This study is the first comprehensive mutational analysis of all genes associated with nonsyndromic albinism, performed in combination with a detailed clinical ophthalmic evaluation of a large series of Italian patients with albinism.
Abstract
Purpose.
The purpose of this study was to identify the molecular basis of albinism in a large cohort of Italian patients showing typical ocular landmarks of the disease and to provide a full characterization of the clinical ophthalmic manifestations.
Methods.
DNA samples from 45 patients with ocular manifestations of albinism were analyzed by direct sequencing analysis of five genes responsible for albinism: TYR, P, TYRP1, SLC45A2 (MATP), and OA1. All patients studied showed a variable degree of skin and hair hypopigmentation. Eighteen patients with distinct mutations in each gene associated with OCA were evaluated by detailed ophthalmic analysis, optical coherence tomography (OCT), and fundus autofluorescence.
Results.
Disease-causing mutations were identified in more than 95% of analyzed patients with OCA (28/45 [62.2%] cases with two or more mutations; 15/45 [33.3%] cases with one mutation). Thirty-five different mutant alleles were identified of which 15 were novel. Mutations in TYR were the most frequent (73.3%), whereas mutations in P occurred more rarely (13.3%) than previously reported. Novel mutations were also identified in rare loci such as TYRP1 and MATP. Mutations in the OA1 gene were not detected. Clinical assessment revealed that patients with iris and macular pigmentation had significantly higher visual acuity than did severe hypopigmented phenotypes.
Conclusions.
TYR gene mutations represent a relevant cause of oculocutaneous albinism in Italy, whereas mutations in P present a lower frequency than that found in other populations. Clinical analysis revealed that the severity of the ocular manifestations depends on the degree of retinal pigmentation.
Albinism is a rare inherited disorder manifested by the complete or partial absence of pigment in the skin, hair, or eyes due to a defect in melanin biosynthesis. It can be classified as oculocutaneous albinism (OCA), when it involves the hair, skin, and eyes, or ocular albinism (OA), when the phenotype is mainly restricted to the eyes and the optic system and therefore is associated with specific ocular changes due to a reduced amount of melanin in the developing eye.1,2 Classic OCA is usually inherited as an autosomal recessive trait due to mutations in four genes known as TYR,3 P (OCA2),4 TYRP1,5 and SLC45A2 (MATP),6 which are responsible for OCA type 1 (OCA1, MIM 203100), type 2 (OCA2, MIM 203200), type 3 (OCA3, MIM 203290), and type 4 (OCA4, MIM 606574), respectively. Two general forms of OA have been distinguished: the X-linked recessive Nettleship-Falls form (OA1; MIM 300500) and autosomal recessive ocular albinism (AROA). OA1 results from mutations in the OA1 locus.7 AROA results from mutations in TYR, P, and possibly TYRP1, thus representing a phenotypically mild variant of OCA.8 OCA is a phenotypic component of syndromic disorders due to different gene mutations, including Hermansky-Pudlak (HPS, MIM 203300), Chediak-Higashi (CHS, MIM 214500), Griscelli (GS, MIM 214450; 607624; 609227), Tietz (TS, MIM 103500), ocular albinism with sensorineural deafness (Waardenburg syndrome type II with ocular albinism; WS2-OA, MIM 103470), Waardenburg (WS, MIM 193500), Cross (oculocerebral syndrome with hypopigmentation, CS, MIM 257800), Prader Willi (PWS, MIM 176270), and Angelman (AS, MIM 105830) syndromes.
The eye and optic system abnormalities are common to all types of albinism and are probably related to the reduction of melanin during embryonic development and early postnatal life.1 Characteristic changes in the optic system include reduced pigmentation of the iris (iris translucency) and of the retinal pigment epithelium, foveal hypoplasia, decreased visual acuity, misrouting of the optic fibers at the chiasm, nystagmus, strabismus, and refractive errors.1The degree of skin and hair hypopigmentation, when present, varies along a wide clinical spectrum of severe to mild phenotypes. The clinical spectrum of OCA varies both within and among genotypes.
OCA1 is generally considered a severe form, due to the absence of tyrosinase activity (OCA1A). Patients show a complete lack of melanin production throughout life with light blue to almost pink irises. Those with mutations determining a decreased tyrosinase activity have a milder form (OCA1B) characterized by blue to green/brown irises.3 OCA2, OCA3, and OCA4 show some pigment accumulation over time in both neural crest (skin, iris, and choroids melanocytes) and neuroectodermic (RPE cells) derived cells. OCA2, OCA3, and OCA4 patients typically have higher visual acuity than OCA1.9 OA primarily affects the eye, sparing hair, or skin, as does AROA. OA and AROA patients show the typical ocular landmarks of albinism, with relatively normal skin and hair pigmentation. Albinism can affect all ethnic backgrounds with an overall prevalence of approximately 1 in 20,000 people.1 Prevalence of the different forms of albinism varies considerably worldwide. Several factors may be involved, including the dissimilar prevalence of different founder gene mutations in different populations.9
OCA2 is considered the most common type of OCA worldwide, with the highest prevalence in Africans and African-American OCA patients, which may, in part, result from the existence of a single common deletion throughout many regions of sub-Sahara Africa.1,10 Recent findings in a large series of non-Hispanic Caucasian patients have shown, instead, that OCA1 is the most frequent cause of OCA in Caucasian patients.11 OCA3 is virtually nonexistent in Caucasians, whereas it affects frequently several African populations (∼1 in 8500).9,10 Mutations of MATP, responsible for OCA4, are extremely rare in Europeans (1 in 85,000), whereas they are associated with the OCA phenotype in 24% of Japanese OCA patients.9,12
At least 230 different pathologic gene mutations have been reported in TYR, 84 in P, 17 in TYRP1, and 42 in SLC45A2 (cf. Albinism Database; http://albinismdb.med.umn.edu/ developed by William Oetting and provided in the public domain by the University of Minnesota, Minneapolis, MN). Common mutations in different loci have been described in different populations. However, the causative roles of some common DNA variations are still controversial. In this respect, the p.R402Q variation in the TYR locus (common among Caucasians and African Americans), which results in a thermolabile tyrosinase polypeptide with decreased catalytic activity at 37°C (the so-called temperature-sensitive [TS] variant), has been considered for years a nonpathologic polymorphism in different populations.13 Instead, different studies have indicated this variant as a possible causative mutation, at least producing an additional negative effect in combination with different heterozygous mutations, even in nonallelic genes.14,15 In agreement with this finding, the p.R402Q variation on one allele has recently been identified in most Caucasian patients showing the AROA phenotype in combination with more severe TYR mutations on the other allele.8 Moreover, the elevated frequency of this variant in nondiagnostic OCA1 alleles in a large series of Caucasian patients has suggested that this variant may contribute to the OCA phenotype in some patients.11 In addition, previous studies reported a high frequency of multiple sequence variations within a single individual.14 As a result of the high clinical and genetic heterogeneity of OCA, it is difficult to clinically distinguish among the different forms of classic OCA.
Indeed, it appears that disease-causing mutations and clinical manifestations correlate poorly. A relationship can be identified only in the case of TYR gene mutations. TYR null mutations producing inactive or incomplete polypeptides result in the most severe OCA1A clinical phenotype, due to the lack of tyrosinase enzyme function, which in turn blocks the first step of the melanin biosynthetic pathway and, thus, the synthesis of melanin in pigmented cells. TYR mutations producing a partially active or hypomorphic tyrosinase enzymes result in the OCA1B milder phenotype.1 Furthermore, genotype–phenotype correlations are not clinically valuable in the case of P, TYRP1, and MATP mutations. The lack of functional assays for the P, TYRP1, and MATP proteins and the limited molecular genetic and clinical data of both TYRP1 and MATP hamper the possibility for a diagnostic and prognostic definition of these forms of albinism.16 Finally, no genotype–phenotype correlations have been identified in the case of mutations of OA1.17
Therefore, the side-by-side comparison of both molecular and clinical characteristics of patients affected by distinct genes and mutations may provide insights into the albinism disease spectrum and also into a more accurate diagnosis and genetic counseling.
In this study, we describe a comprehensive mutational analysis of all genes associated with nonsyndromic albinism in combination with a full clinical ophthalmic evaluation in a large cohort of 45 Italian patients with a clinical diagnosis of OCA.
Materials and Methods
Patient Selection
The diagnostic inclusion criteria of patients were based on the presence of the following ophthalmic characteristic features: photophobia, nystagmus, reduced visual acuity, strabismus, iris translucency, fundus hypopigmentation, and foveal hypoplasia, possibly in combination with various degrees of hypopigmentation of the skin and hair8; VEP has not been considered necessary for the routine diagnosis of albinism.18,19 Syndromic forms of albinism, such as Hermansky-Pudlak, Chediak-Higashi, Griscelli, Tietz, ocular albinism with sensorineural deafness, Waardenburg, Cross, Prader Willi, or Angelman syndromes were excluded on clinical grounds, based on the absence of additional clinical findings such as deafness; immune deficiency; hematologic abnormalities or bleeding diathesis; heart, lung, genitourinary, gastrointestinal or central nervous system involvement; and the presence of obesity and dysmorphic features, as reported at the time of the clinical diagnosis from the ophthalmologist at the referring center.
All patients analyzed showed variable skin and hair involvement with mild or severe degrees of hypopigmentation, representing the clinical spectrum of OCA phenotypes.
Mutation Analyses
We analyzed genomic DNA of 45 Italian patients from 40 independent families, referring to ophthalmic institutes in different Italian regions and having a clinical diagnosis of nonsyndromic albinism. The research adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from each patient, or from the patient's legal representatives in the case of children, after explanation of the nature and possible consequences of the study. To establish a molecular diagnosis, we performed direct DNA sequence analysis of five genes known to be associated with albinism: TYR, P, TYRP1, MATP, and OA1. Patients were initially screened for TYR and P mutations and, if negative, were then screened for TYRP1, MATP, and OA1 gene mutations.
A detailed pedigree analysis of the extended families was performed to determine the mode of inheritance, and in 22 independent families, the segregation of mutations was confirmed by gene sequence analysis in all family members.
Genomic DNA was extracted from patients' peripheral whole blood lymphocytes and, when available, from their parents, by using standard techniques (Qiagen Italy, Milan, Italy). The complete coding sequence and the exon–intron boundaries of the OA1, TYR, P, TYRP1, and MATP genes were amplified by standard PCR (Taq Gold DNA polymerase; Roche, Basel, Switzerland). Tyrosinase exon 1; OA1 exons 1, 3, and 8; and MATP exon 3, were amplified as a pair of overlapping fragments. The exon 1 of P and TYRP1 genes, which are noncoding, was not analyzed. Human chromosome 11 contains a pseudogene, known as the tyrosinase-like gene (TYRL, 11p11.2; MIM 191270), that shares a 98.55% sequence identity with the 3′-region of TYR (∼68 kb), including exons 4 and 5. Thus, the identification of nucleotide variants in TYR by PCR and DNA sequencing is a challenging task and could generate false data due to the co-amplification from both loci. To allow the direct and unequivocal identification of mutations, we used primers for a specific amplification of the TYR locus, as described in Chaki et al.20
The PCR was performed in 35 cycles with 50 ng of genomic DNA at 94°C for 1 minute, at the respective primer annealing temperature for 1 minute, and at 72°C for 1 minute. The primers and reaction conditions of the PCR amplification are available on request. Amplicons were screened for mutations by direct sequencing (Prism Big Dye terminator cycle sequencing V2.0 kit; Applied Biosystems, Inc. [ABI], Foster City, CA), and the reactions were analyzed on a genetic analyzer (Prism 3100; ABI). The sequenced exon and intron–exon boundaries were compared against consensus sequences obtained from the National Centre for Biotechnology Information Database (http://www.ncbi.nlm.nih.gov/), using standard software for DNA sequencing analysis (Autoassembler, ver. 2.1; ABI).
Mutation nomenclature conformed to standard convention.21 The identified nucleotide variations were researched in either the Albinism Database (http://albinismdb.med.umn.edu/) or the Human Gene Mutation Database (http://www.hgmd.cf.ac.uk/ac/index.php/ provided in the public domain by the Institute of Medical Genetics, Cardiff, Wales, UK) to check whether they are described as causative mutations or polymorphisms.
Nine of 13 newly identified sequence variants leading to amino acid missense substitution were tested in other affected and unaffected family members, to verify the segregation with the albino phenotype. Any novel missense variation was considered a possible causative mutation if it was absent in at least 100 control chromosomes, analyzed by denaturing high performance liquid chromatography (dHPLC). All control products displaying a dHPLC pattern similar to the patient's were sequenced. The newly identified frameshift mutations were not tested in control samples, and they were considered to be causative mutations.
Phenotype Analysis
An additional full ophthalmic evaluation, including visual acuity measurements, evaluation of ocular motility and iris translucency, biomicroscopic examination, fundus examination, fundus autofluorescence (FAF), and retinography, was performed in 18 patients harboring mutations of different genes causing albinism. A 4- and 3-point scale according to Summers et al.22 was used to classify iris translucency and macular transparency, respectively.
Autofluorescence was recorded with a standard confocal scanning laser ophthalmoscope (Heidelberg Retina Angiograph II; Heidelberg Engineering, Heidelberg, Germany). To amplify the autofluorescence signal, we aligned the best five images obtained by using the integrated system software and calculated a mean image.
Cross-sectional retinal reflectivity profiles were obtained with optical coherence tomography (OCT3; Carl Zeiss Meditec, Inc., Oberkochen, Germany). Subjects underwent OCT imaging incorporating 512 A-scans over a 3-μm transverse scanning length for an optimal sampling rate of 400 A-scans per second centered on the fovea. According to the manufacturer, the longitudinal resolution of the present model is 8 to 10 μm, and the transverse resolution is approximately 20 μm. The precise location and orientation of each scan were determined with the OCT simultaneous-view video images.
Because nearly all patients were affected by nystagmus, which complicates the recording, OCT scans, and autofluorescence were obtained in only 15 patients. Correlation analysis was performed with Spearman's test.
Results
Mutational Analysis
We analyzed 45 Italian individuals showing ophthalmic characteristic features of albinism including photophobia, nystagmus, reduced visual acuity, strabismus, iris translucency, fundus hypopigmentation, and foveal hypoplasia possibly in combination with various degrees of hypopigmentation of the skin and hair (23 males, 22 females, mean age, 17.6 ± 15 years) from 40 independent families. We found causative DNA variation in 43 (95.6%) of 45 patients analyzed. We identified 2 or more causative mutations in 62.2% of all patients, of which 8 (17.7%) were homozygotes and 20 (44.44%) were compound heterozygotes. In 15 (33.3%) cases the second mutation was not detected. We did not find mutations in the genes studied in two (4.4%) patients (Fig. 1). In three patients, more than two causative mutations were present, either in allelic (two cases) or nonallelic (one case) genes (Table 1). Overall, we detected 35 different, and possibly causative, mutations in TYR, P, TYRP1, and MATP, 15 (42.8%) of which were novel (Table 1). Furthermore, we identified 20 DNA variations in TYR, P, TYRP1, MATP, and OA1 that have been considered as polymorphisms (Supplementary Table S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-6091/-/DCSupplemental).
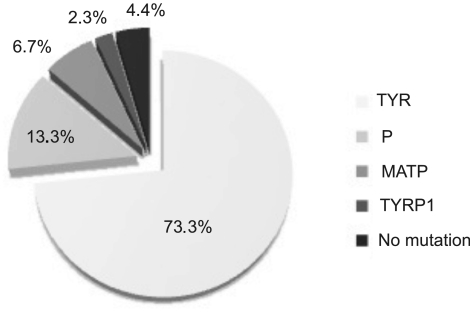
Figure 1.
Distribution of mutated genes involved in albinism based on the results of mutational analysis in our cohort of patients.
Table 1.
Positive Cases in the Mutational Screening
| Gene/Patient | Independent Families | Age | Sex | Mutation 1 |
Mutation 2 |
Other Mutations |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nucleotide Change | AA Change | Parent | Ref | Nucleotide Change | AA Change | Parent | Ref | Ref | |||||
| TYR | |||||||||||||
| P1 | 1 | 7 | F | c.98A>C | p.K33T | M | c.286_287insA | p.F95Fr | F | 23 | |||
| P2 | 2 | 21 | F | c.255T>A | p.Y85X | M | 24 | c.61C>T | p.P21S | 16 | |||
| P3 | 3 | 14 | M | c.255T>A | p.Y85X | M | 24 | c.346C>T | p.R116X | F | 25 | TYR: c.1467_68insT | 26 |
| P4 | 4 | 12 | M | c.255T>A | p.Y85X | M | 24 | NF | |||||
| P5 | 5 | 32 | F | c.255T>A | p.Y85X | M | 24 | NF | |||||
| P6 | 6 | 47 | F | c.619G>A | p.A206T | 27 | c.823G>T | p.V275F | 11 | ||||
| P7 | 7 | M | c.606T>G | p.H202Q | NF | ||||||||
| P8 | 8 | 63 | F | c.823G>T | p.V275F | 11 | c.823G>T | p.V275F | 11 | ||||
| P9 | 9 | 37 | F | c.883G>A | p.G295R | 28 | NF | ||||||
| P10 | 10 | M | c.1037–7T>A | 8 | c.1037–7T>A | 8 | |||||||
| P11 | 11 | 8 | M | c.1037–7T>A | M | 8 | c.1177delG | p.V393Fr | F | ||||
| P12 | 12 | 13 | M | c.1037–7T>A | M | 8 | NF | ||||||
| P13 | 13 | M | c.1037–7T>A | M | 8 | NF | |||||||
| P14 | 13 | F | c.1118C>A | p.T373K | F | 8 | c.1351A>G | p.Y451C | M | ||||
| P15 | 14 | 16 | F | c.1204C>T | p.R402X | M | 8 | c.137G>A | p.C46Y | F | |||
| P16 | 24 | M | c.1204C>T | p.R402X | M | 8 | c.137G>A | p.C46Y | F | ||||
| P17 | 15 | 9 | M | c.1205G>A | p.R402Q | M | 8 | c.74_75 insT | p.V25Fr | ||||
| P18 | 16 | 13 | M | c.1205G>A | p.R402Q | M | 8 | c.137G>A | p.C46Y | ||||
| P19 | 17 | 8 | M | c.1205G>A | p.R402Q | M | 8 | c.573delA | p.Y191Fr | F | |||
| P20 | 18 | 3 | F | c.1205G>A | p.R402Q | 8 | c.832C>T | p.R278X | TYR: c.346C>T (p.R116X) | 25 | |||
| P21 | 19 | F | c.1205G>A | p.R402Q | F | 8 | c.1177delG | p.V393Fr | M | ||||
| P22 | 20 | 15 | F | c.1205G>A | p.R402Q | F | 8 | c.1177delG | p.V393Fr | M | |||
| P23 | 21 | 40 | M | c.1205G>A | p.R402Q | 8 | c.1205G>A | p.R402Q | 8 | ||||
| P24 | 22 | 2 | F | c.1205G>A | p.R402Q | 8 | c.1205G>A | p.R402Q | 8 | ||||
| P25 | 23 | 24 | F | c.1205G>A | p.R402Q | 8 | c.1205G>A | p.R402Q | 8 | ||||
| P26 | 24 | 12 | M | c.1205G>A | p.R402Q | 8 | c.1205G>A | p.R402Q | 8 | ||||
| P27 | 25 | 10 | M | c.1205G>A | p.R402Q | F | 8 | c.1217C>T | p.P406L | M | 8 | ||
| P28 | 26 | 17 | F | c.1205G>A | p.R402Q | M | 8 | c.1217C>T | p.P406L | F | 8 | ||
| P29 | 27 | 9 | M | c.1205G>A | p.R402Q | M | 8 | c.1467_68 insT | p.A490Fr | F | 26 | ||
| P30 | 28 | 1 | F | c.1205G>A | p.R402Q | 8 | NF | ||||||
| P31 | 29 | 11 | M | c.1205G>A | p.R402Q | 8 | NF | ||||||
| P32 | 30 | F | c.1205G>A | p.R402Q | 8 | NF | |||||||
| P33 | 31 | 5 | F | c.1205G>A | p.R402Q | 8 | c.61C>T | p.P21S | M | 16 | |||
| P | |||||||||||||
| P34 | 32 | 33 | M | c.1025A>G | p.G432C | NF | |||||||
| P35 | 33 | 7 | M | c.1327G>A | p.V443I | F | 29 | c.2360C>T | p.A787V | M | 25 | ||
| P36 | 34 | 4 | F | c.2060C>T | p.A687V | NF | |||||||
| P37 | 35 | 41 | F | c.2216T>C | p.I739T | F | c.2216T>C | p.1739T | M | ||||
| P38 | 24 | M | c.2216T>C | p.1739T | F | c.2216T>C | p.1739T | M | |||||
| P39 | 36 | 13 | F | c.2329T>C | p.C777R | F | NF | ||||||
| TYRP1 | |||||||||||||
| P40 | 37 | 16 | F | c.869G>A | p.C290Y | F | c.869G>A | p.C290Y | M | ||||
| MATP | |||||||||||||
| P41 | 38 | F | c.161_171insGGTGGAGGCAG | c.1532C>A | p.A511E | TYR: c.1205G>A (p.R402Q) | 8 | ||||||
| P42 | 39 | M | c.G126T | p.M421 | c.T1280C | p.L427P | |||||||
| P43 | 40 | 4 | M | c.375T>A | p.V126D | F | c.1108G>C | p.G370R | M | ||||
Novel mutations are shaded. NF, not found; M, mother; F, father.
TYR was the most frequently mutated gene in our cohort of patients (33/45; 73.3%), followed by P (6/45; 13.3%), MATP (3/45; 6.7%), and TYRP1 (1/45; 2.3%). No causative mutations were detected in the OA1 gene. A total of 22 missense, 6 nonsense, and 6 frameshift mutations and 1 splicing mutation were identified. Four novel mutations in TYR and four in P were detected. We also identified mutations in rare loci, including six novel mutations in MATP and one in TYRP1 (Supplementary Table S2, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-6091/-/DCSupplemental).
OCA1 (TYR)
TYR mutational analysis showed 22 distinct mutations in 33 patients, including missense, frameshift, nonsense, and splicing variations (Table 1). Thirteen of 23 missense mutations identified were located in the exons 1 and 3.
The most frequent mutated allele identified was the c.G1205A (p.R402Q variation), which was found in 17 patients and 21 alleles. Because of the controversial causative role of this mutation, we also analyzed the other OCA and OA genes in four homozygous patients (P23–P26) and did not identify any additional causative mutation in these distinct loci. Ten patients carrying the p.R402Q variation were compound heterozygous in combination with either described or novel causative mutant alleles. The p.R402Q variant was also found in a patient bearing two causative mutations in MATP. In three further patients (P30–P32) carrying the p.R402Q variation, a second causative mutation was not detected.
We found three novel missense mutations: p.Y451C (c.A1351G), p.C46Y (c.137C>A), and p.H202Q (c.606T>G), all affecting amino acid residues highly conserved across evolution (from Xenopus laevis to Homo sapiens). A fourth novel mutation found in three compound heterozygous patients leads to a frameshift (c.1177delG). In two patients (P3, P19) we found three causative mutations in TYR.
OCA2 (P), OCA3 (TYRP1), and OCA4 (MATP)
Mutations of the P gene were found in six patients. All identified mutations (including four novel ones; Table 1) were missense variations. Two sibling patients (P37, P38) were homozygous for the novel mutation c.2216T>C (p.I739T) and one patient (P35) was compound heterozygous for two known mutations (c.1327G>A, p.V443I and c.2360C>T, p.A787V). In the remaining three individuals, a second mutation was not found (P34, P36, and P39).
The coding sequence of the TYRP1 gene was mutated in only one patient (P40), showing a novel homozygous missense mutation in exon 4 that results in a cysteine-to-tyrosine amino acid change (p.C290Y, c.G869A). The absence of this variation in 220 healthy control chromosomes with the absence of any apparent mutations in the other OCA and OA genes, and also the conservation of the amino acid residue across evolution, suggests that this variation may cause the disease phenotype.
Finally, six different novel mutations were detected in MATP gene in three patients. Patient 42 and 43 were compound heterozygous for two novel missense MATP mutations. Patient 41 showed two novel mutations affecting the first and the seventh exons of MATP and an additional nonallelic TYR gene variation (p.R402Q).
Identification of Polymorphisms
Direct sequencing analysis revealed 20 different polymorphisms in exons or into nearby intronic sequences of the screened genes (Supplementary Table S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-6091/-/DCSupplemental). We found overall eight novel polymorphisms in P, TYRP1, and MATP and none in the TYR gene.
Thirty percent of the exonic polymorphisms resulted in amino acid changes, whereas the remaining ones were silent.
The P gene had a significantly high frequency of missense variations with no apparent pathogenic significance (Supplementary Table S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-6091/-/DCSupplemental). Among them, we identified two novel nucleotide variations: c.1355T>A (p.452V/E) and c.1113C>T (no change D371).
We found two new intronic polymorphisms of the TYRP1 gene with high frequency. IVS7–19InsA was identified in 36.9% and IVS6+20C/T in 32.6% of the patients.
Patient 43 showed a c.1122G>C and a c.G814A substitution affecting exon 5 and 3, respectively, of the MATP gene that lead to the nonpathogenic protein variations p.374F/L and p.E272K.
Two polymorphic intronic variations were found at the OA1 gene: IVS6+10G/C and IVS8+12T/C, both with a frequency of 4.3%.
Phenotype Analysis
A detailed ophthalmic evaluation, including visual acuity measurements, evaluation of ocular motility and iris translucency, fundus examination, fundus autofluorescence (FAF), and optical coherence tomography (OCT), was performed in 18 OCA patients (mean age, 19 ± 14 years) from 16 independent families with mutations in each of the four OCA genes studied (Table 2). Clinical analysis revealed that nystagmus and strabismus were present in most patients independently from the mutated gene. Autofluorescence showed absence of macular pigment in 9 of 15 patients examined, even in those showing an intermediate degree (grade 2) of fundus pigmentation (macular transparency evaluation).
Table 2.
Ophthalmic Features of 18 Patients Analyzed Phenotypically
| Pt. | Gene | Age | Iris Pigmentation | Iris Translucens | Macular Transparency | Foveal Pit | Autofluorescence Macular Pigment | CVA RE | CVA LE | Nystagmus | Strabismus |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | TYR | 14 | LB | 3 | 1 | No | Absent | 20/200 | 20/200 | Yes | No |
| 4 | TYR | 12 | LB | 3 | 1 | No | Absent | 20/100 | 20/100 | Yes | — |
| 9 | TYR | 37 | LB | 2 | 2 | No | ND | 20/200 | 20/200 | Yes | Yes |
| 11 | TYR | 8 | LB | 2 | 2 | — | Absent | 20/200 | 20/200 | Yes | Yes |
| 15 | TYR | 16 | LB | 3 | 2 | No | Absent | 20/600 | 20/600 | Yes | No |
| 16 | TYR | 24 | LB | 3 | 2 | No | Absent | 20/600 | 20/600 | Yes | No |
| 17 | TYR | 9 | LB | 1 | 3 | Yes | ND | 20/100 | 20/50 | No | Yes |
| 18 | TYR | 13 | GB | 1 | 3 | No | Present | 20/25 | 20/25 | No | No |
| 22 | TYR | 15 | LB | 3 | 2 | No | Absent | 20/100 | 20/100 | Yes | Yes |
| 23 | TYR | 40 | LB | 3 | 1 | No | Absent | 20/400 | 20/200 | Yes | Yes |
| 26 | TYR | 12 | LB | 3 | 1 | ND | ND | 20/400 | 20/400 | Yes | Yes |
| 33 | TYR | 5 | LB | 2 | 3 | No | Present | 20/100 | 20/100 | No | Yes |
| 35 | P | 8 | GB | 2 | 2 | Yes | Absent | 20/200 | 20/200 | Yes | Yes |
| 37 | P | 41 | GG | 1 | 3 | No | Present | 20/60 | 20/60 | Yes | Yes |
| 38 | P | 34 | GB | 1 | 3 | Yes | Present | 20/60 | 20/40 | Yes | Yes |
| 40 | TYRP1 | 18 | LB | 1 | ND | Yes | Present | 20/100 | 20/100 | Yes | Yes |
| 41 | MATP | 51 | GB | 3 | 1 | No | Absent | 20/400 | 20/200 | Yes | Yes |
| 43 | MATP | 4 | ND | ND | ND | Yes | Present | 20/100 | 20/200 | Yes | Yes |
The patients carrying P, TYRP1, and MATP mutations showed a milder ocular phenotype than most patients carrying TYR mutations. Those carrying TYR mutations showed high prevalence of light blue (LB) iris pigmentation (11/12 cases) and high degree of iris translucency (7/12 patients with grade 3 iris translucency). Fundus examination and autofluorescence analysis revealed a high or intermediate degree of fundus hypopigmentation (9/12 patients with grade 1 or 2 macular transparency), whereas OCT analysis demonstrated the absence of the foveal pit in 9 of 10 cases. Most patients (7/12) displayed low visual acuity, ranging between 20/600 and 20/200.
Three patients carrying P mutations showed gray (GG) or gray-blue (GB) iris pigmentation with a variable amount of iris pigment and punctate transillumination, a high degree of fundus pigmentation (grade 3 in two of three patients), and the presence of the foveal pit in two of three patients. In two patients, visual acuity was preserved (from 20/60 to 20/40) despite the presence of nystagmus and strabismus.
The only patient carrying mutations of TYRP1 showed a mild ocular phenotype, LB iris, minimal punctuate transillumination, and presence of the foveal pit based on OCT evaluation and a visual acuity of 20/100 in both eyes.
Two patients bearing MATP mutations presented heterogeneous clinical findings, with a more severe ocular involvement and a worse visual acuity in the patient carrying the additional p.R402Q variation (c.G1205A) of the TYR gene.
Correlation analysis revealed that, independent of the gene mutation, reduced iris translucency and macular transparency (as markers of pigmentation) were significantly associated with (1) better visual acuity (P = 0.0007); (2) the presence of macular pigment assessed by autofluorescence analysis (P < 0.0001); (3) the presence of the foveal pit by OCT evaluation (P = 0.04); and (4) the absence of nystagmus (P = 0.05). Importantly, preserved visual acuity correlated with statistical significance (P = 0.0007) with presence of macular pigment, as assessed by autofluorescence analysis. No correlation was found between better visual acuity and the presence of the foveal pit (P = 0.3) or the absence of nystagmus (P = 0.06).
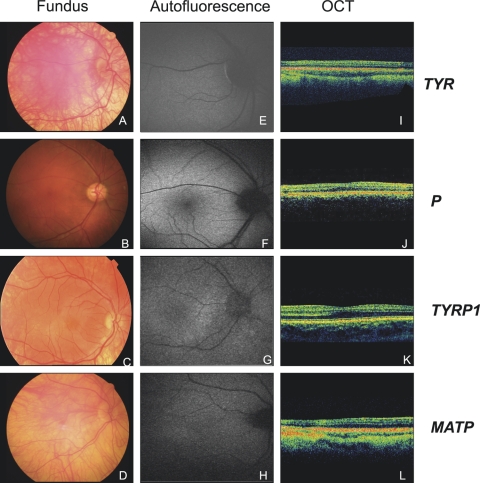
Examples of clinical findings including fundus photography, FAF, and OCT examinations in four patients with mutations in four different OCA genes are reported in Figure 2. As shown in the figure, a mild phenotype was found in two patients carrying P and TYRP1 mutations, which showed presence of the foveal pit, no visible choroidal macula vessels, and the presence of macular pigment evaluated by autofluorescence analysis. Two patients carrying TYR and MATP mutations, respectively, showed absence of the foveal pit, visible choroidal macula vessels, and absence of macular pigment by autofluorescence analysis.
Figure 2.
Fundus photography, FAF, and OCT analysis in four patients with mutations in different OCA genes: P3 (A, E, I), carrying TYR mutation; P36 (B, F, J) bearing a P mutation; P40 (C, G, K) with a TYRP1 mutation; and P41 (D, H, L) showing an MATP mutation (see Table 1). (A–D) Fundus photographs: choroidal vessels are visible in the macula in P3 (A) and P41 (D), but they are not visible in P36 (B) and P40 (C); (E–H) FAF: please note the absence of macular pigment in P3 (E) and P41 (H) and the presence of macular pigment in P36 (F) and P40 (G). (I–L) OCT of the posterior pole crossing the fovea, showing absence of the foveal pit in P3 (I) and P41 (L) and presence of the foveal pit in patients P36 (J) and P40 (K).
Discussion
In this study, we provided the first comprehensive mutational analysis of all genes associated with nonsyndromic albinism, performed in combination with a detailed clinical ophthalmic evaluation of a large series of Italian patients with albinism.
In agreement with previous results in Caucasian patients11 we found that the most frequent mutations are associated with TYR in the Italian population rather than with P. We found 73.3% OCA1, 13.3% OCA2, 2.3% OCA3, and 6.7% OCA4 in 45 Italian patients. In 4.4% of the cases, we were not able to identify any pathologic mutations. In 62.2% of patients we found homozygous or compound heterozygous mutations, whereas in 33.3% of cases we identified mutations in only one allele.
We found a wide spectrum of causative mutations in TYR, P, TYRP1, and MATP and additional DNA polymorphisms in five genes associated with different forms of albinism. Moreover, we identified 15 novel causative mutations in four different loci, expanding the database of OCA-causing mutations.
A missense mutation cluster was identified in putative functional domains of the TYR enzyme.27,30 Consistently, our data showed that many of the missense mutations identified are located in/or alongside the copper-binding sites corresponding to exons 1 and 3. Mutations in these regions are considered to act either by affecting copper binding or by disrupting the substrate-binding site.31 Our data confirm that different mutations within the tyrosinase-coding region underlie OCA1 disorders of varying severity. In one unusual subset of OCA1 patients, OCA1-TS, the mutation generates a temperature-sensitive tyrosinase protein.32 Consequently, melanin synthesis occurs only in the cooler areas of the body, such as the arms and legs. Several TS variants of tyrosinase have been sequenced. One of these (p.R402Q) represented the most common TYR mutation identified in our cohort of Italian families (37% of patients), who showed an evident OCA phenotype with various degrees of severity in ocular phenotype. This finding is in agreement with recent studies.8,14,15 The p.R402Q variation (c.G1205A) was identified in our cohort of patients, either in compound heterozygotes (in combination with known or novel pathogenic mutant alleles) or in patients who did not show a second causative mutation. Interestingly, we also found this variation to be the only identified mutation with a possible causative role in four homozygous patients with an oculocutaneous phenotype, two of whom showed severe visual loss. These data suggest a causative role of the p.R402Q variation of TYR, which has been considered for years a nonpathologic polymorphism.13 Furthermore, we identified this variation as an additional nonallelic mutation in 1 patient (P41) bearing two novel mutations in MATP. This patient showed a more severe ocular involvement in comparison with patients carrying only MATP mutations, thus suggesting an additional negative effect of the p.R402Q mutation in combination with allelic and nonallelic mutations.
OCA2 is the most common type of albinism, especially in black African OCA patients, occurring in approximately 50% of OCA patients worldwide.33 We found mutations of P only in 14% of Italian patient, carrying either known or novel mutations. Unlike from the TYR gene analysis, P gene mutations did not appear to cluster in specific regions but they were found between or in the proximity of transmembrane domain borders in the central region of the protein. Presently, few mutations causing the OCA3 phenotype worldwide have been identified in the TYRP1 gene.5,34–36 TYRP1 mutations causing OCA have been considered virtually absent in Caucasians,11 although a single German patient has been described.35 We identified one patient carrying a novel homozygous missense mutation of TYRP1 (p.C290Y), who showed a mild ocular phenotype. This novel mutation affects an amino acid residue that is highly conserved across evolution. Despite the low frequency of mutations in MATP (OCA4) worldwide12 with the exception of Japan, we found six novel MATP mutations in three Italian patients with a variable OCA phenotype. As expected, based on the selection of patients who showed at least some degree of skin and hair hypopigmentation, we did not find any mutation of the OA1 gene.
We were unable to identify mutations in 4.4% of patients screened, and we found mutations in only one allele in 33.3% (15/45) of cases. In these patients, mutations may be present in intronic or regulatory regions, which were not analyzed in our molecular analysis. Also, large genomic rearrangements cannot be identified with the strategy used in the present study. Finally, in patients with unidentified mutations, the disease may be due to mutations in OCA genes that are still to be identified as OCA-causing genes.
In a previous study,14 we identified more than two causative mutations in three patients (P3, P20, P41), either in allelic or nonallelic genes. Digenic mutation types (mutations in nonallelic genes) have been described in some cases of ocular albinism and Waardenburg syndrome.15,37 Our findings support the hypothesis that heterozygous mutations in nonallelic genes may result in a more severe phenotype.
Our comprehensive analysis of clinical and molecular data confirms that a phenotypic variability is present among patients and that it is generally independent from the mutated gene and the specific mutation. The lack of a sufficient number of patients with each gene mutation did not allow us to draw genotype–phenotype correlations within and among genotypes.
Most of the TYR patients showed a more severe ocular phenotype than did patients bearing mutations of P, TYRP1, or MATP, as previously reported in a genotype–phenotype correlation study.28 However, independent of the gene involved, the severity of the clinical phenotype inversely correlated with the degree of retinal pigmentation, confirming previous findings.38,39 In this respect, we found that visual acuity was more preserved in patients with low iris translucency and macular transparency assessed by autofluorescence analysis. In addition, autofluorescence analysis showed absence of macular pigment (and a poor visual acuity) also in those patients in whom fundus pigmentation assessments revealed an intermediate degree of macular transparency. These data support the use of autofluorescence analysis as a more sensitive analysis to predict the visual phenotype severity compared with fundus macular transparency in albino patients. We also found that low visual acuity was not associated with the presence of the foveal pit, in accordance with the recent report of Marmor et al.40
In conclusion, our clinical evaluation suggests that the degree of ocular pigmentation correlates with preservation of visual function. Our results confirm the wide clinical and genetic heterogeneity of OCA. They also suggest that TYR mutations cause a more severe ocular phenotype in most patients in comparison with P, TYRP1, or MATP mutations. Data obtained from the detailed ophthalmic evaluation also suggest that the severity of ocular phenotype depends on the degree of retinal pigmentation and support the use of autofluorescence analysis of macular pigment as a sensitive prognostic tool to predict the visual function in albino patients.
Finally, as the different OCA forms are indistinguishable on clinical grounds, and also considering both the high frequency of multiple DNA changes in the same patient and the lack of clear cut correlations between the clinical phenotype and molecular genotypes, we concluded that a comprehensive mutational analysis of at least four genes, such as TYR, P, MATP, and TYRP1 is necessary for the differential diagnosis and genetic counseling of patients who have oculocutaneous albinism. We believe that the wide range of patients analyzed in the present study provides valuable information for a better prognostic evaluation of patients with albinism and for a more efficient identification of patients who could benefit from the future experimental development of therapies.41,42
Acknowledgments
The authors thank Luciana Borrelli (TIGEM) for a critical reading of the manuscript.
Footnotes
Supported by Telethon Grant TIGEM P21 and Grant R01EY015136-01 from the National Eye Institute.
Disclosure: A. Gargiulo, None; F. Testa, None; S. Rossi, None; V. Di Iorio, None; S. Fecarotta, None; T. de Berardinis, None; A. Iovine, None; A. Magli, None; S. Signorini, None; E. Fazzi, None; M.S. Galantuomo, None; M. Fossarello, None; S. Montefusco, None; A. Ciccodicola, None; A. Neri, None; C. Macaluso, None; F. Simonelli, None; E.M. Surace, None
References
- 1. King RA, Hearing VJ, Creel DJ, Oetting WS. Albinism. In: Scriver CR, Beaudet AL, Sly WS, Valle D. eds. The Metabolic and Molecular Basis of Inherited Disease. New York: McGraw-Hill; 2001:5587–5627 [Google Scholar]
- 2. Kirkwood BJ. Albinism and its implications with vision. Insight. 2009;34:13–16 [PubMed] [Google Scholar]
- 3. Ray K, Chaki M, Sengupta M. Tyrosinase and ocular diseases: some novel thoughts on the molecular basis of oculocutaneous albinism type 1. Prog Retin Eye Res. 2007;26:323–358 [DOI] [PubMed] [Google Scholar]
- 4. Gardner JM, Nakatsu Y, Gondo Y, et al. The mouse pink-eyed dilution gene: association with human Prader-Willi and Angelman syndromes. Science. 1992;257:1121–1124 [DOI] [PubMed] [Google Scholar]
- 5. Boissy RE, Zhao H, Oetting WS, et al. Mutation in and lack of expression of tyrosinase-related protein-1 (TRP-1) in melanocytes from an individual with brown oculocutaneous albinism: a new subtype of albinism classified as “OCA3”. Am J Hum Genet. 1996;58:1145–1156 [PMC free article] [PubMed] [Google Scholar]
- 6. Fukamachi S, Shimada A, Shima A. Mutations in the gene encoding B, a novel transporter protein, reduce melanin content in medaka. Nat Genet. 2001;28:381–385 [DOI] [PubMed] [Google Scholar]
- 7. Schiaffino MV, Bassi MT, Galli L, et al. Analysis of the OA1 gene reveals mutations in only one-third of patients with X-linked ocular albinism. Hum Mol Genet. 1995;4:2319–2325 [DOI] [PubMed] [Google Scholar]
- 8. Hutton SM, Spritz RA. A comprehensive genetic study of autosomal recessive ocular albinism in Caucasian patients. Invest Ophthalmol Vis Sci. 2008;49:868–872 [DOI] [PubMed] [Google Scholar]
- 9. Gronskov K, Ek J, Brondum-Nielsen K. Oculocutaneous albinism. Orphanet J Rare Dis. 2007;2:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stevens G, van Beukering J, Jenkins T, Ramsay M. An intragenic deletion of the P gene is the common mutation causing tyrosinase-positive oculocutaneous albinism in southern African Negroids. Am J Hum Genet. 1995;56:586–591 [PMC free article] [PubMed] [Google Scholar]
- 11. Hutton SM, Spritz RA. Comprehensive analysis of oculocutaneous albinism among non-Hispanic Caucasians shows that OCA1 is the most prevalent OCA type. J Invest Dermatol. 2008;128:2442–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Inagaki K, Suzuki T, Shimizu H, et al. Oculocutaneous albinism type 4 is one of the most common types of albinism in Japan. Am J Hum Genet. 2004;74:466–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tripathi RK, Giebel LB, Strunk KM, Spritz RA. A polymorphism of the human tyrosinase gene is associated with temperature-sensitive enzymatic activity. Gene Expr. 1991;1:103–110 [PMC free article] [PubMed] [Google Scholar]
- 14. Opitz S, Kasmann-Kellner B, Kaufmann M, Schwinger E, Zuhlke C. Detection of 53 novel DNA variations within the tyrosinase gene and accumulation of mutations in 17 patients with albinism. Hum Mutat. 2004;23:630–631 [DOI] [PubMed] [Google Scholar]
- 15. Morell R, Spritz RA, Ho L, et al. Apparent digenic inheritance of Waardenburg syndrome type 2 (WS2) and autosomal recessive ocular albinism (AROA). Hum Mol Genet. 1997;6:659–664 [DOI] [PubMed] [Google Scholar]
- 16. King RA, Pietsch J, Fryer JP, et al. Tyrosinase gene mutations in oculocutaneous albinism 1 (OCA1): definition of the phenotype. Hum Genet. 2003;113:502–513 [DOI] [PubMed] [Google Scholar]
- 17. Schiaffino MV, d'Addio M, Alloni A, et al. Ocular albinism: evidence for a defect in an intracellular signal transduction system. Nat Genet. 1999;23:108–112 [DOI] [PubMed] [Google Scholar]
- 18. Creel DJ, Summers CG, King RA. Visual anomalies associated with albinism. Ophthalmic Paediatr Genet. 1990;11:193–200 [DOI] [PubMed] [Google Scholar]
- 19. Pott JW, Jansonius NM, Kooijman AC. Chiasmal coefficient of flash and pattern visual evoked potentials for detection of chiasmal misrouting in albinism. Doc Ophthalmol. 2003;106:137–143 [DOI] [PubMed] [Google Scholar]
- 20. Chaki M, Mukhopadhyay A, Ray K. Determination of variants in the 3′-region of the tyrosinase gene requires locus specific amplification. Hum Mutat. 2005;26:53–58 [DOI] [PubMed] [Google Scholar]
- 21. Antonarakis SE. Recommendations for a nomenclature system for human gene mutations. Nomenclature Working Group. Hum Mutat. 1998;11:1–3 [DOI] [PubMed] [Google Scholar]
- 22. Summers CG, Knobloch WH, Witkop CJ, Jr, King RA. Hermansky-Pudlak syndrome: ophthalmic findings. Ophthalmology. 1988;95:545–554 [DOI] [PubMed] [Google Scholar]
- 23. Oetting WS, Mentink MM, Summers CG, Lewis RA, White JG, King RA. Three different frameshift mutations of the tyrosinase gene in type IA oculocutaneous albinism. Am J Hum Genet. 1991;49:199–206 [PMC free article] [PubMed] [Google Scholar]
- 24. Zahed L, Zahreddine H, Noureddine B, et al. Molecular basis of oculocutaneous albinism type 1 in Lebanese patients. J Hum Genet. 2005;50:317–319 [DOI] [PubMed] [Google Scholar]
- 25. Oetting WS, Fryer JP, King RA. Mutations of the human tyrosinase gene associated with tyrosinase related oculocutaneous albinism (OCA1): mutations in brief no. 204. Online. Hum Mutat. 1998;12:433–434 [DOI] [PubMed] [Google Scholar]
- 26. Chintamaneni CD, Halaban R, Kobayashi Y, Witkop CJ, Jr, Kwon BS. A single base insertion in the putative transmembrane domain of the tyrosinase gene as a cause for tyrosinase-negative oculocutaneous albinism. Proc Natl Acad Sci U S A. 1991;88:5272–5276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. King RA, Mentink MM, Oetting WS. Non-random distribution of missense mutations within the human tyrosinase gene in type I (tyrosinase-related) oculocutaneous albinism. Mol Biol Med. 1991;8:19–29 [PubMed] [Google Scholar]
- 28. Miyamura Y, Verma IC, Saxena R, et al. Five novel mutations in tyrosinase gene of Japanese and Indian patients with oculocutaneous albinism type I (OCA1). J Invest Dermatol. 2005;125:397–398 [DOI] [PubMed] [Google Scholar]
- 29. King RA, Willaert RK, Schmidt RM, et al. MC1R mutations modify the classic phenotype of oculocutaneous albinism type 2 (OCA2). Am J Hum Genet. 2003;73:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tripathi RK, Strunk KM, Giebel LB, Weleber RG, Spritz RA. Tyrosinase gene mutations in type I (tyrosinase-deficient) oculocutaneous albinism define two clusters of missense substitutions. Am J Med Genet. 1992;43:865–871 [DOI] [PubMed] [Google Scholar]
- 31. Oetting WS, King RA. Molecular analysis of type I-A (tyrosinase negative) oculocutaneous albinism. Hum Genet. 1992;90:258–262 [DOI] [PubMed] [Google Scholar]
- 32. King RA, Townsend D, Oetting W, et al. Temperature-sensitive tyrosinase associated with peripheral pigmentation in oculocutaneous albinism. J Clin Invest. 1991;87:1046–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oetting WS, King RA. Molecular basis of albinism: mutations and polymorphisms of pigmentation genes associated with albinism. Hum Mutat. 1999;13:99–115 [DOI] [PubMed] [Google Scholar]
- 34. Manga P, Kromberg JG, Box NF, Sturm RA, Jenkins T, Ramsay M. Rufous oculocutaneous albinism in southern African Blacks is caused by mutations in the TYRP1 gene. Am J Hum Genet. 1997;61:1095–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rooryck C, Roudaut C, Robine E, Musebeck J, Arveiler B. Oculocutaneous albinism with TYRP1 gene mutations in a Caucasian patient. Pigment Cell Res. 2006;19:239–242 [DOI] [PubMed] [Google Scholar]
- 36. Forshew T, Khaliq S, Tee L, et al. Identification of novel TYR and TYRP1 mutations in oculocutaneous albinism. Clin Genet. 2005;68:182–184 [DOI] [PubMed] [Google Scholar]
- 37. Ming JE, Muenke M. Multiple hits during early embryonic development: digenic diseases and holoprosencephaly. Am J Hum Genet. 2002;71:1017–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Summers CG, King RA. Ophthalmic features of minimal pigment oculocutaneous albinism. Ophthalmology. 1994;101:906–914 [DOI] [PubMed] [Google Scholar]
- 39. Seo JH, Yu YS, Kim JH, Choung HK, Heo JW, Kim SJ. Correlation of visual acuity with foveal hypoplasia grading by optical coherence tomography in albinism. Ophthalmology. 2007;114:1547–1551 [DOI] [PubMed] [Google Scholar]
- 40. Marmor MF, Choi SS, Zawadzki RJ, Werner JS. Visual insignificance of the foveal pit: reassessment of foveal hypoplasia as fovea plana. Arch Ophthalmol. 2008;126:907–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Surace EM, Domenici L, Cortese K, et al. Amelioration of both functional and morphological abnormalities in the retina of a mouse model of ocular albinism following AAV-mediated gene transfer. Mol Ther. 2005;12:652–658 [DOI] [PubMed] [Google Scholar]
- 42. Gargiulo A, Bonetti C, Montefusco S, et al. AAV-mediated tyrosinase gene transfer restores melanogenesis and retinal function in a model of oculo-cutaneous albinism type I (OCA1). Mol Ther. 2009;17:1347–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]