Abstract
BACKGROUND AND PURPOSE
Zymosan-induced non-septic shock is a multi-factorial pathology that involves several organs including the kidneys, liver and lungs. Its complexity and diversity presents a continuing therapeutic challenge. Given their pleiotropic effect, statins could be beneficial in non-septic shock. One of the molecular mechanisms underlying the anti-inflammatory effect of statins involves the peroxisome proliferator-activated receptor (PPAR) α. We used a zymosan-induced non-septic shock experimental model to investigate the role of PPARα in the anti-inflammatory effects of simvastatin.
EXPERIMENTAL APPROACH
Effects of simvastatin (5 or 10 mg·kg−1 i.p.) were analysed in PPARα knock-out (KO) and PPARα wild type (WT) mice after zymosan or vehicle administration. Organ injury in lung, liver, kidney and intestine was evaluated by immunohistology. PPARα mRNA expression and nuclear factor-κB activation were evaluated in all experimental groups, 18 h after study onset. Cytokine levels were measured in plasma, and nitrite/nitrate in plasma and peritoneal exudate. Nitric oxide synthase, nitrotyrosine and poly ADP-ribose were localized by immunohistochemical methods.
KEY RESULTS
Simvastatin significantly and dose-dependently increased the zymosan-induced expression of PPARα levels in all tissues analysed. It also dose-dependently reduced systemic inflammation and the organ injury induced by zymosan in lung, liver, intestine and kidney. These effects were observed in PPARαWT mice and in PPARαKO mice.
CONCLUSIONS AND IMPLICATIONS
Simvastatin protected against the molecular and cellular damage caused by systemic inflammation in our experimental model. Our results also provide new information regarding the role of PPARα in the anti-inflammatory effects of statins.
Keywords: simvastatin, PPARαKO mice, zymosan, multiple organ failure, systemic inflammation
Introduction
Sepsis is defined as an infection-induced systemic inflammatory response that ultimately leads to the multiple organ dysfunction syndrome (MODS) (Merx et al., 2005). Despite new treatment approaches such as activated protein C or low-dose corticosteroids (Siempos et al., 2008), every third intensive care unit patient in Europe suffers from sepsis (Vincent et al., 2006) and sepsis is the leading cause of death in intensive care units (Angus et al., 2003). Sepsis is a very complex inflammatory syndrome involving activation of inflammatory cascades, cytokine release and endothelial dysfunction (Remick et al., 2007). Therefore, efforts to inhibit individual inflammatory mediators may not be sufficient to arrest the entire inflammatory process. A well-tested experimental model of human MODS is zymosan-induced non-septic shock. Zymosan is a non-bacterial, non-endotoxic agent that produces acute peritonitis and multiple-organ failure characterized by functional and structural changes in the liver, intestine, lung and kidneys (Cuzzocrea et al., 2007).
Statins, such as simvastatin, are lipid-lowering drugs that inhibit 3-hydroxy-3-methylglutaryl co-enzyme A (HMG-CoA) reductase. They are widely prescribed to lower cholesterol and are the first-line treatment for the prevention of coronary artery disease and atherosclerosis (Maron et al., 2000). Simvastatin also exerts important immunomodulatory and anti-inflammatory effects independent of lipid lowering (Alvarez de Sotomayor et al., 2008; Fraunberger et al., 2009). Statins exert pleiotropic effects on endothelium, platelets, smooth muscle cells and inflammation. Indeed, they improve endothelial and microvascular function, and reduce inflammation by decreasing the expression of pro-inflammatory transcriptional factors such as nuclear factor (NF)-κB that result in decreased expression of cytokines, chemokines and inducible NO synthase (iNOS) (Jasińska et al., 2007). Simvastatin inhibits NO production in a murine model of endotoxic shock by reducing iNOS (Giusti-Paiva et al., 2004). A large body of information from retrospective database enquires and observational studies indicate that statins may be helpful in sepsis (Gao et al., 2008). However, the clinical benefit of statin therapy in sepsis has yet to be demonstrated in randomized controlled trials.
The peroxisome proliferator-activated receptor (PPAR)α belongs to the superfamily of nuclear receptors that are ligand-activated transcription factors (Abbott, 2009; Fruchart, 2009; receptor nomenclature follows Alexander et al., 2009). Upon ligand activation, PPARα forms a heterodimer with the 9-cis-retinoic acid receptor (RXR) that regulates gene expression by binding to specific gene promoter response elements. PPARα binds a diverse set of ligands including arachidonic acid metabolites and synthetic fibrate drugs (Sher et al., 1993). In addition, PPARα plays a pivotal role in the regulation of lipid metabolism, and it also exerts pronounced anti-inflammatory activities by negatively interfering with pro-inflammatory signalling pathways including NF-κB (Marx et al., 2004; Okamoto et al., 2005; Paukkeri et al., 2007; Becker et al., 2008). We and others have reported that activation of PPARα resulted in significant anti-inflammatory effects in various experimental models (Becker et al., 2008), including ours (Genovese et al., 2006; 2009; Crisafulli et al., 2009). We also demonstrated that the absence of PPARα increases the degree of multiple organ failure induced by zymosan in mice (Di Paola et al., 2006). With this background, we have here evaluated the role of the nuclear receptor PPARα in the anti-inflammatory effects of simvastatin during systemic inflammation induced by zymosan in a murine model of non-septic shock.
Methods
Animals
All animal care and experimental procedures complied with the European Economic Community regulations on protection of laboratory animals (OJ of E.C. L.358/1, 12/18/1986) and were approved by the local Ethics Committee for Animal Experimentation and the University of Messina Review Board for the care of animals. Mice (4–5 weeks old, 20–22 g) with a targeted disruption of the PPARα gene [PPARα knock-out (KO)] and wild-type (WT) littermate controls (PPARαWT) were purchased from Jackson Laboratories (Harlan Nossan, Milan, Italy). Mice homozygous for the PparatniJGonz targeted mutation are viable, fertile and appear normal in appearance and behaviour. Exon eight, encoding the ligand-binding domain, was disrupted by the insertion of a 1.14 kb neomycin resistance gene in the opposite transcriptional direction. After electroporation of the targeting construct into J1 ES cells, the ES cells were injected into C57BL/6N blastocysts. This strain was created on B6, 129S4 background and was maintained as a homozygote on a 129S4/SvJae background by brother sister mating. The animals were housed in a room at a constant temperature of 22 ± 2°C and 40–60% relative humidity with 12 h light/dark cycles and fed a standard laboratory mice diet and water ad libitum. Every effort was made to minimize both animal number and suffering during the experiments.
Drug treatment and groups
Mice were allocated to two main groups: PPARαWT (n = 40) and PPARαKO (n = 40). Each group was randomly divided into two subgroups: (i) the Sham-operated (SHAM) WT group (n = 20) consisting of PPARαWT mice treated intraperitoneally (i.p.) with the vehicle (saline solution 0.9 % NaCl); (ii) the ZYMOSAN WT (ZYM WT) group (n = 20) consisting of PPARαWT mice treated i.p. with zymosan (500 mg·kg−1, suspended in vehicle); (iii) the SHAM KO group (n = 20) consisting of PPARαKO mice treated i.p. with the vehicle; and (iv) the ZYMOSAN KO (ZYM KO) group (n = 20) consisting of PPARαKO mice treated i.p. with zymosan. One and 6 h after administration of vehicle or zymosan, all four subgroups (n = 80) were treated with simvastatin 5 or 10 mg·kg−1 i.p. We used 10 mg simvastatin to obtain the anti-inflammatory effect and 5 mg simvastatin as a submaximal dose. Eighteen hours after the study onset, lung, liver, kidney, intestine and plasma were obtained from all the animals.
Reverse transcriptase polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from lung, liver and kidney tissues of all the experimental groups, 18 h after the study onset, using Trizol reagent (Invitrogen, Milan, Italy) according to the protocol recommended by the manufacturer. RNAs were treated with DNase (Promega, Milan, Italy) to remove DNA contamination. The concentration of RNA was measured with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). RNA integrity was verified by electrophoresis on denaturing 1% agarose gel (EuroClone, Milan, Italy). Absence of residual DNA was verified by PCR on total RNA without reverse transcription. cDNA was generated from 1 µg of each RNA sample. Reverse transcription was performed at 25°C for 5 min, at 42°C for 30 min and at 85°C for 5 min (iScript cDNA synthesis Kit, Bio-Rad, Milan, Italy). The internal mRNA standard was the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). For the reverse transcription PCR, 3 µL of cDNA were incubated with 14.1 µL de-ionized distilled water, 1 µL MgCl2, 2 µL dNTP, 2.5 µL 10X PCR buffer, 0.4 µL Taq polymerase (Euroclone) and primer pairs for target genes. Primers (PRIMM, San Raffaele Biomedical Science Park, Milan, Italy) were as follows:
PPARα sense: 5′-AAGCCATCTTCACGATGCTG-3′
PPARα antisense: 5′-TCGGAGGTCCCTGAACAGTG-3′
GAPDH sense: 5′-TCACTGGCATGGCCTTCC-3′
GAPDH antisense: 5′-CCCTCAGATGCCTGCTTC-3′
The PPARα and GAPDH cycling conditions were 30 s of denaturation at 94°C, 30 s of annealing at 58°C and 30 s of elongation at 72°C over 30 cycles for all reactions. The PCR products were separated on 2% agarose gel and visualized with ethidium bromide staining. Densitometric evaluation of signal strengths in RT-PCR was performed with Quantity One software (Gel Doc-2000, Bio-Rad).
Acute peritonitis assessment
At 18 h after zymosan or vehicle injection, animals were killed under ether anaesthesia. The abdominal cavity was opened and the peritoneal cavity washed with 3 mL of phosphate buffer saline (PBS; composition in mM: NaCl 137, KCl 2.7, NaH2PO4 1.4, Na2HPO4 4.3, pH 7.4). The peritoneal exudate and washing buffer were removed by aspiration and the total volume was measured. Exudates contaminated with blood were discarded. The peritoneal exudate was centrifuged at 7000×g for 10 min at room temperature. Cells were suspended in PBS and counted with an optical microscope in a Burker chamber after vital staining with Trypan blue.
Myeloperoxidase activity
Myeloperoxidase activity, which is an index of polymorphonuclear (PMN) leukocyte accumulation, was determined as previously described (Cuzzocrea et al., 2007). Ileum and lung tissues, collected at the specified time point, were homogenized in a solution containing 0.5% hexa-decyl-trimethyl-ammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7) and centrifuged for 30 min at 20 000×g at 4°C. An aliquot of the supernatant was allowed to react with a solution of tetra-methyl-benzidine (1.6 mM) and 0.1 mM H2O2. The rate of change in absorbance was measured with a spectrophotometer at 650 nm. Myeloperoxidase activity was defined as the quantity of enzyme degrading 1 µmol of peroxide min−1 at 37°C, and was expressed in units per gram weight of wet tissue.
Preparation of nuclear and cytoplasmic protein extracts
Tissue samples of lung, liver and kidney were obtained, 18 h after the study onset, from all experimental groups and were washed twice with cold PBS. Cytosolic and nuclear extraction was performed with two different lysis buffers. Tissues were homogenized on ice in 500 µL ice-cold hypotonic cytoplasmic buffer [10 mM HEPES pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol (DTT), 0.5 mM phenylmethylsulphonyl fluoride (PMSF) with a protease inhibitor cocktail] using a Politron PT 13 000 D tissue homogenizer (Kinematica, Bohemia, NY, USA). After 15 min incubation on ice, the homogenates were centrifuged at 13 000×g for 1 min at 4°C. Supernatants containing cytoplasmic extracts were stored at −80°C. Nuclear pellets were resuspended in 334 µL of high salt extraction buffer (20 mM HEPES pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5 mM PMSF with a protease inhibitor cocktail), and the tubes were vigorously rocked at 4°C for 30 min on a shaking platform. The nuclear extracts were centrifuged at 13 000×g for 15 min at 4°C (Dignam et al., 1983). The supernatants were frozen in aliquots at −80°C until use. Protein contents were determined by the Bradford method (Bradford, 1976).
NF-κB nuclear translocation assessment by Western blot
Proteins from cytoplasmic and nuclear fractions were added to 2X sodium dodecyl sulphate (SDS) sample buffer [0.125 M Tris-HCl (pH 6.8), 4% SDS, 20% glycerol, 10% β-mercaptoethanol, 0.004% bromphenol blue], and boiled in a water bath for 5 min. Protein samples (40 µg per lane) were separated on denaturing 10% SDS polyacrylamide gel and transferred to a nitrocellulose membrane. Non-specific binding to the membrane was blocked for 1 h at room temperature with 5% milk in Tris buffer saline with 0.1% Tween 20 (T-TBS). Membranes were then incubated at 4°C overnight with primary antibody for nuclear NF-κB p65 (1:1000) and cytosolic IκB-α (1:500) in 1% T-TBS containing 5% non-fat dry milk, washed three times with 0.1% T-TBS solution, and then incubated for 1 h at room temperature with a secondary antibody (anti-rabbit IgG peroxidase conjugated). Laminin and GAPDH were used as internal standard for nuclear and cytosolic extracts respectively. The immunoreactive bands were visualized using an enhanced chemilumunescence system (SuperSignal West Femto Maximum Sensitivity Substrate, Pierce, Rockford, IL, USA). The protein bands were scanned and quantitated with Gel Doc-2000 (Bio-Rad).
Measurement of nitrite/nitrate
Nitrite/nitrate (NO2−/NO3−) production, an indicator of NO synthesis, was measured in plasma and exudate samples collected 18 h after vehicle or zymosan administration. Plasma was incubated with nitrate reductase (0.1 U·mL−1) and nicotinamide adenine dinucleotide phosphate (1 mM) and flavin adenine dinucleotide (50 µM) at 37°C for 15 min, followed by another incubation with lactate dehydrogenase (100 U·mL−1) and sodium pyruvate (10 mM) for 5 min. The nitrite concentration in the samples was measured by the Griess reaction, by adding 100 µL of Griess reagent [0.1% (w/v) naphthylethylenediamide dihydrochloride in H2O and 1% (w/v) sulphanilamide in 5% (v/v) concentrated H2PO4; vol. 1: 1] to the 100 µL sample. The optical density at 550 nm (OD550) was measured using an elisa microplate reader (SLT- Lab Instruments, Salzburg, Austria). Nitrate concentrations were calculated by comparison with OD550 of standard solutions of sodium nitrate prepared in saline solution.
Measurement of cytokines
Tumour necrosis factor (TNF)α and interleukin (IL)-1β levels were measured in plasma samples collected 18 h after zymosan or vehicle administration. The assay was carried out by using a colorimetric, commercial kit (Calbiochem-Novabiochem Corporation, San Diego, CA, USA) according to the manufacturer's instructions. All cytokine determinations were performed in duplicate serial dilutions.
Immunohistochemical localization of iNOS, nitrotyrosine, and poly ADP-ribose (PAR)
Tissues, taken at the end of the experiment, were fixed in 10% (w/v) PBS-buffered formaldehyde, and 8 µm sections were prepared from paraffin-embedded tissues. After de-paraffinization, endogenous peroxidase was quenched with 0.3% (v/v) hydrogen peroxide in 60% (v/v) methanol for 30 min. The sections were permeabilized with 0.1% (w/v) Triton X-100 in PBS for 20 min. Non-specific adsorption was minimized by incubating the section in 2% (v/v) normal goat serum in PBS for 20 min. Endogenous biotin or avidin binding sites were blocked by sequential incubation for 15 min with biotin and avidin (Santa Cruz, Milan, Italy) respectively. Sections were incubated overnight with: (i) rabbit anti-nitrotyrosine antibody (1:500 in PBS, w/v); (ii) with anti-iNOS antibody (1:500 in PBS, w/v); or (iii) with anti-PAR (1:500 in PBS, v/v). Sections were washed with PBS, and incubated with secondary antibody. Specific labelling was detected with a biotin-conjugated goat anti-rabbit IgG and avidin-biotin peroxidase complex. The counter stain was developed with diaminobenzidine (brown) and nuclear fast red (red background). To confirm that the immunoreactions for the nitrotyrosine were specific, we incubated some sections with the primary antibody (anti-nitrotyrosine) in the presence of excess nitrotyrosine (10 mM). Similarly, to confirm the binding specificity for iNOS or PAR, we incubated some sections with only the primary antibody (no secondary) or with only the secondary antibody (no primary). Under these conditions, there was no positive staining in the sections, which indicates the validity of the immunoreaction in all the experiments carried out.
Quantification of organ function and injury
Blood samples were taken 18 h after zymosan or vehicle injection. The blood sample was centrifuged (1610×g for 3 min at room temperature) to separate plasma. All plasma samples were analysed within 24 h in a veterinary clinical laboratory using standard laboratory techniques. The following enzymes were measured in plasma as indicators of multiple organ injury/dysfunction: (i) amylase and lipase as indicators of pancreatic injury; (ii) alkaline phosphatase, aspartate aminotransferase (AST, a non-specific marker for hepatic injury), alanine aminotransferase (ALT, a specific marker for hepatic parenchymal injury) and bilirubin as indicators of liver injury (Baue, 1993); and (iii) creatinine, as an indicator of reduced glomerular filtration rate, and hence, renal failure.
Light microscopy
Lung, liver, kidney and intestine samples were taken 18 h after zymosan or saline injection. The tissue slices were fixed in Dietric solution (14.25% ethanol, 1.85% formaldehyde, 1% acetic acid) for 1 week at room-temperature, dehydrated by graded ethanol and embedded in Paraplast (Sherwood Medical, Mahwah, NJ, USA). Sections (7 µm thick) were de-paraffinized with xylene, stained with haematoxylin/eosin and observed in an Axiovision Ziess microscope (Milan, Italy). The segments of each type of tissue were evaluated by an experienced histopathologist, without knowledge of the treatments. The following morphological criteria were used for scoring lung injury: 0, normal lung; grade 1, minimal oedema or infiltration of alveolar or bronchiolar walls; grade 2, moderate oedema and inflammatory cell infiltration without obvious damage to lung architecture; and grade 3, severe inflammatory cell infiltration with obvious damage to lung architecture. The following morphological criteria were used for scoring kidney injury: the measured parameters were: (i) tubular damage; (ii) monocyte and macrophage infiltration; and (iii) glomerular damage. A semi-quantitative score (0–4) was assigned based on the masked reading. The following morphological criteria were used for scoring liver injury: 0, no damage; 1 (mild), focal oedema and necrosis; 2 (moderate), diffuse swelling, necrosis and hepatocellular injury; 3 (severe), necrosis with evidence of neutrophil infiltration; and 4 (major), widespread necrosis with massive neutrophil infiltration and evidence of haemorrhage. The following morphological criteria were used for intestine injury: 0, no damage; 1 (mild), focal epithelial oedema and necrosis; 2 (moderate), diffuse swelling and necrosis of the villi; 3 (severe), necrosis with the presence of neutrophil infiltrate in the submucosa; and 4 (highly severe), widespread necrosis with massive neutrophil infiltrate and haemorrhage.
Statistical analysis
All values in the figures and text are expressed as mean ± standard error (SEM) of the mean of n observations. For the in vivo studies, n represents the number of animals studied. In the experiments involving histology or immunohistochemistry, the figures shown are representative of at least three experiments performed on different experimental days. The results were analysed by one-way anova followed by a Bonferroni post hoc test for multiple comparisons. A P-value less than 0.05 was considered significant.
Materials
Unless otherwise stated, all compounds were obtained from Sigma-Aldrich Company (Milan, Italy). Primary anti-nitrotyrosine antibody were purchased from Upstate (DBA, Milan, Italy); the primary anti-iNOS, anti-PAR, anti-p65, anti-IκB-α, anti-laminin antibodies and secondary non-specific IgG antibody for Western blot analysis were obtained from Santa Cruz Biotechnology (DBA, Milan, Italy). All the other reagents used for the Western blot were purchased from Bio-Rad Laboratories. Reagents and secondary and non-specific IgG antibody for immunohistochemical analysis were from Vector Laboratories (DBA, Milan, Italy). All other chemicals were of the highest commercial grade available. All stock solutions were prepared in non-pyrogenic saline (0.9% NaCl; Baxter Healthcare Ltd, Thetford, Norfolk, UK).
Results
Effect of simvastatin on PPARα expression in the zymosan-induced non-septic shock model
The effect of simvastatin (5 and 10 mg·kg−1) on PPARα expression was measured by RT-PCR in samples of lung (Figure 1A), liver (Figure 1B) and kidney (Figure 1C) tissues from PPARαWT and PPARαKO mice. Simvastatin induced a dose-dependent increase of PPARα mRNA in all tissues of ZYM WT mice compared with the SHAM WT group. The genetic absence of PPARα was confirmed by the absence of mRNA expression in the SHAM and ZYM KO groups (Figure 1).
Figure 1.
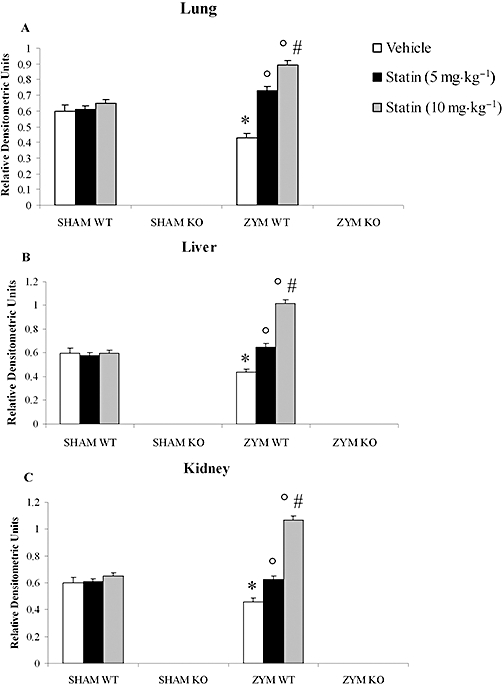
Effects of simvastatin on PPARα mRNA levels in the zymosan-induced non-septic shock model. Total RNA was extracted from lung (A), liver (B) and kidney (C) of all experimental groups, and the levels of mRNA encoding PPARα were determined by reverse transcriptase polymerase chain reaction. Eighteen hours after zymosan administration simvastatin treatment induced a dose-dependent increase of PPARα mRNA levels in the ZYM WT compared with the SHAM WT group. mRNA levels were normalized with respect to the housekeeping gene GAPDH, and gene expression values were expressed as arbitrary units ± SEM of three different experiments. *P < 0.05, significantly different from SHAM groups; °P < 0.05, significantly different from vehicle; #P < 0.05, significantly different from simvastatin 5 mg. KO, knock-out; PPAR, peroxisome proliferator-activated receptor; WT, wild type; ZYM, zymosan.
Role of the functional PPARα gene in the anti-inflammatory effect of simvastatin during zymosan-induced non-septic shock model
To determine whether PPARα was involved in the anti-inflammatory property of simvastatin during zymosan-induced non-septic shock, we evaluated the effect of simvastatin in PPARαKO and PPARαWT mice treated with zymosan or vehicle. Acute peritonitis occurred 18 h after zymosan administration, as shown by the production of turbid exudate (Figure 2A). The total number of peritoneal exudate cells (PEC) (Figure 2B) was determined by Trypan blue staining after i.p. administration of zymosan or vehicle. The number of PMN leukocytes was significantly higher in the ZYM WT group than in the SHAM WT group, which demonstrates the absence of abnormalities in the peritoneal cavity or fluid of the SHAM WT group.
Figure 2.
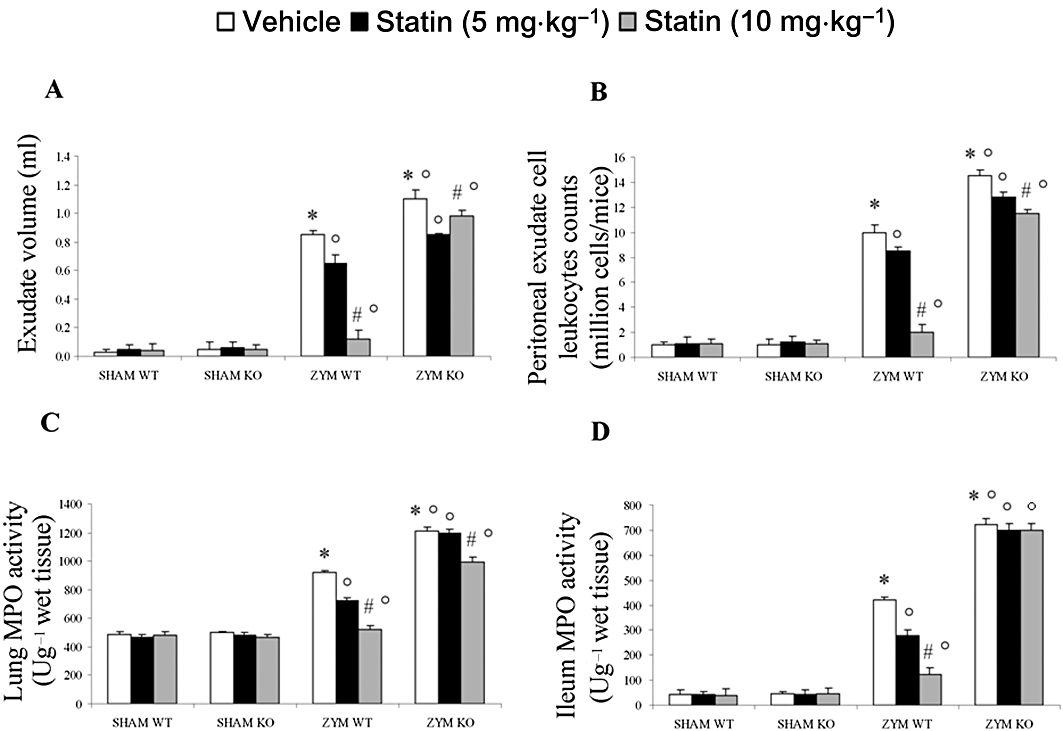
Effect of PPARα on anti-inflammatory activity of simvastatin against zymosan-induced inflammation. Simvastatin reduced the increase in the volume of exudate (A), accumulation of polymorphonuclear cells (PMNs, B) in the peritoneal cavity, and myeloperoxidase (MPO) activity levels in the lung (C) and in the ileum (D) of mice 18 h after zymosan treatment (see Figure 2). The genetic absence of the PPARα receptor blocked the effect of simvastatin treatment in the ZYM KO group. Data are means ± SEM of 10 mice for each group. *P < 0.05, significantly different from SHAM groups; °P < 0.05, significantly different from vehicle; #P < 0.05, significantly different from simvastatin 5 mg. KO, knock-out; PPAR, peroxisome proliferator-activated receptor; WT, wild type; ZYM, zymosan.
Zymosan injection was associated with an increase in PEC counts in ZYM WT group at 18 h, over those in the SHAM WT group (Figure 2B). Because of the increase in PECs after zymosan injection, we prepared cytospin samples of PEC to determine the cell types present in peritoneal exudate. Wright–Giemsa-stained slides of all controls contained mostly mononuclear cells including resident macrophages and lymphocytes and very few PMN neutrophils, as reported by others (Szabóet al., 1997). All cells appeared healthy and intact. Eighteen hours after zymosan administration, almost all cells appeared lysed, and neutrophils could not be distinguished from macrophages because of massive phagocytosis by leukocytes. Therefore, we used carried out cell staining with specific esterases for neutrophil and macrophages in an attempt to differentiate between cell populations in the zymosan-treated animals. In agreement with previous observations (Szabóet al., 1997), we found 90% of mononuclear cells in the peritoneal cavity together with 10% PMNs in all sham animals. In contrast, the cells in the samples from the ZYM WT group could not be identified because of abundant phagocytosis and lysis of cells.
The absence of the functional PPARα receptor in the ZYM KO group was accompanied by significantly enhanced exudate formation (Figure 2A) and PEC counts (Figure 2B). Treatment with simvastatin significantly and dose-dependently reduced exudate formation (Figure 2A) and the PEC count (Figure 2B) in the ZYM WT, but not in the ZYM KO group.
A hallmark of zymosan-induced MODS is the accumulation of neutrophils in the lung and intestine, which augments tissue damage. Therefore, we evaluated the extent of inflammatory cell infiltration in the lung (Figure 2C) and ileum tissues (Figure 2D) by measuring myeloperoxidase activity 18 h after zymosan or vehicle administration. Myeloperoxidase activity in both tissue types was significantly higher in the ZYM KO group than in the ZYM WT group (Figure 2C and D). Treatment with simvastatin significantly and dose-dependently reduced myeloperoxidase activity in ZYM WT, but not in ZYM KO group. On the contrary, there were no differences between the SHAM groups.
Role of the functional PPARα gene in the anti-inflammatory effect of simvastatin on NF-κB activation
Inactive NF-κB is located in the cytosol, complexed with its inhibitory protein, IκB. Activation of NF-κB is characterized by a decrease of cytoplasmic IκB and an increase of nuclear p65. Therefore, we evaluated NF-κB activity by Western blot in lung (Figure 3A and D), liver (Figure 3B and E), and kidney (Figure 3C and F) tissues from PPARαWT and PPARαKO mice. Eighteen hours after zymosan administration, nuclear p65 was higher in the ZYM KO than in the ZYM WT group (Figure 3D–F). Conversely, cytoplasmic expression of IκB-α was lower in the ZYM KO than in the ZYM WT group (Figure 3A–C). These data suggest that PPARα may interfere with NF-κB activation. Simvastatin treatment, in a dose-dependent manner, significantly reduced NF-κB activation, decreasing nuclear p65 and increasing cytoplasmic IκB-α expression in the ZYM group. However, simvastatin did not affect the nuclear and cytoplasmic fraction of NF-κB in the SHAM group (Figure 3).
Figure 3.
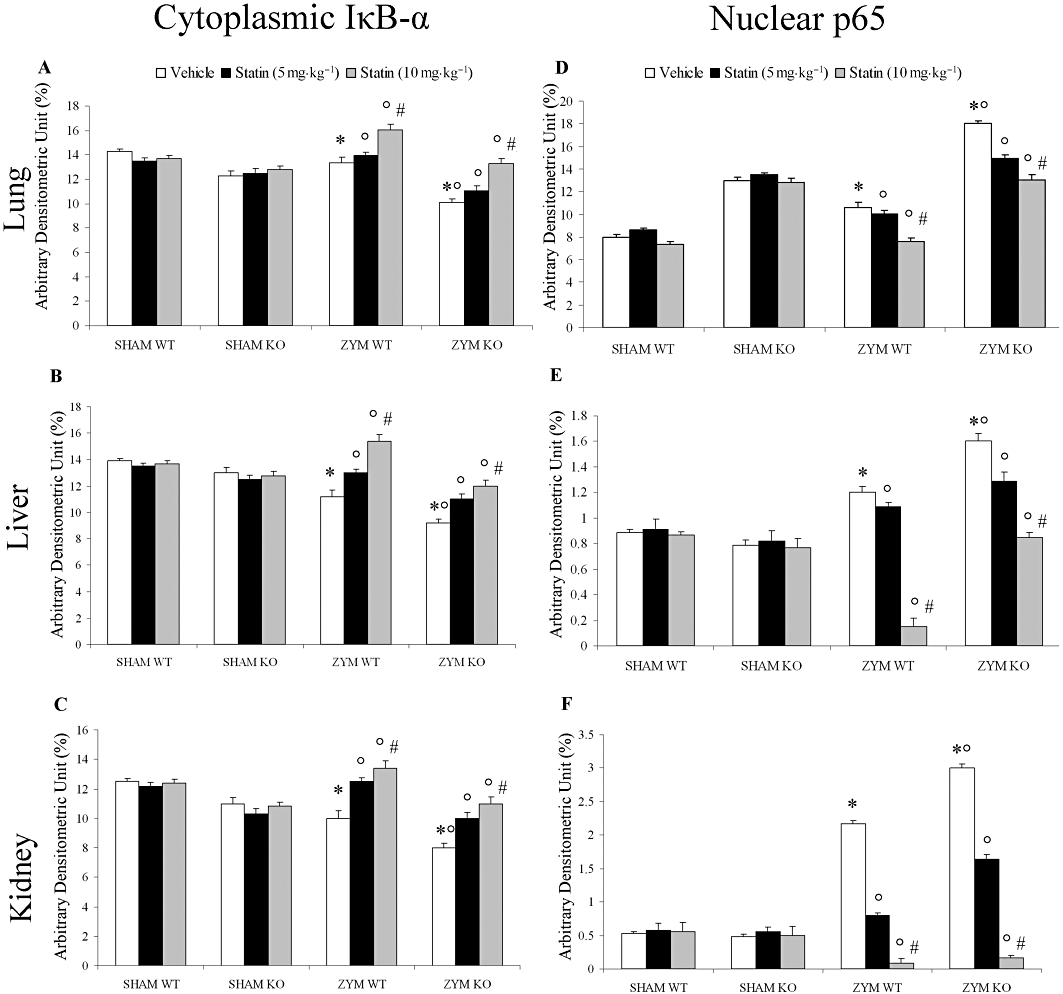
Role of the functional PPARα gene in the anti-inflammatory effect of simvastatin on NF-κB activation. Densitometric analysis of (A, B and C) cytoplasmic IκB-α and (D, E and F) nuclear p65 levels in protein extracts of (A–D) lung, (B–E) liver and (C–F) kidney was performed by Western blot analysis. Tissue lysates (40 µg per lane) were prepared as described. Eighteen hours after zymosan administration, nuclear p65 was higher in PPARαKO than in PPARαWT mice. Simvastatin significantly and dose-dependently reduced nuclear p65 in all three tissue types (D, E and F) in zymosan-treated mice compared with SHAM groups. This result was confirmed by cytoplasmic IκB-α measurement (A, B and C). Bars represent mean ± SEM of three different experiments. *P < 0.05, significantly different from SHAM groups; °P < 0.05, significantly different from vehicle; #P < 0.05, significantly different from simvastatin 5 mg. KO, knock-out; PPAR, peroxisome proliferator-activated receptor; WT, wild type; ZYM, zymosan.
Role of functional PPARα gene in the anti-inflammatory property of simvastatin on NO formation and up-regulation of TNFα and IL-1β levels during zymosan-induced non-septic shock model
The biochemical and inflammatory changes observed in zymosan mice were associated with a significant increase in the volume of exudate and in plasma NO2 levels. In fact, nitrite/nitrate levels were significantly higher in zymosan-treated mice than in sham mice (Figure 4A and B). Eighteen hours after zymosan administration, the volume of exudates and the plasma level of nitrite/nitrate were significantly higher in the ZYM KO group (Figure 4A and B). Simvastatin significantly and dose-dependently reduced the exudate volume and nitrite/nitrate levels in the ZYM WT, but not in the ZYM KO group (Figure 4A and B).
Figure 4.
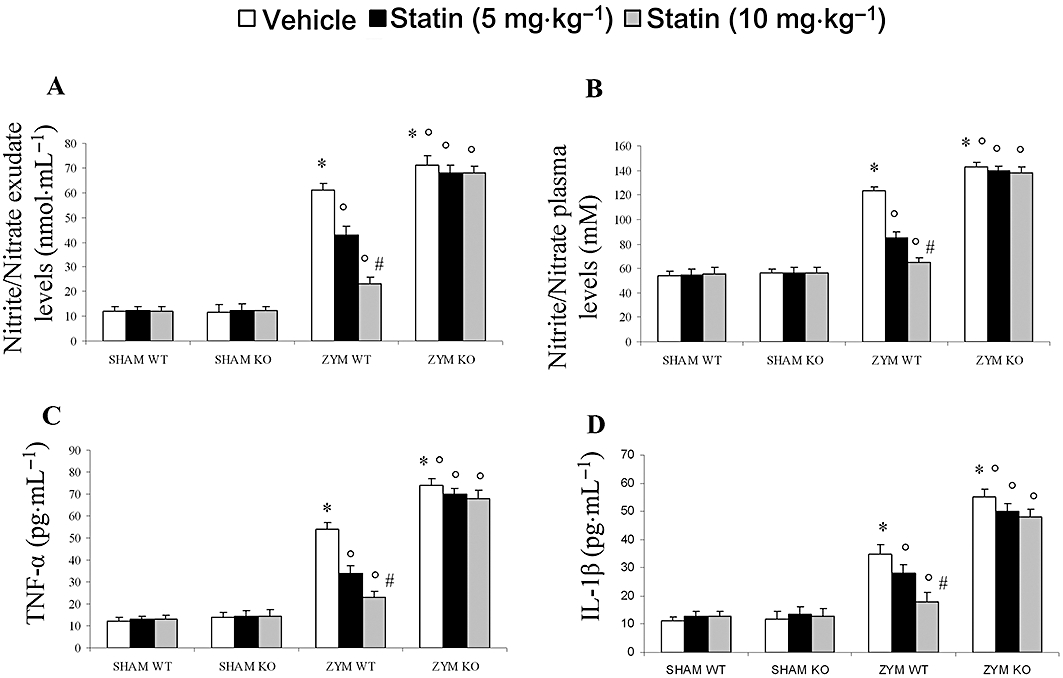
Effect of PPARα on the anti-inflammatory effect of simvastatin against zymosan-induced nitrite/nitrate and cytokine production. Simvastatin dose-dependently reduced the exudate (A) and plasma (B) nitrite/nitrate production as well as the release of plasma TNFα (C), IL-1β (D), 18 h after zymosan treatment in the ZYM WT group. The genetic absence of the PPARα receptor significantly blocked the effect of simvastatin treatment in the ZYM KO group. Data are means ± SEM of 20 mice for each group. *P < 0.05, significantly different from SHAM groups; °P < 0.05, significantly different from vehicle; #P < 0.05, significantly different from simvastatin 5 mg. IL-1β, interleukin 1β; KO, knock-out; PPAR, peroxisome proliferator-activated receptor; TNFα, tumour necrosis factor α; WT, wild type; ZYM, zymosan.
Eighteen hours after zymosan administration, TNFα and IL-1β levels were significantly higher in the plasma of the ZYM KO group than in the ZYM WT and SHAM groups (Figure 4C and D). Simvastatin significantly and dose-dependently inhibited the release of pro-inflammatory cytokines in the ZYM WT but not in the ZYM KO group (Figure 4C and D).
Role of functional PPARα gene in the anti-inflammatory property of simvastatin on nitrosative stress and PAR activation
To determine nitrosative stress, we examined iNOS expression in lung, liver and kidney sections, 18 h after zymosan or vehicle administration (Figure 5). Immunohistochemical analysis with a specific anti-iNOS antibody resulted in positive staining in the lung (Figure 5A, panel a), liver (Figure 5B, panel a) and kidney (Figure 5C, panel a) of the ZYM WT group. This result was more obvious in the ZYM KO group (Figure 5A–C, panels a–b). All immunohistochemical and histological analysis were performed with the highest dose of simvastatin (10 mg·kg−1) and data from the SHAM groups are not shown.
Figure 5.
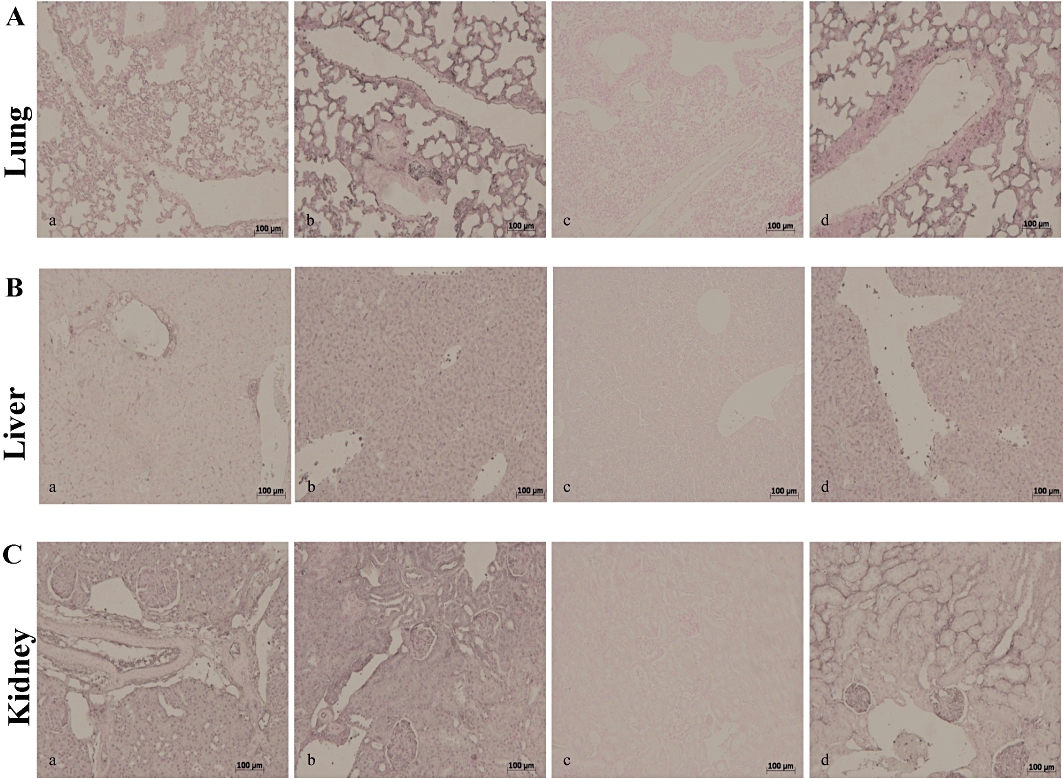
Immunohistochemical localization of iNOS in the mouse lung, liver and kidney. Eighteen hours after zymosan injection, iNOS positive staining was more intense in the lung (A, panel b), liver (B, panel b) and kidney (C, panel b) of the ZYM KO compared with the ZYM WT group (A–C, panel a). Simvastatin inhibited iNOS positive staining in all analysed tissues (A–C, panel c) of the ZYM WT group. The genetic absence of the PPARα receptor blocked the effect of simvastatin treatment (A–C, panel d). The figure is representative of at least three experiments performed on different experimental days. iNOS, inducible NO synthase; KO, knock-out; PPAR, peroxisome proliferator-activated receptor; WT, wild type; ZYM, zymosan.
Simvastatin inhibited iNOS expression in the lung (Figure 5A, panel c), liver (Figure 5B, panel c) and kidney (Figure 5C, panel c) of the ZYM WT, but not of the ZYM KO group (Figure 5A–C, panels a–d). Similarly, immunohistochemical analysis with a specific anti-nitrotyrosine antibody, revealed positive staining in the lung (Figure 6A, panel a), liver (Figure 6B, panel a) and kidney (Figure 6C, panel a) of the ZYM WT and ZYM KO groups (Figure 6A–C, panels a–b). Simvastatin inhibited nitrotyrosine staining in the lung (Figure 6A, panel c), liver (Figure 6B, panel c) and kidney (Figure 6C, panel c) of the ZYM WT, but not of the ZYM KO group (Figure 6A–C, panels a–d). Leukocyte infiltration in tissues has been implicated in the zymosan-induced release of free oxygen and nitrogen radicals, thereby favouring poly (ADP ribose) polymerase (PARP) activation (Peralta-Leal et al., 2009). In our study, immunohistochemistry for PAR, which is an indicator of in vivo PARP activation, resulted in positive PAR staining in the nuclei of inflammatory cells in the lung (Figure 7A, panel a), liver (Figure 7B, panel a) and kidney (Figure 7C, panel a) of the ZYM WT and ZYM KO groups (Figure 7A–C, panels a–b). Simvastatin inhibited PAR staining in the lung (Figure 7A, panel c), liver (Figure 7B, panel c) and kidney (Figure 7C, panel c) from the ZYM WT, but not of the ZYM KO group (Figure 7A–C, panels a–d).
Figure 6.
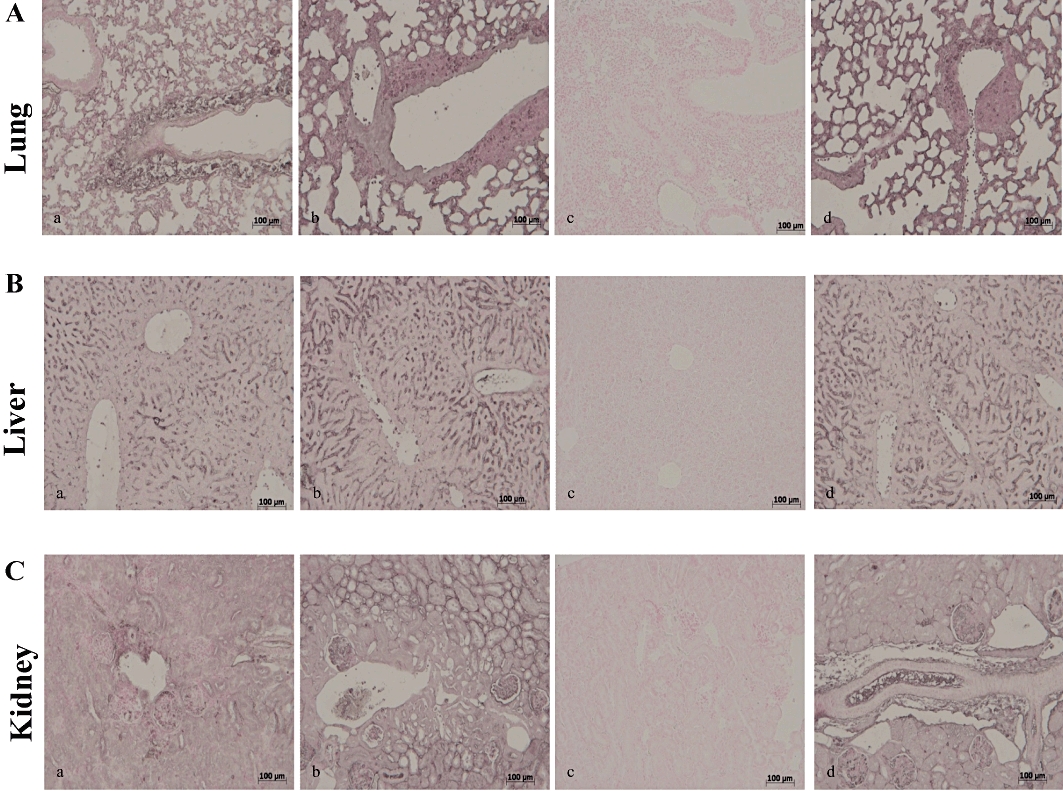
Immunohistochemical localization of nitrotyrosine in mouse lung, liver and kidney. Eighteen hours after zymosan injection, nitrotyrosine positive staining was more intense in the lung (A, panel b), liver (B, panel b) and kidney (C, panel b) of ZYM KO group compared with the ZYM WT group (A–C, panel a). Simvastatin (10 mg·kg−1) inhibited nitrotyrosine positive staining in all analysed tissues (A–C, panel c) of ZYM WT group. The genetic absence of the PPARα receptor blocked the effect of simvastatin treatment (A–C, panel d). The figure is representative of at least three experiments performed on different experimental days. KO, knock-out; PPAR, peroxisome proliferator-activated receptor; WT, wild type; ZYM, zymosan.
Figure 7.
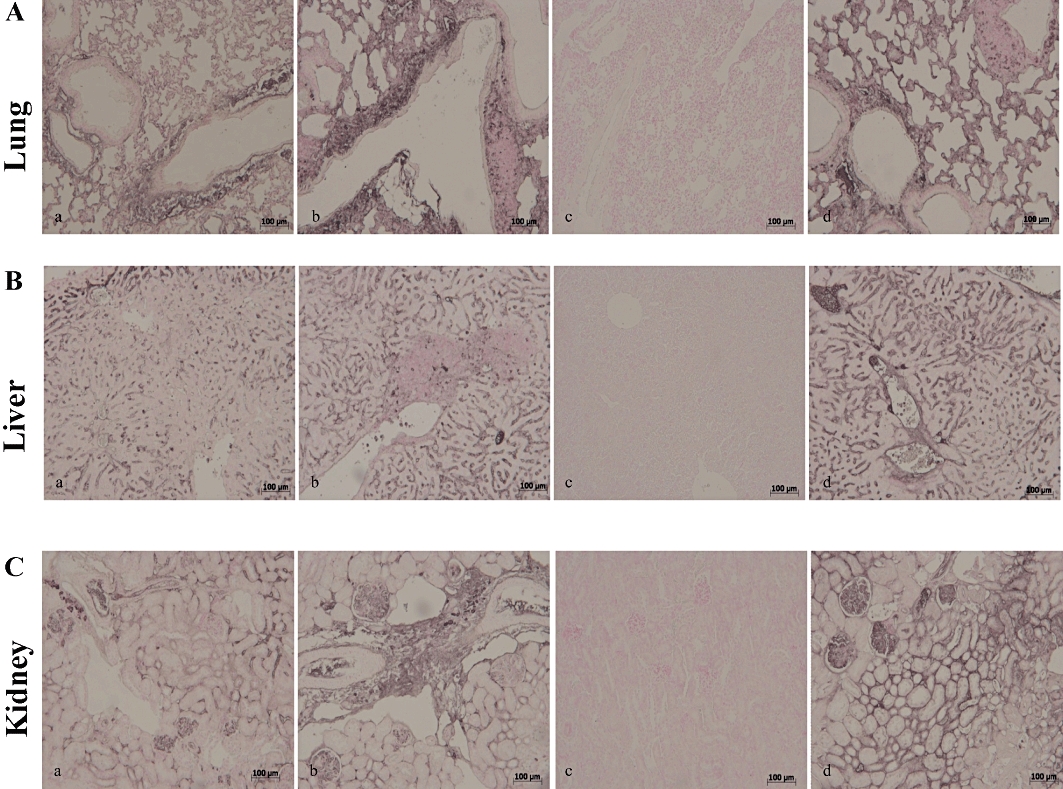
Immunohistochemical localization of PAR in mouse lung, liver and kidney. Eighteen hours after zymosan injection, PAR positive staining was more intense in the lung (A, panel b), liver (B, panel b) and kidney (C, panel b) of the ZYM KO group compared with the ZYM WT group (A–C, panel a). Simvastatin inhibited PAR positive staining in all analysed tissues (A–C, panel c) of the ZYM WT group. The genetic absence of the PPARα receptor blocked the effect of simvastatin treatment (A–C, panel d). The figure is representative of at least three experiments performed on different experimental days. KO, knock-out; PAR, poly ADP-ribose; PPAR, peroxisome proliferator-activated receptor; WT, wild type; ZYM, zymosan.
Role of functional PPARα gene in the anti-inflammatory property of simvastatin on zymosan-induced non-septic shock
Pancreatic injury
To determine the effects of simvastatin treatment on pancreatic injury after zymosan or vehicle administration, we evaluated plasma levels of amylase and lipase. In the SHAM groups, administration of vehicle did not affect plasma levels of amylase or lipase (Figure 8A and B). Plasma levels of amylase and lipase, which are indicative of pancreatic injury, were significantly higher in the ZYM KO than in the ZYM WT and SHAM groups (Figure 8A and B). Simvastatin significantly and dose-dependently prevented the increase in plasma levels of amylase and lipase in the ZYM WT, but not in the ZYM KO group (Figure 8A and B).
Figure 8.
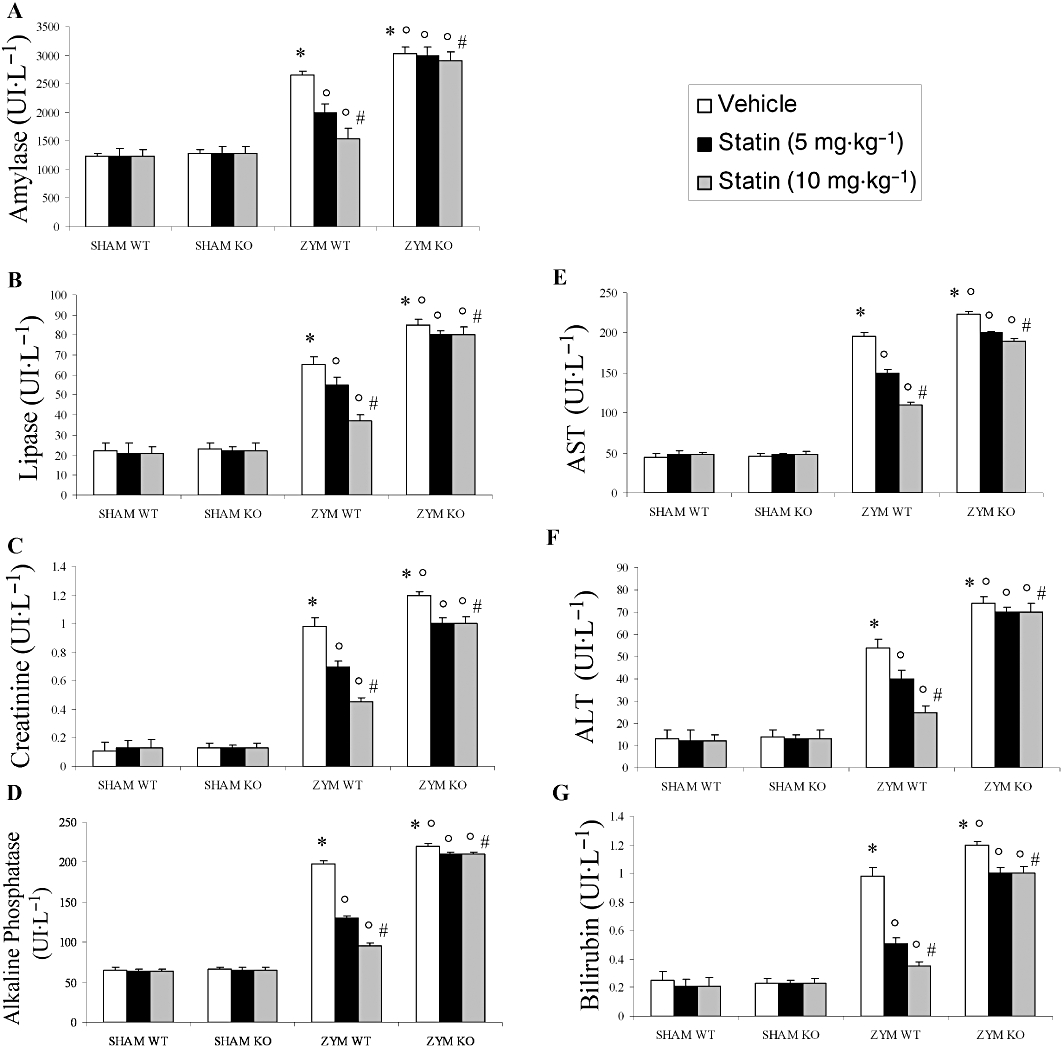
Effect of PPARα on the anti-inflammatory effect of simvastatin against zymosan-induced organ dysfunction. The increase in plasma amylase (A), lipase (B), creatinine (C) (U·L−1), alkaline phosphatase (D), AST (E), ALT (F) and bilirubin (G) at 18 h after zymosan administration (see Figure 8) was inhibited by simvastatin. The genetic absence of the PPARα receptor significantly blocked the effect of simvastatin treatment. Data are means ± SEM of 10 mice for each group. *P < 0.05, significantly different from SHAM groups; °P < 0.05, significantly different from vehicle; #P < 0.05, significantly different from simvastatin 5 mg. KO, knock-out; PPAR, peroxisome proliferator-activated receptor; WT, wild type.
Renal dysfunction
To determine the effects of simvastatin treatment on renal dysfunction after zymosan or vehicle administration, we evaluated plasma levels of creatinine. Eighteen hours after zymosan administration, plasma levels of creatinine, which are indicative of renal dysfunction, were significantly increased in the ZYM KO group relative to the ZYM WT and SHAM groups (Figure 8C). Simvastatin significantly and dose-dependently inhibited the increase of plasma levels of creatinine in the ZYM WT, but not in the ZYM KO group (Figure 8C).
Liver injury
To evaluate the effects of simvastatin treatment on liver injury after zymosan or vehicle administration, we measured the plasma levels of alkaline phosphatase (Figure 8D), AST (Figure 8E), ALT (Figure 8F) and bilirubin (Figure 8G). Eighteen hours after zymosan administration, there was a significant increase in the plasma levels of alkaline phosphatase, AST, ALT and bilirubin, which is indicative of hepatocellular injury in the ZYM KO group compared with the ZYM WT and SHAM groups (Figure 8D–G). Simvastatin significantly and dose-dependently inhibited plasma levels of alkaline phosphatase (Figure 8D), AST (Figure 8E), ALT (Figure 8F), bilirubin (Figure 8G) in the ZYM WT, but not in the ZYM KO group.
Role of functional PPARα gene in the anti-inflammatory property of simvastatin on organ injury caused by zymosan
Histological evaluation of lung (Figure 9A), liver (Figure 9B), kidney (Figure 9C) and intestine (Figure 9D) sections 18 h after zymosan administration revealed marked pathological changes. Lung sections revealed inflammatory infiltration by neutrophils, macrophages and plasma cells (Figure 9A). Hepatic damage was characterized by necrosis of hepatocytes (Figure 9B). In the kidney, there was extravasation of neutrophils as well as tubular injury (Figure 9C). In the intestine, there was infiltration of inflammatory cells, oedema in the space bounded by the villus, and separation of the epithelium from the basement membrane (Figure 9D). Absence of the functional PPARα gene in the ZYM KO group resulted in a significant enhancement of lung (Figure 9A, panel b), liver (Figure 9B, panel b), kidney (Figure 9C, panel b) and intestine (Figure 9D, panel b) injury. Simvastatin reduced lung (Figure 9A, panel c), liver (Figure 9B, panel c), kidney (Figure 9C, panel c) and intestine (Figure D, panel c) injury in the ZYM WT, but not in the ZYM KO group (Figure 9A–D, panels a–d). These effects were quantified with a histological score and are shown in Table 1.
Figure 9.
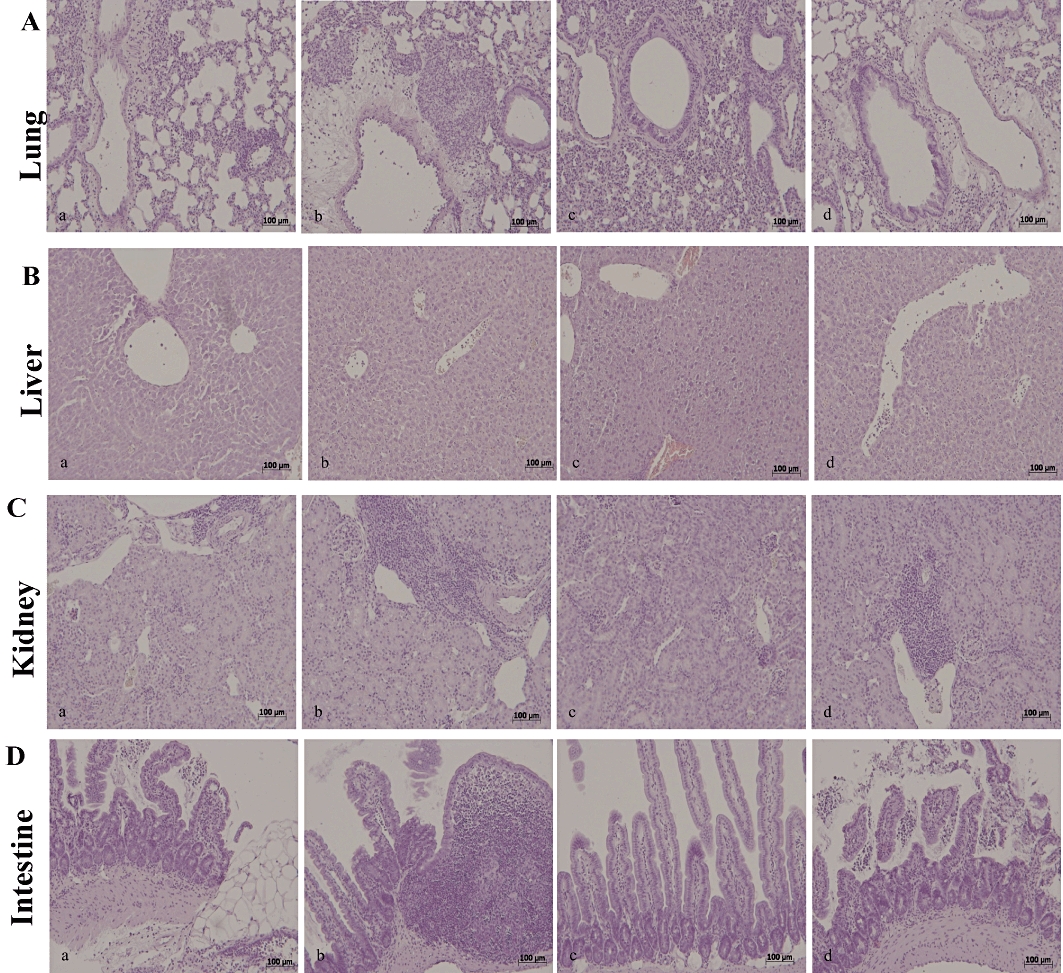
Morphological changes in lung, liver, kidney and intestine, 18 h after zymosan administration. The histological alterations observed at 18 h after zymosan injection in the lung (A, panel b), liver (B, panel b), kidney (C, panel b) and intestine (D, panel b) of the ZYM KO group were more evident than in the ZYM WT group (A–D, panel a). Simvastatin reduced histological alterations in all analysed tissues (A–D, panel c). The genetic absence of the PPARα receptor significantly blocked the effect of simvastatin treatment (A–D, panel d). The figure is representative of at least three experiments performed on different experimental days. KO, knock-out; PPAR, peroxisome proliferator-activated receptor; WT, wild type; ZYM, zymosan.
Table 1.
Histological scoring of lung, liver, kidney and intestine
| Lung | Liver | Kidney | Intestine | |
|---|---|---|---|---|
| SHAM WT | ND | ND | ND | ND |
| SHAM KO | ND | ND | ND | ND |
| SHAM WT + statin (10 mg·kg−1) | ND | ND | ND | ND |
| SHAM KO + statin (10 mg·kg−1) | ND | ND | ND | ND |
| ZYM WT | 2.6 ± 0.12* | 2.74 ± 0.11* | 2.24 ± 0.13* | 2.45 ± 0.1* |
| ZYM KO | 3.4 ± 0.1*° | 3.7 ± 0.14*° | 3.12 ± 0.17*° | 3.22 ± 0.2*° |
| ZYM WT + statin (10 mg·kg−1) | 0.85 ± 0.11*° | 0.62 ± 0.09*° | 0.92 ± 0.09*° | 0.45 ± 0.08*° |
| ZYM KO + statin (10 mg·kg−1) | 3.2 ± 0.12*°° | 3.5 ± 0.12*°° | 2.98 ± 0.11*°° | 2.78 ± 0.1*°° |
The above parameters were evaluated 18h after zymosan (ZYM) injection.
Data are means ± SEM of 20 mice for each group.
P < 0.01 vs SHAM
P < 0.01 vs ZYM WT
P < 0.01 ZYM KO statin vs ZYM WT + statin.
KO, knock-out; ND = not detectable; WT, wild type.
Discussion
This study shows that the PPARα receptor is involved in the anti-inflammatory effect of simvastatin in an experimental model of zymosan-induced multiple organ failure. In particular, exposure of lung, liver, kidney and intestine to simvastatin reduced multiple organ dysfunction, increased PPARα expression, and decreased the development of zymosan-induced acute peritonitis, PMN leukocyte infiltration, activation of transcription factor NF-κB, NO formation, cytokine expression, nitrosative stress and PAR activation.
Statins are competitive inhibitors of HMG-CoA reductase, and thus decrease endogenous cholesterol formation. In addition to their effect on plasma lipid concentrations, statins exert such pleiotropic activities as anti-platelet, anti-oxidant, anti-inflammatory and immunomodulatory activity (Fehrana et al., 2009; Liu et al., 2009). Cross-talk between statins and PPARα was first demonstrated by Martin et al. (2001) in human HepG2 hepatoma cells, and subsequent studies showed that statins increase PPARα mRNA levels (Landrier et al., 2004; Sanguino et al., 2005).
We found that both low and high doses of simvastatin dose-dependently increased PPARα mRNA expression in lung, liver and kidney tissues. PPARα has previously been implicated in the acute anti-inflammatory effect of simvastatin in vitro and in vivo (Paumelle et al., 2006). Our data show that simvastatin significantly reduced the development of acute peritonitis, leukocyte infiltration and exudate formation during zymosan-induced non-septic shock and that these effects were less evident in PPARαKO mice. Moreover, biochemical and histological evaluations showed that simvastatin reduced the multiple organ dysfunction induced by zymosan. The increased expression of PPARα could be one of the possible anti-inflammatory mechanisms of statins involved in the complete recovery of the functionality and structure of organs exposed to zymosan. These data are in line with our previous study demonstrating that the absence of the PPARα functional gene enhances the degree of multiple organ failure induced by zymosan (Di Paola et al., 2006).
A number of experimental studies indicate that PPARα exerts its anti-inflammatory effects by diverse mechanisms. PPARα induced catabolism of lipid pro-inflammatory mediators thus, negatively controlling inflammation (Becker et al., 2008). In addition, PPARα interferes with the activity of the transcription factors AP-1, NF-κB and STAT-1, thereby promoting the signal transduction of cytokines, lipopolysaccharides and interferons (Delerive et al., 2001). Here, we demonstrate that in absence of PPARα gene, zymosan induced a more severe inflammatory response. Simvastatin significantly and dose-dependently reduced NF-κB activation in lung, liver and kidney tissues in zymosan PPARαWT and PPARαKO mice. These data are in line with our previous findings that highlighted the importance of PPARα gene presence in the inflammatory response (Crisafulli and Cuzzocrea, 2009; Crisafulli et al., 2009). In systemic inflammation, various mediators stimulate the overproduction of the inducible isoform iNOS which then produces large amounts of NO that is an important factor in the development of septic and non-septic shock (Heemskerk et al., 2009; Draisma et al., 2010; Fortin et al., 2010). iNOS expression may be induced by cytokine combinations such as TNFα and interferon-γ, via the transcription factor NF-κB. In our experimental model of non-septic shock, simvastatin reduced the zymosan-induced up-regulation of nitrite/nitrate levels, TNFα, IL-1β and iNOS in all tissues analysed. In addition, PARP plays a pivotal role in oxidative/nitrosative stress-related pathology. Moderate activation of PARP can be of physiological importance because it enhances DNA repair (Martin et al., 2008); on the other hand, PARP overactivation results in the tissue damage associated with myocardial reperfusion injury, diabetes and shock (Cuzzocrea and Wang, 2005; Pacher and Szabo, 2008). In particular, the reactive oxygen species/NF-κB/PARP pathway is involved in the multiple inflammatory responses induced in shock. In our experimental model, simvastatin significantly reduced the immunohistochemical distribution of PAR in organs injured by zymosan, and then the activation of the PARP pathway. As observed with the inflammatory markers, the protective effect of the statin was less evident in PPARαKO groups.
In conclusion, we demonstrate that statins exert pleiotropic effects in a model of zymosan-induced non-septic shock by preventing, at both molecular and cellular level, organ damage consequent to a powerful inflammatory response. One of these pleiotropic effects could involve an increased PPARα expression. These and our previous data (Di Paola et al., 2006; Crisafulli et al., 2009) help to clear one of the molecular pathways, whereby simvastatin exerts a beneficial effect on systemic inflammation and organ injury.
Acknowledgments
We thank Jean Ann Gilder for editing the manuscript.
Glossary
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- HMG-CoA
3-hydroxy-3-methylglutaryl co-enzyme A reductase
- IL-1β
interleukin 1β
- iNOS
inducible NO synthase
- MODS
multiple organ dysfunction syndrome
- NF-κB
nuclear factor-κB
- PAR
poly ADP-ribose
- PEC
peritoneal exudate cells
- PPAR
peroxisome proliferator-activated receptor
- PPARαWT
PPARα wild type
- PPARαKO
PPARα knock-out
- PMN
polymorphonuclear
- RXR
9-cis-retinoic acid receptor
- TNFα
tumour necrosis factor α
Conflict of interest
The authors state no conflict of interest.
Supporting Information
Teaching Materials; Figs 1–9 as PowerPoint slide.
References
- Abbott BD. Review of the expression of peroxisome proliferator-activated receptors alpha (PPAR alpha), beta (PPAR beta), and gamma (PPAR gamma) in rodent and human development. Reprod Toxicol. 2009;27:246–257. doi: 10.1016/j.reprotox.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez de Sotomayor M, Vega S, Mingorance C, Marhuenda E, Herrera MD. Effects of HMG-CoA reductase inhibition by simvastatin on vascular dysfunction induced by lipopolysaccharide in rats. Pharmacology. 2008;82:89–96. doi: 10.1159/000135629. [DOI] [PubMed] [Google Scholar]
- Angus DC, Burgner D, Wunderink R, Mira JP, Gerlach H, Wiedermann CJ, et al. The PIRO concept: P is for predisposition. Crit Care. 2003;7:248–251. doi: 10.1186/cc2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baue AE. The multiple organ or system failure syndrome. In: Schlag G, Redl H, editors. Pathophysiology of Shock, Sepsis, and Organ Failure. Berlin: Springer Verlag; 1993. pp. 1004–1018. [Google Scholar]
- Becker J, Delayre-Orthez C, Frossard N, Pons F. Regulation of peroxisome proliferator-activated receptor-alpha expression during lung inflammation. Pulm Pharmacol Ther. 2008;21:324–330. doi: 10.1016/j.pupt.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Crisafulli C, Cuzzocrea S. The role endogenous and exogenous ligands for the peroxisome proliferator-activated receptor alpha (PPAR-alpha) in the regulation of inflammation in macrophages. Shock. 2009;32:62–73. doi: 10.1097/shk.0b013e31818bbad6. [DOI] [PubMed] [Google Scholar]
- Crisafulli C, Bruscoli S, Esposito E, Mazzon E, Di Paola R, Genovese T, et al. PPAR-alpha contributes to the anti-inflammatory activity of 17beta-estradiol. J Pharmacol Exp Ther. 2009;331:796–807. doi: 10.1124/jpet.109.156646. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Wang ZQ. Role of poly(ADP-ribose) glycohydrolase (PARG) in shock, ischemia and reperfusion. Pharmacol Res. 2005;52:100–108. doi: 10.1016/j.phrs.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Esposito E, Muià C, Abdelrahman M, Di Paola R, et al. Glycogen synthase kinase-3beta inhibition attenuates the development of ischaemia/reperfusion injury of the gut. Intensive Care Med. 2007;33:880–893. doi: 10.1007/s00134-007-0595-1. [DOI] [PubMed] [Google Scholar]
- Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol. 2001;169:453–459. doi: 10.1677/joe.0.1690453. [DOI] [PubMed] [Google Scholar]
- Di Paola R, Esposito E, Mazzon E, Genovese T, Muià C, Crisafulli C, et al. Absence of peroxisome proliferators-activated receptors (PPAR)alpha enhanced the multiple organ failure induced by zymosan. Shock. 2006;26:477–484. doi: 10.1097/01.shk.0000230299.78515.2c. [DOI] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draisma A, Dorresteijn MJ, Bouw MP, van der Hoeven JG, Pickkers P. The role of cytokines and inducible nitric oxide synthase in endotoxemia-induced endothelial dysfunction. J Cardiovasc Pharmacol. 2010;55:595–600. doi: 10.1097/FJC.0b013e3181da774b. [DOI] [PubMed] [Google Scholar]
- Fehrana YA, Armstrong PC, Dhanji AR, Tucker AT, Paul-Clark MJ, Mitchell JA, et al. Antiplatelet actions of statins and fibrates are mediated by PPARs. Arterioscler Thromb Vasc Biol. 2009;29:706–711. doi: 10.1161/ATVBAHA.108.183160. [DOI] [PubMed] [Google Scholar]
- Fortin CF, McDonald PP, Fülöp T, Lesur O. Sepsis, leukocytes, and nitric oxide (NO): an intricate affair. Shock. 2010;33:344–352. doi: 10.1097/SHK.0b013e3181c0f068. [DOI] [PubMed] [Google Scholar]
- Fraunberger P, Gröne E, Gröne HJ, Walli AK. Simvastatin reduces endotoxin-induced nuclear factor kappaB activation and mortality in guinea pigs despite lowering circulating low-density lipoprotein cholesterol. Shock. 2009;32:159–163. doi: 10.1097/SHK.0b013e318193c514. [DOI] [PubMed] [Google Scholar]
- Fruchart JC. Peroxisome proliferator-activated receptor-alpha (PPARalpha): at the crossroads of obesity, diabetes and cardiovascular disease. Atherosclerosis. 2009;205:1–8. doi: 10.1016/j.atherosclerosis.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Gao F, Linhartova L, Johnston AM, Thickett DR. Statins and sepsis. Br J Anaesth. 2008;100:288–298. doi: 10.1093/bja/aem406. [DOI] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Di Paola R, Muià C, Crisafulli C, Malleo G, et al. Role of peroxisome proliferator-activated receptor-alpha in acute pancreatitis induced by cerulein. Immunology. 2006;118:559–570. doi: 10.1111/j.1365-2567.2006.02393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese T, Esposito E, Mazzon E, Crisafulli C, Paterniti I, Di Paola R, et al. PPAR-alpha modulate the anti-inflammatory effect of glucocorticoids in the secondary damage in experimental spinal cord trauma. Pharmacol Res. 2009;59:338–350. doi: 10.1016/j.phrs.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Giusti-Paiva A, Martinez MR, Felix JV, da Rocha MJ, Carnio EC, Elias LL, et al. Simvastatin decreases nitric oxide overproduction and reverts the impaired vascular responsiveness induced by endotoxic shock in rats. Shock. 2004;21:271–275. doi: 10.1097/10.shk.0000115756.74059.ce. [DOI] [PubMed] [Google Scholar]
- Heemskerk S, Masereeuw R, Russel FG, Pickkers P. Selective iNOS inhibition for the treatment of sepsis-induced acute kidney injury. Nat Rev Nephrol. 2009;5:629–640. doi: 10.1038/nrneph.2009.155. [DOI] [PubMed] [Google Scholar]
- Jasińska M, Owczarek J, Orszulak-Michalak D. Statins: a new insight into their mechanisms of action and consequent pleiotropic effects. Pharmacol Rep. 2007;59:483–499. [PubMed] [Google Scholar]
- Landrier JF, Thomas C, Grober J, Duez H, Percevault F, Souidi M, et al. Statin induction of liver fatty acid-binding protein (L-FABP) gene expression is peroxisome proliferator-activated receptor-alpha-dependent. J Biol Chem. 2004;279:45512–45518. doi: 10.1074/jbc.M407461200. [DOI] [PubMed] [Google Scholar]
- Liu PY, Liu YW, Lin LJ, Chen JH, Liao JK. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation. 2009;119:131–138. doi: 10.1161/CIRCULATIONAHA.108.813311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation. 2000;101:207–213. doi: 10.1161/01.cir.101.2.207. [DOI] [PubMed] [Google Scholar]
- Martin G, Duez H, Blanquart C, Berezowski V, Poulain P, Fruchart JC, et al. Statin-induced inhibition of the Rho-signaling pathway activates PPARalpha and induces HDL apoA-I. J Clin Invest. 2001;107:1423–1432. doi: 10.1172/JCI10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SA, Lord CJ, Ashworth A. DNA repair deficiency as a therapeutic target in cancer. Curr Opin Genet Dev. 2008;18:80–86. doi: 10.1016/j.gde.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Marx N, Duez H, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors and atherogenesis: regulators of gene expression in vascular cells. Circ Res. 2004;94:1168–1178. doi: 10.1161/01.RES.0000127122.22685.0A. [DOI] [PubMed] [Google Scholar]
- Merx MW, Liehn EA, Graf J, van de Sandt A, Schaltenbrand M, Schrader J, et al. Statin treatment after onset of sepsis in a murine model improves survival. Circulation. 2005;112:117–124. doi: 10.1161/CIRCULATIONAHA.104.502195. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Iwamoto T, Kotake S, Momohara S, Yamanaka H, Kamatani N. Inhibition of NF-kappaB signaling by fenofibrate, a peroxisome proliferator-activated receptor-alpha ligand, presents a therapeutic strategy for rheumatoid arthritis. Clin Exp Rheumatol. 2005;23:323–230. [PubMed] [Google Scholar]
- Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukkeri EL, Leppänen T, Sareila O, Vuolteenaho K, Kankaanranta H, Moilanen E. PPARalpha agonists inhibit nitric oxide production by enhancing iNOS degradation in LPS-treated macrophages. Br J Pharmacol. 2007;152:1081–1091. doi: 10.1038/sj.bjp.0707477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumelle R, Blanquart C, Briand O, Barbier O, Duhem C, Woerly G, et al. Acute antiinflammatory properties of statins involve peroxisome proliferator-activated receptor-alpha via inhibition of the protein kinase C signaling pathway. Circ Res. 2006;98:361–369. doi: 10.1161/01.RES.0000202706.70992.95. [DOI] [PubMed] [Google Scholar]
- Peralta-Leal A, Rodrìguez-Vargas JM, Aguilar-Quesada R, Rodrìguez MI, Linares JL, de Almodòvar MR, et al. PARP inhibitors: new partners in the therapy of cancer and inflammatory diseases. Free Radical Biol & Med. 2009;47:13–26. doi: 10.1016/j.freeradbiomed.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170:1435–1444. doi: 10.2353/ajpath.2007.060872. Erratum in: (2007) Am J Pathol 171: 1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguino E, Roglans N, Alegret M, Sánchez RM, Vázquez-Carrera M, Laguna JC. Atorvastatin reverses age-related reduction in rat hepatic PPARalpha and HNF-4. Br J Pharmacol. 2005;145:853–861. doi: 10.1038/sj.bjp.0706260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher T, Yi HF, McBride OW, Gonzalez FJ. cDNA cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Biochemistry. 1993;32:5598–5604. doi: 10.1021/bi00072a015. [DOI] [PubMed] [Google Scholar]
- Siempos II, Vardakas KZ, Kopterides P, Falagas ME. Adjunctive therapies for community-acquired pneumonia: a systematic review. J Antimicrob Chemother. 2008;62:661–668. doi: 10.1093/jac/dkn283. [DOI] [PubMed] [Google Scholar]
- Szabó C, Lim LH, Cuzzocrea S, Getting SJ, Zingarelli B, Flower RJ, et al. Inhibition of poly (ADP-ribose) synthetase attenuates neutrophil recruitment and exerts antiinflammatory effects. J Exp Med. 1997;186:1041–1049. doi: 10.1084/jem.186.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


