Mizushima explores the physiological roles of self-eating.
Mizushima explores the physiological roles of self-eating.
Eukaryotic cells are great recyclers. When proteins or organelles become damaged or obsolete, cells don't leave this garbage lying around. Instead, the autophagy pathway kicks in to corral it and bring it to the cell's lysosomes, where it's broken down into parts that can be reused elsewhere in the cell. Such cellular recycling not only keeps things tidy, says Noboru Mizushima, it also is important for cellular and organismal survival. He should know, because he's been at the forefront of autophagy research since first encountering it during his postdoctoral studies with Dr. Yoshinori Ohsumi at the National Institute for Basic Biology in Okazaki, Japan (1).
Noboru Mizushima
First trained as a physician, Mizushima encountered autophagy almost by accident when he read about the burgeoning young field in a Japanese-language science magazine. Since then, he's made it his life's work to understand how this process works (2, 3), when it is used (4, 5), and the consequences of its dysregulation (6). We reached him at his office at Tokyo Medical and Dental University to discuss some of the choices he's made in his career, and why cells sometimes choose to eat themselves.
“A major focus of my lab has been using mouse genetics to explore the physiological roles of autophagy.”
HARD CHOICE
As a child, did you know what you wanted to be when you grew up?
Growing up in Tokyo, I was very influenced by my father and my grandfather. My father was a medical doctor and a scientist. He saw patients every week, but he was also very interested in drug development. On the other hand, my grandfather was a physicist. I couldn't understand everything he worked on, but he was doing atomic science. I admired them both very much, so I wanted to be either a scientist or medical doctor. I didn't decide between the two until much later.
Did you have much exposure to biological science in school?
When I was in high school, I was most interested in physics and chemistry. I didn't have much time for biology until I went to medical school at Tokyo Medical and Dental University.
In Japan, we don't do four years of university followed by four years of medical school like they do in the United States. We just have a combined six-year program for medical school. While I was an undergraduate in medical school, I became very interested in immunology and basic research. Then I started thinking I would rather be a scientist than a medical doctor.
But you went through your clinical training anyway?
Yes, and I am very glad that I did because seeing patients had a strong influence on me. Until that time, I hadn't thought very much about whole-body systems, but after my clinical training I became very interested in autoimmune diseases. They're very difficult to treat, but also very interesting because patients show a lot of different symptoms. That is why I decided to do a PhD in immunology, working on T-cell immunology and autoimmune disease.
Was that an unusual career choice to make?
Most Japanese students go to a medical or clinical department after finishing their clinical training. I think this is a serious problem—we have very few MD scientists in Japan, and the number is decreasing. One thing we could do to address this problem is to provide more opportunities for research during medical school.
I am always encouraging medical students in my classes to consider becoming basic scientists. We also have a few students working in my lab, and we have tried to provide them with as many opportunities as possible. It is important that they get this kind of exposure.
BIG CHOICE
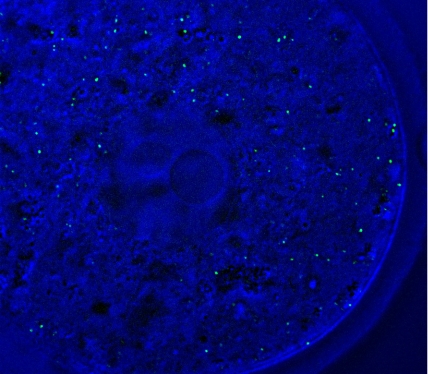
Autophagosomes (green) in a fertilized oocyte.
NOBORU MIZUSHIMA AND SATOSHI TSUKAMOTO
After completing your PhD, did you consider returning to medicine?
That was a critical point in my career. I thought it would be very difficult for me to work as a scientist and as a medical doctor. Both careers were very interesting to me, but finally I decided to focus on basic science. I gave up my medical activities when I started my postdoc.
That is when you first started working on autophagy?
Yes. That was a dramatic shift for me, because I'd been in a medical department and I moved to a yeast research laboratory. I think there were three reasons I made that move. First, I thought immunology was interesting, but the immune system is so complicated that I thought it would be very difficult for me to understand everything about it. The second reason is that I felt it would be hard to survive in an established field like immunology. I wanted to go into a new, less established research area so that I could catch up to others in the field. The third reason was that I was interested in protein recycling and turnover.
I was very lucky to find Dr. Ohsumi's laboratory, where I did my postdoctoral work. At the time, I applied to several laboratories in the United States and other countries. I had never heard anything about autophagy, but I happened to read a very short article about it in a Japanese science magazine. I quickly decided that that was what I wanted to study.
You helped characterize many components of the yeast autophagy pathway, like Apg12?
When I joined Dr. Ohsumi's laboratory, they had already cloned many autophagy genes. Most of them were new genes whose function could not be simply predicted from their sequences. I don't remember why I picked Apg12, but probably it was just because nobody else was working on it yet.
“There may be other autophagy-related diseases that haven't yet been identified.”
The exact function of this protein remains unknown, but it is probably important for elongation of the isolation membrane. The isolation membrane is an intermediate structure during autophagosome formation, in which membrane is extruded until it encloses a portion of cytoplasm. We couldn't actually observe the process of isolation membrane formation until I started studying autophagy in mammalian cells. In yeast everything is very tiny; it's very difficult to see things like the elongation step in yeast cells. In mammalian cells it is much easier.
CHOICE PROBLEMS
Mizushima discussing basic science with undergraduates.
How have your studies on autophagy branched out since your postdoc work?
A major focus of my lab has been using mouse genetics to explore the physiological roles of autophagy. For example, we have found that autophagy is important during embryogenesis. Soon after fertilization but before implantation, fertilized eggs must convert maternal proteins into embryonic proteins. They do this by degrading maternal proteins via autophagy, and recycling the amino acids to make embryonic proteins.
Autophagy is also important later on, in the neonatal stage. During embryogenesis, embryos get nutrients from their mother. But after birth, before they begin nursing, they have to survive by themselves. We know that autophagy is important for adaptation to starvation conditions; it lets us degrade our own proteins when we don't have access to external nutrients. We can eat ourselves to survive. So, in the first few hours after birth, neonates use autophagy to degrade parts of themselves very quickly to produce energy and necessary nutrients.
Finally, we know that autophagy is important for the clearance of abnormal intracellular proteins and organelles. This may be especially important in the context of degenerative conditions like Huntington's, Alzheimer's, or Parkinson's disease. There may be other autophagy-related diseases that haven't yet been identified.
What other problems are you working on now?
Another focus of my lab is the mechanisms by which autophagy is regulated. There are only 30 autophagy genes that directly make up the autophagy pathway, but there are a lot of additional genes and pathways involved in regulating autophagic activity. We are trying to understand these regulatory pathways. Finally, we are also interested in where and how the autophagosome is formed. Our hypothesis is that autophagosomes are generated on a particular domain of the endoplasmic reticulum, because many autophagy proteins accumulate there.
We are doing a lot of things in my lab: cell biology, embryology, neurobiology, metabolism, and endocrinology. Sometimes people advise me to focus on something, to work on a very limited field. But I think a trans-disciplinary approach is very important, because really I am focusing on just one keyword: autophagy. That is what I enjoy.
References
- 1.Mizushima N., et al. 1998. Nature. 395:321, 323. [DOI] [PubMed] [Google Scholar]
- 2.Mizushima N., et al. 2001. J. Cell Biol. 152:657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosokawa N., et al. 2009. Mol. Biol. Cell. 20:1981–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsukamoto S., et al. 2008. Science. 321:117–120. [DOI] [PubMed] [Google Scholar]
- 5.Kuma A., et al. 2004. Nature. 432:1032–1036. [DOI] [PubMed] [Google Scholar]
- 6.Hara T., et al. 2006. Nature. 441:885–889. [DOI] [PubMed] [Google Scholar]