium was collected, the cell debris was pelleted by centrifugation, and the supernatant was aliquoted and stored at −80°C.
Compounds.
INTRON A (IFN) and PEG-INTRON (PEG-IFN) were kindly provided by Schering-Plough (Brussels, Belgium). Poly(IC) was kindly provided by A. Timkovsky (Petersburg Nuclear Physics Institute, St. Petersburg-Gatchina, Russian Federation). Ampligen [poly(I)-poly(C12U)] was a kind gift from Hemispherx Biopharma (Philadelphia, Pa.).
Virus titration from heart homogenate.
Hearts of infected animals were dissected aseptically on day 6 postinfection, when the highest virus titers in the hearts of untreated controls animals were observed in preliminary experiments (data not shown). Heart homogenates 10% (wt/vol) were prepared in minimal essential medium. Vero cells were grown to confluence in microtiter trays, infected with serial dilutions of the homogenates, and incubated for 2 h at 37°C, after which the virus was removed. The virus-induced cytopathic effect was recorded microscopically (at 5 day postinfection, following fixation with 70% ethanol and staining with a 2% Giemsa solution [Merck, Darmstadt, Germany]).
Assessing in vivo toxicity.
Male C3H/HeNHsd mice (weight, 15 g; Harlan Laboratories, Horst, The Netherlands), were treated intraperitoneally (i.p.) for 7 consecutive days with either 5 × 105 U of IFN (n = 6) or 1 × 105 U of PEG-IFN (n = 6) and for 3 consecutive days with either 15 mg of poly(IC) per kg of body weight per day (n = 5) or 20 mg of Ampligen per kg per day (n = 6). The body weights were monitored daily. The body weight changes between the different groups were compared throughout the entire experiment.
Treatment of virus-infected mice.
Four-week-old male C3H/HeNHsd mice (weight, 15 g; Harlan Laboratories) were inoculated i.p. with a 50% cell culture infective dose of CBV3 of 107 (as determined by plaque assay on Vero cells). Infection of this strain of mice with CBV3 (Nancy) results in a nonlethal cardiac disease. Infected animals survived for at least 8 months postinfection (data not shown). IFN (in sterile phosphate-buffered saline [PBS]) was administered i.p. once daily (starting on day 1 before infection and continuing until day 5 postinfection) at a dose of 5 × 105 U. PEG-IFN (in sterile PBS) was administered i.p. once daily (starting on day 1 before infection and continuing until day 5 postinfection) at a dose of 105 U. Poly(IC) was dissolved in sterile PBS and was administered i.p. once daily on days −1, 0, and 1 at a dose of 15 mg/kg/day. Ampligen was dissolved in RNase-free water (Acros Organics, Geel, Belgium) and was administered i.p. once daily on days −1, 0, and 1 at a dose of 20 mg/kg/day. Doses were selected on the basis of (i) our preliminary experience with this model as well as (ii) previous studies with other murine models of viral infection (14, 19). The body weights of all animals were monitored daily. All animals were killed on day 6 postinfection (following ether anesthesia). Half of the hearts were used for virus titration, as described above, and for quantitation of viral RNA. The other hearts were fixed in 10% buffered formaldehyde, embedded in paraffin, and cut into sections of 5 μm. The sections were stained with hematoxylin-eosin (H&E) and examined by light microscopy.
Morphometry.
Because of the relatively homogeneous distribution of the myocarditis lesions in the affected hearts (10), the proportion on the surface of myocarditis lesions may be considered representative of their proportion in the entire volume. The number of myocarditis lesions and their surface proportion were determined with H&E-stained sections of the hearts of untreated and IFN-, PEG-IFN-, poly(IC)-, and Ampligen-treated animals. The surface proportion occupied by myocarditis lesions was determined by means of a conventional point-counting method, as reported earlier (19), by using an ocular grid containing 121 equally spaced points. The surface proportion estimates the percentage of heart tissue that is affected by focal myocarditis. Scoring was done by counting five hit points at five stratified random positions of the grid. The counting was performed on three sections per heart. The counting resulted in a total of 75 points per heart. The sections were evaluated at a magnification of ×200.
Quantitative analysis of CBV3 RNA by Taqman real-time reverse transcription-PCR (RT-PCR).
Total cellular RNA was extracted from 10% heart homogenates by using the QIAamp RNA Mini kit (Qiagen, Hilden, Germany) by the procedure described by the manufacturer. After denaturation at 70°C for 10 min, cDNA was generated at 42°C for 45 min by using 200 U of Moloney murine leukemia virus reverse transcriptase (Gibco, Life Technologies), 40 U of rRNasin RNase inhibitor (Promega Corporation, Madison, Wis.), 5 μM random hexamer primers (Amersham Pharmacia Biotech, Roosendal, The Netherlands), 1 mM deoxynucleoside triphosphates (Gibco, Life Technologies), and buffer containing 250 mM Tris HCl (pH 8.3), 375 mM KCl, and 15 mM Mg2+ (Gibco, Life Technologies). The reaction was terminated by heating at 99°C for 3 min. Real-time PCR was performed on the ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, Calif.). Primers and probes were developed with Primer Express software (Applied Biosystems). The following primers and probe were used and were based on the complete genome sequence of CBV3 (GenBank accession number M16572.1): a forward primer specific for nucleotides 2937 to 2957 (5′-ACGAATCCCAGTGTGTTTTGG-3′), a reverse primer specific for nucleotides 3003 to 2982 (5′-TGCTCAAAAACGGTATGGACAT-3′), and a Taqman probe specific for nucleotides 2960 to 2977 (5′-CGAGGGAAACGCCCCGCC-3′).
The Taqman probe was labeled at the 5′ end with the reporter dye molecule 6-carboxyfluorescein (emission wavelength, 518 nm) and at the 3′ end with the quencher dye 6-carboxytetramethylrhodamine (emission wavelength, 582 nm). The 3′ end of the probe was additionally phosphorylated to prevent extension during PCR. Each PCR was performed in 25 μl of a PCR reagent mixture containing 0.25 μl of each primer (900 nM), 1 μl of the specific Taqman probe (200 nM), 12.5 μl of 2× universal Master Mix (Applied Biosystems, Roche, Branchburg, N.J.), 6 μl of water, and 5 μl of sample. The PCR consisted of a decontamination step (5 min at 50°C), a Taq activation step (10 min at 94°C), and 50 cycles of denaturation (10 s at 94°C) and annealing (1 min at 60°C). For each PCR run, negative template and positive template samples were used. The cycle threshold (Ct) value is defined as the number of PCR cycles for which the signal exceeds the baseline signal, which defines a positive value. The sample was considered positive if the Ct value was <50. The results are expressed as genomic equivalents.
ECG.
ECG recording and analysis were performed by a previously described procedure (24). Briefly, for ECG recording, the mice were placed under stable anesthesia with sodium pentobarbital (70 mg/kg i.p.; Nembutal; Abbott Laboratories, North Chicago, Ill.) and fixed in the supine position, and eight-lead ECGs were recorded from subcutaneous 18-gauge needle electrodes subcutaneously implanted in each limb and two electrodes at precordial positions V2 and V6. ECGs were recorded by using an adapted front-end Siemens mingograph with band-pass filtering between 0.03 and 1,000 Hz. Supplementary amplification and analog-digital conversion were performed with a Powerlab 16S instrument (AD Instruments, Hastings, United Kingdom). Digital recordings (16 bit, 4 kHz/channel) were analyzed with the Chart (version 4.0) program (AD Instruments). The signal-averaged ECG (SAECG) was calculated by using the mouse SAECG extension (version 1.2) program (AD Instruments) and a template-matching algorithm. ECG parameters were calculated by use of standard criteria. To evaluate the physiological impact of CBV3-induced myocarditis in C3H/HeNHsd mice, the sum of the QRS voltage in eight-lead SAECGs was calculated (17). ECGs were recorded on day 1 before infection (day −1) and day 6 postinfection.
Statistics.
Statistical differences in the number of myocarditis lesions or virus titers were assessed by Student's t test.
RESULTS
Effects of Ampligen, poly(IC), IFN, and PEG-IFN on CBV3-induced myocarditis.
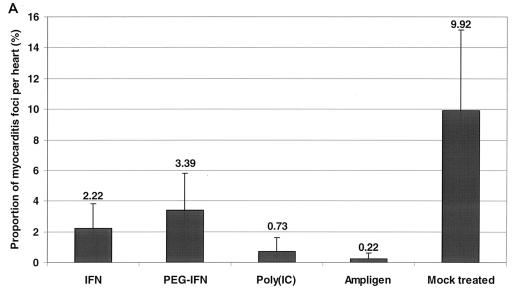
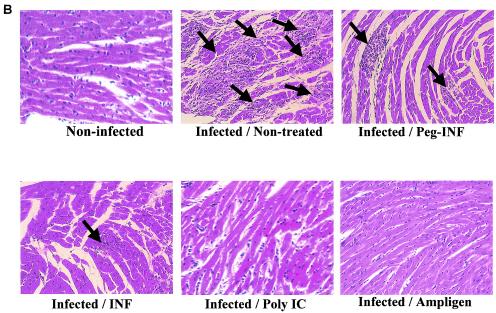
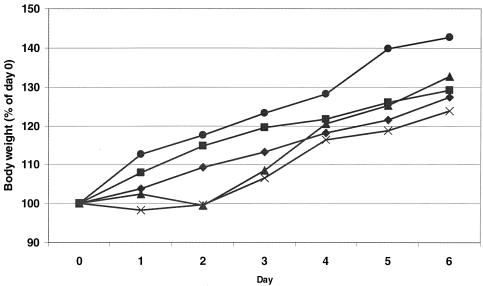
C3H/HeNHsd mice were infected with CBV3 i.p. and treated i.p. once daily for 7 consecutive days (starting on day 1 before infection) with 5 × 105 U of IFN or 1 × 105 U of PEG-IFN. Poly(IC) was administered i.p. at a dose of 15 mg/kg/day and Ampligen was administered i.p. at a dose of 20 mg/kg/day once daily on days −1, 0, and 1 postinfection. Untreated infected animals served as a control group. All mice were killed on day 6 postinfection, and the severity of the myocarditis was assessed by morphometric analysis (Fig. 1A). All treatments resulted in an important reduction in the severity of the CBV3-induced myocarditis that developed compared to that for the untreated control: a 98% reduction was achieved with Ampligen (P = 3.0 × 10−8), a 93% reduction was achieved with poly(IC) (P = 5.6 × 10−5), a 78% reduction was achieved with IFN (P = 0.0002), and a 66% reduction was achieved with PEG-IFN (P = 0.0009). The severity of the lesions in the untreated controls and the protective effects of the different drugs are documented in Fig. 1B. With the best treatment regimens, in which the reductions in the numbers and sizes of the remaining foci of myocarditis were virtually complete, no (or almost no) infiltration was observed. A gradual calcification of the lesions was noted (data not shown) at later times after infection (i.e., days 10 to 33 postinfection). We may assume that in those mice that had received treatment and in which few (or even no) lesions developed, these minor or microlesions will also calcify, like the large lesions in the untreated mice. The body weights of uninfected animals that were treated according to the same schedule as the infected animals were monitored daily over a period of 7 days. Although there was a trend toward growth retardation in all drug-treated animals, no significant difference in body weights compared with those of the untreated controls was calculated (Fig. 2).
FIG. 1.
(A) Effects of treatment with Ampligen, poly(IC), IFN, and PEG-IFN on CBV3-induced myocarditis in C3H/HeNHsd mice. Infected mice were treated i.p. for seven consecutive days (−1, 0, 1, 2, 3, 4, and 5 relative to the time of infection) with 5 × 105 U of IFN (three mice) or 1 × 105 U of PEG-IFN (six mice). Poly(IC) was administered i.p. at 15 mg/kg/day (five mice); and Ampligen was administered i.p. at 20 mg/kg/day once daily on days −1, 0, and 1 relative to the time of infection (six mice). Untreated infected animals (four mice) served as a control group. Animals were killed on day 6 postinfection, and the severity of the myocarditis lesions was assessed by morphometric analysis. (B) Histological sections (H&E staining) of the CBV3-infected hearts of mice treated with Ampligen, poly(IC), IFN, or PEG-IFN. Animals were killed on day 6 postinfection. Arrows indicate myocarditis foci.
FIG. 2.
Effects of Ampligen, poly(IC), IFN, and PEG-IFN on the growth of uninfected C3H/HeNHsd mice. Animals (weight, ≈15 g) were treated i.p. for seven consecutive days with either 5 × 105 U of IFN (six mice) or 1 × 105 U of PEG-IFN (six mice). Poly(IC) at a dose of 15 mg/kg/day once daily (five mice) or Ampligen at a dose of 20 mg/kg/day once daily (six mice) was administered i.p. for three consecutive days. Untreated C3H/HeNHsd mice (n = 4) served as a body weight control group. Body weight was monitored daily. •, untreated controls; ⧫, IFN-treated mice; ▪, PEG-IFN-treated mice; ▴, poly(IC)-treated mice; ×, Ampligen-treated mice.
Effect of Ampligen, poly(IC), IFN, and PEG-IFN on virus titers in heart homogenates.
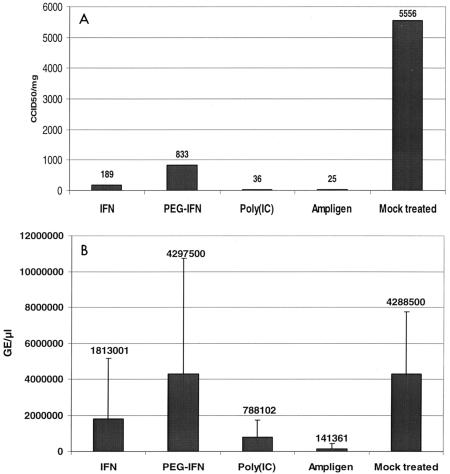
C3H/HeNHsd mice were infected with CBV3 i.p. and treated once daily for 7 consecutive days (starting on day 1 before infection) with Ampligen, poly(IC), IFN, or PEG-IFN according to the treatment schedules described above. In preliminary experiments it was found that the highest virus titers in the hearts were reached at 6 days postinfection. The animals were therefore killed at 6 days postinfection and the virus titers were measured by (i) titration for infectious virus content and (ii) real-time RT-PCR (Fig. 3A and B). PEG-IFN reduced the virus titers by 85%, IFN reduced the titers by 96.6%, poly(IC) reduced the titers by 99.4%, and Ampligen reduced the titers by 99.6% (Fig. 3A). Analysis of the same sample material by the Taqman real-time RT-PCR revealed a significant reduction in viral RNA levels in heart homogenates of animals that had been treated with either poly(IC) (82% reduction of viral RNA levels [P = 0.0186]) or Ampligen (97% reduction in viral RNA levels [P = 0.0086]). Neither IFN nor PEG-IFN caused a significant reduction in viral RNA titers compared to those in the untreated controls (Fig. 3B).
FIG. 3.
(A) Effects of Ampligen, poly(IC), IFN, and PEG-IFN on infectious virus titers (expressed as 50% cell culture infective doses [CCID50]) in the heart tissue of CBV3-infected C3H/HeNHsd mice at day 6 postinfection. Each data set represents the mean ± standard deviation for four mice per group. (B) Effects of Ampligen, poly(IC), IFN, and PEG-IFN on viral RNA titers in heart tissue of CBV3-infected C3H/HeNHsd mice at day 6 postinfection. Each data set represents the mean ± standard deviation for four mice per group. GE, genome equivalents.
Effects of Ampligen and poly(IC) on the ECGs of infected mice.
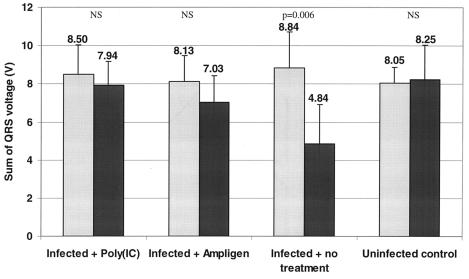
We next studied the effects of Ampligen and poly(IC) (the two compounds that were the most effective in reducing myocarditis lesions as well as infectious virus and viral RNA titers) on the electrical activity of the heart. C3H/HeNHsd mice were infected with CBV3 i.p. and treated once daily for 3 consecutive days (starting at day 1 before infection) with either poly(IC) (n = 6) or Ampligen (n = 6) at a dose of 15 or 20 mg/kg/day, respectively. Eight-lead ECGs were recorded from subcutaneous 18-gauge needles in each limb and in precordial positions for mice in the supine position. ECGs were recorded on the day before infection (day −1) and day 6 postinfection. As the sum of the QRS voltages decreases with the stage of myocardial necrosis (17), we compared the sum of the QRS voltages per group on day −1 and day 6. Conduction and repolarization abnormalities were observed in infected, untreated mice (Fig. 4), and the difference between the values of the sums of the QRS voltages on day −1 and day 6 was reduced by 46% (P = 0.006) (Fig. 5). The differences in the sums of the QRS voltages on day −1 and day 6 were, as expected, not significantly different for the uninfected controls (P = 0.834). Also, no significant difference in the sums of the QRS voltages between day −1 and day 6 was noted for infected mice that were treated with Ampligen (P = 0.196) or poly(IC) (P = 0.504).
FIG. 4.
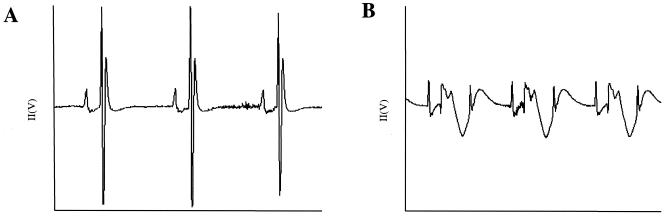
Example ECGs for C3H/HeNHsd mice with CBV3-induced myocarditis. ECGs were recorded on day 1 before infection (A) and day 6 postinfection (B). (A) The ECG on day −1 was normal. (B) The ECG on day 6 was abnormal, and conduction was present as advanced second-degree atrioventricular block (2:1 AVB), a QRS prolongation, and a diminished overall QRS amplitude. Heterogeneous ventricular repolarization is also suggested by the QT prolongation.
FIG. 5.
Effects of poly(IC) and Ampligen on ECG parameters in CBV3-infected mice at day 6 postinfection. ECGs were recorded on 1 day before infection (day −1) and 6 day postinfection. The sums of the QRS voltages on either day −1 (gray bars) or day 6 (black bars) were calculated for infected animals that had been either left untreated (six mice), treated with poly(IC) (six mice; 15 mg/kg/day i.p. once daily for three consecutive days [−1, 0, and 1]), or treated with Ampligen (six mice; 20 mg/kg/day i.p. once daily for three consecutive days [−1, 0, and 1]). Untreated uninfected control mice (n = 5) were included as well. NS, not significant.
Effects of delayed start of treatment with Ampligen and poly(IC) on CBV3-induced myocarditis.
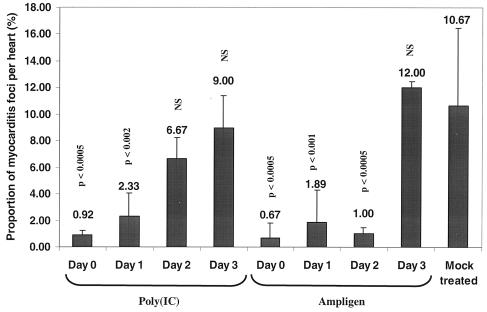
We next studied whether a delayed start of treatment with Ampligen or poly(IC) would still be effective in reducing the severity of CBV3-induced myocarditis in the murine model. Myocardial lesions in a parallel group of untreated control animals were observed as early as 2 days postinfection (data not shown). C3H/HeNHsd mice were infected with CBV3 i.p.; and treatment was initiated on day 0, 1, 2, or 3 after infection and was continued for three consecutive days. In a typical experiment viral RNA was detectable in the hearts of infected mice by real-time quantitative RT-PCR as early as 24 h postinfection (data not shown). Between day 1 and day 2 postinfection the virus titers in the hearts increased by a factor 220. All mice were killed on day 6 postinfection, and the severity of the myocarditis was monitored by morphometric analysis (Fig. 6). Both Ampligen and poly(IC) caused an important reduction in the severity the CBV3-induced myocarditis that developed when treatment was started on either day 0 or day 1. No statistically significant reduction was noted when treatment with poly(IC) was started on day 2 postinfection. Ampligen, however, was still protective when treatment was started on day 2 postinfection, when control animals had already developed myocarditis lesions, as assessed in a parallel group (data not shown). Delay of the start of treatment with Ampligen until 3 days postinfection, however, was no longer more effective (Fig. 6).
FIG. 6.
Effect of delayed start of treatment with poly(IC) or Ampligen on CBV3-induced myocarditis in C3H/HeNHsd mice. Treatment of infected mice was initiated at day 0, 1, 2, or 3 postinfection and was continued for three consecutive days. Poly(IC) was administered at 15 mg/kg/day i.p. once daily (four mice), and Ampligen was administered at a dose of 20 mg/kg/day according to the same treatment schedule used for poly(IC) (three mice). The animals were killed at day 7 postinfection, and the severities of the myocarditis lesions were assessed by morphometric analysis. Untreated uninfected control mice (n = 4) were included as well. NS, not significant.
DISCUSSION
In the murine model for acute myocarditis, as used here, treatment with the interferon inducer Ampligen or poly(IC), as well as with IFN or PEG-IFN, resulted in a marked reduction in the severity of myocarditis. Even a delayed start of treatment with Ampligen (i.e., until the time that the first myocardial lesions started to develop in the untreated animals when the virus was present in the hearts) resulted in a marked protective effect. Ampligen and poly(IC) proved more effective than both IFN and PEG-IFN. This may be related to the fact that the IFN and PEG-IFN used were of human origin and may thus be expected to be less effective than the species-specific murine interferon induced by interferon inducers. We do not yet have an explanation why Ampligen exhibited activity superior to that of poly(IC).
The impact of direct virus-induced damage in the pathogenesis of CBV3-induced myocarditis and the potential benefit of reducing the level of viremia early during infection have recently been reported (12, 28). The reduction in the severity of the myocarditis scores as a result of treatment with all four compounds used in the present study was corroborated by a marked reduction in virus titers in heart tissue. Quantitative RT-PCR also revealed a significant reduction in viral RNA levels in the hearts of animals that had been treated with interferon inducers, although no significant reduction in viral RNA loads was observed in mice that had been treated with IFN or PEG-IFN. The higher efficiencies of both Ampligen and poly(IC) compared to those of IFN and PEG-IFN in reducing infectious virus titers as well as viral RNA titers were mirrored by the fact that the interferon inducers were also more effective than IFN or PEG-IFN in protecting the mice against myocarditis.
ECGs have successfully been used to monitor the physiological alterations of the heart functions of animals with myocarditis (7, 30, 33) and have been shown to be instrumental in assessing the severity of myocarditis (11). In the present study, conduction and repolarization abnormalities and an important reduction in the total QRS complex voltages were noted in the CBV-infected untreated animals at day 6 postinfection. The preservation of the histological structures of the hearts of CBV-infected animals that had been treated with Ampligen or poly(IC) was reflected in a normal ECG and a lack of alteration of the total QRS voltage.
Our data indicate that the efficient reduction of viral replication, achieved here by the interferon inducers Ampligen and poly(IC), is sufficient to protect mice against CBV3-induced myocarditis. Given (i) the potency of Ampligen in the murine model of CBV3-induced myocarditis, (ii) the fact that the start of treatment with Ampligen can be significantly delayed for several days after infection before the protective activity is lost, and (iii) the fact that there exists a significant amount of experience with Ampligen in the clinical setting, our data may have clinical consequences. Ampligen demonstrates minimal toxic effects, in contrast to the parent compound, poly(IC). The latter produces liver toxicity, blood dyscrasias, and antibodies to double-stranded DNA and RNA, with a resultant lupus-like syndrome that persists even upon elimination of the poly(IC). The reduced toxicity is the major rationale for the study of Ampligen as a drug for human use. We recently demonstrated (unpublished data) that a reduction in the inflammation process following administration of the immunosuppressive agent mycophenolate mofetil also results in a marked decrease in the severity of myocarditis, according to the myocarditis scores in CBV3-infected mice, even when use of the immunosuppressive agent resulted in an increase in the infectious virus titer in heart tissue. Use of a combination consisting of an inhibitor of viral replication (such as Ampligen) and mycophenolate mofetil may likely represent an ideal treatment strategy. The question remains, however, how such a treatment regimen can be implemented in the clinical setting. Once extensive lesions have developed in the heart, the proposed treatment may no longer be expected to ensure complete cure of disease. Therefore, an early diagnosis of virus-induced myocarditis will be mandatory for a treatment regimen based on an interferon inducer, such as Ampligen, and immunosuppressants to yield the greatest clinical benefit.
Acknowledgments
This work was supported by the Geconcerteerde Onderzoeksactie (grant GOA 0/12) of the KULeuven.
We appreciate the editorial assistance of D. Brabants and I. Aerts and the technical assistance of W. Zeegers.
REFERENCES
- 1.Baboonian, C., M. J. Davies, J. C. Booth, and W. J. McKenna. 1997. Coxsackie B viruses and human heart disease. Curr. Top Microbiol. Immunol. 223:31-52. [DOI] [PubMed] [Google Scholar]
- 2.Baboonian, C., and T. Treasure. 1997. Meta-analysis of the association of enteroviruses with human heart disease. Heart 78:539-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black, P. L., K. M. McKinnon, S. L. Wooden, and M. A. Ussery. 1996. Antiviral activity of biological response modifiers in a murine model of AIDS. Requirement for augmentation of natural killer cell activity and synergy with oral AZT. Int. J. Immunopharmacol. 18:633-650. [DOI] [PubMed] [Google Scholar]
- 4.Druker, B. J., C. L. Sawyers, R. Capdeville, J. M. Ford, M. Baccarani, and J. M. Goldman. 2001. Chronic myelogenous leukemia. Hematology 1:87-112. [DOI] [PubMed] [Google Scholar]
- 5.Field, A. K., A. A. Tytell, E. Piperno, G. P. Lampson, M. M. Nemes, and M. R. Hilleman. 1972. Poly I:C, an inducer of interferon and interference against virus infections. Medicine (Baltimore) 51:169-174. [DOI] [PubMed] [Google Scholar]
- 6.Guidotti, L. G., A. Morris, H. Mendez, R. Koch, R. H. Silverman, B. R. Williams, and F. V. Chisari. 2002. Interferon-regulated pathways that control hepatitis B virus replication in transgenic mice. J. Virol. 76:2617-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gwathmey, J. K., S. Nakao, P. C. Come, M. E. Goad, J. R. Serur, A. V. Alsm, and W. H. Abelmann. 1992. An experimental model of acute and subacute viral myocarditis in the pig. J. Am. Coll. Cardiol. 19:864-869. [DOI] [PubMed] [Google Scholar]
- 8.Heim, A., C. Brehm, M. Stille-Siegener, G. Mullerm, S. Hakem, R. Kandolf, and H. R. Figulla. 1995. Cultured human myocardial fibroblasts of pediatric origin: natural human interferon-alpha is more effective than recombinant interferon-alpha 2a in carrier-state coxsackievirus B3 replication. J. Mol. Cell. Cardiol. 27:2199-2208. [DOI] [PubMed] [Google Scholar]
- 9.Heim, A., I. Grumbach, P. Pring-Akerblom, M. Stille-Siegener, G. Muller, R. Kandolf, and H. R. Figulla. 1997. Inhibition of coxsackievirus B3 carrier state infection of cultured human myocardial fibroblasts by ribavirin and human natural interferon-alpha. Antivir. Res. 34:101-111. [DOI] [PubMed] [Google Scholar]
- 10.Herskowitz, A., L. J. Wolgram, N. R. Rose, and K. W. Beisel. 1987. Coxsackievirus B3 murine myocarditis: a pathologic spectrum of myocarditis in genetically defined inbred strains. J. Am. Coll. Cardiol. 9:1311-1319. [DOI] [PubMed] [Google Scholar]
- 11.Herzum, M., R. Weller, H. Jomaa, F. Wietrzychowski, S. Pankuweit, P. Mahr, and B. Maisch. 1995. Left ventricular hemodynamic parameters in the course of acute experimental coxsackievirus B3 myocarditis. J. Mol. Cell. Cardiol. 27:1573-1580. [DOI] [PubMed] [Google Scholar]
- 12.Horwitz, M. S., A. La Cava, C. Fine, E. Rodrigez, A. Ilic, and N. Sarvetnick. 2000. Pancreatic expression of interferon-gamma protects mice from lethal coxsackievirus B3 infection and subsequent myocarditis. Nat. Med. 6:693-697. [DOI] [PubMed] [Google Scholar]
- 13.Hosenpud, J. D., R. J. Novick, L. E. Bennet, B. M. Keck, B. Fiol, and O. P. Daily. 1996. The registry of transplantation: thirteenth official report. J. Heart Lung Transplant. 15:655-674. [PubMed] [Google Scholar]
- 14.Kandolf, R., A. Canu, and P. H. Hofschneider. 1985. Coxsackie B3 virus can replicate in cultured human foetal heart cells and is inhibited by interferon. J. Mol. Cell. Cardiol. 17:167-181. [DOI] [PubMed] [Google Scholar]
- 15.Karjalainen, J., M. Viitasalo, R. Kala, and J. Heikkila. 1984. 24-Hour electrocardiographic recordings in mild acute infectious myocarditis. Ann. Clin. Res. 16:34-39. [PubMed] [Google Scholar]
- 16.Kearney, M. T., J. M. Cotton, P. J. Richardson, and A. J. Shah. 2001. Viral myocarditis and dilated cardiomyopathy: mechanisms, manifestations, and management. Postgrad. Med. J. 77:4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishimoto, C., A. Matsumori, M. Ohmae, N. Tomioka, and C. Kawai. 1984. Electrocardiographic findings in experimental myocarditis in DBA/2 mice: complete atrioventricular block in the acute stage, low voltage of the QRS complex in the subacute stage and arrhytmias in the chronic stage. J. Am. Coll. Cardiol. 6:1461-1468. [DOI] [PubMed] [Google Scholar]
- 18.Leyssen, P., C. Drosten, M. Paning, N. Charlier, J. Paeshuyse, E. De Clercq, and J. Neyts. 2003. Interferons, interferon inducers, and interferon-ribavirin in treatment of flavivirus-induced encephalitis in mice. Antimicrob. Agents Chemother. 47:777-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liekens, S., E. Verbeken, M. Vandeputte, E. De Clercq, and J. Neyts. 1999. A novel animal model for hemangiomas: inhibition of hemangioma development by the angiogenesis inhibitor TNP-470. Cancer Res. 59:2376-2383. [PubMed] [Google Scholar]
- 20.Lutton, C. W., and C. J. Gauntt. 1985. Ameliorating effect of IFN-beta and anti-IFN-beta on coxsackievirus B3-induced myocarditis in mice. J. Interferon Res. 5:137-146. [DOI] [PubMed] [Google Scholar]
- 21.Matsumori, A., C. S. Crumpacker, and W. H. Abelmann. 1987. Prevention of viral myocarditis with recombinant human leukocyte interferon alpha A/D in a murine model. J. Am. Coll. Cardiol. 9:1320-1325. [DOI] [PubMed] [Google Scholar]
- 22.Matsumori, A., N. Tomioka, and C. Kawai. 1988. Protective effect of recombinant alpha interferon on coxsackievirus B3 myocarditis in mice. Am. Heart J. 115:1229-1232. [DOI] [PubMed] [Google Scholar]
- 23.Nakashima, H., Y. Honda, and T. Katayama. 1994. Serial electrocardiographic findings in acute myocarditis. Intern. Med. 33:659-666. [DOI] [PubMed] [Google Scholar]
- 24.Nuyens, D., M. Stengl, S. Dugarmaa, T. Rossenbacker, V. Compernolle, Y. Rudy, J. F. Smits, W. Flameng, C. E. Clancy, L. Moons, M. A. Vos, M. Dewerchin, K. Benndorf, D. Collen, E. Carmeliet, and P. Carmeliet. 2001. Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nat. Med. 7:1021-1027. [DOI] [PubMed] [Google Scholar]
- 25.Okada, I., A. Matsumori, Y. Matoba, M. Tominaga, T. Yamada, and C. Kawai. 1992. Combination treatment with ribavirin and interferon for coxsackievirus B3 replication. J. Lab. Clin. Med. 120:569-573. [PubMed] [Google Scholar]
- 26.Pehamberger, H. 2002. Perspectives of pegylated interferon use in dermatological oncology. Recent Results Cancer Res. 160:158-164. [DOI] [PubMed] [Google Scholar]
- 27.Poynard, T., J. McHutchison, M. Manns, C. Trepo, K. Lindsay, Z. Goodman, M. J. Ling, and J. Albrecht. 2002. Impact of pegylated interferon alpha-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology 122:1303-1313. [DOI] [PubMed] [Google Scholar]
- 28.Rose, N. R. 2000. Viral damage or ‘molecular mimicry'—placing the blame in myocarditis. Nat. Med. 6:631-632. [DOI] [PubMed] [Google Scholar]
- 29.Sievert, W. 2002. Management issues in chronic viral hepatitis: hepatitis C. J. Gastroenterol Hepatol. 17:415-422. [DOI] [PubMed] [Google Scholar]
- 30.Terasaki, F., Y. Kitaura, T. Hayashi, Y. Nakayama, H. Deguchim, and K. Kawamura. 1990. Arrhythmias in coxsackie B3 virus myocarditis. Continuous electrocardiography in conscious mice and histopathology of the heart with special reference to the conduction system. Heart Vessels Suppl. 5:45-50. [PubMed] [Google Scholar]
- 31.Thompson, K. A., D. R. Strayer, P. D. Salvato, C. E. Thompson, N. Klimas, A. Molavi, A. K. Hamill, Z. Zheng, D. Ventura, and W. A. Carter. 1996. Results of a double-blind placebo-controlled study of the double-stranded RNA drug polyI:polyC12U in the treatment of HIV infection. Eur. J. Clin. Microbiol. Infect. Dis. 15:580-587. [DOI] [PubMed] [Google Scholar]
- 32.Wang, Y. S., S. Youngster, M. Grace, J. Bausch, R. Bordens, and D. F. Wyss. 2002. Structural and biological characterization of pegylated recombinant interferon alpha-2a and its therapeutic implications. Adv. Drug Deliv. Rev. 54:547-570. [DOI] [PubMed] [Google Scholar]
- 33.Yang, Y. Z., Q. Guo, T. S. Zhou, J. Zhang, L. Li, P. Y. Jin, W. Z. Wu, J. Y. Shen, J. H. Yang, B. Z. Peng, et al. 1993. Electrophysiological and pathological observations on experimental coxsackie B-3 viral myocarditis in mice. Clin. Med. J. (England) 106:100-104. [PubMed] [Google Scholar]