Abstract
Mutators may present an enhanced risk for the emergence of antibiotic resistance in bacteria during chemotherapy. Using Escherichia coli mutators as a model, we evaluated their ability to develop resistance to antibiotics routinely used for the treatment of urinary tract infections (UTIs). Under conditions that simulate therapeutic drug concentrations in humans, low-level resistance to trimethoprim, gentamicin, and cefotaxime emerged more frequently in mutators than normal strains. Resistance to trimethoprim in both cell types arose from a single point mutation in folA (Ile94→Leu) and cefotaxime resistance resulted from loss of outer membrane porin OmpF. The mechanisms of gentamicin resistance could not be defined, but resistance did not result from mutations in ribosomal protein L6 (rplF). Although similar mechanisms of low-level antibiotic resistance probably arise in these strains, mutators are a risk factor because the increased generation of mutants with low-level resistance enhances the opportunity for subsequent emergence of high-level resistance.
Bacteria exhibiting elevated mutation frequencies (mutators) have been found among natural isolates of pathogenic organisms, including Escherichia coli, Salmonella enterica, Pseudomonas aeruginosa, Helicobacter pylori, and Neisseria meningitidis (9). Mutators are frequently deficient in the methyl-directed mismatch repair (MMR) system, a postreplicative DNA repair pathway that identifies and corrects mismatched DNA duplexes (9).
In the presence of antibiotics, mutators are predicted to benefit the populations in which they occur, since resistant variants can be rapidly selected (9, 17). Indeed, in some cases the frequencies of mutations for high-level antibiotic resistance are 1,000-fold higher in mutators than normal strains, indicating the increased adaptability of these strains (9, 21). Generation of multiple chromosomal mutations, which occurs more readily in mutators, may also assist development of high-level resistance when stepwise acquisition of several mutations is required (23). Mutators also have increased potential to generate mutants expressing low-level antibiotic resistance. These mutants may persist long enough to acquire subsequent resistance mechanisms via horizontal gene transfer (4). In addition, MMR-deficient strains present reduced barriers to interspecies genetic exchange (9) and therefore may be important in the acquisition of mobile resistance determinants. In view of their superior mutability and enhanced gene acquisition properties, mutators could therefore have an important role in the emergence and spread of antibiotic resistance within bacterial populations.
The likelihood that mutators are indeed a risk factor in the treatment of infectious diseases is suggested by the correlation between high mutation frequency and antibiotic resistance in P. aeruginosa recovered from the lungs of cystic fibrosis patients (26). Nevertheless, the response of mutators to antibiotics at the concentrations therapeutically achievable in humans has not been determined. In this paper we report on the effects of antibiotics used for the treatment of human urinary tract (UT) infections (UTIs) on E. coli mutators, focusing on mutational responses to pharmacokinetic peak antibiotic concentrations (Cmaxs) and trough antibiotic concentrations (Cmins). Our studies provide a relevant model of the in vivo behavior of mutators because E. coli is the primary UT pathogen in humans (34) and E. coli mutators occur at a high frequency among human uropathogenic isolates (10). We conclude that mutators can present an enhanced risk for emergence of antibiotic resistance during therapy of UTIs caused by E. coli.
(Portions of this work were presented at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 27 to 30 September 2002 [K. Miller, A. J. O'Neill, and I. Chopra, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1-965, 2002].)
MATERIALS AND METHODS
Bacterial strains.
The E. coli strains used in this study are described in Table 1.
TABLE 1.
E. coli strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| 1411 | lac13 lacZ118 proB trp nalA rpsL | 21 |
| 1412 | lac13 lacZ118 proB trp nalA rpsL uvrD210 | 21 |
| 1413 | lac13 lacZ118 proB trp nalA rpsL mutS3 | 21 |
| 1417 | lac13 lacZ118 proB trp nalA rpsL mutH34 | 21 |
| CSH109 | pro araΔ(gpt-lac)5 rpsL | 20 |
| CSH115 | pro araΔ (gpt-lac)5 rpsL mutS::mini-Tn10 | 20 |
| SM1411 | lac13 lacZ118 proB trp nalA rpsL ΔacrAB | 27 |
| AG100 | uncb+argE3 thi-1 rpsL xyl mtl galK supE44 λ | 24 |
| AG112 | uncb+argE3 thi-1 rpsL xyl mtl galK supE44 λ marOR | 24 |
| JF 568 | aroA357 ilv-277 metB65 his-53 purE41 proC24 cyc-1 xyl-14 lacY29 rpsL177 tsx-63 | 8 |
| JF 703 | aroA357 ilv-277 metB65 his-53 purE41 proC24 cyc-1 xyl-14 lacY29 rpsL177 tsx-63 ompF | 8 |
Antibiotics, chemicals, and growth media.
Chemicals were from Sigma-Aldrich (Poole, United Kingdom) unless stated otherwise in the text. All antibiotics except ciprofloxacin (CIP) and trimethoprim (TMP) were from Sigma-Aldrich; TMP (monotrim) was purchased from Solvay Healthcare (Southampton, United Kingdom), and CIP was a gift from Bayer AG (Leverkusen, Germany). Mueller-Hinton broth (MHB) and agar (MHA) were from Fisher (Loughborough, United Kingdom), and Iso-Sensitest broth (ISB) and agar (ISA) were from Oxoid (Basingstoke, United Kingdom).
Determination of susceptibilities to antibiotics and chemicals.
The MICs of cefotaxime (CTX), CIP, gentamicin (GEN), nitrofurantoin (NIT), tetracycline (TET), cetyltrimethylammonium bromide (CTAB), and ethidium bromide (EB) were determined by serially diluting the antibiotics twofold in MHA and inoculating the dilutions on MHB at 104 CFU/spot. For TMP, MHA and MHB were replaced by ISA and ISB, respectively. The plates were incubated aerobically or anaerobically (GasPak 150 anaerobic jar system; Becton Dickinson, Oxford, United Kingdom) for 18 h at 37°C, and the MIC was defined as the lowest concentration of inhibitor that allowed no visible growth.
Determination of MPCs.
The mutant prevention concentration (MPC) is the lowest drug concentration that prevents bacterial colony formation from a culture containing ≥1010 CFU (38). Accordingly, to determine MPCs, late-logarithmic-phase cultures of E. coli growing in either MHB or ISB were harvested by centrifugation (6,000 × g, 10 min) and resuspended in fresh growth medium at a final concentration of 1011 CFU/ml. Aliquots (100 μl) were incorporated into MHA plates or ISA plates (for TMP) containing serial twofold drug dilutions in the range of 0.5 to 64 times the MIC of each drug. MPCs were determined following aerobic or anaerobic incubation at 37°C for 72 h.
Determination of frequencies of mutation to antibiotic resistance.
The frequencies of mutation to antibiotic resistance were determined as described previously (21) with Mueller-Hinton or Iso-Sensitest growth media. Following aerobic or anaerobic incubation, the mutation frequencies were expressed as the number of resistant mutants recovered as a fraction of the total number of viable bacteria (21).
PCR amplification and sequencing of the mar (multiple antibiotic resistance) regulon, folA, and rplF.
Preparation of chromosomal DNA and PCR amplifications were performed as described previously (32). Oligonucleotide primers designed with the Oligo (version 6.0) program (Molecular Biology Insights, Cascade, Colo.) were used to amplify the resistance-determining (25) regions of the marR and marO genes of the mar regulon (5′-AGCCTTGCATCGCATTGAA and 5′-GCGTCAGTATTGCGTCTGG); the folA gene, including the promoter (5′-GAACCGGAAACGAAACC and 5′-CTTCATCCACCGACTTCAC); and rplF (5′-TGTACCACCAGGCGAGTT and 5′-GGGTCTGGGTATCGCAGTTGT). PCR products were purified by using Microcon PCR Centrifugal Filter Devices (Millipore, Watford, United Kingdom) according to the instructions of the manufacturer. DNA sequencing was performed by Lark Technologies (Saffron Walden, United Kingdom).
Fluorometric determination of intracellular EB.
The level of accumulation of EB was measured as described previously (6), with the exception that the cells were washed twice in uptake buffer and lysed with BugBuster reagent (Novagen, Madison, Wis.).
Determination of protein concentration.
Protein concentrations were determined by the method of Bradford (5).
Outer membrane preparation and analysis of proteins.
Outer membranes were prepared essentially as described previously (30). Outer membrane proteins (OMPs) were analyzed on sodium dodecyl sulfate-polyacrylamide gels (8%) containing 6 M urea to achieve optimum resolution of the porin proteins (30). Gels were stained with Coomassie brilliant blue. The identities of the OMPs were determined by N-terminal sequencing and comparison of the sequence data with those available in the SWISS-PROT database.
Crude DHFR preparation.
The method used for the preparation of crude dihydrofolate reductase (DHFR) was adapted from the method of Adrian and Klugman (1). Sonication was replaced by chemical lysis with the BugBuster protein reagent, followed by exchange of the BugBuster reagent for reaction buffer (50 mM sodium phosphate buffer [pH 7.4]) containing 10 mM β-mercaptoethanol and 1 mM EDTA and concentration fivefold with a Centricon Plus-20 centrifugal filter device (Millipore).
TMP IC50 determination.
The 50% inhibitory concentrations (IC50s) of TMP were determined by using an adaptation of the DHFR assay described by Thillet et al. (36). Reactions were carried out with 200-μl volumes of a reaction mixture comprising 50 μM dihydrofolate, 100 μM NADPH, 100 mM potassium phosphate (pH 7), 100 mM potassium chloride, and 20 μg of the whole soluble protein fraction containing DHFR, which was added last to start the reaction. The reaction mixture was added to various concentrations of TMP in a 96-well microtiter tray. The activity of DHFR (and therefore the susceptibility of DHFR to TMP) was measured by determination of the drop in absorbance at 340 nm after 3 min with a SpectraMax Plus 384 microplate reader (Molecular Devices, Sunnyvale, Calif.). IC50s were established by determination of the concentration of TMP required to bring about a 50% reduction of DHFR activity relative to that for the untreated control.
RESULTS AND DISCUSSION
Mutant selection windows and selection indices for mutators.
Selection of single-step chromosomal antibiotic-resistant mutants occurs in a mutant selection window in which the drug concentration lies between the MIC and MPC (38). The breadth of the mutant selection window, or the mutant selection index, is calculated by determination of the MPC/MIC ratio (38). Agents with high selection indices are likely to be more vulnerable to select for resistance when they are used as monotherapy if drug concentrations cannot be maintained above the MPC in vivo (38).
MPCs have not been determined for mutators. We therefore used E. coli mutators as a model system and studied their responses to the antibiotics commonly used for the treatment of UTIs. The UT is invaded predominantly by aerobic bacteria in the course of an infection (34). However, anaerobes can also be recovered from patients with UTIs (3, 33), indicating that anaerobic sites exist within the UT. It was therefore relevant to determine MPCs for E. coli under aerobic and anaerobic conditions. To our knowledge MPCs have not previously been reported for anaerobes or facultative organisms under anaerobic conditions.
Mutant selection windows and selection indices were therefore experimentally defined for E. coli mutators by determination under aerobic and anaerobic conditions of the MICs and MPCs (Table 2) of a set of antimicrobial drugs used as single agents for the treatment of UTIs (16, 22). The majority of studies were performed with strains 1411, 1412, 1413, and 1417 (Table 1). However, since these strains contain a gyrA (Ser83→Leu) mutation (21), they were not used for further work with CIP. Consequently, MIC and MPC data for CIP were generated with CIP-sensitive mutator strain CSH115 (mutS) and its parent, CSH109 (Table 1).
TABLE 2.
MICs and MPCs for parent and mutator strains under aerobic and anaerobic conditions
| Condition and strain | Mutator allele | TMP
|
CTX
|
TET
|
GEN
|
CIP
|
NIT
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml) | MPC (μg/ml) | MPC/MIC ratio | MIC (μg/ml) | MPC (μg/ml) | MPC/MIC ratio | MIC (μg/ml) | MPC (μg/ml) | MPC/MIC ratio | MIC (μg/ml) | MPC (μg/ml) | MPC/MIC ratio | MIC (μg/ml) | MPC (μg/ml) | MPC/MIC ratio | MIC (μg/ml) | MPC (μg/ml) | MPC/MIC ratio | ||
| Aerobic | |||||||||||||||||||
| 1411 | —a | 0.125 | 4 | 32 | 0.032 | 0.5 | 16 | 1 | 3 | 3 | 0.25 | 2 | 8 | NDb | ND | ND | 5 | 64 | 16 |
| 1412 | uvrD | 0.25 | 4 | 16 | 0.063 | 1 | 16 | 1 | 3 | 3 | 0.25 | 4 | 16 | ND | ND | ND | 5 | 64 | 16 |
| 1413 | mutS | 0.25 | 4 | 16 | 0.063 | 1 | 16 | 1 | 3 | 3 | 0.5 | 4 | 8 | ND | ND | ND | 5 | 64 | 16 |
| 1417 | mutH | 0.25 | 8 | 32 | 0.063 | 0.5 | 8 | 1 | 3 | 3 | 0.25 | 2 | 8 | ND | ND | ND | 5 | 64 | 16 |
| CSH109 | — | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.016 | 0.125 | 8 | ND | ND | ND |
| CSH115 | mutS | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.016 | 0.2 | 12.5 | ND | ND | ND |
| Anaerobic | |||||||||||||||||||
| 1411 | — | 0.064 | 8 | 128 | 0.032 | 0.1 | 3.125 | 1 | 3 | 3 | 1 | 125 | 125 | ND | ND | ND | 5 | 64 | 16 |
| 1412 | uvrD | 0.125 | 8 | 64 | 0.032 | 0.1 | 3.125 | 1 | 3 | 3 | 2 | 125 | 62.5 | ND | ND | ND | 5 | 64 | 16 |
| 1413 | mutS | 0.125 | 8 | 64 | 0.016 | 0.1 | 6.25 | 1 | 3 | 3 | 2 | 125 | 62.5 | ND | ND | ND | 5 | 64 | 16 |
| 1417 | mutH | 0.125 | 8 | 64 | 0.032 | 0.1 | 3.125 | 1 | 3 | 3 | 2 | 125 | 62.5 | ND | ND | ND | 5 | 64 | 16 |
| CSH109 | — | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.016 | 0.125 | 8 | ND | ND | ND |
| CSH115 | mutS | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.016 | 0.2 | 12.5 | ND | ND | ND |
—, no mutator allele.
ND, not determined.
The MICs and MPCs of TET and NIT were not influenced by mutator status or by aerobic or anaerobic growth (Table 2). For example, TET MICs and MPCs were 1 and 3 μg/ml, respectively, for all strains grown aerobically or anaerobically; and those of NIT were 5 and 64 μg/ml, respectively. The MICs and MPCs of TMP, CTX, and CIP did not differ more than twofold when the MICs and MPCs for the mutators were compared to those for their parent strains under either aerobic or anaerobic growth conditions (Table 2). However, compared to the results obtained under aerobic conditions, MICs, MPCs, and selection indices (MPC/MIC ratio) obtained under anaerobic conditions all increased for GEN for both mutators and their parental hosts (Table 2). For example, selection indices increased from values of 8 to 16 under aerobic conditions to values of 62.5 to 125 under anaerobic conditions. The increased aminoglycoside MICs for the E. coli strains grown anaerobically probably reflect poor drug uptake under anaerobic conditions (29). The basis for the increased MPCs of GEN under anaerobic conditions is unclear, but it is unlikely to involve the MMR pathway, since similar MPC increases were observed for mutators and their parents. Nevertheless, GEN became more vulnerable to selection for resistance under anaerobic conditions. In contrast to GEN, selection indices for CTX were lower under anaerobic conditions (3.125 to 6.25) than under aerobic conditions (8 to 16), reflecting a decrease in the MPCs of CTX from 0.5 to 1 μg/ml under aerobic conditions to 0.1 μg/ml under anaerobic conditions (Table 2). The mechanism for the decreased MPCs of CTX under anaerobic conditions is unclear, but as for the increased GEN MPCs, it is unlikely to involve the MMR pathway, since similar decreases in the CTX MPCs were observed under anaerobic conditions for mutators and their parent strains.
MPCs, pharmacokinetic parameters of anti-UTI drugs, and prediction of resistance emergence.
Measurement of the MPC is useful for predicting the in vivo emergence of drug resistance when the MPC is considered along with the pharmacokinetic data for antimicrobial agents (38). During chemotherapy it may be impossible to maintain drug concentrations above the MPC, particularly as Cmin is approached. Consequently, when MPCs exceed achievable drug concentrations at any point in the dosing schedule, there is the potential for selection of drug-resistant mutants in vivo.
Pharmacokinetic data for anti-UTI drugs (Table 3) were compared with the MPCs (Table 2) to predict the likelihood of mutant selection in vivo for both mutator and parent E. coli strains during treatment of UTIs. The pharmacokinetic data that we considered were Cmaxs and Cmins in the urine of both healthy patients and renal failure patients and Cmaxs and Cmins in the serum of healthy patients after application of standard therapeutic regimens for the treatment of UTIs. Renal failure can be due to a number of causes. However, the pharmacokinetic data accessible from the literature (Table 3) do not address drug levels in relation to specific renal symptoms. Concentrations in serum are surrogates of concentrations in renal tissue for patients without renal failure (12).
TABLE 3.
Pharmacokinetic parameters for anti-UTI drugs in humans
| Drug | Serum (renal tissue)
|
Urine
|
Urine (renal failure patients)
|
|||
|---|---|---|---|---|---|---|
| Cmin (μg/ml) | Cmax (μg/ml) | Cmin (μg/ml) | Cmax (μg/ml) | Cmin (μg/ml) | Cmax (μg/ml) | |
| TMP | 1 (15)a | 2 (15) | 50 (13) | 100 (13) | 8 (13) | 16 (13) |
| CTX | 1 (15) | 20.5 (15) | 9 (28) | 180 (28) | 0.1 (28) | 50 (28) |
| TET | 0.5 (15) | 3 (15) | 180 (11) | 300 (11) | NRb (31) | NR (31) |
| GEN | 1 (15) | 12.5 (15) | 16 (29) | 125 (29) | 50 (29) | 1,000 (29) |
| CIP | 0.2 (15) | 2.4 (15) | 7 (37) | 82 (37) | 0.6 (37) | 6.8 (37) |
| NIT | 0.02 (15) | 1.3 (15) | 5 (15) | 100 (15) | NR (15) | NR (15) |
Reference numbers are given in parentheses.
NR, not relevant (not indicated for use in renal failure patients).
Selection for TMP and GEN resistance in mutators and normal strains may occur in the renal tissue of healthy patients at both aerobic and anaerobic sites since MPCs (TMP MPCs, 4 to 8 μg/ml; GEN MPCs, 2 to 125 μg/ml) (Table 2) usually exceeded the Cmaxs in serum (Cmax in serum for TMP, 2 μg/ml; Cmax in serum for GEN, 12.5 μg/ml) and always exceeded the Cmins in serum (Cmin in serum for TMP, 1 μg/ml; Cmin in serum for GEN, 1 μg/ml) (Table 3). For GEN this extended to anaerobic sites in the UTs of healthy individuals since the MPC (125 μg/ml) exceeded the Cmin in urine (16 μg/ml). For CTX there is the potential for the selection of resistance in both mutator and normal strains at aerobic sites in the UTs of individuals with renal failure since the MPCs (0.5 to 1.0 μg/ml) exceeded the Cmin of CTX in urine (for renal failure patients) (0.1 μg/ml). Under aerobic and anaerobic conditions, TET MPCs (3 μg/ml) (Table 2) exceeded the Cmins in serum (renal tissue) (0.5 μg/ml). However, in this case the MPC cannot be used as a predictor of resistance at the Cmin because the TET MIC (1 μg/ml) was already greater than Cmin (0.5 μg/ml); i.e., there was no selection pressure for the generation of TET-resistant mutants at the Cmin in serum. The Cmax of NIT in urine (100 μg/ml) was greater than the MPCs for strains 1411, 1412, 1413, and 1417 (64 μg/ml) and as such would prevent mutant selection. The Cmin of NIT in urine (5 μg/ml), the Cmin of NIT in serum (0.02 μg/ml), and Cmax (1.3 μg/ml) were all below the MPCs for strains grown aerobically or anaerobically (64 μg/ml) (Tables 2 and 3). However, in these cases the NIT concentrations (which are less than or equal to the MICs) were insufficient to inhibit growth, and therefore, there was no selection pressure for the emergence of resistance to NIT.
Mutation frequencies for resistance at drug concentrations below the MPC.
The data presented above predict the emergence of resistance to GEN, TMP, and CTX in E. coli during therapy for UTIs. However, since the MPCs of these drugs were no greater for mutators than for the parent strain, the mutants were expected to express similar levels of antibiotic resistance, probably reflecting similar genotypes. Indeed, the MICs of individual drugs for the mutants, whether they were derived from mutator or normal hosts, were similar and no greater than twice the concentrations initially used for mutant selection (data not shown).
Although the types of mutations arising in mutators and normal strains may be similar, the frequency at which resistant mutants arise in mutators may be considerably elevated for certain antibiotics (21). We therefore examined the frequencies of mutations for resistance to GEN, TMP, and CTX under appropriate aerobic or anaerobic conditions at those pharmacokinetic Cmaxs or Cmins of the drugs in the UT that fell below the MPCs of these agents (Table 4). Apart from selection for resistance with the Cmin of GEN in urine (16 μg/ml) under anaerobic conditions (Table 4), mutators gave rise to resistant mutants at frequencies 10- to 100-fold greater than those for their parents (Table 4).
TABLE 4.
Mutation frequencies under aerobic and anaerobic conditions at concentrations of TMP, GEN, and CTX expected in vivo
| Strain | Mutation frequencya
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Aerobic
|
Anaerobic
|
|||||||
| TMP
|
GEN Cmin in serum (renal tissue) of 1 μg/ml | CTX Cmin in urine (renal failure patients) of 0.1 μg/ml | TMP
|
GEN
|
||||
| Cmax in serum (renal tissue) of 2 μg/ml | Cmin in serum (renal tissue) of 1 μg/ml | Cmax in serum (renal tissue) of 2 μg/ml | Cmin in serum (renal tissue) of 1 μg/ml | Cmax in serum (renal tissue) of 12.5 μg/ml | Cmin in urinary (renally competent patients) of 16 μg/ml | |||
| 1411 | (2.46 ± 0.60) × 10−10 | (1.14 ± 0.42) × 10−9 | (2.56 ± 1.43) × 10−9 | (6.35 ± 3.35) × 10−9 | (2.96 ± 2.43) × 10−11 | (2.96 ± 1.28) × 10−10 | (2.82 ± 1.30) × 10−11 | (3.72 ± 1.85) × 10−12 |
| 1412 | (1.42 ± 0.90) × 10−8 | (1.91 ± 0.65) × 10−8 | (1.59 ± 0.85) × 10−8 | (2.02 ± 2.18) × 10−8 | (4.52 ± 3.95) × 10−9 | (1.22 ± 0.79) × 10−8 | (1.38 ± 0.25) × 10−9 | (6.25 ± 5.63) × 10−12 |
| 1413 | (8.80 ± 2.80) × 10−8 | (1.49 ± 0.37) × 10−8 | (7.30 ± 3.05) × 10−8 | (1.25 ± 0.83) × 10−8 | (1.50 ± 1.11) × 10−9 | (3.33 ± 0.73) × 10−8 | (9.93 ± 0.49) × 10−10 | (1.64 ± 1.02) × 10−12 |
| 1417 | (7.72 ± 2.28) × 10−8 | (3.61 ± 0.45) × 10−8 | (2.84 ± 1.32) × 10−8 | (3.50 ± 1.55) × 10−8 | (6.46 ± 4.48) × 10−10 | (4.47 ± 0.68) × 10−8 | (4.18 ± 0.73) × 10−10 | (1.18 ± 0.84) × 10−12 |
In each case the selective concentration is less than the MPC of the antibiotic.
Nature of mutations conferring resistance to GEN, TMP, and CTX.
The experiments described above demonstrate that CTX-, GEN-, and TMP-resistant mutants are selected among E. coli mutators following exposure to antibiotics at concentrations arising during chemotherapy for UTIs. We determined the mechanisms of resistance to CTX, GEN, and TMP in mutators and compared them with the mechanisms arising in mutants derived from parent strain 1411. Possible resistance mechanisms were alteration of DHFR for TMP (18, 19), loss of OMPs and/or increased efflux pump activity for CTX (14, 25), and mutation in ribosomal protein L6 for GEN (7).
Two TMP-resistant mutants derived from each of the starting strains 1411, 1412, 1413, and 1417 were selected at random for further analysis. KM13 and KM14 were derived from strain 1411, KM15 and KM16 were derived from 1412, KM17 and KM18 were derived from 1413, and KM19 and KM20 were derived from 1417. The folA genes and their promoter regions of mutants KM13 to KM20 and their parents were sequenced following PCR amplification of folA. All mutants carried a single point mutation in folA (Ile94→Leu). In vitro TMP inhibition of DHFR from mutator strain 1412 (TMP IC50, 0.156 μg/ml) and one of the TMP-resistant mutants (KM15; TMP IC50, 4.7 μg/ml) confirmed that resistance was mediated by the altered DHFR.
Two CTX-resistant mutants derived from each of the starting strains 1411, 1412, 1413, and 1417 were selected at random for analysis of resistance mechanisms. KM21 and KM22 were derived from strain 1411, KM23 and KM24 were derived from strain 1412, KM25 and KM26 were derived from strain 1413, and KM27 and KM28 were derived from strain 1417. The chromosomally encoded mar locus in E. coli determines low-level resistance to a number of chemically unrelated antimicrobial agents, including β-lactam antibiotics (2). Mutations within mar, at either marO or marR, lead to increased levels of expression of mar-specific transcripts, which cause decreased levels of expression of the porin OmpF and increased levels of expression of the AcrAB efflux pump (25). However, sequencing of marOR of CTX mutants KM21 to KM28 revealed no marOR mutations (data not shown).
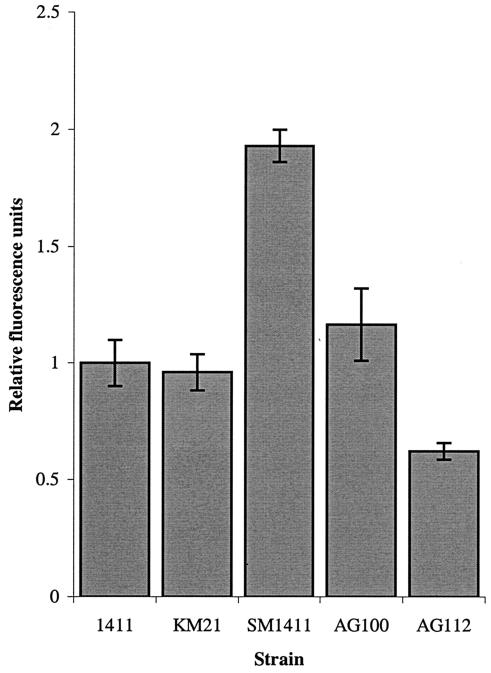
EB is a substrate for many multidrug resistance (MDR) efflux pumps in E. coli, including AcrAB (35); and accumulation of this agent, determined fluorometrically, indicates MDR activity. Steady-state accumulation of EB was enhanced in acrAB-knockout strain SM1411 and decreased in marOR mutant AG112 (Fig. 1). However, EB accumulation levels were not altered in a representative CTX-resistant mutant, KM21 (Fig. 1). In addition, the MICs of EB and CTAB for mutants KM21 to KM27 were inconsistent with the MIC profiles of the MDR mutants characterized (35). Representative data for mutant KM21 and the MDR mutants are shown in Table 5. The data indicate that the susceptibilities to EB and CTAB are unaltered in mutant KM21 but that the susceptibilities of mutants SM1411 and AG112 differ from those of their parent strains.
FIG. 1.
Accumulation of EB by various E. coli strains. Fluorescence data have been normalized relative to the fluorescence for strain 1411 and are the means of at least four determinations. Bars represent standard deviations.
TABLE 5.
Susceptibilities of E. coli strains to EB and CTAB
| Strain | Relevant genotype | MIC (μg/ml)
|
|
|---|---|---|---|
| EB | CTAB | ||
| 1411 | 1,024 | 256 | |
| KM21 | 1411 Ctxr mutant | 1,024 | 256 |
| SM1411 | 1411 ΔacrAB | 16 | 64 |
| AG100 | 1,024 | 256 | |
| AG112 | AG100 marR | >1,024 | 512 |
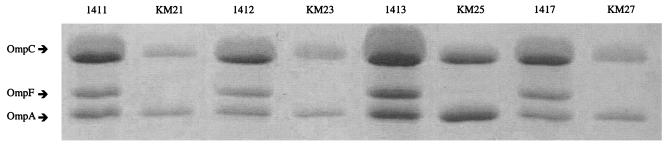
Loss of OMPs in response to selection pressure during β-lactam therapy can occur in the absence of increased levels of MDR pump expression (14). We examined this possibility for the CTX-resistant mutants generated here. OMPs from the parent strains (1411, 1412, 1413, and 1417) and one resistant mutant of each genetic background were analyzed. The parent strains exhibited three prominent OMPs in the molecular mass range of 32 to 36 kDa (Fig. 2). These were identified as OmpA, OmpC, and OmpF, respectively (Fig. 2), on the basis of their N-terminal amino acid sequences, which were identical to those found in the SWISS-PROT database: OmpA, (M)KKTAIAIAV; OmpC, (M)KVKVLSLLV; and OmpF, (M)MKRNILAVI. Each CTX-resistant mutant lacked the OmpF porin (Fig. 2).
FIG. 2.
Outer membrane protein profiles of KM21, KM23, KM25, and KM27 and their parent strains (strains 1411, 1412, 1413, and 1417, respectively).
Two GEN-resistant mutants derived from each of the starting strains 1411, 1412, 1413, and 1417 were selected at random for further analysis. KM29 and KM30 were derived from strain 1411, KM31 and KM32 were derived from 1412, KM33 and KM34 were derived from 1413, and KM35 and KM36 were derived from 1417. The GEN-resistant mutants showed no alterations in porin profiles (data not shown), suggesting that altered permeability is not the mechanism of resistance. Mutation in the rplF gene, which encodes the L6 ribosomal protein implicated in aminoglycoside interactions, confers low-level GEN resistance in other species (7). However, sequencing of rplF from the GEN-resistant E. coli strains revealed no mutations.
Concluding remarks.
Mutators with defects in MMR appear to be an important clinical source of drug-resistant bacteria (9, 21, 26). Using a model for the in vivo behavior of E. coli mutators during exposure to anti-UTI drugs, we demonstrated that they have the potential to generate mutants with low-level resistance to TMP, GEN, and CTX during standard schedules of dosing with these drugs. Although the types of mutations arising in mutators and normal strains appear to be similar, the increased frequency at which resistance arises in mutators is cause for concern. Since bacterial populations in excess of 1010 organisms can occur in patients with UTIs (3), E. coli mutators are indeed a likely source of low-level antibiotic-resistant mutants in vivo.
The emergence of mutant populations possessing low-level antibiotic resistance increases the chance of survival of the bacterial population in the host during chemotherapy and provides an important platform for the development of high-level antibiotic resistance (4). We therefore conclude that E. coli mutators present an enhanced risk for the emergence of antibiotic resistance during treatment for UTIs. Our results agree with those of Oliver et al. (26), who suggested that mutators are also risk factors in the treatment of P. aeruginosa infections. Whether mutators of other clinical species pose a similar threat has yet to be established.
Acknowledgments
Keith Miller was supported by a research grant to Ian Chopra from the British Society for Antimicrobial Chemotherapy.
REFERENCES
- 1.Adrian, P. V., and K. P. Klugman. 1997. Mutations in the dihydrofolate reductase gene of trimethoprim-resistant isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 41:2406-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alekshun, M. N., and S. B. Levy. 1997. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob. Agents Chemother. 41:2067-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannon, J., M. H. Hatem, and M. Noone. 1998. Anaerobic infections of the urinary tract: are they being missed? J. Clin. Pathol. 51:709-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baquero, F. 2001. Low-level antibacterial resistance: a gateway to clinical resistance. Drug Resist. Updates 4:93-105. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Brenwald, N. P., M. J. Gill, and R. Wise. 1998. Prevalence of a putative efflux mechanism among fluoroquinolone-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2032-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckel, P., A. Buchberger, A. Bock, and H. G. Wittmann. 1977. Alteration of ribosomal protein L6 in mutants of Escherichia coli resistant to gentamicin. Mol. Gen. Genet. 158:47-54. [DOI] [PubMed] [Google Scholar]
- 8.Chai, T., and J. Foulds. 1978. Two bacteriophages which utilize a new Escherichia coli major outer membrane protein as part of their receptor. J. Bacteriol. 135:164-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chopra, I., A. J. O'Neill, and K. Miller. 2003. The role of mutators in the emergence of antibiotic-resistant bacteria. Drug Resist. Updates 6:137-145. [DOI] [PubMed] [Google Scholar]
- 10.Denamur, E., S. Bonacorsi, A. Giraud, P. Duriez, F. Hilali, C. Amorin, E. Bingen, A. Andremont, B. Picard, F. Taddei, and I. Matic. 2002. High frequency of mutator strains among human uropathogenic Escherichia coli isolates. J. Bacteriol. 184:605-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finch, R. G. 1997. Tetracyclines, p. 475-484. In F. O'Grady, H. P. Lambert, R. G. Finch, and D. Greenwood (ed.), Antibiotic and chemotherapy, 7th ed. Churchill Livingstone, New York, N.Y.
- 12.Frimodt-Moller, N. 2002. Correlation between pharmacokinetic/pharmacodynamic parameters and efficacy for antibiotics in the treatment of urinary tract infection. Int. J. Antimicrob. Agents 19:546-553. [DOI] [PubMed] [Google Scholar]
- 13.Hughes, D. T. D. 1997. Diaminopyrimidines, p. 346-356. In F. O'Grady, H. P. Lambert, R. G. Finch, and D. Greenwood (ed.), Antibiotic and chemotherapy, 7th ed. Churchill Livingstone, New York, N.Y.
- 14.Jaffe, A., Y. A. Chabbert, and O. Semonin. 1982. Role of porin proteins OmpF and OmpC in the permeation of beta-lactams. Antimicrob. Agents Chemother. 22:942-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kucers, A., and N. M. Bennet. 1989. The use of antibiotics, 4th ed. Heinemann Medical Books, Edinburgh, United Kingdom.
- 16.Liu, Y. C., W. K. Huang, T. S. Huang, and C. M. Kunin. 1999. Detection of antimicrobial activity in urine for epidemiologic studies of antibiotic use. J. Clin. Epidemiol. 52:539-545. [DOI] [PubMed] [Google Scholar]
- 17.Martinez, J. L., and F. Baquero. 2000. Mutation frequencies and antibiotic resistance. Antimicrob. Agents Chemother. 44:1771-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maskell, J. P., A. M. Sefton, and L. M. C. Hall. 2001. Multiple mutations modulate the function of dihydrofolate reductase in trimethoprim-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:1104-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews, D. A., J. T. Bolin, J. M. Burridge, D. J. Filman, K. W. Volz, B. T. Kaufman, C. R. Beddell, J. N. Champness, D. K. Stammers, and J. Kraut. 1985. Refined crystal-structures of Escherichia coli and chicken liver dihydrofolate-reductase containing bound trimethoprim. J. Biol. Chem. 260:381-391. [PubMed] [Google Scholar]
- 20.Miller, J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Miller, K., A. J. O'Neill, and I. Chopra. 2002. Response of Escherichia coli hypermutators to selection pressure with antimicrobial agents from different classes. J. Antimicrob. Chemother. 49:925-934. [DOI] [PubMed] [Google Scholar]
- 22.Naber, K. G. 2000. Survey on antibiotic usage in the treatment of urinary tract infections. J. Antimicrob. Chemother. 46:49-52. [PubMed] [Google Scholar]
- 23.Normark, B. H., and S. Normark. 2002. Evolution and spread of antibiotic resistance. J. Intern. Med. 252:91-106. [DOI] [PubMed] [Google Scholar]
- 24.Oethinger, M., W. V. Kern, A. S. Jellen-Ritter, L. M. McMurry, and S. B. Levy. 2000. Ineffectiveness of topoisomerase mutations in mediating clinically significant fluoroquinolone resistance in Escherichia coli in the absence of the AcrAB efflux pump. Antimicrob. Agents Chemother. 44:10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oethinger, M., I. Podglajen, W. V. Kern, and S. B. Levy. 1998. Overexpression of the marA or soxS regulatory gene in clinical topoisomerase mutants of Escherichia coli. Antimicrob. Agents Chemother. 42:2089-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver, A., R. Canton, P. Campo, F. Baquero, and J. Blazquez. 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288:1251-1253. [DOI] [PubMed] [Google Scholar]
- 27.O'Neill, A. J., J. M. Bostock, A. M. Moita, and I. Chopra. 2002. Antimicrobial activity and mechanisms of resistance to cephalosporin P1, an antibiotic related to fusidic acid. J. Antimicrob. Chemother. 50:839-848. [DOI] [PubMed] [Google Scholar]
- 28.Patel, K. B., D. P. Nicolau, C. H. Nightingale, and R. Quintiliani. 1995. Pharmacokinetics of cefotaxime in healthy-volunteers and patients. Diagn. Microbiol. Infect. Dis. 22:49-55. [DOI] [PubMed] [Google Scholar]
- 29.Phillips, I., and K. P. Shannon. 1997. Aminoglycosides and aminocyclitols, p. 164-201. In F. O'Grady, H. P. Lambert, R. G. Finch, and D. Greenwood (ed.), Antibiotic and chemotherapy, 7th ed. Churchill Livingstone, New York, N.Y.
- 30.Rahaman, S. O., J. Mukherjee, A. Chakrabarti, and S. Pal. 1998. Decreased membrane permeability in a polymyxin B-resistant Escherichia coli mutant exhibiting multiple resistance to beta-lactams as well as aminoglycosides. FEMS Microbiol. Lett. 161:249-254. [DOI] [PubMed] [Google Scholar]
- 31.Reddy, J. 1981. Tetracycline antibiotics should be avoided in patients with renal-disease. N. Z. Med. J. 94:396. [PubMed] [Google Scholar]
- 32.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Segura, J. W., P. P. Kelalis, W. J. Martin, and L. H. Smith. 1972. Anaerobic bacteria in the urinary tract. Mayo Clin. Proc. 47:30-33. [PubMed] [Google Scholar]
- 34.Stamm, W. E. 1992. Approach to the patient with urinary tract infection, p. 788-798. In S. L. Gorbach, J. G. Bartlett, and N. R. Blacklow (ed.), Infectious diseases. The W. B. Saunders Co., Philadelphia, Pa.
- 35.Sulavik, M. C., C. Houseweart, C. Cramer, N. Jiwani, N. Murgolo, J. Greene, B. DiDomenico, K. J. Shaw, G. H. Miller, R. Hare, and G. Shimer. 2001. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob. Agents Chemother. 45:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thillet, J., J. Absil, S. R. Stone, and R. Pictet. 1988. Site-directed mutagenesis of mouse dihydrofolate-reductase-mutants with increased resistance to methotrexate and trimethoprim. J. Biol. Chem. 263:12500-12508. [PubMed] [Google Scholar]
- 37.Wilson, A. P. R., and R. N. Gruneberg. 1997. Ciprofloxacin: 10 years of clinical experience, 1st ed. Maxim Medical, Oxford, United Kingdom.
- 38.Zhao, X. L., and K. Drlica. 2002. Restricting the selection of antibiotic-resistant mutant bacteria: measurement and potential use of the mutant selection window. J. Infect. Dis. 185:561-565. [DOI] [PubMed] [Google Scholar]