Abstract
Two ketolides, three macrolides, and one azalide were tested in vitro against 17 isolates of the B. burgdorferi s.l. complex. As measured in micrograms per milliliter, activity was highest for cethromycin (MIC at which 90% of the tested isolates were inhibited [MIC90], 0.0019 μg/ml) and telithromycin (MIC90, 0.0078 μg/ml). Electron-microscope analysis and time-kill studies also supported enhanced effectiveness of both ketolides.
Borrelia burgdorferi is susceptible to macrolides in vitro (5, 8, 9, 18, 20), and important clinical indications for macrolides in the therapy of acute Lyme diseases (LD) include pregnancy, β-lactam allergy, and treatment of children <14 years of age (17, 23, 24, 26). Recently new clarithromycin derivatives, the ketolides, proved highly active in vitro against atypical microorganisms, such as Chlamydia pneumoniae, Mycoplasma pneumoniae, and Legionella species (1, 3, 6, 22). If effective in vitro and in vivo against borreliae, ketolides should be considered for treatment of LD as well. Here, we investigated under standardized conditions the in vitro activities of ketolides in comparison to those of macrolides and one azalide against 17 isolates of the B. burgdorferi complex, including all three genospecies pathogenic for humans, in addition to one Borrelia valaisiana and one Borrelia bissettii tick isolate.
The clinical, geographic, and genotypic characteristics of the strains tested (Table 1) have been published elsewhere (8, 10, 12). Except for reference strain B31 (ATCC 35210), low-passage isolates (10 to 20 passages) were tested using microtiter trays carrying lyophilized antimicrobial agents (Merlin-Diagnostika GmbH, Bornheim-Hersel, Germany). The test ranges appear in Table 1. Ceftriaxone and apramycin served as controls with known high activity and no activity, respectively, against borreliae (12, 13). MICs were determined after 72 h using a colorimetric assay, as recently described in detail (9, 13). Minimal borreliacidal concentrations (MBCs) were determined under stringent conditions (100% killing in liquid medium) at 72 h. Aliquots (20 μl) from all vials without detectable growth were diluted (1:1,000) below the MIC in Barbour-Stoenner-Kelly medium (BSK) and inspected for regrowth after 3 weeks of subculture (9, 10, 12). For each isolate and substance, independent experiments were performed on different days, with MICs and MBCs reported as the median of all three experiments. Moreover, time-kill studies with B. burgdorferi strain PKa-1 and Borrelia afzelii strain FEM1 exposed to telithromycin, cethromycin, and erythromycin for 120 h and electron-microscope analysis of B. garinii PSth cultures in the log phase of growth treated with 0.0312 μg of cethromycin/ml for 72 h were performed as described elsewhere (10, 13, 19). To detect possible differences in MIC and MBC data for the borrelial genospecies, the Kruskall-Wallis test was applied using BIAS, version 5.03 (Epsilon Verlag, Hochheim, Germany), for statistical calculation. Finally, possible antibiotic-medium interactions were investigated after 24 h of preincubation of the antibiotic-BSK preparation followed by conventional MIC determination for another fastidious organism, S. pneumoniae ATCC 49619 (Table 1). Testing was performed in triplicate, following NCCLS protocols (15) except for use of a preincubated antibiotic-BSK preparation.
TABLE 1.
Antibiotic susceptibility of 17 B. burgdorferi isolates and medium control organism to macrolides, one azalide, ketolides, and ceftriaxone as determined in BSKa
| Isolate or parameter | Value (μg/ml) for antimicrobial agentb
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Erythromycin
|
Roxithromycin
|
Clarithromycin
|
Azithromycin
|
Telithromycin
|
Cethromycin
|
Ceftriaxone
|
||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| B. burgdorferi | ||||||||||||||
| B31 (ATCC 35210) | 0.0156 | >0.5 | 0.0156 | 0.5 | 0.0039 | 0.5 | 0.0019 | 0.12 | ≤0.0002 | 0.06 | ≤0.0002 | 0.06 | 0.0625 | 1 |
| 297 | 0.0039 | >0.5 | 0.0019 | 0.25 | 0.0019 | 0.25 | ≤0.0002 | 0.5 | ≤0.0002 | 0.12 | ≤0.0002 | 0.03 | 0.0625 | 0.5 |
| LW2 | 0.0312 | >0.5 | 0.0156 | 0.5 | 0.0039 | >0.5 | 0.0009 | 0.5 | ≤0.0002 | 0.12 | ≤0.0002 | 0.03 | 0.0312 | 2 |
| Z25 | 0.0078 | >0.5 | 0.0039 | 0.5 | 0.0019 | 0.5 | 0.0009 | 0.5 | ≤0.0002 | 0.12 | ≤0.0002 | 0.12 | ≤0.0156 | 2 |
| PKa-I | 0.0156 | >0.5 | 0.0078 | >0.5 | 0.0156 | >0.5 | 0.0004 | 0.5 | ≤0.0002 | 0.06 | ≤0.0002 | 0.06 | ≤0.0156 | 2 |
| B. garinii | ||||||||||||||
| PTrob | 0.0078 | >0.5 | 0.0078 | >0.5 | 0.0039 | 0.25 | 0.0009 | 0.5 | 0.0004 | 0.12 | ≤0.0002 | 0.03 | 0.0312 | 2 |
| JP2 | 0.0156 | >0.5 | 0.0156 | >0.5 | 0.0039 | 0.5 | 0.0019 | 0.25 | ≤0.0002 | 0.06 | ≤0.0002 | 0.03 | ≤0.0156 | 0.5 |
| A87SB | 0.0078 | >0.5 | 0.0039 | 0.25 | 0.0019 | 0.12 | 0.0004 | 0.03 | ≤0.0002 | 0.25 | ≤0.0002 | 0.12 | ≤0.0156 | 1 |
| ZQ1 | 0.0156 | >0.5 | 0.0078 | >0.5 | 0.0039 | 0.5 | 0.0009 | 0.25 | 0.0009 | 0.12 | ≤0.0002 | 0.03 | ≤0.0156 | 2 |
| PSth | 0.0625 | >0.5 | 0.0625 | >0.5 | 0.0312 | >0.5 | 0.0156 | 0.5 | 0.0078 | 0.25 | 0.0019 | 0.12 | 0.0625 | 2 |
| B. afzelii | ||||||||||||||
| EB1 | 0.0625 | >0.5 | 0.0625 | >0.5 | 0.0312 | >0.5 | 0.0156 | 0.5 | 0.0078 | 0.25 | 0.0078 | 0.25 | 0.0625 | 2 |
| EB2 | 0.0156 | >0.5 | 0.0156 | >0.5 | 0.0078 | 0.5 | 0.0039 | 0.25 | 0.0009 | 0.12 | 0.0004 | 0.12 | ≤0.0156 | 1 |
| FEM1 | 0.0156 | >0.5 | 0.0078 | 0.12 | 0.0039 | 0.12 | 0.0019 | 0.12 | ≤0.0002 | 0.03 | ≤0.0002 | 0.03 | ≤0.0156 | 1 |
| VS461 | 0.0078 | >0.5 | 0.0039 | >0.5 | 0.0019 | 0.5 | 0.0004 | 0.25 | ≤0.0002 | 0.12 | ≤0.0002 | 0.12 | 0.0625 | 1 |
| FAC-1 | 0.0312 | >0.5 | 0.0156 | >0.5 | 0.0078 | 0.12 | 0.0019 | 0.06 | ≤0.0002 | 0.12 | ≤0.0002 | 0.12 | ≤0.0156 | 0.5 |
| B. valaisiana | ||||||||||||||
| VS116 | 0.0156 | >0.5 | 0.0078 | 0.5 | 0.0039 | >0.5 | 0.0009 | 0.06 | 0.0004 | 0.12 | ≤0.0002 | 0.06 | 0.0312 | 2 |
| B. bissettii | ||||||||||||||
| 25015 | 0.0312 | >0.5 | 0.0312 | 0.5 | 0.0078 | 0.5 | 0.0039 | 0.03 | 0.0009 | 0.5 | ≤0.0002 | 0.06 | 0.0312 | 2 |
| Range | 0.0039-0.0625 | 0.25->0.5 | 0.0009-0.0625 | 0.125->0.5 | 0.0009-0.0312 | 0.12->0.5 | ≤0.0002-0.0156 | 0.0156-0.5 | ≤0.0002-0.0078 | 0.0156-0.5 | ≤0.0002-0.0078 | 0.0156-0.25 | ≤0.0156-0.0625 | 0.25-2 |
| MIC90 or MBC90c | 0.0625 | >0.5 | 0.0625 | >0.5 | 0.0312 | >0.5 | 0.0156 | 0.5 | 0.0078 | 0.25 | 0.0019 | 0.125 | 0.0625 | 2 |
| Medium control, S. pneumoniae (ATCC 49619)d | ||||||||||||||
| Median MIC in BSK | 0.0625 | 0.0312 | 0.0156 | 0.0156 | 0.0019 | 0.0019 | 0.0625 | |||||||
| MIC Range in BSK | 0.0312-0.0625 | 0.0156-0.0625 | 0.0078-0.0156 | 0.0078-0.0156 | 0.0019-0.0039 | 0.0009-0.0019 | 0.0625 | |||||||
| NCCLS range | 0.0312-0.012 | NA | 0.0312-0.12 | 0.0625-0.25 | 0.0039-0.312e | 0.0039-0.0156f | 0.0312-0.12 | |||||||
Antimicrobial susceptibility was determined on three different days, and MIC and MBC values for each isolate were reported as the median of three experiments:
The test ranges (in μg/ml) were as follows: for erythromycin, roxithromycin, clarithromycin, azithromycin, telithromycin, and cethromycin, 0.0002 to 0.5; for ceftriaxone, 0.0156 to 32.
MBC90, MBC required to kill 90% of the isolates.
To investigate significant antibiotic-medium interaction, MICs for S. pneumoniae (ATCC 49619) were determined on three different days, referring to the NCCLS method (15) except for the use of BSK and pre-incubation of the antibiotic-medium test preparation for 24 h before testing. Results were reported as the median of all three experiments.
Tentative NCCLS range.
Tentative range provided by manufacturer.
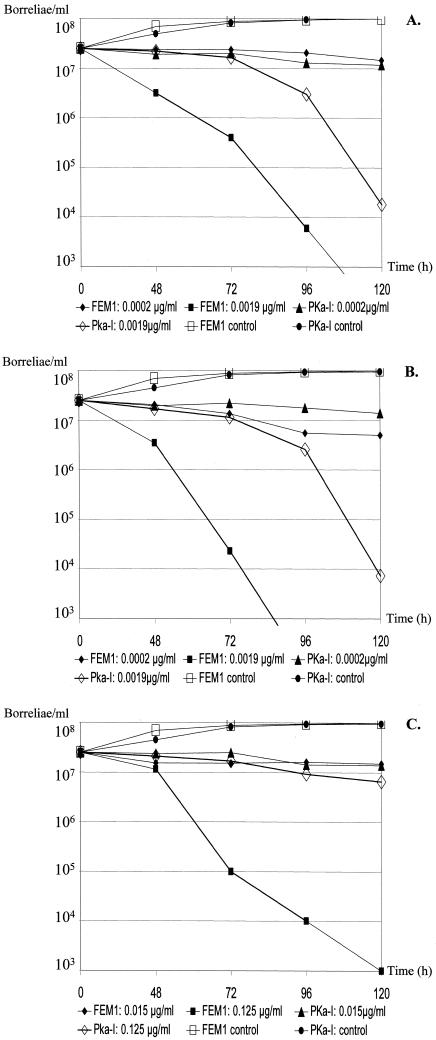
Apramycin was ineffective against the 17 borrelial strains. Table 1 summarizes the in vitro activities of the other antimicrobial agents. MICs and MBCs of each antimicrobial agent for the same isolate spanned a maximum range of ±1 log2 unit dilution around the median only. The ketolides were the most potent against borrelial isolates on a micrograms-per-milliliter basis. For all agents except cethromycin and telithromycin, the MIC at which 90% of isolates were inhibited (MIC90) and the MBC at which 90% of the isolates were killed were ≥0.01 μg/ml and >0.25 μg/ml, respectively. Statistical analysis, including all measured MICs and MBCs (n = 816), did not show significant differences in the tested genospecies. In our time-kill experiments (Fig. 1A to C), exposure to the ketolides for PKa-1 and FEM1 at three log2 unit dilutions above the MIC led to a >3 log10 unit (99.9%) reduction of morphologically intact motile cells after 48 to 120 h. Reduction was more pronounced for FEM1 than for PKa-1. Erythromycin clearly was less effective than the ketolides. Electron-microscopic analysis of strain PSth exposed to cethromycin at 0.0312 μg/ml (4 log2 unit dilutions above the MIC90) further substantiated in vitro activity by showing spheroblast formation and severe cell disintegration (data not shown).
FIG. 1.
(A to C) Time-kill curves for B. burgdorferi s.l. isolates PKa-1 and FEM1 with telithromycin (A) and cethromycin (B) at the MIC (0.0002 μg/ml) and eight times the MIC (0.0019 μg/ml), as well as with erythromycin (C) at the MIC (0.0156 μg/ml) and eight times the MIC (0.125 μg/ml). Experiments were performed on different days by investigation of growth using conventional cell counts, and data were reported as the means from two experiments.
Test results for S. pneumoniae ATCC 49619 in BSK appear in Table 1. The conventional MICs for apramycin (data not shown), erythromycin, and ceftriaxone were in the range published for these agents by the NCCLS (15, 16). The MICs for azithromycin, clarithromycin, telithromycin, and cethromycin, however, were at the lower range limits or were one to two log2 unit dilutions below the ranges specified by the NCCLS (15) and the tentative ranges indicated by the manufacturer for cethromycin.
In our study, the rank order of activity by classical macrolides and azalides against borreliae clearly corresponds to the effectiveness of these agents as revealed by in vitro susceptibility studies and clinical treatment trials to date (2, 4, 5, 7, 8, 9, 11, 23, 24), demonstrating higher in vitro effectiveness for azithromycin (MIC90, 0.0156 μg/ml) than for erythromycin (MIC90, 0.0625 μg/ml), roxitromycin (MIC90, 0.0625 μg/ml), and clarithromycin (MIC90, 0.0312 μg/ml). Median MICs of the different substances, however, tended to vary over a 10-fold range between individual strains, with the B. garinii isolate PSth and the B. afzelii isolate EB1 showing the highest MICs for both the classical macrolides and the ketolides. In contrast to the recent findings of Sicklinger et al. (20), we could not show significant differences in MBCs for the different genospecies tested against macrolides or ketolides, possibly owing to differences in test methodology and inoculum. Instead, our in vitro findings point to interstrain variability of the in vitro susceptibilities of B. burgdorferi to macrolides rather than to intergenospecies-specific variations as observed for other antimicrobial agents (8, 9, 11, 13). Testing of S. pneumoniae ATCC 49619 clearly demonstrated increased activities of some macrolides in BSK. This side effect of BSK also was noted by other authors (2, 21). However, further investigations are necessary to assess possible consequences for in vitro susceptibility testing of these agents against B. burgdorferi.
Classical macrolides and azalides frequently fail in the therapy of early LD (7, 14, 17, 26), and clinical relapse has been observed following conclusion of treatment (14, 17, 26). Moreover, it has been speculated that resistance may develop in borreliae preexposed to erythromycin owing to resistant subpopulations (25). Based upon our findings, however, the ketolides were superior in vitro on a micrograms-per-milliliter basis when tested alongside classical macrolides under identical test conditions in BSK. This is further substantiated by our time-kill experiments (Fig. 1A to C) and by electron microscopy. Moreover, maximum concentrations of ketolides in plasma after regular oral dosage (1, 6, 27) are 90 to 270 times higher than the MIC90s against borreliae in our study, and tissue concentrations exceed by 10-fold the maximum plasma concentrations of both drugs in controls (1, 6, 27). Therefore, the potential role of ketolides in the treatment of LD merits further evaluation.
REFERENCES
- 1.Balfour, J. A., and D. P. Figgitt. 2001. Telithromycin. Drugs 61:815-829. [DOI] [PubMed] [Google Scholar]
- 2.Boerner, J., K. Failing, and M. M. Wittenbrink. 1995. In vitro antimicrobial susceptibility testing of Borrelia burgdorferi: influence of test conditions on minimal inhibitory concentration (MIC) values. Zentbl. Bakteriol. 283:49-60. [DOI] [PubMed] [Google Scholar]
- 3.Chu, D. T. W. 1995. Recent developments in 14- and 15-membered macrolides. Expert Opin. Investig. Drugs 4:65-96. [Google Scholar]
- 4.Dever, L. L., J. H. Jorgensen, and A. G. Barbour. 1992. In vitro antimicrobial susceptibility testing of Borrelia burgdorferi: a microdilution MIC method and time-kill studies. J. Clin. Microbiol. 30:2692-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dever, L. L., J. H. Jorgensen, and A. G. Barbour. 1993. Comparative in vitro activities of clarithromycin, azithromycin, and erythromycin against Borrelia burgdorferi. Antimicrob. Agents Chemother. 37:1704-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dougherty, T. J., and J. F. Barrett. 2001. ABT 773: a new ketolide antibiotic. Expert Opin. Investig. Drugs 10:343-351. [DOI] [PubMed] [Google Scholar]
- 7.Hansen, K., A. Hovemark, A. M. Lebech, K. Lebech, I. Olson, L. Halkier-Sorensen, E. Olsson, and E. Asbrink. 1992. Roxithromycin in Lyme borreliosis: discrepant results of an in vitro and in vivo animal susceptibility study and a clinical trial in patients with erythema migrans. Acta Dermatol. Venerol. 72:297-300. [PubMed] [Google Scholar]
- 8.Hunfeld, K.-P., P. Kraiczy, T. A. Wichelhaus, V. Schäfer, and V. Brade. 2000. New colorimetric microdilution method for in vitro susceptibility testing of Borrelia burgdorferi against antimicrobial substances. Eur. J. Clin. Microbiol. Infect. Dis. 19:27-32. [DOI] [PubMed] [Google Scholar]
- 9.Hunfeld, K.-P., P. Kraiczy, T. A. Wichelhaus, V. Schäfer, and V. Brade. 2000. Colorimetric in vitro susceptibility testing of penicillins, cephalosporins, macrolides, streptogramins, tetracyclines and aminoglycosides against Borrelia burgdorferi isolates. Int. J. Antimicrob. Agents 15:11-17. [DOI] [PubMed] [Google Scholar]
- 10.Hunfeld, K.-P., T. A. Wichelhaus, E. Kekoukh, P. Kraiczy, and V. Brade. 2001. In vitro susceptibility of the Borrelia burgdorferi sensu lato complex to ABT-773, a novel ketolide. J. Antimicrob. Chemother. 48:447-449. [DOI] [PubMed] [Google Scholar]
- 11.Hunfeld, K.-P., P. Kraiczy, E. Kekoukh, V. Schäfer, and V. Brade. 2002. Standardised in vitro susceptibility testing of Borrelia burgdorferi against well-known and newly developed antimicrobial agents—possible implications for new therapeutic approaches to Lyme disease. Int. J. Med. Microbiol. 291(suppl. 33):125-137. [DOI] [PubMed] [Google Scholar]
- 12.Hunfeld, K.-P., R. Rodel, and T. A. Wichelhaus. 2003. In vitro activity of eight oral cephalosporins against Borrelia burgdorferi. Int. J. Antimicrob. Agents 21:125-137. [DOI] [PubMed] [Google Scholar]
- 13.Kraiczy, P., J. Weigand, T. A. Wichelhaus, P. Heisig, H. Backes, V. Schäfer, G. Acker, V. Brade, and K.-P. Hunfeld. 2001. In vitro activity of fluoroquinolones against the spirochete Borrelia burgdorferi. Antimicrob. Agents Chemother. 45:2486-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luft, B. J., R. J. Dattwyler, R. C. Johnson, S. W. Luger, E. M. Bosler, D. W. Rahn, E. J. Masters, E. Grunwaldt, and S. D. Gadgil. 1996. Azithromycin compared with amoxicillin in the treatment of erythema migrans. A double-blind, randomized, controlled trial. Ann. Intern. Med. 124:785-791. [DOI] [PubMed] [Google Scholar]
- 15.NCCLS. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard-6th ed., M7-A6. NCCLS, Wayne, Pa.
- 16.Odland, B. A., M. E. Erwin, and R. N. Jones. 2000. Quality control guidelines for disk diffusion and broth microdilution antimicrobial susceptibility tests with seven drugs for veterinary applications. J. Clin. Microbiol. 38:453-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oschmann, P., and R. Kaiser. 1999. Therapy and prognosis, p. 112-119. In P. Oschmann, P. Kraiczy, J. Halperin, and V. Brade (ed.), Lyme borreliosis and tick-borne encephalitis. UNI-Med Verlag AG, International Medical Publishers, Bremen, Germany.
- 18.Preac-Mursic, V., B. Wilske, G. Schierz, E. Süβ, and B. Groβ. 1989. Comparative in vitro activity of the new macrolides against Borrelia burgdorferi. Eur. J. Clin. Microbiol. Infect. Dis. 8:651-653. [DOI] [PubMed] [Google Scholar]
- 19.Preac-Mursic, V., W. Marget, U. Busch, D. Pleterski-Riegler, and S. Hagl. 1996. Kill kinetics of Borrelia burgdorferi and bacterial findings in relation to the treatment of Lyme borreliosis. Infection 24:9-16. [DOI] [PubMed] [Google Scholar]
- 20.Sicklinger, M., R. Wienecke, and U. Neubert. 2003. In vitro susceptibility testing of four antibiotics against Borrelia burgdorferi: a comparison of results for the three genospecies Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto. J. Clin. Microbiol. 41:1791-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sohaskey, C. D., and A. G. Barbour. 1999. Esterases in serum-containing growth media counteract chloramphenicol acetyltransferase activity in vitro. Antimicrob. Agents Chemother. 43:655-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strigl, S., P. M. Roblin, T. Reznik, and M. R. Hammerschlag. 2000. In vitro activity of ABT 773, a new ketolide antibiotic, against Chlamydia pneumoniae. Antimicrob. Agents Chemother. 44:1112-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strle, F., V. Maraspin, S. Lotric-Furlan, E. Ruzic-Sabljic, T. Jurca, and J. Cimperman. 1996. Azithromycin and doxycycline for treatment of Borrelia culture-positive erythema migrans. Infection 24:64-68. [DOI] [PubMed] [Google Scholar]
- 24.Strle, F., V. Preac-Mursic, J. Cimperman, E. Ruzic, V. Maraspin, M. Jereb. 1993. Azithromycin versus doxycycline for treatment of erythema migrans: clinical and microbiological findings. Infection 21:83-88. [DOI] [PubMed] [Google Scholar]
- 25.Terekhova, D., M. L. Sartakova, G. P. Wormser, I. Schwartz, and F. C. Cabello. 2002. Erythromycin resistance in Borrelia burgdorferi. Antimicrob. Agents Chemother. 46:3637-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wormser, G. P., R. B. Nadelman, R. J. Dattwyler, D. T. Dennis, E. D. Shapiro, A. C. Steere, T. J. Rush, D. W. Rahn, P. K. Coyle, D. H. Persing, D. Fish, and B. J. Luft. 2000. Practice guidelines for the treatment of Lyme disease. The Infectious Diseases Society of America. Clin. Infect. Dis. 31(Suppl. 1):1-14. [DOI] [PubMed] [Google Scholar]
- 27.Yassin, H. M., and L. L. Dever. 2001. Telithromycin: a new ketolide antimicrobial for treatment of respiratory tract infections. Expert Opin. Investig. Drugs 10:353-367. [DOI] [PubMed] [Google Scholar]