Abstract
Antibiotics that are excreted into the intestinal tract promote antibiotic resistance by exerting selective pressure on the gut microbiota. Using a beagle dog model, we show that an orally administered targeted recombinant β-lactamase enzyme eliminates the portion of parenteral ampicillin that is excreted into the small intestine, preventing ampicillin-induced changes to the fecal microbiota without affecting ampicillin levels in serum. In dogs receiving ampicillin, significant disruption of the fecal microbiota and the emergence of ampicillin-resistant Escherichia coli and TEM genes were observed, whereas in dogs treated with ampicillin in combination with an oral β-lactamase, these did not occur. These results suggest a new strategy for reducing antimicrobial resistance in humans.
The gastrointestinal microbiotas of humans and animals form a complex but relatively stable ecosystem (2). Antimicrobials that are excreted into the intestinal tract may cause profound disruption of the indigenous microbiota (18, 20, 23, 25), resulting in a number of adverse effects such as antibiotic-associated diarrhea and Clostridium difficile colitis (20). In addition, the selective pressure exerted by antimicrobials in the intestinal tract contributes to the acquisition and overgrowth of antimicrobial-resistant pathogens, including vancomycin-resistant enterococci (VRE), Candida species, and multiresistant gram-negative bacilli (28). Overgrowth of these microorganisms may facilitate resistance gene transfer (11, 26) and increase the risk of person-to-person transmission. For example, patients with high-density stool colonization with VRE were shown to have significantly higher rates of environmental contamination than those with low-density colonization (4).
β-Lactams, including ampicillin, are among the most widely used classes of antimicrobials, and many of these agents are excreted into the intestinal tract at high concentrations. Previous studies have demonstrated that the normal intestinal microbiota contains various degrees of antibiotic resistance genes (1, 8, 17, 22) and that healthy individuals may harbor intestinal bacteria that can produce TEM β-lactamases (19, 21, 23). It has further been shown that the resistance patterns of enteric bacteria change in response to increased levels of exposure to antibiotics (9) and that selective pressure from ampicillin treatment can result in increased levels of ampicillin-resistant bacteria (7).
In previous experiments, we demonstrated that the β-lactamase enzyme of Bacillus licheniformis resisted proteolysis and maintained activity in the small intestines of rodents (unpublished data) and that it degrades ampicillin in the jejuna of beagle dogs in a dose-dependent manner (6).
In this study, it was hypothesized that oral administration of a β-lactamase enzyme in conjunction with parenteral β-lactam antibiotic administration would result in inactivation of the portion of antibiotic excreted into the intestinal tract, thereby resulting in preservation of the indigenous microbiota and prevention of the emergence of ampicillin-resistant Escherichia coli and TEM β-lactamase genes.
MATERIALS AND METHODS
Eighteen male laboratory beagle dogs were included in the study. The dogs had permanently fistulated jejuna (5, 30) and were obtained from Harlan-Winkelmann GmbH (Borchen, Germany) and the National Laboratory Animal Centre (University of Kuopio, Finland). The experimental protocol was approved by the local ethics committee for animal experiment action in Helsinki, Finland, and was conducted in accordance with the valid guidelines (www.hmso.gov.uk).
Targeted recombinant β-lactamase (TRBL) was used for peroral (p.o.) treatment and was dosed in enteropellets (6). Similar pellets without the enzyme were used as p.o. placebo treatment. The dogs were randomized into three treatment groups: ampicillin (40 mg/kg of body weight) intravenously (i.v.) plus placebo p.o. (n = 6), ampicillin (40 mg/kg) i.v. plus TRBL (0.6 mg/kg) p.o. (n = 6), and placebo i.v. plus placebo p.o. (n = 6). Ampicillin sodium (1 g; A-PEN inject; Orion Pharma, Espoo, Finland) or placebo (100 ml; Natrosteril [9 mg/ml], 0.9% infusion; Orion Pharma) was administered intravenously four times a day 30 min after feeding, and TRBL- or placebo-containing capsules were administered orally 20 and 27 min after feeding. The total duration of treatment was 14 days. Jejunal, serum, and fecal samples were collected once a day before (day 0), during (days 1, 4, 10, and 14), and after (day 28) the treatment period. Serum and jejunal samples were collected 1 h after the first antibiotic administration of the day. Fecal samples were taken from fresh defecated stool (within 1 h of defecation). The samples were frozen at −70°C until they were analyzed. The treatments were not blinded, but diagnostic testing was; i.e., the individuals running the tests did not know from which group the samples originated.
Diagnostic methods. (i) Measurement of ampicillin concentrations.
High-performance liquid chromatography (United Laboratories Ltd., Helsinki, Finland) was performed to measure ampicillin concentrations by a modification of a previously described technique (29). The lower limits of detection were 1.0 μg/ml for serum samples and 0.5 μg/ml for jejunal samples.
(ii) TGGE.
To assess the bacterial diversity in the gastrointestinal tract, a molecular approach based on the sequence variability of the 16S rRNA gene was applied by using temperature gradient gel electrophoresis (TGGE), as described previously (14). The gel was stained with AgNO3 and developed by the technique of Cairns and Murray (3). Samples taken from the same individual on different days of the experiment were analyzed simultaneously, and the results for samples from each sampling day were compared to those for samples obtained on day 0; thus, similarities (in percent) between samples could be computed.
(iii) Analysis of ampicillin resistance.
The emergence of ampicillin resistance in the study dogs was evaluated by performing quantitative PCR of TEM β-lactamase genes and by standard culture techniques. In order to determine the percentage of TEM-producing bacteria in the samples, the quantity of TEM-producing organisms was compared to the total number of bacteria in the fecal samples, as determined by quantitative real-time PCR of the V6 to V8 region of the 16S rRNA gene. The following PCR primers for the amplification of the TEM gene were used: TemA (5′-ATAAAATTCTTGAAGAC-3′), TemB (5′-TTACCAATGCTTAATCA-3′), TemE (5′-TCGTCGTTTGGTATGGC-3′), and TemH (5′-AGGAAGAGTATGAGTAT-3′). Both amplification reactions were monitored by using SYBR Green (Molecular Probes, AA Leiden, The Netherlands) as a fluorogenic marker. The total count of bacteria was set equal to 100%.
For standard culture techniques, fecal samples were serially diluted (10−1 to 10−7) in prereduced peptone-yeast extract broth (pH 7.0) and plated onto several selective and nonselective agar media for enumeration of total counts and the main groups of anaerobic and aerobic bacteria and yeasts. Additionally, 10 coliformic (gram-negative, aerobic, fermentative) colonies were selected at random from a sample of each of the different dilutions of blood (5% sheep) and cystine lactose electrolyte-deficient plates whenever optimal growth was detected (most often, 10−5 to 10−6). Also, if different phenotypes were visible, all different phenotypes were picked. The bacteria were identified by established methods (13). The susceptibilities of the coliform isolates to ampicillin (10 μg), cefotaxime (30 μg), and meropenem (10 μg) were determined by the disk diffusion method approved by the National Committee for Clinical Laboratory Standards (16).
Statistical analysis.
The data analyses were performed with Statistical Analysis Systems (SAS) software (version 8.1, 2000; SAS Institute Inc.). The PROC MIXED program in SAS software was used to analyze the repeated measurements of the percent similarities of the fecal flora determined by TGGE. Due to the nonnormal distribution of the data for ampicillin concentrations and TEM gene levels, nonparametric analysis of variance was used to compare those values between the treatment groups and between the values obtained on different days during the study period. The results were considered statistically significant if P was <0.01. For the statistical analysis, the ampicillin concentration and the TEM gene level were assigned values of zero if they were below the limit of detection.
RESULTS
No significant difference in serum ampicillin concentrations was present between the two ampicillin-treated groups (P = 0.98) (Table 1). The group treated with ampicillin and placebo had significantly higher jejunum ampicillin concentrations during the treatment period than the group treated with ampicillin and TRBL (P < 0.0001) (Table 2).
TABLE 1.
Effects of enterocoated β-lactamase pellets on serum ampicillin concentrations in different test groupsa
| Sampling day | Mean serum AMP concn (μg/ml)
|
Median serum AMP concn (μg/ml)
|
||||
|---|---|---|---|---|---|---|
| AMP + TRBL | AMP + pl | pl + pl | AMP + TRBL | AMP + pl | pl + pl | |
| 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 | 0 | 0 |
| 1 | 21.7 ± 3.5 | 22.0 ± 1.7 | 0 ± 0 | 19.8 | 21.9 | 0 |
| 4 | 19.1 ± 2.1 | 16.1 ± 1.0 | 0 ± 0 | 19.4 | 16.4 | 0 |
| 10 | 15.6 ± 1.0 | 15.8 ± 1.6 | 0 ± 0 | 16.4 | 15.5 | 0 |
| 14 | 15.7 ± 2.4 | 16.9 ± 2.4 | 0 ± 0 | 14.9 | 14.0 | 0 |
| Overall mean | 18.0 | 17.7 | 0 | |||
| Overall median | 16.8 | 16.9 | 0 | |||
The values for each test group are presented as the mean ± standard error of the mean and the median serum ampicillin concentrations during the treatment period (days 1 to 14). AMP, ampicillin; pl, placebo. The overall difference in the serum ampicillin concentration between the group treated with ampicillin and TRBL and the group treated with ampicillin and placebo is nonsignificant.
TABLE 2.
Effects of enterocoated β-lactamase pellets on jejunum ampicillin concentrations in different test groupsa
| Sampling day | Mean jejunal AMP concn (μg/ml)
|
Median jejunal AMP concn (μg/ml)
|
||||
|---|---|---|---|---|---|---|
| AMP + TRBL | AMP + pl | pl + pl | AMP + TRBL | AMP + pl | pl + pl | |
| 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 | 0 | 0 |
| 1 | 0 ± 0 | 61.4 ± 23.1 | 0 ± 0 | 0 | 87.0 | 0 |
| 4 | 0 ± 0 | 8.0 ± 8.0 | 0 ± 0 | 0 | 0 | 0 |
| 10 | 5.8 ± 5.8 | 14.9 ± 6.3 | 0 ± 0 | 0 | 19.5 | 0 |
| 14 | 0 ± 0 | 21.0 ± 9.0 | 0 ± 0 | 0 | 14.5 | 0 |
| Overall mean | 1.5 | 26.3 | 0 | |||
| Overall median | 0 | 15.0b | 0 | |||
The values for each test group are presented as the mean ± standard error of the mean and median jejunum ampicillin concentrations during the treatment period (days 1 to 14). AMP, ampicillin; pl, placebo.
The overall difference in the median jejunum ampicillin concentrations between the group treated with ampicillin and TRBL and the group treated with ampicillin and placebo is significant (P < 0.0001).
Oral TRBL preserved the fecal microflora during ampicillin treatment.
The group treated with ampicillin and placebo had a significant reduction of the overall percent similarity of the fecal flora, as determined by TGGE, in comparison to those for the other two groups (P < 0.0001) (Fig. 1). The overall percent similarity of the fecal flora determined by TGGE for the group treated with ampicillin and TRBL did not differ from that for the group treated with placebo i.v. and placebo p.o. (P = 0.54).
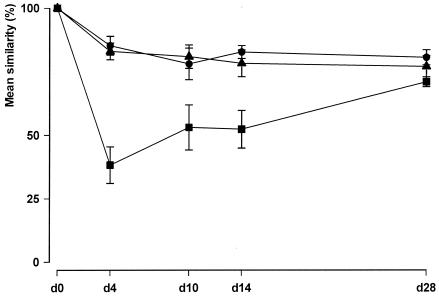
FIG. 1.
Effects of enterocoated β-lactamase pellets on the percent similarity of the fecal flora determined by TGGE. Solid circle, ampicillin at 40 mg/kg i.v. plus TRBL p.o.; solid square, ampicillin at 40 mg/kg i.v. plus placebo p.o.; solid triangle, placebo i.v. plus placebo p.o. The values for each test group are presented as the mean ± standard error of the mean percent similarity of the fecal flora determined by TGGE on different sampling days during the experimental period. The results for samples from each sampling day were compared to those for the samples obtained on day 0 (d0). The percent similarity of the fecal flora between the group receiving ampicillin and placebo and the two other treatment groups differed significantly (P < 0.0001) during the treatment period at days 4, 10, and 14.
By conventional bacterial cultivation, the total counts of aerobic bacteria (9.1 log10 versus 10.2 log10 [P = 0.003]) and anaerobic bacteria (10.3 log10 versus 10.9 log10 [P = 0.041]) were significantly lower in the group treated with ampicillin and placebo than in the group treated with ampicillin and TRBL during the intervention. The counts of streptococci, enterococci, clostridia, and anaerobic gram-positive cocci in particular decreased; and the proportions of both aerobic and anaerobic gram-negative rods increased. Otherwise, no marked changes were recorded. Dogs receiving ampicillin and TRBL or placebo alone had only minor overall changes and some occasional changes for a single species.
Oral TRBL prevented the emergence of ampicillin resistance.
The TEM gene level was very low (8 of 18 dogs) or undetectable (10 of 18 dogs) at day 0 in all of the dogs. Dogs receiving ampicillin and placebo developed a significant increase in TEM gene levels compared to dogs receiving ampicillin and TRBL or placebo i.v. and placebo p.o. (P < 0.001) (Fig. 2). The difference in the percentages of TEM genes between the last two groups was not significant (P = 0.38). Two weeks after the treatment period the TEM gene concentrations were near the baseline levels in all treatment groups. One of the six dogs in the group receiving ampicillin and TRBL developed a transient elevation in the small intestine ampicillin concentration on day 10, and this coincided with a reduction in the percent similarity of the fecal flora determined by TGGE and an elevation in the proportion of TEM genes (Fig. 3). By day 14, these abnormal levels had returned to the previous baseline levels.
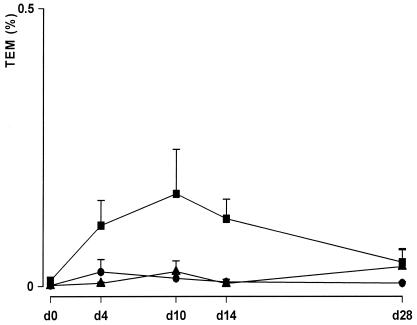
FIG. 2.
Effects of enterocoated β-lactamase pellets on the percentage of TEM genes in feces analyzed by quantitative real-time PCR. Solid circle, ampicillin at 40 mg/kg i.v. plus TRBL p.o.; solid square, ampicillin at 40 mg/kg i.v. plus placebo p.o.; solid triangle, placebo i.v. plus placebo p.o. The values for each test group are presented as the mean ± standard error of the mean percentage of DNA for ampicillin resistance (TEM gene) in fecal samples compared with the total DNA count on different sampling days during the experimental period. The TEM gene levels during the treatment period in the group receiving ampicillin and placebo differed significantly (P < 0.001) from those in the other two treatment groups (at days 4, 10, and 14). The results for the group receiving ampicillin and TRBL do not include those for one dog (Fig. 3).
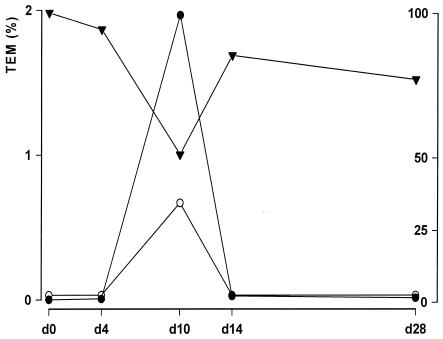
FIG. 3.
Results for the exceptional dog in the ampicillin and TRBL group. Open circle, jejunum ampicillin concentration (0 to 100 μg/ml); solid inverted triangle, percent similarity of fecal flora determined by TGGE (0 to 100%); solid circle, percentage of the TEM gene (0 to 2%). The values for the percentage of the TEM gene are presented on the left y axis, and the values for the jejunum ampicillin concentration and the percent similarity of fecal flora are presented on the right y axis.
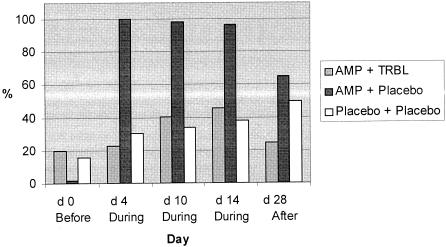
A total of 860 coliformic colonies were isolated (9.6 isolates per sample). E. coli was the predominant species (99% of all isolates). At the baseline the majority (69 to 83%) of isolates in all groups were sensitive to all of the antibiotics tested. Among the dogs in the group receiving ampicillin and placebo, one dog harbored ampicillin-resistant E. coli at the baseline, but as the intervention started, all six dogs became ampicillin-resistant E. coli carriers (Fig. 4). The proportion of ampicillin-resistant strains of all fecal coliforms isolated rose dramatically, from 2% at the baseline to 100% on day 4, and remained high throughout the intervention. Two weeks after the intervention (day 28), the proportion of ampicillin-resistant strains was still 65% of the isolates in this study group (Fig. 4). Among the dogs in the group receiving ampicillin and TRBL, three dogs harbored ampicillin-resistant E. coli at the baseline; but no additional dogs became carriers during the intervention, and the proportion of fecal ampicillin-resistant strains at no time exceeded 46%. On day 28, the distribution of the different resistance patterns was restored and became similar to that at the baseline.
FIG. 4.
Effects of enterocoated β-lactamase pellets on the proportion of strains of coliform isolates from different treatment groups resistant to ampicillin (10 μg) during the experimental period. The duration of the treatment period was 14 days. Light gray bars, ampicillin plus TRBL; dark gray bars, ampicillin plus placebo; open bars, placebo i.v. and placebo p.o.
Among the dogs in the group receiving placebo i.v. and placebo p.o., two dogs were carriers of ampicillin-resistant E. coli at the baseline and three additional dogs became carriers during the intervention. The proportion of fecal ampicillin-resistant strains grew steadily, from 16% on day 0 to 50% on day 28.
DISCUSSION
We found that an orally administered targeted recombinant β-lactamase inactivated the portion of parenteral ampicillin that was excreted into the small intestines of beagle dogs and prevented ampicillin-induced changes in the fecal microbiota. High levels of ampicillin-resistant E. coli emerged in dogs treated with ampicillin but not in those treated with ampicillin in combination with oral β-lactamase. These results suggest that β-lactamase therapy may be an effective means of reducing the selective pressure that parenteral β-lactam antibiotics exert on the intestinal microbiota. Because the intestinal tract is a major reservoir for the emergence and dissemination of antimicrobial-resistant pathogens and β-lactam antibiotics are among the most widely used antimicrobials, this new treatment could potentially provide an important aid in reducing the rates of antimicrobial resistance in humans.
Our data suggest that oral β-lactamase administration will not reduce the efficacy of parenteral antibiotic therapy when extraintestinal infections are treated. The serum ampicillin concentrations of the group receiving ampicillin and TRBL did not differ from those in the group receiving ampicillin and placebo. This suggests that TRBL was not absorbed from the intestinal tract by the systemic circulation during the 2-week period of treatment. This is a noteworthy result, signifying that by using this combination treatment (i.v. antibiotic and p.o. TRBL) the systemic concentration of the antimicrobial agent can be maintained at a therapeutic level. The TRBL in the dosage formulation was targeted to be released from the enteropellets in the lower part of the small intestine in a time- and pH-dependent manner, which ensured the absorption of the antibiotic prior to TRBL activity in the gut.
The data are consistent with those from previous studies that demonstrate that ampicillin treatment may have a marked effect on the intestinal microbiota (15, 20, 23, 25) and that the population of antimicrobial-resistant pathogens may expand within days after the onset of antibiotic therapy (9). Thirty-three percent of the dogs used in this study carried ampicillin-resistant E. coli at low but detectable levels at the baseline. This finding is consistent with those from previous studies indicating that carriage of ampicillin-resistant E. coli may be detected in ≥50% of healthy persons or domestic animals (10, 12, 27). Standard culture techniques and PCR analysis of TEM β-lactamase genes demonstrated the rapid expansion of ampicillin-resistant E. coli in the ampicillin-treated dogs, but the baseline level of resistance was recovered as the percent similarity of the fecal flora, as determined by TGGE, returned to the baseline level. The minor increases in the proportion of ampicillin-resistant coliforms that were observed in the group receiving placebo i.v. and placebo p.o. and the group receiving ampicillin and TRBL were most likely due to cross-contamination from the heavily colonized group receiving ampicillin and placebo, as the dogs were not totally isolated from each other and were handled by the same personnel. This finding is consistent with the concept that overgrowth of pathogens in the intestinal tract may facilitate transmission among hospitalized patients (4).
This study had some limitations. It was not definitively shown that immunologic or other nonspecific effects associated with administration of the β-lactamase enzyme did not contribute to the treatment effect. In mice, however, we have demonstrated that inactivation of the enzyme with tazobactam, an effective inhibitor of β-lactamase, results in a loss of efficacy (unpublished data). The efficacy of the treatment may be reduced if gastric emptying or intestinal motility is altered, as may commonly occur in hospitalized patients. Even a transient elevation in the intestinal antibiotic concentration may affect the composition of the gut microbiota and the level of resistance, as was demonstrated for one of the dogs in the group receiving ampicillin and TRBL. However, the indigenous microbiota of this individual dog rapidly recovered, and the level of ampicillin resistance also rapidly returned to the baseline level.
In summary, oral administration of β-lactamase in a controlled-release formulation preserved the intestinal microbiota during ampicillin treatment and prevented an increase in the rates of ampicillin resistance. These results suggest that β-lactamase treatment could provide an important new strategy to reducing the emergence of resistance to β-lactam antibiotics in clinical settings. In addition, we have demonstrated in mice that oral β-lactamase administration in conjunction with parenteral piperacillin treatment preserves resistance to colonization with nosocomial pathogens such as VRE and extended-spectrum β-lactamase-producing Klebsiella pneumoniae (24). We are also developing a targeted β-lactamase that can be used to inactivate cephalosporins in the gastrointestinal tract.
Acknowledgments
This work was supported by Ipsat Therapies Oy/Ltd.
We thank laboratory technician Seppo Lasanen for conscientious work during the study and Anne Bryk for skillful technical assistance. Merja Rautio is gratefully acknowledged for generous advice.
REFERENCES
- 1.Barbosa, T. M., and S. B. Levy. 2000. The impact of antibiotic use on resistance development and persistence. Drug Resist. Updates 3:303-311. [DOI] [PubMed] [Google Scholar]
- 2.Batt, R. M. 1996. Enteric bacteria: friend or foe? Eur. J. Comp. Gastroenterol. 1:19-24. [DOI] [PubMed] [Google Scholar]
- 3.Cairns, M. J., and V. Murray. 1994. Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. BioTechniques 17:915-919. [PubMed] [Google Scholar]
- 4.Donskey, C. J., T. K. Chowdhry, M. T. Hecker, C. K. Hoyen, J. A. Hanrahan, A. M. Hujer, R. A. Hutton-Thomas, C. C. Whalen, R. A. Bonomo, and L. B. Rice. 2000. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N. Engl. J. Med. 343:1925-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harmoinen, J., J. Mättö, M. Rinkinen, M. Wilsson-Rahmberg, and E. Westermarck. 2001. Permanent jejunum fistula: promising method for obtaining small intestinal chyme without disturbing intestinal function. Comp. Med. 51:252-256. [PubMed] [Google Scholar]
- 6.Harmoinen, J., K. Vaali, P. Koski, K. Syrjänen, O. Laitinen, K. Lindevall, and E. Westermarck. 2003. Enzymic degradation of β-lactam antibiotic, ampicillin, in the gut: a novel treatment modality. J. Antimicrob. Chemother. 51:361-365. [DOI] [PubMed] [Google Scholar]
- 7.Heritage, J., B. Ransome, P. A. Chambers, and M. H. Wilcox. 2001. A comparison of culture and PCR to determine the prevalence of ampicillin-resistant bacteria in the fecal flora of general practice patients. J. Antimicrob. Chemother. 48:287-292. [DOI] [PubMed] [Google Scholar]
- 8.Hoiby, N. 2000. Ecological antibiotic policy. J. Antimicrob. Chemother. 46:59-62. [PubMed] [Google Scholar]
- 9.Houndt, T., and H. Ochman. 2000. Long-term shifts in patterns of antibiotic resistance in enteric bacteria. Appl. Environ. Microbiol. 66:5406-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macias, A. E., L. E. Herrera, J. M. Munoz, and H. Medina. 2002. Antimicrobial resistance of fecal Escherichia coli from healthy children. Induced by antibiotic use? Rev. Investig. Clin. 54:108-112. [PubMed] [Google Scholar]
- 11.Monroe, S., and R. Polk. 2000. Antimicrobial use and bacterial resistance. Curr. Opin. Microbiol. 3:496-501. [DOI] [PubMed] [Google Scholar]
- 12.Moss, S., and A. J. Frost. 1984. The resistance to chemotherapeutic agents of Escherichia coli from domestic dogs and cats. Aust. Vet. J. 61:82-84. [DOI] [PubMed] [Google Scholar]
- 13.Murray, P. R., E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.). 1999. Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 14.Muyzer, G., E. C. de Waal, and G. A. Uitterlinden. 1993. Profiling of complex populations by denaturating gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakaya, R., T. Chida, and H. Sibaoka. 1981. Antimicrobial agents and intestinal microflora. Bifidobact. Microflora 1:25-37. [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial disk susceptibility tests; approved standard, 7th ed. NCCLS document M2-A7. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.Newman, M. J., and A. Seidu. 2002. Carriage of antimicrobial resistant Escherichia coli in adult intestinal flora. West Afr. J. Med. 21:48-50. [PubMed] [Google Scholar]
- 18.Nord, C. E., and A. Heimdahl. 1986. Impact of orally administered antimicrobial agents on human oropharyngeal and colonic microflora. J. Antimicrob. Chemother. 18:159-164. [DOI] [PubMed] [Google Scholar]
- 19.Nord, C. E., and M. Hedberg. 1990. Resistance to β-lactam antibiotics in anaerobic bacteria. Rev. Infect. Dis. 12:S231-S234. [DOI] [PubMed] [Google Scholar]
- 20.Nord, C. E., L. Kager, and A. Heimdahl. 1984. Impact of antimicrobial agents on the gastrointestinal microflora and the risk of infections. Am. J. Med. 15:99-106. [DOI] [PubMed] [Google Scholar]
- 21.Normand, E. H., N. R. Gibson, S. W. J. Reid, S. Carmichael, and D. J. Taylor. 2000. Antimicrobial-resistance trends in bacterial isolates from companion-animal community practice in the UK. Prev. Vet. Med. 46:267-278. [DOI] [PubMed] [Google Scholar]
- 22.Sorum, H., and M. Sunde. 2001. Resistance to antibiotics in the normal flora of animals. Vet. Res. 32:227-241. [DOI] [PubMed] [Google Scholar]
- 23.Stark, C. A., C. Edlund, S. Sjöstedt, G. Kristensen, and C. E. Nord. 1993. Antimicrobial resistance in human oral and intestinal anaerobic microfloras. Antimicrob. Agents Chemother. 37:1665-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stiefel, U., N. J. Pultz, J. Harmoinen, P. Koski, K. Lindevall, M. S. Helfand, and C. J. Donskey. 2003. Oral administration of β-lactamase preserves colonization resistance of piperacillin-treated mice. J. Infect. Dis. 188:1605-1609. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan, Å., C. Edlund, and C. E. Nord. 2001. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect. Dis. 1:101-114. [DOI] [PubMed] [Google Scholar]
- 26.Summers, A. O. 2002. Generally overlooked fundamentals of bacterial genetic and ecology. Clin. Infect. Dis. 34:S85-S92. [DOI] [PubMed] [Google Scholar]
- 27.Tvede, M., N. Hoiby, L. A. Larsen, A. Raben, and A. V. Astrup. 2001. Antibiotic resistance of E. coli isolated from healthy persons. Ugeskr. Laeger. 3:4868-4871. [PubMed] [Google Scholar]
- 28.Vollaard, E. J., and H. A. L. Clasener. 1994. Colonization resistance. Antimicrob. Agents Chemother. 38:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vree, T. B., Y. A. Hekster, A. M. Baars, and E. van der Kleijn. 1978. Rapid determination of amoxycillin and ampicillin in body fluids of many by means of high-performance liquid chromatography. J. Chromatogr. 145:496-501. [DOI] [PubMed] [Google Scholar]
- 30.Wilsson-Rahmberg, M., and O. Jonsson. 1997. Method for long-term intestinal access in the dog. Lab. Anim. 31:231-240. [DOI] [PubMed] [Google Scholar]