Corneal epithelial barrier function provides the first line of cellular defense against chemical, biological, and physical insults from the environment to the eye, ensuring retention of the transparent and refractive properties of the cornea. Here, the authors show that the Krüppel-like transcription factor KLF4 contributes to ocular surface barrier function by upregulating the expression of functionally related subsets of cell junctional proteins and basement membrane components.
Abstract
Purpose.
Previously, the authors showed that Klf4-conditional null (Klf4CN) corneas display epithelial fragility. Here, they investigated the mechanism by which Klf4 regulates corneal epithelial barrier function.
Methods.
Klf4CN mice were generated by breeding Le-Cre with Klf4-LoxP mice. Fluorescein staining was used to test the corneal barrier function. RT-PCR, immunoblots, and immunofluorescence were used to detect the expression of cell junctional proteins. The effect of Klf4 on promoter activities was measured by transient cotransfection assays. Trans-epithelial electrical resistance (TEER) was used to measure the barrier-forming ability of control or anti-KLF4 siRNA–treated cells.
Results.
Increased fluorescein staining and decreased tight junction protein Tjp1 expression demonstrated that the Klf4CN corneal epithelial barrier function is defective. Expression of desmosomal components Dsp, Dsg-1a, and Dsg-1b was downregulated in the Klf4CN corneas, and their corresponding promoter activities were upregulated by Klf4 in transient cotransfection assays. Hemidesmosomal α3- and β4-integrin levels were not affected even though there were fewer hemidesmosomes in the Klf4CN corneas. The basement membrane components laminin-α5, -α3, -β3, and -β1–1 were downregulated, suggesting that the disrupted basement membrane is responsible for fewer hemidesmosomes in the Klf4CN cornea. Tight junction proteins OCLN1 and TJP1were downregulated in anti-KLF4 siRNA–treated cells, which failed to develop epithelial barrier function as measured by TEER.
Conclusions.
Klf4 contributes to corneal epithelial barrier function by upregulating the expression of functionally related subsets of cell junctional proteins and basement membrane components.
The transparent cornea, the anterior-most refractive ocular tissue comprising an outer stratified squamous epithelium, an inner monolayered endothelium, and a central collagenous stroma sparsely populated by keratocytes, is critical for clear vision.1 The corneal epithelial barrier defends the eye against chemical, biological, and physical insults from the environment.2 Loss of corneal epithelial barrier function is associated with debilitating ocular surface disorders such as dry eye, ocular cicatricial pemphigoid, and Meesmann's dystrophy.3
The mouse corneal epithelium consists of approximately 8 to 10 layers of cells derived from the head surface ectoderm. Even though corneal development begins in the early embryonic stages, corneal epithelial stratification takes place after eyelid opening.4,5 Different cell-cell junctional complexes exist at different depths of these layers.6 Tight junctions at the superficial layers are necessary for proper barrier function.7–9 Desmosomes in the spinous cell layers, adherens junctions throughout the different layers, and hemidesmosomes in the basal cell layers provide structural stability and help resist the shearing forces in the corneal epithelium.6,10,11 The gap junctions in the basal cell layers allow intercellular communications, synchronizing cellular responses in facilitating quick wound healing.12 Proper expression of each of these cell junctional components is essential for the corneal epithelial structural integrity.
Development of the corneal epithelial barrier function is regulated at multiple levels, starting with gene expression. Although different transcription factors have been identified as involved in epidermal, intestinal, and alveolar epithelial barrier formation, information regarding the role of gene regulation in corneal epithelial barrier formation is scant.13–15 Even though many previous studies have explored the role of diverse transcription factors such as Pax6, AP2-alpha, HMGN1, basonuclin, Sp1, AP1, NF1, Oct1, and p300 in the cornea, relatively few of them focused extensively on the regulation of epithelial barrier formation.16–21 Thus, our knowledge of the role of gene regulation in development and maintenance of the corneal barrier function remains incomplete.
Serial analysis of gene expression identified the highly expressed transcription factors in the mouse cornea.22 Chief among them was Klf4, a member of the conserved family of Krüppel-like factors (KLFs) that regulates a wide array of events including cell proliferation, differentiation, maintenance of pluripotent stem cells, development, and apoptosis.23 Klf4-null mice develop normally until birth but die within 15 hours after birth by dehydration caused by defective epidermal barrier function.24 The Klf4 conditional null (Klf4CN) mice derived by mating Klf4-LoxP25 with Le-Cre26 mice develop multiple ocular defects including corneal epithelial fragility, stromal edema, a smaller, vacuolated lens, and loss of conjunctival goblet cells.27 We have identified the changes in gene expression underlying the Klf4CN corneal phenotype by comparing the gene expression patterns of the wild-type and Klf4CN corneas by microarray analysis.28 Klf4CN stromal edema was associated with an increase in the stromal collagen fibril diameter and interfibrillar spacing and with a significant reduction in the stromal proteoglycan levels, brought about by an increase in the matrix metalloproteinase levels.29 Here we show that KLF4 contributes to corneal epithelial barrier function by upregulating the expression of corneal epithelial cell junctional proteins and components of the basement membrane.
Materials and Methods
Conditional Deletion of Klf4 and Measurement of Corneal Epithelial Permeability Barrier Function by Fluorescein Uptake
Klf4CN mice were generated on a mixed background by mating Klf4loxP/loxP, Le-Cre/− mice with Klf4loxP/loxP mice as described earlier.27 Mice studied here were maintained in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee of the University of Pittsburgh and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Corneal epithelial permeability was tested by instilling a drop of 1% sodium fluorescein solution on the surfaces of wild-type or Klf4CN eyes, rinsing with PBS after 1 minute, and photographing under UV light. All studies reported here were performed with 8- to 10-week-old adult mice.
Immunoblots and Immunofluorescence
Equal amounts of total protein extracted by homogenizing dissected corneas in urea lysis buffer (8.0 M urea, 0.08% Triton X-100, 0.2% sodium dodecyl sulfate, 3% β-mercaptoethanol, and proteinase inhibitors) and quantified by the bicinchoninic acid method (Pierce, Rockford, IL) were electrophoresed in sodium dodecyl sulfate-polyacrylamide gels, transferred to polyvinylidene difluoride membranes, and subjected to immunoblot analysis. Mouse anti-TJP1 (Zymed Laboratories, Carlsbad, CA), rabbit anti-occludin, anti-desmoglein, anti-desmoplakin (Santa Cruz Biotechnologies, Santa Cruz, CA), and anti-actin antibody (Sigma Chemical Company, St. Louis, MO) were used as primary antibodies at 1:500, 1:2000, 1:500, 1:500, and 1:2000 dilution, respectively, in PBST. Horseradish peroxidase-coupled anti-rabbit immunoglobulin G (Amersham Biosciences, Piscataway, NJ) was used as a secondary antibody at a 1:2500 dilution. Immunoreactive bands were visualized by chemiluminescence using Super Signal West Pico solutions (Pierce).
For immunofluorescence, 10-μm-thick cryosections from OCT-embedded eyeballs were fixed in freshly prepared buffered 4% paraformaldehyde for 30 minutes, blocked with 10% sheep serum in PBST for 1 hour at room temperature in a humidified chamber, washed twice with PBST for 5 minutes each, incubated with a 1:100 dilution of the primary antibody for 1 hour at room temperature, washed thrice with PBST for 10 minutes each, incubated with secondary antibody (Alexa Fluor 555-coupled goat anti-rabbit IgG antibody; Molecular Probes, Carlsbad, CA) at a 1:300 dilution for 1 hour at room temperature, washed thrice with PBST for 10 minutes each, mounted with antifade reagent with DAPI (Prolong Gold; Molecular Probes), and observed with a fluorescence microscope. All images presented within each composite figure in this manuscript have been processed in a similar manner using image editing software (Photoshop or Illustrator; Adobe, Mountain View, CA).
Reporter Vectors, Cell Culture, and Transient Transfection Assays
Mouse genomic DNA was used to amplify different promoter fragments cloned upstream of the luciferase reporter gene (pGL3-Basic Vector; Promega, Madison, WI) to generate the reporter vectors. The sequence of oligonucleotide primers used for PCR is given in (Supplementary Table S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-6134/-/DCSupplemental). Full-length Klf4 in pCI-Klf4 was transiently expressed using the CMV promoter. Human skin keratinocyte NCTC cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, penicillin, and streptomycin at 37°C in a humidified chamber containing 5% CO2 in air. Simian virus SV40-transformed human corneal epithelial (HCE) cells were grown at 37°C in DMEM/F-12 (1:1) supplemented with 10% fetal bovine serum, 0.5% (vol/vol) dimethyl sulfoxide, cholera toxin (0.1 μg/mL), epidermal growth factor (10 ng/mL), insulin (5 μg/mL), and gentamicin (40 μg/mL) in a humidified chamber containing 5% CO2 in air. Mid-log phase cells in 12-well plates were transfected with 0.25 μg pDsg1a-Luc or pDsg1b-Luc or pDsp-Luc, along with 10 ng pRL-SV40 (Promega; for normalization of transfection efficiency) and 0.25 μg pCI or pCI-Klf4, using 1.5 μL transfection reagent (FuGENE 6; Roche Molecular Biochemicals, Indianapolis, IN). After 2 days, cells were washed with cold PBS and lysed with 500 μL passive lysis buffer (Promega). The lysate was clarified by centrifugation, and 50 μg protein in the lysate was analyzed using a dual-luciferase assay kit (Promega) and a microplate luminometer (Synergy-II; Biotek Instruments, Winooski, VT). The measurement was integrated over 10 seconds, with a delay of 2 seconds. Results from at least three independent experiments, normalized for transfection efficiency using the SV40 promoter-driven Renilla luciferase activity, were used to obtain mean promoter activities, and standard deviation (SD). Fold-activation was determined by dividing mean promoter activity by the promoter activity without added pCI/pCI-Klf4.
Development of HCE Cell Lines Stably Transfected with Control or Anti-KLF4 siRNA and Measurement of Trans-epithelial Electrical Resistance
HCE cells transfected with linearized vectors expressing control or anti-KLF4 siRNAs under the control of the U6 promoter were selected by G418 for 15 days. G418-resistant individual clones were further expanded and tested for the expression of KLF4 by qRT-PCR. Anti-KLF4 siRNA–treated clones in which KLF4 expression was suppressed by >70% compared with wild-type or control siRNA–treated clones were selected and used in experiments reported here. For measurement of trans-epithelial electrical resistance (TEER), wild-type, control, or anti-KLF4-siRNA–transfected HCE cells were grown to a confluent stage on permeable supports (pore size, 0.4μm, surface area, 1.13 cm2; Transwell [Corning Costar Inc. Corning, NY]), starting at a density of 105 cells/cm2. TEER was measured every 24 hours for 15 days using a commercially available electrode chamber (EndOhm-12; World Precision Instruments, Sarasota, FL) and epithelial voltohmmeter (EVOM; World Precision Instruments). Background electrical resistance, including filter and medium, was constantly low (20 Ω/cm2) and was subtracted from the experimental values. TEER was expressed as Ω/cm2.
Results
Klf4CN Corneal Epithelial Fragility and Loss of Barrier Function
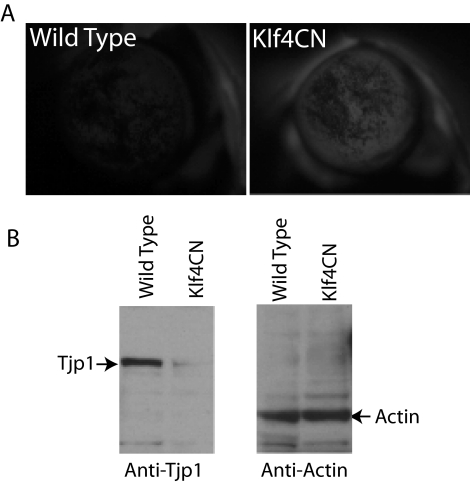
Previous histologic examination as well as transmission and scanning electron microscopic examination suggested that Klf4CN mice display corneal epithelial fragility.27 To more directly test the effect of deletion of Klf4 on corneal epithelial barrier function, we stained the wild-type and Klf4CN eyes with sodium fluorescein. The sodium fluorescein dye penetrated the Klf4CN corneas more than the wild-type corneas, consistent with the Klf4CN corneal epithelial fragility and loss of barrier function (Fig. 1A).
Figure 1.
Loss of Klf4CN corneal epithelial barrier function. (A) Klf4CN corneas are fluorescein stained more intensely than the wild-type counterparts, indicating a loss of barrier function. (B, left) Immunoblot with anti-Tjp1 antibody shows that the tight junction protein Tjp1 is downregulated in the Klf4CN cornea. Right: the Tjp1 blot was stripped of the primary antibody and reprobed with anti-actin antibody to ensure that equal amounts of protein were loaded in both lanes. Reactive bands in the expected position are indicated in both.
Tight junctions, normally present in the most superficial cell layers in stratified squamous epithelia, are responsible for the epithelial barrier function.30 In view of the extensive desquamation observed, we expected the superficial cell layers to be missing and consequently the tight junctions to be significantly downregulated in the Klf4CN cornea.27 Indeed, immunoblots demonstrated that the expression of Tjp1 (ZO1), a component of the tight junctions, is significantly downregulated, consistent with the loss of barrier function in Klf4CN corneas (Fig 1B).
Klf4CN corneal Epithelial Desmosomal Components Are Downregulated
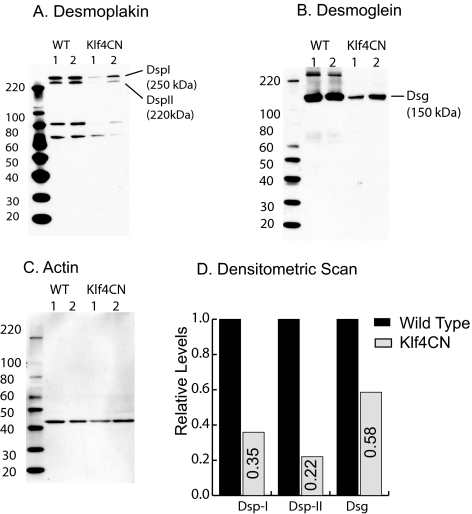
To detect the underlying cause of Klf4CN corneal epithelial fragility, we examined the expression levels of different junctional proteins in the microarray data for comparison of gene expression between wild-type and Klf4CN corneas.28 Although most of the adherens junction and gap junction components remained unchanged in these corneas, almost all the desmosomal components were significantly downregulated in the Klf4CN compared with the wild-type corneas.28 Immunoblots with equal amounts of total protein from the wild-type and Klf4CN corneas probed with anti-desmoplakin (Fig. 2A) or anti-desmoglein (Fig. 2B) antibody confirmed this reduction. This blot was stripped of the primary antibody and reprobed with anti–actin antibody, which confirmed equal loading of wild-type and Klf4CN corneal lysates (Fig. 2C). Densitometric scans allowed us to correct for the minor loading differences between samples and indicated that the levels of desmoglein, desmoplakin-I, and desmoplakin-II were reduced to 58%, 35%, and 22% of the wild-type levels, respectively, in the Klf4CN corneas (Fig. 2D).
Figure 2.
Immunoblots showing downregulation of desmoglein and desmoplakin in the Klf4CN cornea. (A) Immunoblot of the wild-type and Klf4CN whole corneal extract probed with anti-Dsp antibody. (B) The blot in (A) was stripped of the primary antibody and reprobed with anti-Dsg antibody. (C) The blot from (B) was stripped of the primary antibody and reprobed with anti-actin antibody to ensure equal loading. (D) Fold change in expression of Dsg, Dsp-I, and Dsp-II estimated by densitometric scan of the immunoblots in (A–C) after normalizing for loading differences based on actin levels.
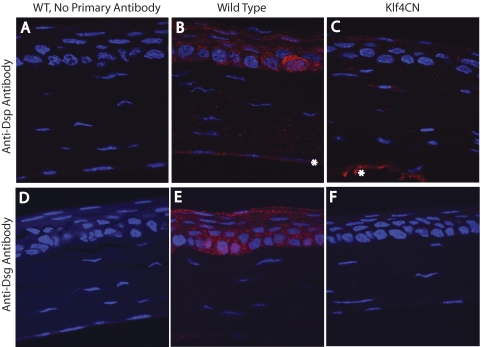
To confirm the downregulation of desmosomal components, we performed immunofluorescence with wild-type and Klf4CN corneal sections. Strong Dsg- or Dsp-specific signal was observed in the wild-type (Figs. 3B, 3E) but not the Klf4CN corneal epithelia (Figs. 3C, 3F), confirming the significant downregulation of desmoglein and desmoplakins in the Klf4CN corneal epithelia. Sections processed in a similar manner without the primary antibody served as negative controls (Figs. 3A, 3D). Taken together, these results suggest that the reduced expression of the desmosomal components is responsible for the Klf4CN corneal epithelial fragility and the resultant loss of epithelial barrier function.
Figure 3.
Immunofluorescence showing the downregulation of desmoglein and desmoplakin in the Klf4CN cornea. (A) Wild-type (WT), no primary antibody control. (B) Wild-type corneal section stained with anti-Dsp antibody. (C) Klf4CN corneal section probed with anti-Dsp antibody. (D) Wild-type (WT), no primary antibody control. (E) Wild-type corneal section stained with anti-Dsg antibody. (F) Klf4CN corneal section probed with anti-Dsg antibody. (B) Although the endothelial staining (*) is genuine, it appears to be an artifact of iridocorneal fusion (which we observed occasionally in Klf4CN eyes) in (C) (*).
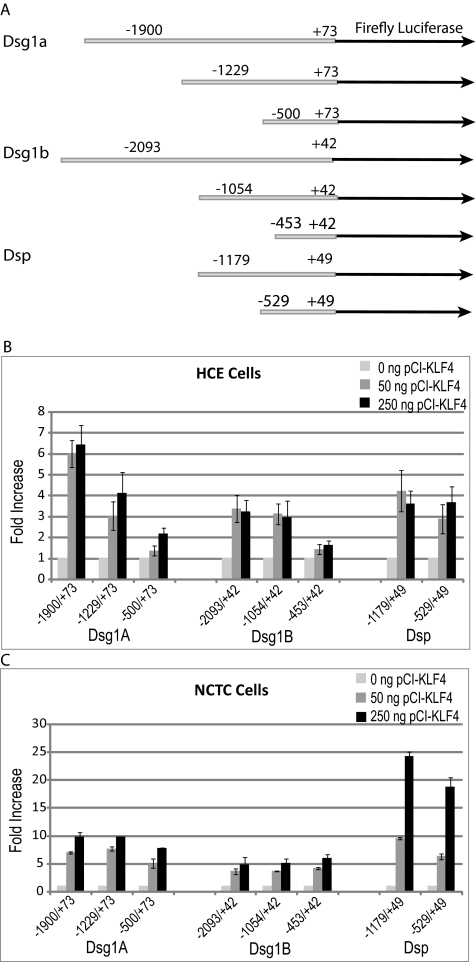
Klf4 Upregulates Mouse Dsg-1a, Dsg-1b, and Dsp Promoter Activities in Transient Cotransfection Assays
To test whether Klf4 stimulates the promoter activities of desmosomal components, we used transient cotransfection assays in in vitro cultured HCE and NCTC cells. Different lengths of Dsg1a, Dsg1b, and Dsp proximal promoter fragments were amplified from the mouse genomic DNA and cloned upstream of the luciferase reporter gene in pGL3 vector, as shown (Fig. 4A). On cotransfection with pCI-Klf4, Dsg1a, Dsg1b, and Dsp promoter activities were upregulated 6-, 3-, and 4-fold respectively, in HCE cells (Fig. 4B) and 6- to 11-fold, 4- to 6-fold, and 21- to 25-fold, respectively, in NCTC cells (Fig. 4C). These results establish that Klf4 stimulates the Dsg1a, Dsg1b, and Dsp promoter activities and suggest that the Klf4-responsive elements reside within the −500/+73 bp, −453/+42 bp, and −529/+49 bp regions of the Dsg1a, Dsg1b, and Dsp proximal promoters, respectively (Fig. 4). Consistent with this, examination of the Dsg1a, Dsg1b and Dsp proximal promoters yielded several potential Klf4-binding sites (5′ C/T C/T C C G/A C G/A 3′)31 (Supplementary Fig. S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-6134/-/DCSupplemental). Based on these results, we conclude that Klf4 contributes to corneal epithelial barrier function by upregulating the expression of desmosomal components.
Figure 4.
Activation of Dsg1a, Dsg1b, and Dsp promoters by Klf4. (A) Schematic depiction of the different reporter constructs used. (B, C) Stimulation of Dsg1a, Dsg1b, and Dsp promoter activities by cotransfection of increasing amounts of pCI-Klf4, as shown, in HCE (B) and NCTC (C) cells.
Defective Basement Membrane May Account for the Reduced Hemidesmosomes in the Klf4CN Cornea
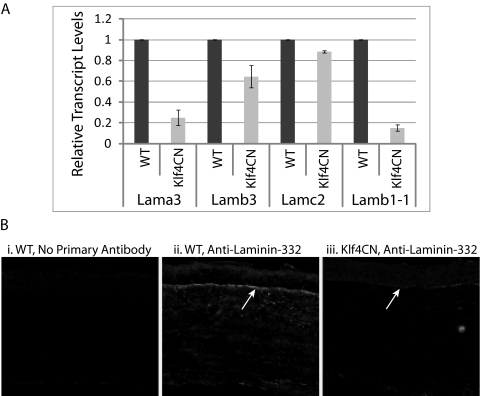
Previously, we have shown that the Klf4CN corneal basal epithelial cells do not form as many hemidesmosomes as their wild-type counterparts.27 Examination of the microarray data comparing gene expression patterns between wild-type and Klf4CN corneas indicated no significant change in the expression levels of integrins, the individual membrane-bound components of the hemidesmosomes28 (Supplementary Table S2, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-6134/-/DCSupplemental). Consistent with these data, our current RT-PCR analyses yielded no significant difference in the expression of α3- and β4-integrins between wild-type and Klf4CN corneas, whereas lama5 was significantly downregulated in the Klf4CN corneas (Supplementary Fig. S2, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-6134/-/DCSupplemental). In contrast, real-time qRT-PCR revealed that the basement membrane components laminin-α3 (lama3), laminin-β3 (lamb3), and laminin-β1–1 (lamb1–1) were significantly downregulated, whereas laminin-γ2 (lamc2) remained relatively unaffected in the Klf4CN corneas (Fig. 5A). To further explore whether disrupted basement membrane was the underlying cause for the formation of fewer hemidesmosomes, we performed immunofluorescence with anti–laminin-332 antibody, which confirmed the downregulation of laminin-332 expression and disruption of the basement membrane in Klf4CN corneas (Fig. 5B).
Figure 5.
Defective basement membrane is responsible for the presence of fewer hemidesmosomes in Klf4CN corneas. (A) Real time qRT-PCR analysis of expression levels of laminin-α3 (lama3), -β3 (lamb3), -γ2 (lamc2), and β1–1 (lamb1–1) transcripts in wild-type and Klf4CN corneas, with GAPDH as the endogenous control. (B) Immunofluorescence of wild-type (middle) and Klf4CN (right) corneal sections with anti-laminin-332 antibody shows that the Klf4CN corneal epithelial basement membrane is defective. Negative control (no primary antibody) is shown in the left panel.
Loss of Barrier-Forming Ability in Anti-KLF4 siRNA–Treated HCE Cells
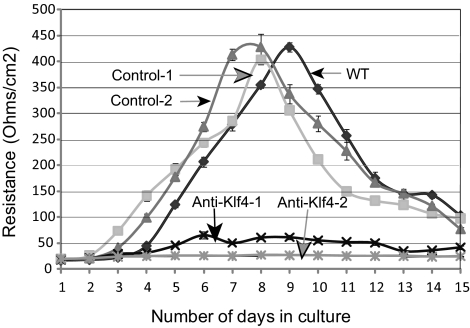
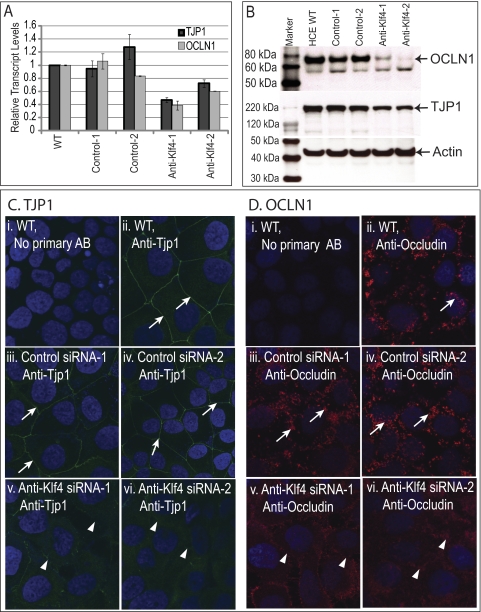
We examined the TEER of the confluent monolayer of the wild-type, control siRNA, and anti-KLF4 siRNA–treated HCE cells grown on permeable supports (Transwell plates; Corning Costar) as a measure of epithelial barrier function. Although both the wild-type and the control siRNA–treated cells started developing measurable electrical resistance by the fourth day in culture, reaching a peak of ∼400 Ω/cm2 within 8 days, two different clones of anti-KLF4 siRNA–treated cells failed to do so (Fig. 6). To understand the molecular basis for this inability to develop barrier function, we quantified the expression levels of tight junction proteins TJP1 and OCLN1 in the wild-type and KLF4-knockdown HCE cells by qRT-PCR and immunoblots. Expression of TJP1 and OCLN1 transcripts and of the corresponding proteins was downregulated in the anti-KLF4 siRNA–transfected cells compared with wild-type or control siRNA–transfected HCE cells (Figs. 7A, 7B). Consistent with these results, immunofluorescence with anti–Tjp1 or anti–occludin antibody demonstrated a significant downregulation of Tjp1 and occludin in the anti-KLF4 siRNA–treated HCE cells compared with the wild-type and control siRNA–treated cells (Figs. 7C, 7D). Taken together, these results demonstrate that the downregulation of KLF4 results in the loss of epithelial barrier formation in vitro, accompanied by decreased expression of tight junction proteins.
Figure 6.
Loss of epithelial barrier–forming ability in anti-KLF4 siRNA treated cells. TEER developed by wild-type, control siRNA, or anti-KLF4 siRNA–treated HCE cells cultured on permeable supports (Transwell plates; Corning Costar) was estimated every day for 15 days, as a measure of epithelial barrier–forming ability.
Figure 7.
Downregulation of tight junction components in the anti-KLF4 siRNA–treated HCE cells compared with wild-type or control siRNA treated–HCE cells. (A) Real-time qRT-PCR analysis of TJP1 and OCLN1 transcript levels in wild-type, control-, or anti-KLF4 siRNA–treated HCE cells, using GAPDH as an endogenous control. (B) Immunoblot analysis of total lysate from wild-type, control-, or anti-KLF4 siRNA–treated HCE cells, probed with anti-OCLN1, anti-TJP1, or anti-actin antibody. Immunofluorescence with anti-Tjp1 (C) or anti-occludin (D) antibody shows that the expression of TJP1 and OCLN1 detected in the cell-cell junctions of wild-type or control siRNA-transfected HCE cells (white arrows) is decreased in the anti-KLF4 siRNA transfected HCE cells (white arrowheads).
Discussion
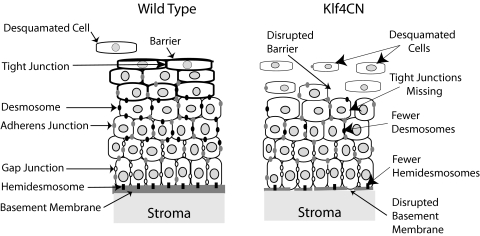
Multiple types of cell junctional complexes exist in different depths of the corneal stratified squamous epithelium, where they play important and diverse roles in normal corneal homeostasis and wound healing.6,32,33 In the present investigation, we identified the role of Klf4 in regulating the expression of three different desmosomal components and the extracellular laminin-332, a component of the basement membrane required for the formation of hemidesmosomes. Our results, summarized in Figure 8, show that Klf4 contributes to corneal epithelial barrier formation by regulating the expression of functionally related subsets of genes.
Figure 8.
Summary of the roles of Klf4 in corneal epithelial barrier formation. Schematic shows that in the Klf4CN cornea, superficial epithelial cell tight junction barrier is disrupted, wing cell desmosomes are decreased, and, beneath the basal epithelial cells, the basement membrane is disrupted, resulting in fewer hemidesmosomes.
The results presented in this report show that lama3, lamb3, lamb1–1, and lama5 are downregulated in the Klf4CN corneas. Although it is likely that Klf4 directly regulates their corresponding promoter activities, we do not have direct evidence to confirm it. Previous studies have demonstrated that KLF4 upregulates LAMA3A,34 LAMA135, and LAMC136 promoter activities and that KLF6 upregulates LAMA1 promoter activity.35 In another study, Klf4 downregulated lama1 promoter activity while Klf5 upregulated it in the mouse intestinal epithelial cells, corroborating the complementary expression of Klf4 and Klf5 along the intestinal crypt-villus axis and the parallel expression of Klf5 and lama1 in the crypt region.37 Together, these studies suggest that the effects of different Klfs on various laminin promoter activities are promoter and context dependent.
Hemidesmosomes help attach the basal epithelial cells to the basement membrane through the interaction of cellular integrins with the basement membrane components.38–40 Both α3β1- and α6β4-integrin heterodimers serve as receptors for various components of the basement membrane beneath the corneal epithelium such as laminin-332, collagen-1 or -IV, and fibronectin.6,38,39,41,42 The present report shows that though the hemidesmosomal integrins Itga3 and Itgb4 are relatively unaffected, the basement membrane components lama3, lamb3, lama5, and lamb1–1, which serve as ligands for hemidesmosomal integrins,38,39are downregulated, consistent with the reduced number of hemidesmosomes and disruption of the Klf4CN corneal basement membrane detected by transmission electron microscopy and periodic acid-Schiff reagent.27–29
Disruption of epithelial cell-basement membrane interactions is an important initial step in the course of epithelial-to-mesenchymal transition (EMT), a fundamental morphogenetic process involved in many tissue remodeling events.43,44 Our observation of the disrupted basement membrane in the Klf4CN cornea, when considered along with the role of Klf4 as a tumor suppressor,45 suggests that the Klf4CN corneal epithelial cells may be undergoing the initial stages of EMT. This possibility is further supported by the observation that the Klf4CN corneal epithelial cells display reduced desmosome-mediated cell-cell adhesion, another early event in the course of EMT,46 and is consistent with a recent report that Klf4 inhibits EMT in mammary epithelial cells through the regulation of E-cadherin gene expression.47
Although transient transfection assays reported here demonstrate that Klf4 directly regulates the expression of desmosomal components, our results do not rule out the possibility of an additional indirect component as well. Rapid re-epithelialization during corneal wound healing requires remodeling of cell junctional complexes.32 It is possible that the initial desquamation of the superficial epithelial cells in the Klf4CN cornea27 is perceived by the underlying cells as a wound that must be healed, triggering additional signals to downregulate the cell junctional complexes and basement membrane components. In such a scenario, the observed reduction in the desmosomal components Dsp, Dsg1a, and Dsg1b and the basement membrane components laminin-α5, -α3, and -β1–1 could also reflect an indirect effect as part of the wound-healing response. Upregulation of MMPs in the Klf4CN cornea, frequently associated with corneal wound healing, is consistent with this possibility.29,48 Disruption of the basement membrane, a direct outcome of the reduced expression of laminins, could be exacerbated by the elevated expression of matrix metalloproteinases in the Klf4CN corneas reported earlier.29
In summary, our results show that Klf4 contributes to corneal epithelial barrier formation by regulating the expression of functionally related subsets of genes encoding desmosomal and tight junction components and laminins required for the formation of the basement membrane. Taken together with our previous reports,27–29 these results establish the role of Klf4 as an important transcription factor that regulates the maturation and maintenance of the ocular surface.
Footnotes
Supported by the NEI K22 Career Development Award EY016875 (SKS); startup funds from the Department of Ophthalmology, University of Pittsburgh School of Medicine (SKS); NEI/NIH Core Grant for Vision Research 5P30 EY08098-19; Research to Prevent Blindness; the Eye and Ear Foundation, Pittsburgh; and the intramural research program of the NEI, NIH.
Disclosure: S. Swamynathan, None; D. Kenchegowda, None; J. Piatigorsky, None; S. Swamynathan, None
References
- 1. Hay ED. Development of the vertebrate cornea. Int Rev Cytol. 1979;63:263–322 [DOI] [PubMed] [Google Scholar]
- 2. Kinoshita S, Adachi W, Sotozono C, et al. Characteristics of the human ocular surface epithelium. Prog Retin Eye Res. 2001;20:639–673 [DOI] [PubMed] [Google Scholar]
- 3. Klintworth GK. The molecular genetics of the corneal dystrophies—current status. Front Biosci. 2003;8:687–713 [DOI] [PubMed] [Google Scholar]
- 4. Wolosin JM, Budak MT, Akinci MA. Ocular surface epithelial and stem cell development. Int J Dev Biol. 2004;48:981–991 [DOI] [PubMed] [Google Scholar]
- 5. Zieske JD. Corneal development associated with eyelid opening. Int J Dev Biol. 2004;48:903–911 [DOI] [PubMed] [Google Scholar]
- 6. Suzuki K, Saito J, Yanai R, et al. Cell-matrix and cell-cell interactions during corneal epithelial wound healing. Prog Retin Eye Res. 2003;22:113–133 [DOI] [PubMed] [Google Scholar]
- 7. Balda MS, Matter K. Tight junctions at a glance. J Cell Sci. 2008;121:3677–3682 [DOI] [PubMed] [Google Scholar]
- 8. Sugrue SP, Zieske JD. ZO1 in corneal epithelium: association to the zonula occludens and adherens junctions. Exp Eye Res. 1997;64:11–20 [DOI] [PubMed] [Google Scholar]
- 9. Ban Y, Dota A, Cooper LJ, et al. Tight junction-related protein expression and distribution in human corneal epithelium. Exp Eye Res. 2003;76:663–669 [DOI] [PubMed] [Google Scholar]
- 10. Kottke MD, Delva E, Kowalczyk AP. The desmosome: cell science lessons from human diseases. J Cell Sci. 2006;119:797–806 [DOI] [PubMed] [Google Scholar]
- 11. Stepp MA, Spurr-Michaud S, Gipson IK. Integrins in the wounded and unwounded stratified squamous epithelium of the cornea. Invest Ophthalmol Vis Sci. 1993;34:1829–1844 [PubMed] [Google Scholar]
- 12. Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388 [DOI] [PubMed] [Google Scholar]
- 13. Koster MI, Roop DR. Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol. 2007;23:93–113 [DOI] [PubMed] [Google Scholar]
- 14. Lui WY, Lee WM. Mechanisms of reorganization of cell-cell junctions in the testis. Front Biosci. 2008;13:6775–6786 [DOI] [PubMed] [Google Scholar]
- 15. Galambos C, Demello DE. Regulation of alveologenesis: clinical implications of impaired growth. Pathology. 2008;40:124–140 [DOI] [PubMed] [Google Scholar]
- 16. Zaniolo K, Leclerc S, Cvekl A, et al. Expression of the alpha4 integrin subunit gene promoter is modulated by the transcription factor Pax-6 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:1692–1704 [DOI] [PubMed] [Google Scholar]
- 17. Sivak JM, West-Mays JA, Yee A, Williams T, Fini ME. Transcription factors Pax6 and AP-2alpha interact to coordinate corneal epithelial repair by controlling expression of matrix metalloproteinase gelatinase B. Mol Cell Biol. 2004;24:245–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dwivedi DJ, Pontoriero GF, Ashery-Padan R, Sullivan S, Williams T, West-Mays JA. Targeted deletion of AP-2alpha leads to disruption in corneal epithelial cell integrity and defects in the corneal stroma. Invest Ophthalmol Vis Sci. 2005;46:3623–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Birger Y, Davis J, Furusawa T, Rand E, Piatigorsky J, Bustin M. A role for chromosomal protein HMGN1 in corneal maturation. Differentiation. 2006;74:19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davis J, Duncan MK, Robison WG, Jr, Piatigorsky J. Requirement for Pax6 in corneal morphogenesis: a role in adhesion. J Cell Sci. 2003;116:2157–2167 [DOI] [PubMed] [Google Scholar]
- 21. Zhang X, Tseng H. Basonuclin-null mutation impairs homeostasis and wound repair in mouse corneal epithelium. PLoS One. 2007;2:e1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Norman B, Davis J, Piatigorsky J. Postnatal gene expression in the normal mouse cornea by SAGE. Invest Ophthalmol Vis Sci. 2004;45:429–440 [DOI] [PubMed] [Google Scholar]
- 23. McConnell BB, Ghaleb AM, Nandan MO, Yang VW. The diverse functions of Kruppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays. 2007;29:549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360 [DOI] [PubMed] [Google Scholar]
- 25. Katz JP, Perreault N, Goldstein BG, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swamynathan SK, Katz JP, Kaestner KH, Ashery-Padan R, Crawford MA, Piatigorsky J. Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells. Mol Cell Biol. 2007;27:182–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Swamynathan SK, Davis J, Piatigorsky J. Identification of candidate Klf4 target genes reveals the molecular basis of the diverse regulatory roles of Klf4 in the mouse cornea. Invest Ophthalmol Vis Sci. 2008;49:3360–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Young RD, Swamynathan S, Boote C, et al. Stromal edema in Klf4 conditional null mouse cornea is associated with altered collagen fibril organization and reduced proteoglycans. Invest Ophthalmol Vis Sci. 2009;50:4155–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17:1063–1072 [DOI] [PubMed] [Google Scholar]
- 31. Shields JM, Yang VW. Identification of the DNA sequence that interacts with the gut-enriched Kruppel-like factor. Nucleic Acids Res. 1998;26:796–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood). 2001;226:653–664 [DOI] [PubMed] [Google Scholar]
- 33. Suzuki K, Tanaka T, Enoki M, Nishida T. Coordinated reassembly of the basement membrane and junctional proteins during corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2000;41:2495–2500 [PubMed] [Google Scholar]
- 34. Miller KA, Eklund EA, Peddinghaus ML, et al. Kruppel-like factor 4 regulates laminin alpha 3A expression in mammary epithelial cells. J Biol Chem. 2001;276:42863–42868 [DOI] [PubMed] [Google Scholar]
- 35. Niimi T, Hayashi Y, Sekiguchi K, Kitagawa Y. The Sp family of transcription factors regulates the human laminin alpha1 gene in JAR choriocarcinoma cells. Biochim Biophys Acta. 2006;1759:573–579 [DOI] [PubMed] [Google Scholar]
- 36. Higaki Y, Schullery D, Kawata Y, Shnyreva M, Abrass C, Bomsztyk K. Synergistic activation of the rat laminin gamma1 chain promoter by the gut-enriched Kruppel-like factor (GKLF/KLF4) and Sp1. Nucleic Acids Res. 2002;30:2270–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Piccinni SA, Bolcato-Bellemin AL, Klein A, et al. Kruppel-like factors regulate the Lama1 gene encoding the laminin alpha1 chain. J Biol Chem. 2004;279:9103–9114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Belkin AM, Stepp MA. Integrins as receptors for laminins. Microsc Res Tech. 2000;51:280–301 [DOI] [PubMed] [Google Scholar]
- 39. Stepp MA. Corneal integrins and their functions. Exp Eye Res. 2006;83:3–15 [DOI] [PubMed] [Google Scholar]
- 40. Litjens SH, de Pereda JM, Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006;16:376–383 [DOI] [PubMed] [Google Scholar]
- 41. Stepp MA, Spurr-Michaud S, Tisdale A, Elwell J, Gipson IK. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci U S A. 1990;87:8970–8974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114:567–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Levayer R, Lecuit T. Breaking down EMT. Nat Cell Biol. 2008;10:757–759 [DOI] [PubMed] [Google Scholar]
- 44. Nakaya Y, Sukowati EW, Wu Y, Sheng G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol. 2008;10:765–775 [DOI] [PubMed] [Google Scholar]
- 45. Rowland BD, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006;6:11–23 [DOI] [PubMed] [Google Scholar]
- 46. Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829 [DOI] [PubMed] [Google Scholar]
- 47. Yori JL, Johnson E, Zhou G, Jain MK, Keri RA. Kruppel-like factor 4 inhibits epithelial-to-mesenchymal transition through regulation of E-cadherin gene expression. J Biol Chem. 285:16854–16863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fini ME, Stramer BM. How the cornea heals: cornea-specific repair mechanisms affecting surgical outcomes. Cornea. 2005;24:S2–S11 [DOI] [PubMed] [Google Scholar]