Abstract
As part of the CANCER Antimicrobial Surveillance Program in North America, a Pseudomonas aeruginosa isolate, strain 07-406, was shown to possess a metallo-β-lactamase, designated VIM-7. blaVIM-7 is located on a 24-kb plasmid which can be readily transferred into Enterobacteriaceae and other pseudomonads. This is the first report of a mobile metallo-β-lactamase gene, blaVIM-7, being detected within the United States.
In 1999, a novel family of class B metallo-β-lactamases, the VIM family (VIM-1 to VIM-6 enzymes) of Pseudomonas aeruginosa and Acinetobacter spp. in Europe, was initially described (4, 5, 10) The VIM family was subsequently found in strains of Serratia marcescens and Acinetobacter spp. in Korea (VIM-2) (15) and P. aeruginosa (VIM-3), Pseudomonas putida, and Pseudomonas stutzeri spp. (VIM-2) in Taiwan (12, 14, 15). More recently, VIM variants have been found in Escherichia coli (VIM-1) (6) and P. aeruginosa (VIM-4; EMBL accession no. AY135661 and AF531419) (8) in Greece, in Klebsiella pneumoniae (VIM-5; EMBL accession no. AY144612) in Turkey, and in P. putida (VIM-6; EMBL accession no. AY165025) in Singapore. The blaVIM gene, like the blaIMP gene, is carried on mobile gene cassettes inserted into class 1 integrons and located chromosomally or on resident plasmids (3). The class 1 integrons are the most common mechanisms by which bacteria are able to move resistant gene cassettes from one bacterium to another. The process involves recombination between 59-bp elements on the gene cassette and attI1 sites on the integron (2).
P. aeruginosa, isolate 07-406, was cultured from a 58-year-old female with a history of autoimmune hepatitis that required a liver transplant in 1988. In May 2001, she was diagnosed with left-breast carcinoma. Six days after admission of the patient, a diffuse pneumonitis was noted, and an invasive pulmonary sample was taken by bronchoscopy that revealed P. aeruginosa pneumonia. The organism was resistant to all tested antimicrobials except polymyxin B (MIC, ≤2 μg/ml) by local tests. P. aeruginosa isolate 07-406 gave a positive result with the metallo-β-lactamase Etest strip, the imipenem MIC being reduced from 256 to 4 μg/ml in the presence of EDTA (11).
The genomic library was created by partial Sau3A restriction, cloned into pK18, and expressed in E. coli strain DH5α [supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1] as previously described (9). Recombinants were screened on agar containing ampicillin plus the serine β-lactamase inhibitor BRL42715 (GlaxoSmithKline), ensuring that any resistance detected was not mediated by a serine-type β-lactamase (1). Twelve colonies harboring identical inserts were isolated, and one colony demonstrating imipenemase activity (extinction coefficient, −7,000) inhibited by EDTA was chosen randomly for further study. E. coli DH5α carrying pMATVIM-7 conferred resistance to nearly all β-lactams except for aztreonam and the carbapenems; P. aeruginosa 07-406 was resistant to all β-lactams (Table 1). blaVIM-7 is found on a 24-kb plasmid which can be readily transferred by electroporation into Enterobacteriaceae and other pseudomonads (data not shown).
TABLE 1.
MICs of β-lactams for P. aeruginosa 07-406, E. coli DH5α, and E. coli DH5α containing pMATVIM-7
| β-Lactam | MIC (μg/ml)
|
||
|---|---|---|---|
| P. aeruginosa 07-406 | E. coli DH5α | E. coli DH5α (pMATVIM-7) | |
| Ceftazidime | >256 | 0.125 | 128 |
| Cefotaxime | >256 | 0.125 | 128 |
| Cefepime | >256 | 0.06 | 8 |
| Cefoxitin | >256 | 2 | 4 |
| Ceftriaxone | >256 | 0.125 | 32 |
| Imipenem | >256 | 0.06 | 0.5 |
| Meropenem | >256 | 0.06 | 0.125 |
| Piperacillin | >256 | 0.5 | 32 |
| Ampicillin | >256 | 1 | 256 |
| Amoxicillin | >256 | 2 | 256 |
| Oxacillin | >256 | 4 | 32 |
| Aztreonam | >256 | 0.06 | 0.125 |
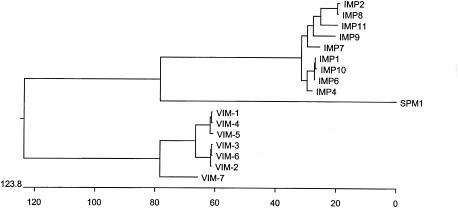
The clone harbored the recombinant plasmid pMATVIM-7, containing a 1,540-bp insert that was sequenced on both strands. Primer sequences used for both sequencing the insert pMATVIM-7 and back probing are shown in Table 2. Sequence analysis of the pMATVIM-7 insert revealed the presence of a 795-bp open reading frame showing high homology with a number of previously cloned metallo-β-lactamases (Fig. 1). The phylogenetic tree (Fig. 2), which is based on a CLUSTAL W multiple alignment of the putative amino acid sequence of the β-lactamase from P. aeruginosa strain 07-406 with other metallo-β-lactamases, demonstrates that it clusters more closely to the VIM family than the IMP family of metallo-β-lactamases. VIM-7 has 77% amino acid identity to VIM-1, whereas VIM-1 to VIM-6 have 89 to 99% identity (4, 7, 13). These amino acid variations among VIM-7 and the other VIM-type enzymes cluster in the leader sequence but are also found throughout the mature protein. The amino acid changes within the mature protein are often changes involving functionally different residues, namely, Q48K, S61K, D64G, S192R, Y195F, N216D, E225K, and H219R. The most likely site for cleavage occurs between amino acid positions 26 and 27 (YSA-QP), which would leave a mature peptide of 25,392 Da with a pI of 5.71. This pI is in close agreement with the experimental pI of 6.6 (data not shown).
TABLE 2.
Primers used to sequence pMATVIM-7 and to back probe to the P. aeruginosa 07-406 chromosome to verify origins of clones
| Primer name | Nucleotide sequence reading 5′→3′ |
|---|---|
| M13F | GTAAAACGACGGCCAGTG |
| pK18R | GCAAGGCGATTAAGTTGG |
| PVIM-F | GCCGTGCGCGTACTGACTGTTTG |
| PVIM-R | CAAACAGTCAGTACGCGCACGGC |
| FPF | ATTCGCAGCTTTCTGGTTGG |
| FPR | TAGCCTTGAGCGCAGCGTTG |
FIG. 1.
Nucleotide sequence of the 1,546-bp insert of recombinant plasmid pMATVIM-7 containing the blaVIM-7 coding sequence. The start codon of blaVIM-7 is indicated with a horizontal arrow, and the stop codon is indicated with an asterisk. The deduced amino acid sequence is reported below the gene sequence. The conserved core and inverse core sites are enclosed in boxes, and the attI1 site is highlighted in italicized capital letters.
FIG. 2.
Phylogenetic tree showing the relatedness of VIM-7 to other mobile metallo-β-lactamases. The phylogenetic tree is based on a CLUSTAL W alignment of a metallo-β-lactamase proteins generated by using the PAM250 MATRIX.
Analysis of the DNA sequence (with the Lasergene DNASTAR software package) of the pMATVIM-7 insert (Fig. 1) indicates that blaVIM-7 is preceded by a ribosome binding site (AGGAG) 6 bp upstream of the start codon. The presence of a conserved core site 13 bp upstream of the ribosome binding site, together with an inverse core site 7 bp after the stop codon and a 59-bp element, demonstrates that blaVIM-7 is harbored on a gene cassette. Additionally, an attI1 site identified immediately upstream of the gene cassette containing the promoter of the intI gene suggests that blaVIM-7, like other metallo-β-lactamases, is harbored on an integron (Fig. 3).
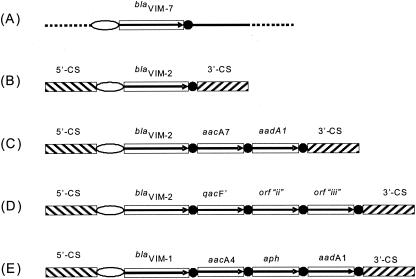
FIG. 3.
Genetic context of VIM-7 and VIM metallo-β-lactamases. A schematic map of the recombinant plasmid PMATVIM-7 carrying blaVIM-7 (accession no. AJ536835 [A]) is compared with examples of structures of blaVIM-2-containing class 1 integrons isolated in France (accession no. AF191564 [B]) and Korea (accession no. AF369871 [C] and accession no. AY030343 [D]) and with the blaVIM-1 integron isolated from Italy (accession AJ278514 [E]). Hatched rectangles indicate 5′ and 3′ CSs, black circles indicate 59-bp elements, and open ellipses indicate attI1 sites. Open reading frames of the various resistance genes are boxed, with an arrow indicating the direction of transcription. The solid line in pMATVIM-7 indicates the clones' insert from P. aeruginosa 07-406, and dotted lines indicate the cloning vector pK18.
The gene encoding the enzyme described in this paper, blaVIM-7, like the blaVIM-1 and blaVIM-3 genes, is harbored on a gene cassette which gives it the potential to move readily from one genome to another (Fig. 3). The presence of an attI1 site upstream of blaVIM-7 also strongly suggests that the blaVIM-7 cassette is part of an integron, which characteristically consists of an integrase gene followed by a recombination site (attI1 site). The blaVIM-7 genetic locus does differ in that no additional antimicrobial resistance genes were found directly downstream of blaVIM-7. The 600 bp of DNA downstream of blaVIM-7 in the recombinant plasmid pMATVIM-7 displays no homology with any antimicrobial resistance genes or the 3′ conserved sequence (CS) found in most class 1 integrons. The 3′ CS is a section of DNA conserved among many class 1 integrons, consisting of the remnants of a quaternary ammonium compound resistance gene, qacEΔ1, fused to a sulfonamide resistance gene, su1. It is possible that this section is present further downstream of the blaVIM-7 gene that is not represented in the clone pMATVIM-7. However, the 3′ CS is not found in all class 1 integrons (e.g., Tn402) and is not required for mobility.
VIM-7, unlike the other enzymes of the VIM group, is markedly divergent (Fig. 2). VIM-7 shows 77% identity with VIM-1, whereas the other enzymes vary from each other by less than 11%. Many of the changes unique to VIM-7 were changes in amino acids possessing different functions and may well alter the biochemical properties of the enzyme. However, though divergent from other VIM-like proteins, VIM-7 shares even less homology with other metallo-β-lactamases and therefore may be considered part of the VIM family. These data and the genetic context of the blaVIM-7 gene indicate that VIM-7 is only distantly related to the other VIM-like metallo-β-lactamases. It is therefore unlikely that VIM-7 has arrived on the North American continent via immediate dissemination from Europe or East Asia, where the other VIM enzymes have been reported. It is probable that VIM-7 has arisen independently within the United States, possibly by the therapeutic use of broad-spectrum β-lactams.
This is the first report of a mobile metallo-β-lactamase, VIM-7, being found in the bacterial population of the United States. blaVIM-7 has been shown to be highly mobile and can be expressed in Enterobacteriaceae as well as Pseudomonas spp. Given that these enzymes can hydrolyze all known classes of therapeutic β-lactams and that there is no clinically available metallo-β-lactamase inhibitor, mobile metallo-β-lactamases will pose a serious threat to broad-spectrum β-lactam therapies within the United States.
Nucleotide sequence accession number.
The nucleotide sequence reported here has been registered with the EMBL database under accession no. AJ536835.
REFERENCES
- 1.Coleman, K., D. R. J. Griffin, J. W. J. Page, and P. A. Upshon. 1989. In vitro evaluation of BRL 42715, a novel β-lactamase inhibitor. Antimicrob. Agents Chemother. 33:1580-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall, R. M. 1998. Antibiotic resistance in gram-negative bacteria: the role of gene cassettes and integrons. Drug Resist. Updates 1:109-119. [DOI] [PubMed] [Google Scholar]
- 3.Laraki, N., M. Galleni, I. Thamm, M. L. Riccio, G. Amicosante, J.-M. Frère, and G. M. Rossolini. 1999. Structure of In31, a blaIMP-containing Pseudomonas aeruginosa integron phyletically related to In5, which carries an unusual array of gene cassettes. Antimicrob. Agents Chemother. 43:890-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauretti, L., M. L. Riccio, A. Mazzariol, G. Cornaglia, G. Amicosante, R. Fontana, and G. M. Rossolini. 1999. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 43:1584-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mavroidi, A., A. Tsakris, E. Tzelepi, S. Pournaras, V. Loukova, and L. S. Tzouvelekis. 2000. Carbapenem-hydrolysing VIM-2 metallo-β-lactamase in Pseudomonas aeruginosa from Greece. J. Antimicrob. Chemother. 46:1041-1042. [DOI] [PubMed] [Google Scholar]
- 6.Miriagou, V., E. Tzelepi, D. Gianneli, and L. S. Tzouvelekis. 2003. Escherichia coli with a self-transferable, multiresistant plasmid coding for metallo-β-lactamase VIM-1. Antimicrob. Agents Chemother. 47:395-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poirel, L., T. Naas, D. Nicolas, L. Collet, S. Bellais, J.-D. Cavallo, and P. Nordmann. 2000. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 44:891-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pournaras, S., A. Tsakris, M. Maniati, L. S. Tzouvelekis, and A. N. Maniatis. 2002. Novel variant (blaVIM-4) of the metallo-β-lactamase gene blaVIM-1 in a clinical strain of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 46:4026-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toleman, M. A., A. M. Simm, T. A. Murphy, A. C. Gales, D. J. Biedenbach, R. N. Jones, and T. R. Walsh. 2002. Molecular characterization of SPM-1, a novel metallo-β-lactamase isolated in Latin America: report from the SENTRY antimicrobial surveillance programme. J. Antimicrob. Chemother. 50:673-679. [DOI] [PubMed] [Google Scholar]
- 10.Tsakris, A., S. Pournaras, N. Woodford, M.-F. Palepou, G. S. Babini, J. Douboyas, and D. M. Livermore. 2000. Outbreak of infections caused by Pseudomonas aeruginosa producing VIM-1 carbapenemase in Greece. J. Clin. Microbiol. 38:1290-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh, T. R., A. Bolmström, A. Qwärnström, and A. Gales. 2002. Evaluation of a new Etest for detecting metallo-β-lactamases in routine clinical testing. J. Clin. Microbiol. 40:2755-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan, J.-J., P.-R. Hsueh, W.-C. Ko, K.-T. Luh, S.-H. Tsai, H.-M. Wu, and J.-J. Wu. 2001. Metallo-β-lactamases in clinical Pseudomonas isolates in Taiwan and identification of VIM-3, a novel variant of the VIM-2 enzyme. Antimicrob. Agents. Chemother. 45:2224-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan, J. J., W. C. Ko, C. L. Chuang, and J. J. Wu. 2002. Metallo-β-lactamase-producing Enterobacteriaceae isolates in a university hospital in Taiwan: prevalence of IMP-8 in Enterobacter cloacae and first identification of VIM-2 in Citrobacter freundii. J. Antimicrob. Chemother. 50:503-511. [DOI] [PubMed] [Google Scholar]
- 14.Yum, J. H., K. Yi, H. Lee, D. Yong, K. Lee, J. M. Kim, G. M. Rossolini, and Y. Chong. 2002. Molecular characterization of metallo-β-lactamase-producing Acinetobacter baumannii and Acinetobacter genomospecies 3 from Korea: identification of two new integrons carrying the bla(VIM-2) gene cassettes. J. Antimicrob. Chemother. 49:837-840. [DOI] [PubMed] [Google Scholar]
- 15.Yum, J. H., D. Yong, K. Lee, H. S. Kim, and Y. Chong. 2002. A new integron carrying VIM-2 metallo-β-lactamase gene cassette in a Serratia marcescens isolate. Diagn. Microbiol. Infect. Dis. 42:217-219. [DOI] [PubMed] [Google Scholar]