This study shows that ILK can trans-dominantly control cooperative integrin signaling events used to regulate the organization of the actin cytoskeleton in differentiated HTM cells. Alterations in ILK activity are likely to affect how TM cells in vivo respond to ECM-mediated signals, thereby altering the functional properties of the TM, such as contractility.
Abstract
Purpose.
To determine whether integrin-linked kinase (ILK) controls the organization of the actin cytoskeleton in the trabecular meshwork (TM) by regulating integrin co-signaling.
Methods.
The cell binding domain and the Heparin II (Hep II) domain of fibronectin were used to activate α5β1 and α4β1 integrin signaling, respectively, in differentiated human TM (HTM) cells. The role of ILK was determined using either ILK small interfering RNA (siRNA) to knockout ILK expression or the ILK inhibitors, KP392 and QLT0267. The knockdown of ILK expression was verified by Western blot analysis. The presence of actin stress fibers and focal adhesions was determined by labeling cultures with phalloidin and anti-talin or ILK antibodies, respectively.
Results.
Cell spreading in differentiated HTM cells required ILK, since ILK siRNA and the ILK inhibitors significantly reduced cell spreading, actin polymerization, and the localization of talin and ILK in focal adhesions (FAs). Both cell spreading and the localization of talin and ILK to FAs in differentiated HTM cells could be rescued by inducing α4β1 integrin signaling with a recombinant Hep II domain of fibronectin, even though α4β1 integrins were not found in FAs. In the absence of ILK inhibition, the Hep II domain had minimal effect on α5β1 integrin-mediated spreading.
Conclusions.
This study suggests that cooperative α5β1/α4β1 integrin signaling may be regulated by ILK trans-dominantly and that alterations in ILK activity may affect actin cytoskeleton organization and contractility in the TM.
Recent studies show that the contractile properties of human trabecular meshwork (HTM) cells regulate intraocular pressure (IOP) by increasing aqueous humor outflow and that cell-matrix signaling events mediated by the Heparin II (Hep II) domain of fibronectin and α4β1 integrins1–3 may be involved. In vitro, the Hep II domain activates an α4β1 integrin signaling pathway that triggers the disruption of the actin cytoskeleton in confluent cultures of both differentiated HTM cells and an immortalized TM-1 cell line, which is consistent with its functional role in vivo.1,2,4 Activation of this α4β1 integrin signaling pathway requires co-signaling with either α1β1 or α2β1 integrins, which are receptors for types I and IV collagens.3 TM-1 cells interacting with a fibronectin substrate, presumably via α5β1 integrin, appeared to be refractile to activation of α4β1 integrins via the Hep II domain, suggesting that integrin crosstalk in HTM cells is regulated.
Most cells express more than one integrin and cross-talk among different integrins as well as between integrins and other receptors impacts contractility and cell spreading.5 Co-signaling between integrins can result in both antagonistic, or trans-dominant inhibition, and cooperative signaling. For instance, α4β1 integrin activation inhibits α5β1 integrin mediated stress fiber and focal adhesion formation in melanoma cells6 and suppresses metalloprotease expression transduced by α5β1 integrin in synovial fibroblasts.7 In Chinese hamster ovary cells, αIIbβ3 integrin engagement down-regulates the activity of α5β1 and α2β1 integrins.8 In contrast, co-signaling between α5β1 and α4β1 integrins in proliferating HTM cells induces the formation of actin stress fibers and cell spreading.9
Integrin signaling has been shown to be regulated by a number of cytoplasmic proteins, including integrin-linked kinase (ILK). ILK is an adaptor protein containing an ankyrin repeat domain, a pleckstrin homology-like motif, and a pseudokinase-like domain.10 ILK interacts with the cytoplasmic domain of β1 and β3 integrins and localizes to focal adhesions.10–12 ILK also interacts with phosphatidylinositol-3,4,5-trisphospate (PIP3)13,14 and plays a major structural role in integrin mediated adhesion complexes through interactions with a heterotrimeric complex of paxillin, PINCH, and α- or β-parvin referred to as the IPP complex,15,16 as well as kindlin-217–19 and mitogen-inducible gene (Mig)-2.17
Since previous studies have shown that activation of α4β1 integrin signaling in HTM cells can have opposite effects on actin organization and hence contractility,4,9 we wanted to determine whether there were any cytoplasmic signaling molecules that could be regulating α4β1 integrin signaling. Here, we show that ILK is involved in regulating α4β1 integrin signaling in HTM cells plated on the cell binding domain of fibronectin. The present studies show that ILK inhibits α5β1/α4β1 integrin co-signaling in a trans-dominant fashion. This is the first evidence that ILK can regulate cooperative integrin signaling in HTM cells.
Methods
Cell Culture
The A7–1 (71-yr-old) and N27TM-1 (27-yr-old) HTM cell strains isolated as described previously20,21 were grown until differentiated/quiescent as described previously.22 Cells were used in experiments within three days after achieving differentiation. The N27TM-1 cell strain was used in the small interfering RNA (siRNA) experiments. All other experiments were done with the A7–1 cell strain. The procurement of human tissue was done with the consent of the donors according to the tenets of the Declaration of Helsinki.
Preparation of Recombinant Proteins
Recombinant Hep II domain (type III 12 to 14 repeats of fibronectin), the cell binding domain (CBD; type III 7 to 10 repeats of fibronectin), and the IIICS domain of fibronectin were made as described previously.2,23 Recombinant vascular cell adhesion molecule (VCAM)-1 consisting of the seven extracellular domains was kindly provided by Deane Mosher (University of Wisconsin, Madison, WI) and was prepared as previously described.24
ILK siRNA
Human ILK siRNA (ON-TARGETplus SMARTpool L-004,499-00-0005) and control siRNA (ON-TARGETplus Non-targeting Pool D-001810-10-05) were obtained from Dharmacon/Thermo Fisher Scientific (Lafayette, CO). N27TM-1 cells were transfected with 25 or 50 nM siRNA using a cationic lipid-based reagent (Lipofectamine 2000; Invitrogen Corp., Carlsbad, CA) according to the manufacturer's reverse transfection protocol. Cells were incubated for 48 hours before performing a spreading assay (see below).
Western Blot Analyses
ILK expression levels were determined with Western blot analysis of cell lysates. Cells were lysed with ice cold lysis buffer (25 mM Hepes, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM NaF, 1% NP-40, 0.25% deoxycholate, and protease inhibitors). Lysates (10 μg) were resolved on a 10% SDS-PAGE and transferred to a membrane (Immobilon-P; Millipore Corp., Billerica, MA). The membrane was blocked and incubated with primary and secondary antibody as previously described.9 ILK was detected using an ILK polyclonal antibody (Cell Signaling Technologies, Danvers, MA) diluted 1:1000. GAPDH, the loading control, was detected with a rabbit anti-GAPDH antibody (Abcam Inc., Cambridge, MA). Bound antibody was detected with an enhanced chemiluminescence kit (ECL Plus Western blotting detection kit; Amersham Biosciences, Piscataway, NJ) and the area of the bands was measured using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).
Spreading Assay
Serum-starved cells were replated on coverslips coated with 10 μg/mL of the CBD of fibronectin and allowed to spread for 3 hours as previously described.9 All spreading assays were performed in the presence of cycloheximide to prevent endogenous synthesis of extracellular matrix proteins. All peptides, inhibitors, and antibodies were added at the time of replating the cells. The ILK inhibitors KP392 (100 μM) and QLT0267 (20 μM) were supplied by Quadra Logic Technology (QLT; Vancouver, BC, Canada). The phosphotidylinositol 3-kinase (PI3K) inhibitor LY294002 (10 or 25 μM) and the α4 integrin blocking antibody (clone P1H4; 25 μg/mL) were purchased from EMD Biosciences, Inc. (San Diego, CA) and Millipore Corp, respectively. Dimethyl sulfoxide (DMSO) served as the control for the ILK and PI3K inhibitors.
Immunofluorescence Microscopy
HTM cells were permeabilized, fixed, and blocked as previously described.9 Blocked cells were incubated for 1 hour with mouse anti-vinculin (Sigma Aldrich Co., St. Louis, MO), anti-ILK (Millipore Corp.), anti-talin (Millipore Corp.), anti-paxillin (BD Biosciences, San Jose, CA), or anti-FAK (BD Biosciences) monoclonal antibody. Antibodies were diluted 1:3000 (vinculin) or 1:1000 (ILK, talin, paxillin, and FAK) in 0.1% BSA in PBS. Cells were then incubated simultaneously with Alexa Fluor 546–conjugated goat anti-mouse secondary antibody (4 μg/mL; Invitrogen) and Alexa Fluor 488–conjugated phalloidin (0.67 unit/mL; Invitrogen) in 0.1% BSA/PBS for 1 hour. For localization of α4β1 integrin, HTM cells were fixed in 100% methanol for 15 minutes at −20°C, blocked as above, and then incubated for 1 hour with mouse anti-α4 integrin antibody (clone HP2/1; Millipore Corp.) at 1:1000 in 0.1% BSA/PBS followed by 1 hour incubation with the Alexa Fluor 546–conjugated goat anti-mouse secondary antibody. Fluorescent images were acquired using an epifluorescence microscope (Axioplan 2; Carl Zeiss, Inc., Thornwood, NY) equipped with a digital camera (Axiocam HRm; Zeiss) together with image acquisition software (AxioVision version 4.5; Zeiss). In cells treated with the ILK inhibitors, cell spreading was determined by counting spread cells containing polymerized actin versus cells lacking polymerized actin or rounded cells from 8 to 12 different fields of view per coverslip (n = 100 cells/treatment). Cell area for the siRNA experiments was measured from four to six different fields of view per coverslip using the image acquisition software (n = 123 cells/treatment).
Cell Adhesion Assay
The cell adhesion assay was performed with the A7–1 cells as previously described.9 Briefly, serum-starved HTM cells were replated into 96 well plates in the presence of 10 μg/mL integrin adhesion blocking antibodies or control mouse IgG (Sigma-Aldrich). All wells were precoated with 47 nM of the CBD for 1 hour at 37°C. Bound cells were fixed and stained overnight with 0.5% toluidine blue in 4% paraformaldehyde. Bound dye was then redissolved in 2% SDS and detected at 600 nm using a microplate reader. Rat β1 integrin antibody mAb13 and rat α5 integrin antibody mAb16 were kindly provided by Steve Akiyama (National Institutes of Health, Research Triangle Park, NC). The αv integrin monoclonal antibody M9 was purchased from Millipore Corp.
Fluorescence-Activated Cell-Sorting (FACS) Analysis
FACS analysis of the A7–1 cells was performed as previously described9 on a cytometer (FACScan; BD Biosciences). The α4 monoclonal HP2/1, α5 polyclonal, β1 monoclonal Hb1.1, and α5β1 monoclonal HA5 antibodies were obtained from Millipore Corp.
Data Analysis
All comparisons were made as a percentage of the control or percentage of spread cells. Data are presented as mean ± SEM. Statistical comparisons were done using two-tailed Student's t-test with P < 0.05 considered significant.
Results
Cell Spreading and Actin Polymerization Are Not Affected by the Hep II Domain in Differentiated HTM Cells Plated on the CBD of Fibronectin
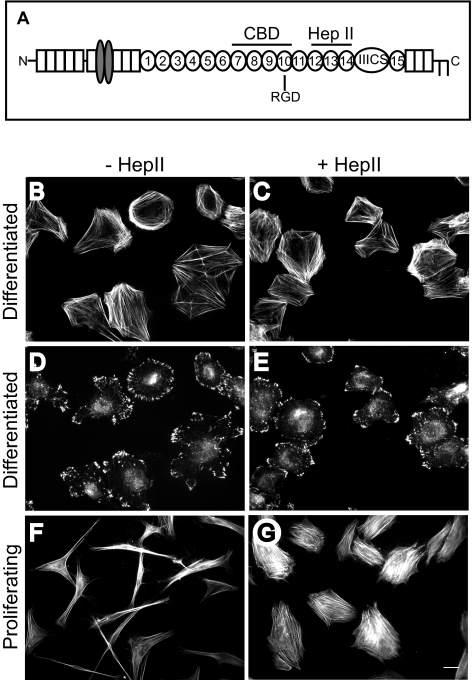
Previous studies have suggested that activation of an α4β1 integrin–signaling pathway by the Hep II domain of fibronectin can have opposing effects on contractility and actin polymerization depending on the matrix substrate, cell cycle stage (quiescent/differentiated versus proliferating), and cross-talk with other integrins engaged on the cell surface.3,4,9 To determine whether there was a cell cycle specific factor that could explain these different responses to the Hep II domain, we preformed a spreading assay by replating cultures of either proliferating or differentiated HTM cells that had reached quiescence onto the CBD of fibronectin in the absence or presence of the Hep II domain. The CBD of fibronectin was used to minimize effects from other signaling domains of fibronectin (Fig. 1A). Figures 1B–1G show that the differentiated HTM cells responded differently than proliferating HTM cells in this assay and were refractile to the Hep II domain. In the absence of the Hep II domain, differentiated HTM cells showed a polygonal morphology that typically contained cortical actin structures with some stress fibers and numerous focal adhesions containing vinculin (Figs. 1B, 1D). Addition of the Hep II domain to these differentiated HTM cultures had no apparent effect on cell spreading or actin stress fiber formation (Figs. 1C, 1E). In contrast, the Hep II domain induced actin stress fiber formation and cell spreading in proliferating HTM cells (Figs. 1F, 1G) as previously shown.9 Thus, there appears to be a cell cycle specific factor which is regulating integrin signaling.
Figure 1.
Effect of Hep II domain on differentiated and proliferating HTM cells. (A) Schematic of fibronectin showing the cell binding domain (CBD) and the Hep II domain. (B–E) Cell spreading in differentiated A7–1 HTM cells plated on the CBD occurs in the absence (B, D) or presence (C, E) of 472 nM of the Hep II domain of fibronectin. (F, G) In contrast, cell spreading in proliferating A7–1 HTM cells requires the addition of the Hep II domain. Cells were labeled with phalloidin (B, C, F, G) to visualize F-actin or vinculin (D, E) to visualize focal contacts and adhesions. Bar, 20 μm.
To verify the cell cycle stage of these cells, FACS analysis was performed on the replated differentiated and proliferating HTM cells. These results showed that 88.4% of differentiated cells were in G1/Go phase, with only 9.3% in S phase (data not shown). Replating the cells for 3 hours on fibronectin showed only small changes in cell cycle distribution with 79.9% of cells in G1/G0 and 17.8% of cells in S phase. This was different from proliferating HTM cells where 55.8% of the cells were in G1/G0 phase while 44.2% were in S phase.
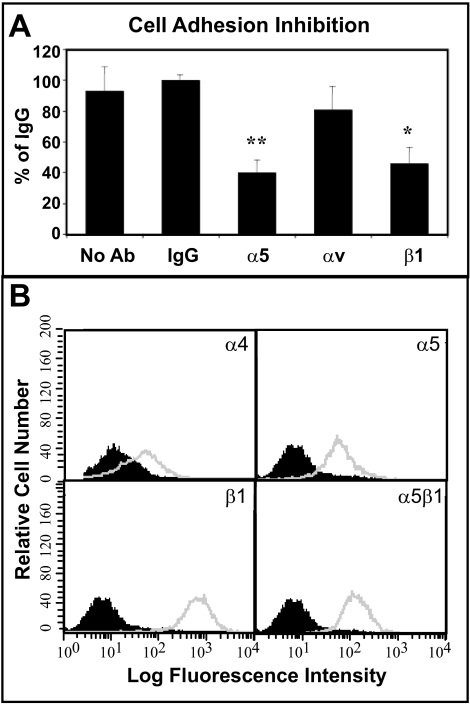
To rule out the possibility that changes in integrin expression were responsible for the different responses to the Hep II domain, FACS analyses was done. These studies indicated that differentiated HTM cells, like proliferating cells,9 expressed the α4 and α5 integrin subunits as well as β1 integrin subunit (Fig. 2B). Cell binding studies using antibodies against β1, α5 and αv integrins or a control IgG to block cell attachment were done to demonstrate that both proliferating and differentiating cells used the same integrins to attach to the CBD of fibronectin. Previous studies with proliferating HTM cells showed that these cells used α5β1 integrins to attach to the CBD of fibronectin.9 This study showed that differentiated HTM cells, like proliferating HTM cells, also used α5β1 integrin to adhere to the CBD of fibronectin (Fig. 2A). Together, these data indicate that actin polymerization in differentiated HTM cells was mediated by α5β1 integrins when plated on the CBD of fibronectin, and that the Hep II domain did not have any effect even though its α4β1 integrin receptor was expressed.
Figure 2.
HTM cell adhesion via α5β1 integrin and integrin expression. (A) The α5 and β1 integrin blocking antibodies significantly inhibited cell attachment to the CBD of fibronectin (*P = 0.0004, **P < 0.0001 compared to IgG control). The αv integrin blocking antibody had no significant effect. Differentiated A7–1 cells were plated on the CBD for 1 hour in the absence or presence of integrin blocking antibodies or control IgG. Bound cells were colorimetrically quantified using toluidine blue. Data are the mean percentage of IgG ± SEM. (B) FACS analysis of integrin levels in differentiated A7–1 cells. Cells were labeled with antibodies against various integrin subunits. Non-immune IgG controls are shown as black peaks, integrin expression is shown in gray.
Polymerization of Actin and Cell Spreading Is ILK Dependent
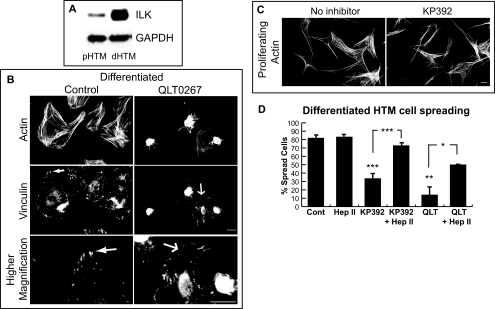
To determine whether ILK was involved in mediating the α5β1 integrin signaling pathway seen in differentiated HTM cells, ILK expression levels were analyzed by Western blot analysis. As shown in Figure 3A, differentiated HTM cells expressed more ILK than proliferating HTM cells. GAPDH levels were similar, indicating that the higher levels of ILK in differentiated HTM cells was not due to loading differences. We then replated cells on the CBD of fibronectin in the absence or presence of the ILK inhibitors QLT0267 or KP392, to determine whether ILK was involved in α5β1 integrin-mediated actin polymerization in HTM cells. Addition of 20 μM QLT0267 to the medium inhibited differentiated HTM cell spreading by 70% (P < 0.03; Figs. 3B, 3D) when compared to control cells (Figs. 3B, 3D). Similar results were seen with KP392 (Fig. 3D). Differentiated HTM cells that did spread in the presence of QLT0267 exhibited a decrease in stress fiber formation (Fig. 3B) and showed focal contacts rather than focal adhesions (Fig. 3B). In contrast, proliferating HTM cell spreading and actin polymerization was unaffected by KP392 (Fig. 3C). Thus, differentiated HTM cells, but not proliferating cells use ILK to polymerize actin filaments on the CBD of fibronectin.
Figure 3.
Differentiated, but not proliferating HTM cells spread in an ILK-dependent manner. (A) The level of ILK expression was higher in the differentiated cell lysate. Western blot analysis was done with equal concentrations of differentiated (dHTM) and proliferating (pHTM) N27TM-2 cell lysate (10 μg). GAPDH levels, which served as a loading control, were similar. (B) Cell spreading and focal adhesion formation in differentiated A7–1 cells plated on the CBD for 3 hours in the absence or presence of the ILK inhibitor QLT0267 (20 μM). Cells were labeled with phalloidin to visualize F-actin or vinculin to visualize focal contacts and adhesions. Solid arrows: well developed focal adhesions; open arrows: smaller focal adhesions or contacts. (B, bottom panels) higher magnifications of upper panels. Bar, 20 μm. (C) Cell spreading in proliferating A7–1 cells plated on the CBD for 3 hours in the absence or presence of KP392 (100 μM) was unaffected. Bar, 20 μm. (D) Cell spreading in the presence of KP392 (100 μM; n = 5 experiments) and QLT0267 (20 μM; n = 2 experiments) was significantly reduced (***P < 0.001 and **P < 0.03, respectively) compared to control cells. Soluble Hep II domain (472 nM) significantly increased cell spreading in the presence of the ILK inhibitors KP392 (***P < 0.001) and QLT0267 (*P < 0.05) when compared to ILK inhibitors alone. Data are the mean percentage of cells spread with F-actin ± SEM.
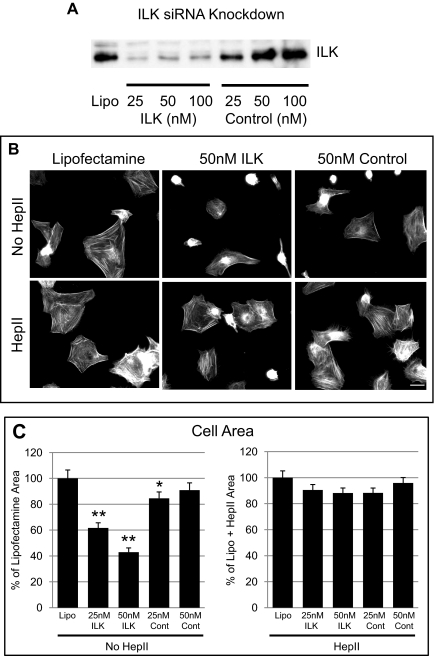
To further demonstrate that ILK was involved in promoting actin polymerization in differentiated HTM cells, ILK siRNA was used to knock down ILK expression levels before a spreading assay. As shown in Figure 4A, there was a major knock down of ILK expression with ILK siRNA when compared to lipofectamine only or to the non-specific control siRNA. By densitometry, there was approximately 100, 96.8, and 91.6% ILK knock down with 25, 50, and 100 nM ILK siRNA, respectively, compared to lipofectamine only. Similar results were seen with two other HTM cell lines (data not shown). Figure 4B shows that cell spreading and actin polymerization in differentiated HTM cells treated with 50 nM ILK siRNA for 48 hours was impaired compared to lipofectamine-treated controls. Measurements of cell area further verified this observation and showed a reduction in the area of cells of 38.4 ± 4.0% (P < 0.0001) and 57 ± 3.2% (P < 0.0001) with 25 and 50 nM ILK siRNA, respectively, compared to lipofectamine only–treated cells (Fig. 4C). The non-specific control siRNA at 25 nM also had a small inhibitory effect on cell spreading (15.4 ± 4.9%, P < 0.05), but not to the same extent as the ILK siRNA.
Figure 4.
ILK siRNA knocks down ILK expression. (A) Levels of ILK expression in differentiated N27TM-2 cells treated for 48 hours with ILK siRNA. The blot is representative of the same experiment in three different cell strains. (B) Cell spreading was impaired in HTM cells treated with 50 nM of ILK siRNA compared to control siRNA or lipofectamine 2000 unless the Hep II domain was present. Cells were plated on the CBD for 3 hours in the absence or presence of the Hep II domain and labeled with phalloidin to visualize F-actin. Bar, 20 μm. (C) Treatments of 25 and 50 nM ILK siRNA significantly decreased cell spreading. Statistically significant (*P < 0.05, **P < 0.0001). Data are the mean ratio of cell area compared to Lipofectamine only control (set to 100%) ± SEM for non-Hep II domain–treated cells and compared to lipofectamine + Hep II for cells treated with the Hep II domain. Cell spreading was quantified by measuring the area of the cells in four to six fields of view from a triplicate experiment (n = 123).
ILK Inhibits α4β1 Integrin Signaling
To determine whether ILK regulated signaling through α4β1 integrin, cells transfected with ILK siRNA or treated with ILK inhibitors were replated in the presence of soluble Hep II domain. As shown in Figure 4B, addition of the Hep II domain re-established the formation of cortical actin and restored cell spreading to 90.5 ± 4.2% and 88.2 ± 3.9% in cells treated with 25 and 50 nM ILK siRNA, respectively, compared to lipofectamine cells treated with the Hep II domain (Fig. 4C).
The Hep II domain also induced cell spreading with cortical actin and focal adhesions in the presence of both ILK inhibitors KP392 and QLT0267. As shown in Figure 3D, only 36% and 14% of cells spread when treated with the KP392 and QLT0267 inhibitors, respectively. However, the addition of soluble Hep II domain increased cell spreading to 73% (P < 0.001) and 50% (P < 0.05), respectively, when compared to ILK inhibitors alone (Fig. 3D). The Hep II domain by itself had no effect on cell spreading as 84% of both control and Hep II domain–treated cells spread. This suggests that ILK activity trans-dominantly inhibits signaling from the Hep II domain to induce differentiated HTM-cell spreading on the CBD.
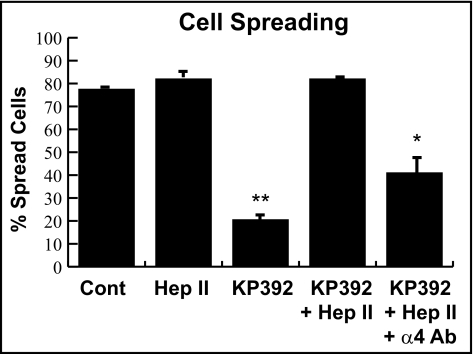
To demonstrate that the Hep II domain used α4β1 integrin to promote actin polymerization when ILK was inhibited, α4β1 integrin–blocking antibody was added to differentiated HTM cells plated on the CBD of fibronectin in the presence or absence of the Hep II domain or the ILK inhibitor KP392. The ILK inhibitors were used instead of the ILK siRNA for the remainder of experiments because it has been shown that siRNA knock down of ILK affects PINCH and α-parvin protein expression levels as well,25 which could complicate data interpretation. As quantified in Figure 5, the ILK inhibitor KP392 reduced cell spreading to 21% (P < 0.025 compared to control) and the presence of the Hep II domain recovered cell spreading to 82%. Addition of the α4β1 integrin–blocking antibody partially blocked cell spreading induced by the Hep II domain in the presence of KP392 and only 41% of cells spread. This was significant reduction compared to control cells (P < 0.03) and represented a twofold decrease.
Figure 5.
Hep II domain–mediated spreading is dependent on α4β1 integrin. Differentiated A7–1 cells were plated on the CBD in the absence or presence of the ILK inhibitor KP392 (100 μM) and/or 472 nM of the Hep II domain. Whereas inhibition of ILK function with KP392 significantly decreased cell spreading compared to control cells (**P < 0.025, n = 2 experiments), the inhibition with KP392 could be overcome with the addition of the Hep II domain. The recovery of cell spreading by the addition of the Hep II domain could be blocked with a soluble α4 integrin–blocking antibody (25 μg/mL; clone P1H4). Compared to untreated control cells, the percent of cell spreading in HTM cells treated with KP392, the Hep II domain, and P1H4 was significantly different (*P < 0.03). Data are the mean percentage of cells spread with F-actin ± SEM.
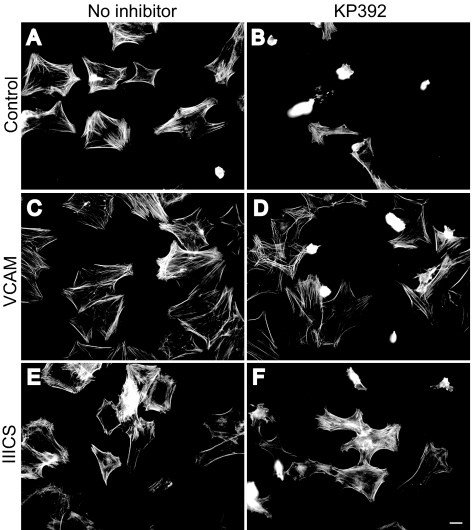
To further establish that α4β1 integrin was involved in mediating actin polymerization when ILK was inhibited, cells were plated in the presence of either VCAM-1 or the IIICS domain of fibronectin, both documented α4β1 integrin ligands.26–30 If either VCAM-1 (Figs. 6C, 6D) or the IIICS domain of fibronectin (Figs. 6E, 6F) were added to the medium instead of the Hep II domain, cell spreading could be restored in the presence of the ILK inhibitor KP392 (compare Fig. 6B with Figs. 6D and 6F).
Figure 6.
α4β1 integrin ligands induce cell spreading when ILK is inhibited. Differentiated A7–1 cells were plated on the CBD in the absence (A, C, E) or presence of the ILK inhibitor KP392 (100 μM; B, D, F); with or without soluble extracellular domain of VCAM-1 (236 nM; C, D); or the IIICS domain of fibronectin (472 nM; E, F). Cells were labeled with phalloidin to visualize F-actin. Bar, 20 μm.
Differentiated HTM Cell Spreading Requires PI3K
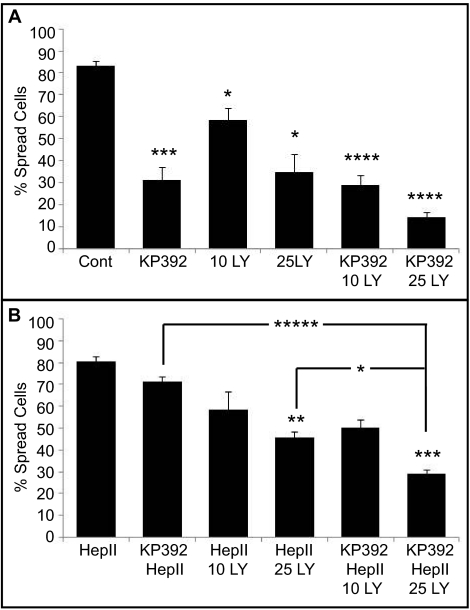
We next wanted to determine the signaling pathway used by ILK to regulate α4β1 integrin activity. For this, cells were treated with the PI3K inhibitor LY294002 (10 or 25 μM) in the spreading assay, because PI3K has been shown to be an upstream activator of ILK.13,31 As shown in Figure 7A, the PI3K inhibitor prevented cell spreading on CBD of fibronectin in a dose dependent fashion and incubation of cells with 10 μM and 25 μM LY294002 reduced cell spreading to 58.6% and 35% (P < 0.05 compared to control), respectively. Cell spreading was further reduced to 14% in the presence of both the PI3K (25 μM) and the ILK inhibitor KP392.
Figure 7.
PI3K inhibition leads to differentiated HTM cell rounding. (A) Differentiated A7–1 cells were plated on the CBD in the presence of the ILK inhibitor KP392 or the PI3K inhibitor LY294002 (LY) or in combination. Treatment with LY294002 significantly inhibited cell spreading compared to control cells (25 μM; *P < 0.05; n = 3 experiments). Statistically significant from control cells (*P < 0.05, ***P < 0.015, ****P < 0.007). (B) Spreading differentiated A7–1 cells were incubated with the Hep II domain in combination with KP392 or LY294002. Treatment with 25 μM of the PI3K inhibitor significantly decreased cell spreading induced by 472 nM of the Hep II domain in the presence of 100 μM of the ILK inhibitor compared to cells treated with both the Hep II domain and the ILK inhibitor (*****P < 0.002). Statistically significant from Hep II–treated cells or as indicated by brackets (*P < 0.05, **P < 0.04, ***P < 0.015, ****P < 0.007, *****P < 0.002). Data are the mean percentage of cells spread with F-actin ± SEM.
The PI3K inhibitor also blocked the ability of the Hep II domain to rescue spreading in the presence of the ILK inhibitor, suggesting that signaling events mediated by the Hep II domain may also require PI3K. In the presence of 10 μM LY294002 and the Hep II domain, only 58.7% of the cells spread and at 25 μM LY294002, only 45.7% of the cells spread in the presence of the Hep II domain (Fig. 7B). A slight increase in cell spreading was observed in cells treated with 25 μM LY294002 and the Hep II domain compared to cells treated with 25 μM LY294002 only, but this increase of 11% was not significant. Only 29% of cells spread when treated with the Hep II domain and both the PI3K inhibitor (25 μM) and ILK inhibitor. These data suggest that both α5β1 integrin and Hep II domain signaling through α4β1 integrin used PI3K to induce cell spreading.
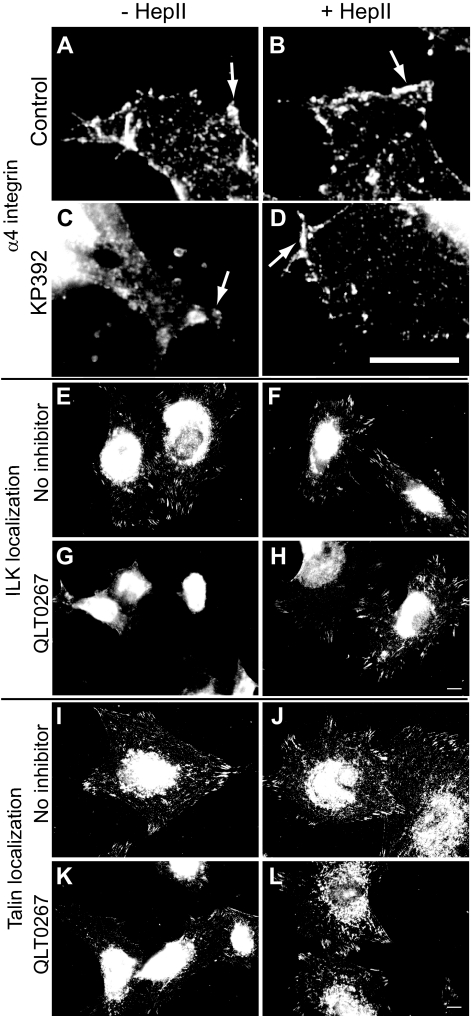
α4β1 Integrin Is Absent from Focal Adhesion Complexes Containing ILK and Talin
Because α4β1 integrin signaling could rescue actin polymerization and focal adhesion formation in the presence of the ILK inhibitor, immunofluorescence microscopy was used to determine whether α4β1 integrin localized to focal adhesions. Figure 8A shows that α4β1 integrins were not found in focal adhesions, unlike ILK and talin (Figs. 8E and 8I, respectively). Instead, α4β1 integrins appeared to be distributed throughout the cytoplasm with some discrete clustering along the cell periphery and in cellular protrusions. In cells treated with the ILK inhibitors KP392 or QLT0267, the clustering of α4β1 integrins along the edges of cell protrusions was reduced (Fig. 8C) and there was a loss of ILK and talin localized to focal adhesions (Figs. 8G and 8K, respectively).
Figure 8.
α4β1 integrin, ILK and talin localization. Differentiated A7–1 cells plated on the CBD were labeled with an α4β1 integrin (A–D), ILK (E–H), or talin (I–L) antibody. α4β1 integrins appeared clustered along the edges of the cells treated with Hep II domain (472 nM; B), KP392 and Hep II domain (D), or left untreated (A). Cells treated with KP392 (100 μM; C) did not show any labeling along the edges. Arrows indicate areas of α4 integrin localization. ILK and talin were observed in focal adhesions in differentiated A7–1 cells plated on the CBD in the absence (E, F, I, and J), but not the presence, of the ILK inhibitor QLT0267 (20 μM; G, H, K, and L). Addition of the Hep II domain (472 nM; H, L) restored the localization of ILK and talin in focal adhesions. Bars: (A–D) 10 μm; (E–L) 20 μm.
The addition of the Hep II domain to KP392 or QLT0267-treated cells restored the localization of α4β1 integrin to cell protrusions (Fig. 8D) and ILK and talin to focal adhesions (Figs. 8H and 8L, respectively). Similar results to ILK and talin were seen with other focal adhesion proteins, including vinculin, paxillin, and FAK (data not shown). Together, these data suggest that cross-talk between α5β1 and α4β1 integrins recruited talin and ILK to focal adhesions even when ILK activity is inhibited.
Discussion
This study demonstrates for the first time that ILK mediates trans-dominant integrin signaling in HTM cells and plays a critical role in regulating cooperative signaling between α5β1 and α4β1 integrins. Inhibition of ILK expression with siRNA or the ILK inhibitors KP392 or QLT0267 allowed activation of a cooperative α5β1/α4β1 integrin signaling pathway that regulated cell spreading, actin polymerization, and focal adhesion formation. When ILK was active, however, cell spreading was dependent solely on α5β1 integrin signaling. This role of ILK appeared to be cell cycle dependent because α5β1/α4β1 integrin cooperative signaling was constitutively active in proliferating HTM cells9 and was unaffected by the ILK inhibitors, most likely because of low ILK expression in these cells. These observations are consistent with the role of ILK in cell cycle progression and differentiation32–35 and suggest that the level of ILK expression regulates cooperative α5β1/α4β1 integrin signaling.
Activation of this α5β1/α4β1 integrin pathway in differentiated HTM cells was not specific for a particular ligand, and all three α4β1 integrin ligands tested (VCAM-1, IIICS domain, Hep II domain) triggered cell spreading, actin polymerization, and focal adhesion formation when ILK activity was disrupted. In addition, α4β1 integrin–blocking antibodies partially blocked the Hep II-mediated rescue of differentiated cell spreading when ILK activity was disrupted. Only partial blockage was seen with the α4β1 integrin–blocking antibody, most likely because insufficient antibody was used for complete blockage.
This α5β1/α4β1 integrin signaling pathway did not involve the localization of α4β1 integrin into focal adhesions. This is not the first time that α4β1 integrin signaling has been observed to occur outside of focal adhesions and had been previously seen in Chinese hamster ovary (CHO) cells.36 This suggests that α4β1 integrin regulates α5β1 integrin signaling and focal adhesion formation through separate membrane sites via an intermediate inside-out signaling pathway. Interestingly, both the Hep II–mediated cell spreading in the presence of the ILK inhibitor and the α5β1 integrin mediated–cell spreading via the CBD of fibronectin required active PI3K. This suggests that although the α5β1 and α4β1 integrin signaling may use separate pathways, these pathways may merge at, or are upstream of, PI3K.
Exactly how ILK is regulating integrin signaling is unknown. When ILK is inactive, Hep II–mediated signaling through α4β1 integrin appears to “re-activate” β1 integrins through an outside-in signaling pathway which would be in keeping with ILK's role in outside-in signaling.37 Recent studies suggest that ILK may trigger an active conformation of β1 integrin38 by promoting the interaction of talin with the cytoplasmic tails of the β1 subunit. Integrin β cytoplasmic tails that are unable to bind talin have been shown to lose their ability to mediate trans-dominant integrin inhibition.39–41 In support of this idea, the disruption of ILK activity blocked the recruitment of talin to focal adhesions mediated by α5β1 integrin signaling. Recruitment of talin to focal adhesions could be recovered if the cooperative α5β1/α4β1 integrin pathway was activated with the Hep II domain.
In summary, our results suggest that ILK can trans-dominantly control cooperative integrin signaling events used to regulate the organization of the actin cytoskeleton in differentiated HTM cells. Alterations in ILK activity are likely to affect how TM cells in vivo respond to ECM-mediated signals, thereby altering the functional properties of the TM, such as contractility. For instance, it is well established that the contractile state of the actin network in the TM in vivo can regulate aqueous humor outflow from the eye and IOP.42–46 Given the importance of the ECM in regulating the function of the TM, these studies show that is it critical we understand not only the molecular pathways involved in ECM-mediated signaling events but how the cell cycle state may alter these events. Such understanding will enable us to develop more specific treatments for disease.
Footnotes
Supported in part by a NEI-funded Core Grant P30 EY016665 (Department of Ophthalmology and Visual Sciences); the NEI Grants EY012515, EY017006, EY020490 (DMP) and EY016695 (NS); National Cancer Institute of Canada (NCIC; SD); and the Canadian Institute for Health Research (CIHR; SD). NS is a recipient of a Research Award from the Retina Research Foundation.
Disclosure: J.A. Faralli, P; J.R. Newman, None; N. Sheibani, None; S. Dedhar, None; D.M. Peters, P
References
- 1. Gonzalez JM, Hu Y, Gabelt BT, Kaufman PL, Peters DM. Identification of the active site in the heparin II domain of fibronectin that increases outflow facility in cultured monkey anterior segments. Invest Ophthalmol Vis Sci. 2009;50:235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Santas AJ, Bahler C, Peterson JA, et al. Effect of heparin II domain of fibronectin on aqueous outflow in cultured anterior segments of human eyes. Invest Ophthalmol Vis Sci. 2003;44:4796–4804 [DOI] [PubMed] [Google Scholar]
- 3. Schwinn MK, Gonzalez JM, Gabelt BT, Sheibani N, Kaufman PL, Peters DM. Heparin II domain of fibronectin mediates contractility through an α4β1 co-signaling pathway. Exp Cell Res. 2010;316:1500–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gonzalez JM, Faralli JA, Peters JM, Newman JR, Peters DM. Effect of heparin II domain of fibronectin on actin cytoskeleton and adherens junctions in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006;47:2924–2931 [DOI] [PubMed] [Google Scholar]
- 5. Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4:E65–E68 [DOI] [PubMed] [Google Scholar]
- 6. Moyano JV, Maqueda A, Casanova B, Garcia-Pardo A. α4β1 integrin/ligand interaction inhibits α5β1-induced stress fibers and focal adhesions via down-regulation of RhoA and induces melanoma cell migration. Mol Biol Cell. 2003;14:3699–3715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huhtala P, Humphries MJ, McCarthy JB, Tremble PM, Werb Z, Damsky CH. Cooperative signaling by alpha 5 beta 1 and alpha 4 beta 1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J Cell Biol. 1995;129:867–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diaz-Gonzalez F, Forsyth J, Steiner B, Ginsberg MH. Trans-dominant inhibition of integrin function. Mol Biol Cell. 1996;7:1939–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peterson JA, Sheibani N, David G, Garcia-Pardo A, Peters DM. Heparin II domain of fibronectin uses α4β1 integrin to control focal adhesion and stress fiber formation, independent of syndecan-4. J Biol Chem. 2005;280:6915–6922 [DOI] [PubMed] [Google Scholar]
- 10. Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, et al. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379:91–96 [DOI] [PubMed] [Google Scholar]
- 11. Li F, Zhang Y, Wu C. Integrin-linked kinase is localized to cell-matrix focal adhesions but not cell-cell adhesion sites and the focal adhesion localization of integrin-linked kinase is regulated by the PINCH-binding ANK repeats. J Cell Sci. 1999;112:4589–4599 [DOI] [PubMed] [Google Scholar]
- 12. Nikolopoulos SN, Turner CE. Integrin-linked kinase (ILK) binding to paxillin LD1 motif regulates ILK localization to focal adhesions. J Biol Chem. 2001;276:23499–23505 [DOI] [PubMed] [Google Scholar]
- 13. Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Nat Acad Sci U S A. 1998;95:11211–11216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pasquali C, Bertschy-Meier D, Chabert C, et al. A chemical proteomics approach to phosphatidylinositol 3-kinase signaling in macrophages. Mol Cell Proteomics. 2007;6:1829–1841 [DOI] [PubMed] [Google Scholar]
- 15. Grashoff C, Thievessen I, Lorenz K, Ussar S, Fassler R. Integrin-linked kinase: integrin's mysterious partner. Curr Opin Cell Biol. 2004;16:565–571 [DOI] [PubMed] [Google Scholar]
- 16. Wu C, Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol. 2001;155:505–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mackinnon AC, Qadota H, Norman KR, Moerman DG, Williams BD. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr Biol. 2002;12:787–797 [DOI] [PubMed] [Google Scholar]
- 18. Montanez E, Ussar S, Schifferer M, et al. Kindlin-2 controls bidirectional signaling of integrins. Gene Dev. 2008;22:1325–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tu Y, Wu S, Shi X, Chen K, Wu C. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell. 2003;113:37–47 [DOI] [PubMed] [Google Scholar]
- 20. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthal Vis Sci. 1979;18:1043–1049 [PubMed] [Google Scholar]
- 21. Polansky JR, Wood IS, Maglio MT, Alvarado JA. Trabecular meshwork cell culture in glaucoma research: evaluation of biological activity and structural properties of human trabecular cells in vitro. Ophthalmol. 1984;91:580–595 [DOI] [PubMed] [Google Scholar]
- 22. Filla MS, Liu X, Nguyen TD, et al. In vitro localization of TIGR/MYOC in trabecular meshwork extracellular matrix and binding to fibronectin. Invest Ophthalmol Vis Sci. 2002;43:151–161 [PubMed] [Google Scholar]
- 23. Aukhil I, Joshi P, Yan Y, Erickson HP. Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. J Biol Chem. 1993;268:2542–2553 [PubMed] [Google Scholar]
- 24. Li Z, Calzada MJ, Sipes JM, et al. Interactions of thrombospondins with α4β1 integrin and CD47 differentially modulate T cell behavior. J Cell Biol. 2002;157:509–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fukuda T, Chen K, Shi X, Wu C. PINCH-1 is an obligate partner of integrin-linked kinase (ILK) functioning in cell shape, modulation, motility, and survival. J Biol Chem. 2003;278:51324–51333 [DOI] [PubMed] [Google Scholar]
- 26. Elices MJ, Osborn L, Takada Y, et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584 [DOI] [PubMed] [Google Scholar]
- 27. Garcia-Pardo A, Wayner EA, Carter WG, Ferreira OC., Jr Human B lymphocytes define an alternative mechanism of adhesion to fibronectin. The interaction of the alpha 4 beta 1 integrin with the LHGPEILDVPST sequence of the type III connecting segment is sufficient to promote cell attachment. J Immunol. 1990;144:3361–3366 [PubMed] [Google Scholar]
- 28. Guan JL, Hynes RO. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor α4β1. Cell. 1990;60:53–61 [DOI] [PubMed] [Google Scholar]
- 29. Humphries MJ, Komoriya A, Akiyama SK, Olden K, Yamada KM. Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J Biol Chem. 1987;262:6886–6892 [PubMed] [Google Scholar]
- 30. Wayner EA, Garcia-Pardo A, Humphries MJ, McDonald JA, Carter WG. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989;109:1321–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lynch DK, Ellis CA, Edwards PA, Hiles ID. Integrin-linked kinase regulates phosphorylation of serine 473 of protein kinase B by an indirect mechanism. Oncogene. 1999;18:8024–8032 [DOI] [PubMed] [Google Scholar]
- 32. Akhtar N, Marlow R, Lambert E, et al. Molecular dissection of integrin signalling proteins in the control of mammary epithelial development and differentiation. Development. 2009;136:1019–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gkretsi V, Bowen WC, Yang Y, Wu C, Michalopoulos GK. Integrin-linked kinase is involved in matrix-induced hepatocyte differentiation. Biochem Biophys Res Commun. 2007;353:638–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haase M, Gmach CC, Eke I, Hehlgans S, Baretton GB, Cordes N. Expression of integrin-linked kinase is increased in differentiated cells. J Histochem Cytochem. 2008;56:819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weaver MS, Toida N, Sage EH. Expression of integrin-linked kinase in the murine lens is consistent with its role in epithelial-mesenchymal transition of lens epithelial cells in vitro. Mol Vis. 2007;13:707–718 [PMC free article] [PubMed] [Google Scholar]
- 36. Kassner PD, Alon R, Springer TA, Hemler ME. Specialized functional properties of the integrin alpha 4 cytoplasmic domain. Mol Biol Cell. 1995;6:661–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase—essential roles in physiology and cancer biology. J Cell Sci. 2008;121:3121–3132 [DOI] [PubMed] [Google Scholar]
- 38. Honda S, Shirotani-Ikejima H, Tadokoro S, et al. Integrin-linked kinase associated with integrin activation. Blood. 2009;113:5304–5313 [DOI] [PubMed] [Google Scholar]
- 39. Bodeau AL, Berrier AL, Mastrangelo AM, Martinez R, LaFlamme SE. A functional comparison of mutations in integrin βcytoplasmic domains: effects on the regulation of tyrosine phosphorylation, cell spreading, cell attachment and β1 integrin conformation. J Cell Sci. 2001;114:2795–2807 [DOI] [PubMed] [Google Scholar]
- 40. Calderwood DA, Tai V, Di Paolo G, De Camilli P, Ginsberg MH. Competition for talin results in trans-dominant inhibition of integrin activation. J Biol Chem. 2004;279:28889–28895 [DOI] [PubMed] [Google Scholar]
- 41. Tadokoro S, Shattil SJ, Eto K, et al. Talin binding to integrin β tails: a final common step in integrin activation. Science. 2003;302:103–106 [DOI] [PubMed] [Google Scholar]
- 42. Kaufman PL. Pharmacologic trabeculocanalotomy: facilitating aqueous outflow by assaulting the meshwork cytoskeleton, junctional complexes, and extracellular matrix. Arch Ophthalmol. 1992;110:34–36 [DOI] [PubMed] [Google Scholar]
- 43. Peterson JA, Tian B, McLaren JW, Hubbard WC, Geiger B, Kaufman PL. Latrunculins' effects on intraocular pressure, aqueous humor flow, and corneal endothelium. Invest Ophthalmol Vis Sci. 2000;41:1749–1758 [PubMed] [Google Scholar]
- 44. Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632.[Erratum appears in Invest Ophthalmol Vis Sci. 2001 Jul;42(8):1690.] Invest Ophthalmol Vis Sci. 2001;42:1029–1037 [PubMed] [Google Scholar]
- 45. Robinson JC, Kaufman PL. Phalloidin inhibits epinephrine's and cytochalasin B's facilitation of aqueous outflow. Arch Ophthalmol. 1994;112:1610–1613 [DOI] [PubMed] [Google Scholar]
- 46. Tian B, Kaufman PL, Volberg T, Gabelt BT, Geiger B. H-7 disrupts the actin cytoskeleton and increases outflow facility. Arch Ophthalmol. 1998;116:633–643 [DOI] [PubMed] [Google Scholar]