Fundamentally, proteins undergo changes when exposed to alkaline conditions. These changes range from aggregation, changes in solubility, elimination reactions involving the amino acid side chains, cross-linking with lipids (or other nonprotein constituents), and hydrolysis. Data presented here suggest that the hydrolytic truncation and aggregation of proteins occur as a result of alkali exposure. Proteomic analysis of alkali-exposed tissue has identified plexin D1, dynein, and other proteins. Further studies will demonstrate the feasibility of such proteins serving as biomarkers for alkali-induced damage.
Abstract
Purpose.
To determine whether exposure to alkaline chemicals results in predictable changes in corneal protein profile. To determine whether protein profile changes are indicative of severity and duration of alkali exposure.
Methods.
Enucleated bovine and porcine (n = 59 each) eyes were used for exposure to sodium, ammonium, and calcium hydroxide, respectively. Eyes were subjected to fluorescein staining, 5-bromo-2′-deoxy-uridine (BrdU) labeling. Excised cornea was subjected to protein extraction, spectrophotometric determination of protein amount, dynamic light scattering and SDS-PAGE profiling, mass spectrometric protein identification, and iTRAQ-labeled quantification. Select identified proteins were subjected to Western blot and immunohistochemical analyses.
Results.
Alkali exposure resulted in lower protein extractability from corneal tissue. Elevated aggregate formation was found with strong alkali exposure (sodium hydroxide>ammonium, calcium hydroxide), even with a short duration of exposure compared with controls. The protein yield after exposure varied as a function of postexposure time. Protein profiles changed because of alkali exposure. Concentration and strength of the alkali affected the profile change significantly. Mass spectrometry identified 15 proteins from different bands with relative quantification. Plexin D1 was identified for the first time in the cornea at a protein level that was further confirmed by Western blot and immunohistochemical analyses.
Conclusions.
Exposure to alkaline chemicals results in predictable and reproducible changes in corneal protein profile. Stronger alkali, longer durations, or both, of exposure resulted in lower yields and significant protein profile changes compared with controls.
Eye injuries may range in severity from minor scratches and small penetrating wounds to irreversible blindness. Chemical exposure and resultant injuries account for a small, yet significant, number of ocular injuries that occur at home, work, agricultural or industrial settings, and even warfare.1 Traumatic exposure to chemicals may be unintentional or intentional.1,2 With the advent of industrialization, chemicals are often stored or transported through populated areas,2 creating the possibility for accidents; therefore, changes associated with exposure to chemicals must be studied so we may better understand the resultant damage and design better medical treatment.
The cornea is often the first ocular structure injured after chemical exposure. The corneal epithelium is the transparent anterior boundary of the eye, past which are other layers such as the stroma; posteriorly it is bordered by a single layer of endothelial cells. The cornea is composed of layered proteinaceous fibers that give the cornea its elastic structure and strength. The arrangement and spacing of theses proteins are critical in conferring transparency. The response of these structural and enzymatic proteins to environmental challenges such as alkali damage are likely to affect protein solubility and extractability.3
Many physical and chemical factors determine the type of tissue damage that results from chemical exposure.2,4,5 Although acidic agents, primarily organic acids, can penetrate the eye, alkaline agents more rapidly penetrate the cornea and saponify cellular membrane lipids.6–9 The acidic injuries tend to result in local coagulation-necrosis, whereas alkali injuries tend to be gelatinous and liquefactive.8–12 The change in pH of the tissue has been attributed to render biochemical changes and is thus directly related to the severity of damage that has been incurred after exposure to alkaline chemicals.8,13 Controlling ocular surface inflammation after alkali burns is very important for further rehabilitation.14,15 The rapidity of alkali penetration and its destructive properties make it an area of interest for study. In the industrial setting, both stronger alkali such as sodium hydroxide and relatively weaker alkali such as ammonium hydroxide are used. Therefore, studying a spectrum of alkali encompassing both stronger and weaker alkali is expected to provide important insights into protein profile changes associated with damage processes.
The damage induced by individual chemical exposures differs in severity, tissue interaction, and reversibility, making it difficult to initiate treatment because of a lack of understanding of the host tissue's response to the irritant. Healing processes reverse some of the immediate molecular changes after exposure, rendering detection of such changes difficult and thus poorly understood. This study attempts to characterize the very initial modifications of the protein profile after the burn onset in a relatively “static” model of protein synthesis–deficient eyes. In a “dynamic” in vivo model, the protein profile would vary very quickly with the onset of the secondary immune reaction and the enzymatic remodeling of the cornea. The objective of this study was to determine exposure-responsive protein changes without the added complexity of concomitant changes caused by cellular protein synthesis processes.
Changes to proteins in the eyes are expected to provide insight into the damage processes/empiric estimates of changes in proteins exposed to alkali and potentially may serve as an outcome measure for determining the most appropriate treatment after chemical injuries. This study simulates traumatic ocular injuries and uses gel electrophoresis, dynamic light scattering, and mass spectrometric analyses to identify changes to the corneal protein content after different alkali exposures. Porcine and bovine eyes have been used as model systems because of their anatomic similarities and were followed with human eyes only for a limited subset of experiments. We used tissue where DNA synthesis/repair and protein synthesis were severely compromised. The results, therefore, are predominantly attributed to exposure to alkaline chemicals.
Methods
Tissue Procurement
Porcine and bovine eyes (Supplementary Fig. S1A, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental) were procured from the University of Miami (Miami, FL) and Just Meats Company (Chillecothe, OH). Experiments were performed adhering to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and all experimental procedures followed Institutional Animal Care and Use Committee–approved protocols. Human eyes were procured from the Lion's Eye Bank at Bascom Palmer Eye Institute. All eyes were used within 72 hours of enucleation after institutional review board approval and adherence to the tenets of the Declaration of Helsinki. All corneal specimens were collected using sharp scissors and fine forceps, after alkali-exposure (or control) setup.
Fluorescein Staining and Measurement of Central Corneal Thickness
For the purposes of this experiment, a subset of enucleated eyes were stained using fluorescein sodium solution (Akorn Inc., Buffalo Grove, IL) and were washed after 1 minute using sterile phosphate-buffered saline (PBS) solution to ensure corneal epithelium integrity. Fluorescein-treated cornea after PBS wash did not reveal any damage (Supplementary Figs. S1B–E, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental). Central corneal thickness measurements were attempted using a hand-held pachymeter (Pachette3; DGH Technology Inc., Exton, PA). However, measurements were unsuccessful; for some reason, instruments consistently showed errors when attempted on enucleated eyes.
Analyses of Cell Proliferation (BrdU Incorporation Assay) and Protein Synthesis (35S-Methionine Incorporation Assay)
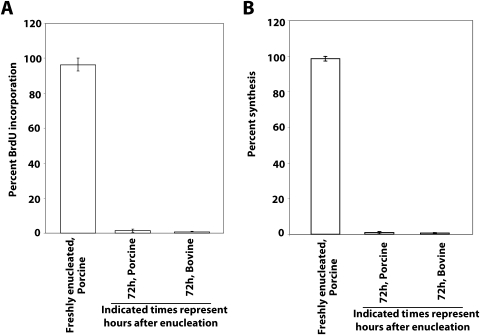
Cell proliferation was studied using a 5-bromo-2′-deoxy-uridine (BrdU) labeling and detection kit (catalog no. 8401202; Thermo Scientific, Waltham, MA), after suitable modification of previously published procedures.16,17 The corneas were excised from freshly enucleated eyes or after 72 hours of incubation and were placed immediately into a BrdU-containing labeling solution with complete minimum essential medium (MEM). All incubations were carried out at room temperature. After 60 minutes in the labeling solution, the eyes were washed three times in MEM without BrdU and were incubated for an additional 20 minutes in MEM, with one change each for 10 minutes in fresh MEM without BrdU. Corneas were then fixed in 1× PBS containing 4% paraformaldehyde at 4°C for 2 to 3 hours, placed in 15% and 30% sucrose solution for 2 hours each, and embedded in optimal cutting temperature (OCT) compound (Tissue Tek II; Laboratory Tek, Naperville, IL), and 8-μm sections were cut. Sections were washed once in PBS and blocked with 0.2% bovine serum albumin (BSA) in PBS for 30 minutes. Slides were processed for immunofluorescence with antibodies to BrdU provided in the kit, as recommended by the manufacturer. At least three corneal sections at each point were used for estimation of BrdU immunoreactivity. Image analysis was performed with ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html), and data were subjected to analysis. Freshly incorporated porcine corneas showed the highest BrdU incorporation. The estimate from fresh porcine cornea was taken as 100% to normalize the incorporation for other corneas (Fig. 1A). Porcine and bovine eyes after enucleation and within 1 hour (porcine eyes) or after 72 hours of incubation (porcine and bovine eyes) were subjected to determination of 35S-labeled methionine incorporation after standard biochemical protocol (EXPRE35S35S Protein Labeling Mix [Perkin Elmer, Waltham, MA] containing 14 mCi [518 MBq] [35S] methionine-specific activity in 50 mM tricine [pH 7.4] buffer; Perkin Elmer). The excised cornea (∼50 mg) was incubated with 10-μm protein labeling mixture (∼14 μCi radioactivity) for labeling. The excised cornea was placed immediately in the labeling solution with complete MEM. All incubations were carried out at room temperature. After 60 minutes in the labeling solution, the excised corneas were washed three times in fresh nonradioactive MEM and incubated for an additional 20 minutes (three incubations) in fresh nonradioactive MEM and given a final wash with PBS. Radioactivity counting was performed after placement in 10 mL scintillation fluid in a counter (Tri-Carb 1900CA; Packard Instrument Co., Downer Grove, IL). The maximum count was taken as 100%, and scintillation fluid alone was used as a negative control. The maximum incorporation found in the corneas of fresh porcine eyes (subjected to 35S-methionine incorporation within 1 hour of enucleation) was taken as 100% and was used for plotting. After 72 hours of incubation, corneal pieces had significantly diminished or no methionine incorporation, indicating lack of protein synthesis (Fig. 1B).
Figure 1.
Evaluation of DNA and protein synthesis in enucleated porcine and bovine eyes. (A) The freshly enucleated porcine eye within 1 hour of euthanatization was procured and subjected to determination of BrdU. Similar BrdU incorporation estimation was performed with porcine and bovine eyes (72 hours after enucleation) and plotted as a percentage of the maximum incorporation found in the corneas of porcine eyes subjected to BrdU incorporation within 1 hour of enucleation. (B) As indicated, the eyes were subjected to determination of 35S-labeled methionine incorporation. The maximum incorporation found in the corneas of fresh porcine eyes (within 1 hour of enucleation) subjected to 35S-methionine incorporation was taken as 100% and used for plotting. Comparative incorporations of label in porcine and bovine eyes are shown as indicated. Results are the mean ± SD of three independent determinations.
Exposure to Alkali and Protein Extractions
All enucleated eyes were washed in 1× PBS and stored at 4°C before experiments were performed. Including transportation, the total incubation time from enucleation to use of the eyes was 72 hours. Porcine and bovine eyes were placed in an in-house constructed acrylic resin (Plexiglas; Altuglas International, Philadelphia, PA) chamber/apparatus (Supplementary Figs. S2F, S2G, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental). The acrylic resin (Plexiglas) chamber ensured the safety of the experimenter and contained alkali solutions that might have splashed onto the ocular tissue. Three different alkali solutions—sodium hydroxide (NaOH), ammonium hydroxide (NH4OH), and calcium hydroxide [Ca(OH)2]—were used in three different concentrations—11 M, 6 M, or 0.25 M concentrations—and were splashed onto the eye using a syringe, as shown in Figure 2A. Approximately 5 mL each alkali solution was splashed onto the fully exposed cornea, ensuring that the entire cornea was covered. After a single exposure, excess alkali was drained off using an absorbent paper, and each cornea was immediately excised into six sections, with each assigned to a predesignated time interval (30 seconds, 60 seconds, 12 minutes, 30 minutes, 8 hours, and 24 hours) for exposure and were immediately washed in water unless stated otherwise. For 8- and 24-hour experiments, approximately 10 μL water was placed every 90 minutes to prevent corneas from drying. A repeat experiment was made for each exposure for 30- and 60-second exposure intervals if a time lag was experienced. After the preset time of exposure to the alkali, the corneal tissue was washed three times using distilled water (pH strips were used to confirm neutralization). Control experiments were performed using splash with distilled water. Once excised, corneal specimens were minced and subjected to homogenization using a handheld homogenizer for 1 minute, followed by 1 minute of rest (this was repeated three times), proteins were then extracted using a suitable volume of buffer: 125 mM Tris-HCl (pH 7.0), 100 mM NaCl, 0.1% Triton X-100, 0.1% detergent (Genapol C-100; Merck KGaA, Darmstadt, Germany), and 0.1% sodium dodecyl sulfate (SDS). This detergent combination was found to be optimal for corneal protein extraction in a previous study.3 Insoluble materials were removed by centrifugation at 10,000g for 10 minutes at 4°C. Protein extracts were prepared for at least three independent sets for each sample and were stored at −80°C until further use. Soluble proteins were quantified by the Bradford assay18,19 using commercially available reagents (Bio-Rad, Hercules, CA) with purified BSA as standard. In addition to the Bradford method, initial confirmatory estimations for soluble proteins were also performed using Biuret,20 Lowry,21 and bicinchoninic acid22 methods, respectively, for a given volume of corneal extract (Supplementary Fig. 2A, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental). Estimations showed agreement among different biochemical methods; therefore, further determination of protein amounts used only the Bradford method. Protein estimations may show great variations among different methods because of buffer composition or materials inherent in the biological samples.23,24 Biochemical estimation was used to determine the protein amount loaded on SDS-PAGE.
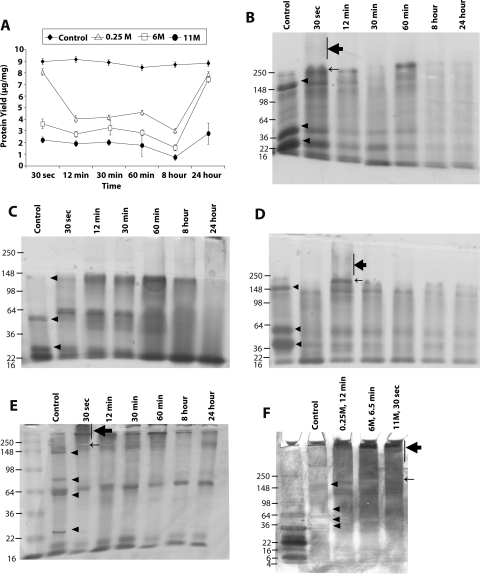
Figure 2.
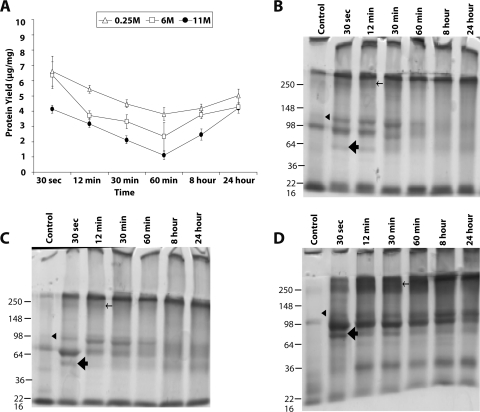
Representative bovine corneal normalized protein yield and profile after exposure to sodium hydroxide. (A) Representative protein yield (μg/mg) after exposure of cornea to distilled water (control) (♦), 0.25 M (▵), 6 M (□), and 11 M (●) concentrations of NaOH at the indicated time intervals. Recovered protein amounts estimated by Bradford's method were normalized to initial amount of wet tissue used. Results are the mean ± SD of three independent estimates. Results were significantly different from 0.0 by the one-sample t-test (*P < 0.05). (B–D) SDS-PAGE profile of total extracted protein after exposure to 11 M (B), 6 M (C), and 0.25 M (D) NaOH. An equal amount of protein (10 μg) for each predetermined time exposure, as indicated, was separated on 10% SDS-PAGE and subsequently stained with Coomassie blue. (E) Longer electrophoresis of the gel as in (B). (F) The control or sodium hydroxide–exposed proteins (10 μg) were loaded on a 4% to 20% gradient gel and subjected to electrophoresis for 3 hours and subsequently stained with silver staining. (E, F, lane 1) Molecular weight markers. Arrowheads: protein bands that decrease in intensity after NaOH exposure. Bar, thick arrow: smeared protein aggregates. Thin arrow: new protein band observed after NaOH exposure.
Gel Electrophoresis
When protein concentrations were determined after alkali exposure, protein extracts along with controls were subjected to electrophoresis using 10% SDS-PAGE with Tris-glycine buffer with a constant voltage of 80 V for approximately 3 hours. Longer run electrophoresis was carried out for 4.5 hours. Unless stated otherwise, protein samples (10 μg) were loaded onto individual lanes with Laemmli buffer.25 At the end of electrophoresis, gels were stained with Coomassie blue (Gel Code Blue; Thermo Scientific Corporation; Rockford, IL) to visualize protein bands. A 4% to 15% gradient gel electrophoresis was also performed using a gel system (PHAST; GE Healthcare Biosciences, Piscataway, NJ; Supplementary Fig. S2B, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental). When evaluated with silver staining, at the end of electrophoresis, gel was subjected to staining using silver staining kit (Silver BULLit; Amresco, Solon, OH) according to the manufacturer's recommended protocol.
Neutralization of Alkali Exposure with a Weak Acid
To simulate the treatment of alkali injuries in real-time situations, chemicals (lime juice [catalog no. 1031071864; Mrs. Biddle's Key Lime Juice, Miami, FL] and vinegar [catalog no. BDH3096; VWR, West Chester, PA]) that are commonly found in household/natural environments and would neutralize alkali caused by acidity were used. After alkali exposure for preset time intervals, eyes were subjected to neutralization with 1 mL of 1:2 diluted solution (with water) of lime juice or vinegar for 1 minute at room temperature. Titration of weak acids revealed the washing solutions to be approximately 0.25 M. Tissues were washed with distilled water to ensure cessation of the treatment. Once rinsed, the corneal tissue was homogenized and extracted with buffer, as described. Protein solutions were centrifuged at 10,000g, and the supernatant was carefully transferred to a fresh tube. After measurement of protein concentrations, samples were frozen at −80°C.
Western, Dot Blot, and ELISA Analyses
Unless stated otherwise, protein extracts (10 μg) were separated on a 10% SDS-PAGE and transferred onto a polyvinylidene fluoride (PVDF) membrane using precut blotting sandwiches (Ready Gel, catalog no. 162-0219; Bio-Rad, Hercules, CA) using 80 V constantly for 40 minutes in 2.5 mM Tris-19.2 mM glycine-0.01% SDS (wt/vol) buffer, pH 8.3, containing 20% methanol. For dot blots, the protein solutions (5 μg/spot) were directly spotted onto a PVDF membrane (BioTrace, catalog no. 66594; Pall Corporation, Washington, NY). Before spotting, membranes were treated for 5 minutes in methanol and 5 minutes in buffer with 20% methanol, as described. The membrane was blocked with 5% nonfat dry milk (catalog 170-6404) in 1× Tris-buffered saline (TBS; 15 mM Tris (hydroxymethyl) aminomethane, 2.7 mM KCl, 0.14 M NaCl, pH 7.6; catalog no. 170-6404; Bio-Rad Laboratories]. Blots were incubated overnight at 4°C with a 1:1000 dilution of goat polyclonal and mouse monoclonal antibodies to human plexin D1 and dynein heavy chain 9 (catalog no. ab28762 ab6305; Abcam Inc., Cambridge, MA), respectively. Membranes were washed three times with 1× TBS solution and incubated with 1:5000 dilution of secondary antibodies (anti-goat raised in donkey) conjugated with horseradish peroxidase (Santa Cruz Biotechnology Inc., Santa Cruz, CA) for 2 hours at room temperature and then were washed three times with 1× TBS and subjected to chemiluminescence detection using ECL kit (catalog no. 32106; Thermo Scientific, Rockford, IL). ELISA was performed with 1 μg total protein; each sample in triplicate was placed in a plate (9018 plate; Corning, Corning, NY) and was incubated for 1 hour at 37°C. The supernatant was discarded, and the plate was washed with PBS. The plates were blocked with 0.2% BSA for 1 hour, washed with PBS, and incubated for an additional hour with primary antibodies (plexin D1 and dynein) and subsequently with the secondary antibody coupled with alkaline phosphatase for 1 hour. A control ELISA estimate was also performed in an identical manner using mouse monoclonal glyceraldehyde 3-phosphate dehydrogenase (GAPDH; catalog MAB374; Chemicon International, Inc., Temecula, CA) antibody that revealed equal immunoreactivity for equal amounts of protein load. The plates were washed with PBS and incubated with phosphatase substrate (100 μL/well) in diethanolamine buffer (pH 7.5), and absorbance was measured at 405 nm on a plate reader.
Mass Spectrometry
To identify the proteins and perform quantification, protein bands separated on 10% SDS-PAGE were excised. Gel slices were destained with 50% acetonitrile/water and were suspended in 0.5 M triethyl ammonium bicarbonate (TEAB; number 17902; Sigma Chemical Co., St. Louis, MO), pH 8.5, and reduced with 10 mM Tris-(2-carboxyethyl) phosphine (Sigma Chemical Co., St. Louis, MO). The proteins were subsequently alkylated in the dark using 55 mM solution of iodoacetamide (catalog no. RPN6302V; GE Healthcare Inc.) and in-gel digested with sequencing-grade modified trypsin (catalog no. V5113; Promega Corporation, Madison, WI; 0.1 μg/15 μL in 15 mM N-ethylmorpholin) overnight at 37°C. The peptides were extracted twice with 50 μL 0.1% trifluoroacetic acid/60% acetonitrile and finally with 30 μL acetonitrile and were dried in a vacuum concentrator (SpeedVac; Thermo Fisher Scientific, Pittsburgh, PA). The extracted peptides were incubated with 8-plex reagent (iTRAQ; Applied Biosystems, Foster City, CA). Separate peptides isolated from different bands were subjected to incubation with reagents 113, 114, 115, and 116 (4381557, 4381557, 4381557, 4381560; Applied Biosystems) from peptides derived from a protein band of control cornea and with reagents 117, 118, 119, and 121 (4381561, 4381562, 4381563, 4381564) for peptides derived from alkali-exposed corneal protein bands in 0.5 M TEAB containing 60% vol/vol isopropanol. Incubation mixtures were dried in a vacuum concentrator (SpeedVac; Thermo Fisher Scientific), mixed together and loaded onto a slurry of 500 μL cation exchange buffer in 12 mM ammonium formate in 25% acetonitrile at pH 2.5 to 3.0 and subjected to chromatographic separation. Mass spectrometry was performed (ABI 4800 MALDI TOF-TOF; Applied Biosystems). The only protein IDs accepted were those that had a local false discovery rate estimation of <5%, calculated using the Proteomics System Performance Evaluation Pipeline program.26 These methods have been described in greater detail elsewhere greater detail elsewhere (Supplementary Mass spectrometry methods, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental).
Dynamic Light Scattering
To quantify protein size and differences in aggregation/degradation, dynamic light scattering was performed on a light-scattering device (DynaPro; Wyatt Technology Corporation, Santa Barbara, CA) in a total volume of 12 μL containing 1 μg of each protein sample in 1× PBS. Data acquisition and analysis were performed using Dynamics software (version 5.26.02) on a computer (Optiplex GX110; Dell, Austin, TX) that was connected to the microsampler.
Immunohistochemistry
Histologic evaluations were made according to previously published protocols for human corneal/ocular tissues.27 The excised cornea or globe was fixed in 4% paraformaldehyde in 1× PBS buffer for 2 to 3 hours or overnight, and paraffin-embedded sections (8 μm) were prepared and stained with commercially procured goat polyclonal antibodies to plexin D1 (Abcam Inc.) and rabbit monoclonal antibodies to glyceraldehyde-3-phosphate dehydrogenase (GAPDH; catalog no. 2118; Cell Signaling Technologies, Danvers, MA). The sections were deparaffinized and washed with PBS, blocked with 0.2% BSA, and subsequently incubated with 1:200 dilution of primary antibodies. The presence of proteins was verified by fluorescence using 1:1000 dilution of secondary anti-goat and anti-rabbit antibodies conjugated with AlexaFluor 488 and 594, respectively (Invitrogen-Molecular Probes, Eugene, OR). To ensure identical processing and uniform exposure, control samples (without primary antibody-treated sections) were examined side by side on the same slide. Fluorescence images were obtained with a laser scanning confocal microscope (model TCS-SP5; Leica Microsystems, Exton, PA). A series of 1-μm x-y (en face) images were collected. Confocal microscopic panels were composed with image-analysis software (Photoshop 8.0; Adobe Systems, San Jose, CA).
Results
For this work enucleated bovine and porcine eyes (Supplementary Fig. S1A, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental) within 72 hours of enucleation were used unless stated otherwise. Our attempts to obtain central corneal thickness on enucleated eyes were unsuccessful. The eyes were kept in a moist chamber immediately on enucleation. To determine the integrity of corneal epithelium, a subset of eyes was exposed to fluorescein, and images were taken after PBS wash (Supplementary Figs. S1B–E, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental). In each estimate an equivalent non–alkali-exposed eye served as a control. Very little BrdU incorporation was found to have occurred in eyes 72 hours after enucleation (Fig. 1A), and incorporation of exogenous methionine in proteins also revealed very little incorporation into proteins (Fig. 1B), suggesting severely impaired DNA synthesis/repair and protein synthesis in these eyes. Eyes were exposed to alkali in an acrylic resin (Plexiglas) chamber (Supplementary Figs. S1F, S1G, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental).
Protein Yield and Profile Changes as a Consequence of Exposure to Strong Alkali
Bovine eyes exposed to sodium hydroxide showed distinct differences in normalized protein yield (amount of protein recovered per unit amount of tissue, expressed throughout this text as μg/mg) for different concentrations of alkali. Control corneal extract consistently showed near uniform extraction of 9.0 ± 0.3 μg/mg normalized protein yield from the tissue. Alkali exposure for bovine corneal proteins showed four distinct kinetic profiles of soluble protein recovery. Initial protein recovery on exposure to alkali consistently decreased up to a certain time point (12 minutes; Fig. 2A), followed by a second kinetic profile (up to 60 minutes) during which the recovery of proteins showed either stationary or slightly increased yield. A third profile between 60 minutes and 8 hours showed a decrease, and in a fourth profile a protein yield increased as a function of time (8–24 hours). The protein yield was lowest at each time point for the strongest alkali exposure; thus, 11 M showed the least recovery, and 0.25 M showed the most recovery (Fig. 2A).
Protein profile of 10 μg equal load was performed using gel electrophoresis for each alkali concentration to determine the protein band pattern. For 11 M NaOH, the protein profile of tissue subjected to exposure was distinctly different from that of the control (Fig. 2B). The 11 M NaOH exposure showed distinct decreases in intensity of three bands in the molecular weight ranges 22 to 36 kDa, 36 to 50 kDa, and ∼148 kDa (arrowheads in the control lane) and the appearance of a high molecular weight band (∼250 kDa or >148 kDa; thin arrow) and aggregate, giving the appearance of smear (vertical bar, thick arrow) in the molecular weight range 148 to >250 kDa (Fig. 2B). Although the protein band pattern could vary slightly because of the quality of the gels given slight differences in casting, the overall difference in pattern is distinct and not attributed to variations in the gel. The treatment of 6 M NaOH showed some changes similar to those of 11 M NaOH, such as the decreased intensity of bands compared with control and the appearance of bands in the ∼98-kDa region (for exposure times of 12 minutes and greater). However, the appearance of a smear at 30 seconds was absent from 6 M NaOH–treated corneal proteins (Fig. 2C). The 0.25 M NaOH treatment (Fig. 2D) showed similar changes; however, at this concentration, the appearance of smear (bar, thick arrow) and the ∼98-kDa protein band shifted to 12 minutes after initial exposure (Fig. 2D). These shifts in smear and the ∼98-kDa band were consistent with the cumulative exposure effect of alkali. In 11 M NaOH exposure, 30 seconds alone was sufficient to show the band (Figs. 2B, 2E), whereas in the 0.25-M NaOH exposure, the band appeared at 12 minutes and in the 6-M exposure it appeared between those times (∼6.5 minutes; Fig. 2F). The bovine corneal protein profile attributed to NaOH exposure showed similar changes when subjected to silver staining (data not shown), with minor differences. As in bovine eyes, NaOH exposure resulted in decreased protein yields in porcine eyes, with the curve resembling a depressed horseshoe shape (Supplementary Fig. S3A, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental). The yields of normalized protein with control porcine cornea were 12.5 ± 0.5 μg/mg tissue. The protein profiles for 11 M NaOH–treated corneal extracts (Supplementary Fig. S3B, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental) showed aggregation at the >250-kDa region (bar, thick arrow), the appearance of a new protein band (thin arrow), and the disappearance of protein bands in exposed tissues (arrowheads).
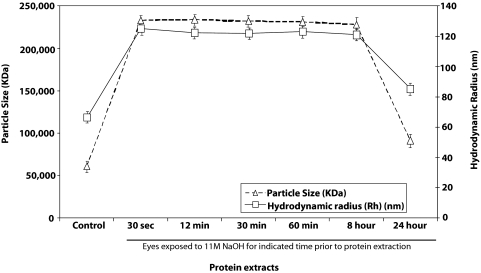
We used dynamic light scattering of the protein extracts to determine the average particle mass (kDa) and hydrodynamic radius (Rh; nm) using cumulative bimodal analysis for corneal protein exposed to 11 M NaOH for different durations compared with water-exposed controls. Dynamic light scattering showed an increase in average particle size and average hydrodynamic radius to alkali exposure within 30 seconds of exposure, suggesting rapid protein aggregate formation on subjection to NaOH exposure (Fig. 3). The representative dynamic light scattering estimate for 11 M–exposed protein extracts showed the dominance of similarly sized particles and of similar average hydrodynamic radii between 30 seconds and 8 hours. However, particle size and hydrodynamic radius appeared to have decreased between 8 and 24 hours (Fig. 3). The dynamic light scattering estimates of average cumulative bimodal molecular weight corroborated a rapid increase in particle size followed by a decrease in average particle size between 8 and 24 hours of exposure. The average particle size at 24 hours remained slightly larger than that of the controls (Fig. 3).
Figure 3.
Representative particle size and hydrodynamic radius using dynamic light-scattering measurements. Dynamic light scattering was performed on total protein extracts (1 μg in a volume of 12 μL) and was analyzed in cumulative bimodal mode. Average particle size has been provided in kilodaltons (kDa), and hydrodynamic radius (Rh, in nm) has been presented for controls and proteins exposed to alkali for the indicated time periods.
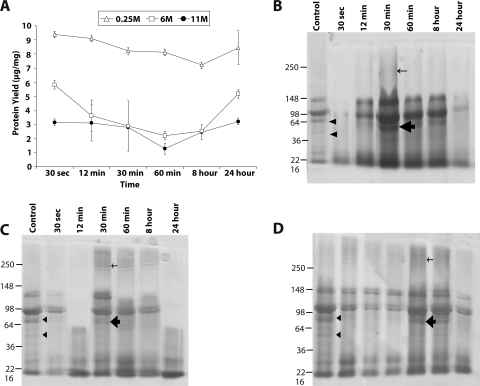
The ammonium hydroxide is a milder alkali than sodium hydroxide, and the increased normalized protein yield for the corneal protein exposed to the former is evident (Fig. 4A). The curve for the 11 and 6 M ammonium hydroxide resembles the depressed horseshoe shape; however, that for 0.25 M is similar to that of control, and the normalized protein yield is closer to control as well (Figs. 2A, 4A). The protein profile for ammonium hydroxide–treated tissue (Figs. 4B–D) is different from that of NaOH-treated tissue (Figs. 2B–D). In 11 M and 6 M NH4OH-treated tissue (Figs. 4B, 4C), a new band is observed between the 98- and 250-kDa range, indicated by a thin arrow, together with a thick band in tissue treated for 30 minutes. However, when exposed to 0.25 M, the higher molecular weight protein band and the protein band (Figs. 4B, 4C, thick arrow) appeared at 60 minutes (in contrast to 30 minutes, as with 11 and 6 M), suggesting that cumulative exposure (strength of alkali multiplied by time) plays a role in protein profile change (Fig. 4D). As with bovine corneal protein, the porcine normalized protein yield (Supplementary Fig. S4A, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental) showed reduced recovery that can be explained in terms of cumulative alkali exposure. Protein profiles were also found to undergo changes on 11 M NH4OH exposure. The control bands (Supplementary Fig. S4A, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental, arrowheads) underwent a decrease because of the exposure to alkali, whereas bands appeared in the alkali-exposed tissue but not in controls (Supplementary Fig. S4B, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental, thin arrows, thick arrows).
Figure 4.
Representative bovine corneal normalized protein yield and profile after exposure to ammonium hydroxide. (A) Representative protein yield (μg/mg) after exposure of cornea to 0.25 M (▵), 6 M (□), and 11 M (●) concentrations of NH4OH at the indicated time intervals. The recovered protein amounts estimated by Bradford's method were normalized to the initial amount of wet tissue used. Results are the mean ± SD of three independent estimates. Results were found to be significantly different from 0.0 by the one-sample t-test (*P < 0.05). (B–D) SDS-PAGE profile of total extracted protein after exposure to 11 M (B), 6 M (C), and 0.25 M (D) NH4OH, respectively. An equal amount of protein (10 μg) for each predetermined time exposure, as indicated, was separated on 10% SDS-PAGE and subsequently stained with Coomassie blue. Arrowheads: protein bands that decrease in intensity after NH4OH exposure. Protein bands that appear after exposure to NH4OH in high (thin arrows) and low (thick arrows) molecular weight regions, respectively.
Calcium hydroxide is a relatively sticky alkali; however, the normalized protein yield after exposure to this alkali showed an intermediate recovery (recovery lower than NH4OH but higher than NaOH), with a horseshoe profile (Fig. 5A). Similar to NH4OH recovery, the lowest normalized protein yield was recorded after 60 minutes of exposure (Fig. 5A). Protein profiles showed changes due to calcium hydroxide exposure; however, there were no dramatic changes between different time points (Figs. 5B–D). With 30 minutes of exposure, a weak band in control appeared to get intense in the exposed tissues (Figs. 5B–D, arrowheads), whereas a very thin band between 98 and 250 kDa started to appear (Figs. 5B–D, thin arrows). Compared with controls, a thick band indicated by thick arrow also appeared in all exposed lanes (Figs. 5B–D). The 8- to 24-hour protein profiles showed some changes in protein bands in locations (between arrowheads and thick arrows; Figs. 5B–D). Once again porcine cornea showed changes, but with important differences; normalized protein yield profiles were similar to those of bovine cornea (Supplementary Fig. S5A, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental). In protein profiles, although a protein band (arrowhead) was downregulated, new protein bands appeared in lanes from exposed tissues (thin arrows, thick arrows). Like those observed in bovine cornea exposed to calcium hydroxide (Fig. 5), the protein profile changes in porcine cornea were less dramatic, and the 8- to 24-hour protein profile showed decreased intensity in protein bands in regions below the arrowhead (Supplementary Fig. S5B, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental) compared with profile changes caused by exposure to sodium hydroxide (Fig. 3, Supplementary Fig. S3, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental).
Figure 5.
Representative bovine corneal normalized protein yield and profile after exposure to calcium hydroxide. (A) Representative protein yield (μg/mg) after exposure of cornea to 0.25 M (▵), 6 M (□), and 11 M (●) concentrations of Ca(OH)2 at the indicated time intervals. Recovered protein amounts estimated by Bradford's method were normalized to the initial amount of wet tissue used. Results are the mean ± SD of three independent estimates. Results were found to be significantly different from 0.0 by the one-sample t-test (*P < 0.05). (B–D) SDS-PAGE profile of total extracted protein after exposure to (B) 11 M, (C) 6 M, and (D) 0.25 M Ca(OH)2. An equal amount of protein (10 μg) for each predetermined time exposure, as indicated, was separated on a 10% SDS-PAGE and subsequently stained with Coomassie blue. Arrowhead: protein band that decreased in intensity after Ca(OH)2 exposure. Protein bands that appeared after exposure to Ca(OH)2 in high (thin arrow) and low (thick arrow) molecular weight regions, respectively.
The two acidic substances found to be in most households, lime juice and vinegar, were used to halt the damage by 11 M NaOH (Fig. 6). Treatment with lime juice showed some improvement in normalized protein yield/recovery (Fig. 6A). At 30 seconds, the protein yield increased from approximately 2.5 ± 0.2 μg/mg to 4.0 ± 0.2 μg/mg tissue. At 8 hours, the protein recovery was most dramatic, from <1 μg/mg to 3.8 μg/mg tissue (Figs. 2A, 6A). The protein profile also showed improvement, particularly for shorter durations, the protein profiles overall are similar to control proteins than that were exposed to NaOH without lime juice treatment (Fig. 6B). However, lime juice treatment seemed to be effective in restoring the protein profile (similar to profile for control) for exposures below 60 minutes and was increasingly less effective beyond 60 minutes (Fig. 6B). The aggregate proteins that appeared at 30 seconds of exposure (Fig. 2B) shifted to 12 minutes in lime juice–treated corneal extract (Fig. 6B). The 1-minute treatment with lime juice or vinegar alone used here before wash with water did not result in any change in protein yield or any discernible protein changes (data not shown).
Figure 6.
Representative bovine corneal normalized protein yield and profile after sodium hydroxide exposure and subsequent treatment. (A) Representative normalized protein yield (μg/mg) after corneal exposure to 11 M NaOH for indicated time intervals followed by treatment with lime juice. Results were significantly different from 0.0 by the one-sample t-test (*P < 0.05). (B) SDS-PAGE profile of total extracted protein after 11 M NaOH and lime juice treatment. (C) Representative normalized protein yield (μg/mg) after corneal exposure to 11 M NaOH for the indicated time intervals followed by treatment with vinegar. (D) Representative SDS-PAGE profile of total extracted protein after 11 M NaOH and subsequent vinegar treatment. (A, C) The recovered protein amounts estimated by Bradford's method were normalized to the initial amount of wet tissue used. Results are mean ± SD of three independent estimates. (B, D) An equal amount of protein (10 μg) for the indicated exposure time was separated by 10% SDS-PAGE and stained with Coomassie blue.
Vinegar juice–treated corneas showed greater normalized protein yield (Fig. 6C) than lime juice–treated corneas (Fig. 6B). The lowest point for protein recovery for the lime juice–treated cornea was approximately 12 minutes (Fig. 6A), whereas that for the vinegar-treated cornea was approximately 30 minutes (Fig. 6C) and was different from that for untreated 11-M NaOH–exposed cornea (Fig. 2A). With vinegar, the lowest yield increased from 1 μg/mg to 5 μg/mg (Fig. 6C); however, as evident by the protein profile (Fig. 6D), vinegar did not block or counter the effect of alkali, but provided a semblance of greater protein recovery. Lime juice–treated porcine cornea, similar to bovine cornea, showed improved protein yield and profile (Supplementary Figs. S6A, S6B, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental). The protein profile even for 8 to 24 hours, in contrast to bovine protein, showed better representation of protein bands than nontreated controls (Supplementary Fig. S6B, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental). In porcine cornea, as in bovine cornea, the vinegar-treated cornea showed an increased normalized protein yield (Supplementary Fig. S6C, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental) compared with the lime juice–treated cornea (Supplementary Fig. S6B, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental). The lowest point for protein recovery for both lime juice–treated corneas (Supplementary Fig. S6A, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental) and vinegar-treated corneas (Supplementary Fig. S6C, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental) was 8 hours, which paralleled the protein yield patterns of untreated 11-M NaOH exposed bovine cornea (Fig. 2A).
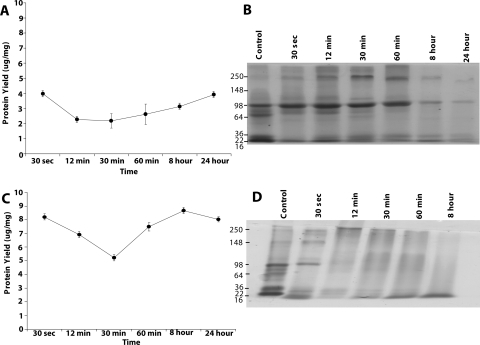
In preliminary experiments with human eyes, protein recovery showed some initial loss in the normalized protein yield at 12 minutes, followed by a slightly higher yield at 30 minutes compared with nontreated controls (Fig. 7A). Overall, the 11 M NaOH–exposed corneal protein recovery and SDS-PAGE profiles were similar (data not shown) to those observed for bovine eyes (Figs. 2A and 2B, respectively). The characteristic aggregation of protein was observed after 30 seconds of exposure to 11 NaOH, with decreased and increased intensity for specific bands (Fig. 7B), which was comparable to that for bovine cornea (Fig. 2B). Select corneal protein bands after 30 seconds in treated and control tissue were excised and subjected to tandem mass spectrometry.
Figure 7.
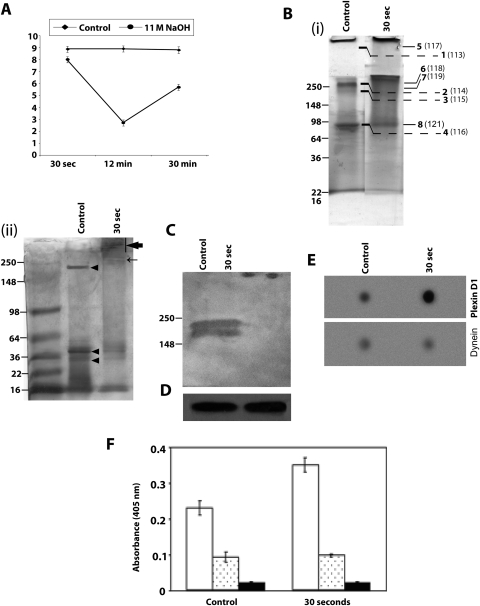
Representative human corneal normalized protein yield and profile after exposure to sodium hydroxide. (A) Representative protein yield (μg/mg) after exposure of the cornea to distilled water (control) (♦) or 11 M NaOH (●) at the indicated time intervals. Recovered protein amounts estimated by Bradford's method were normalized to the initial amount of wet tissue used. Results are the mean ± SD of three independent estimates and are significantly different from 0.0 by the one-sample t-test (*P < 0.05). (B) (i) Representative SDS-PAGE profile of total extracted protein (10 μg) after exposure to 11 M NaOH for 30 seconds and control, as stained with Coomassie blue. Gel bands shown with numbers were excised from locations, as indicated. Bands 1 to 4 are from the control, and bands 5 to 8 are from alkali-exposed tissue. They were labeled with 113 to 116 plex and 117 to 121 plex reagents and subjected to quantitative mass spectrometry. Bold numbers denote gel band number, and numbers within parentheses indicate 8 plex reagent treatment. (ii) Representative SDS-PAGE as in (i) except with 5 μg protein and stained with silver stain. Arrowheads: protein bands that decrease in intensity after NaOH exposure. Bar, thick arrow: smeared protein aggregates. Thin arrow: new protein band observed after NaOH exposure. (C) Western blot analysis of control and 11 NaOH–treated (30-second exposure) proteins (10 μg) with polyclonal goat anti–human plexin D1 antibody and a horseradish peroxidase–coupled donkey anti–goat antibody. (D) The same Western blot as in (C) probed with anti-GAPDH. (E) Representative dot blot determination of immunoreactivity for select identified proteins (plexin D1 and dynein, as indicated) in control and alkali-exposed corneal tissue. Control and 30-second NaOH-exposed protein extracts (1 μg) were spotted onto a PVDF membrane, air dried, blocked (with 5% milk), and probed with antibodies to plexin D1 or dynein, and an appropriate horseradish peroxidase–coupled secondary antibody. (F) ELISA was performed with 1 μg control or 30-second NaOH exposed tissue-derived proteins, as indicated, for plexin D1, dynein heavy chain 9 antibody with secondary antibodies conjugated with alkaline phosphatase. Purified bovine serum albumin (1 μg) served as a negative control. Plexin D1 (hollow bar) and dynein (dotted bar) ELISA estimates. Solid bar: immunoreactivity for BSA with a mixture of plexin D1 and dynein antibodies, with the same concentration used individually on corneal extracts. Results are the mean ± SD of three independent experiments.
For this purpose, the protein bands from identical locations in control and exposed tissue (bands 1–4 were labeled with 113–116 and bands 5–8 were labeled with 117–119 and 121 of 8-plex labels) were subjected to tandem mass spectrometry, as described in Methods. The proteins identified from these bands, together with their areas for different 8-plex labels, are provided in Table 1. Plexin D1, a protein, was identified for the first time in cornea at the protein level (Table 1). Control and treated corneal proteins were probed with goat antibody against human plexin D1, revealing two bands (∼212 and 195 kDa), known isoforms of plexin D1 (Fig. 7C). The partially transferred gel was subjected to Coomassie blue staining showing equal loading of the protein after transfer (Fig. 7D). Further probing the blot with GAPDH also showed equal intensity in the two lanes (data not shown). We performed dot blot and ELISA analyses to determine the relative quantity of proteins in nontreated control and alkali-exposed tissue–derived protein (Figs. 7E, 7F), which showed increased plexin D1 immunoreactivity in 11 M NaOH–exposed tissue compared with control and was consistent with 8 plex–labeled quantitative mass spectrometry (Table 1; Supplementary Table S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental).
Table 1.
Proteins Identified in Control and Alkali-Treated Cornea
| Accession No.* | Protein | MWt(KDa)† | Ratio | Total Area in Control (113–116) | Total Area in Treated (117–121) | Percentage Coverage |
|---|---|---|---|---|---|---|
| P01834 | Ig kappa chain C region | 11.6 | 2.8 | 5084.97 | 1791.94 | 7.6 |
| Q15653 | NF-kappaB inhibitor beta variant | 38, 36 | 2.5 | 4598.96 | 1847.18 | 3.0 |
| P13645 | Cytokeratin-10 | 58.8 | 2.4 | 3414.53 | 1410.43 | 5.2 |
| AAT49050 | Immunoglobulin heavy constant gamma 1-like protein | 52 | 2.3 | 33482.23 | 14520.06 | 22.6 |
| P30838 | Aldehyde dehydrogenase | 50 | 2.2 | 24074.86 | 10986.91 | 4.6 |
| Q9NYC9 | Dynein heavy chain 9 | 512, 503 | 1.0 | 32429.21 | 32429.95 | 1.5 |
| XP_002348035 | Hypothetical protein | 100.3 | 0.8 | 101778.2 | 122117.23 | 2.1 |
| P81947 | Alpha tubulin | 50.1 | 0.8 | 5816.66 | 7140.06 | 3.3 |
| Q14694 | Ubiquitin carboxyl-terminal hydrolase 10 | 87 | 0.7 | 9731.67 | 14329.4 | 1.9 |
| P02768 | Human Serum Albumin | 69, 47 | 0.7 | 20632.69 | 27517.97 | 6.0 |
| Q9Y4D7 | Plexin-D1 | 212, 196 | 0.7 | 9731.67 | 14329.4 | 1.9 |
| Q8IZT6 | Abnormal spindle-like microcephaly-associated protein | 409, 218 | 0.6 | 18310.43 | 32429.75 | 1.6 |
| O60938 | Keratocan | 40.5 | 0.5 | 22538.42 | 46965.53 | 9.7 |
| P51884 | Lumican | 38.4 | 0.3 | 15852.29 | 60204.61 | 16.6 |
| P07585 | Decorin variant A | 39.7, 27.3, 23, 19, 8.2 | 0.2 | 23272.06 | 114922.21 | 8.6 |
| Q15582 | Transforming growth factor, 68-kDa variant | 74.7 | 0.2 | 9240.24 | 55649.9 | 2.6 |
NCBI accession number is expressed in italics, and SwissProt accession number is provided. Ratio refers to total area is control divided by total area in alkali-treated group.
Molecular weights for all isoforms have been listed. The protein bands 1–8 depicted in Figure 7B were subjected to iTRAQ labeling (1–4 labeled with 113–116, 5–8 labeled with 117–119 and 121) and tandem mass spectrometry for identification and relative quantification of proteins. The area for each individual tag is presented in Supplementary Table S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental.
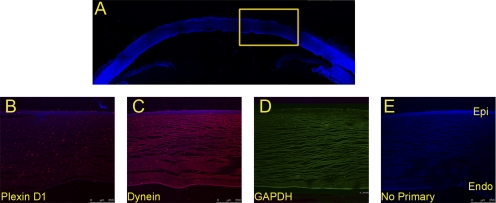
Similar immunoreactivity was observed for dynein immunoreactivity in control and NaOH-treated cornea (Figs. 7E, 7F), which is also consistent with iTRAQ-labeled quantitative mass spectrometry (Table 1; Supplementary Table S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental). We used immunohistochemical detection (Fig. 8) to determine the localization of plexin D1 and dynein heavy chain (Figs. 8B, 8C), the two proteins identified by 8-plex quantitative mass spectrometry (Table 1). Immunohistochemistry revealed the presence of plexin D1 (Fig. 8B) and dynein (Fig. 8C) in the corneal epithelium. Although both proteins are present in other regions of the cornea, plexin D1 is present in relatively higher amounts in the epithelium than in the stroma. In contrast, GAPDH is present throughout the cornea in a diffused manner (Supplementary Fig. S7, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental; Figs. 8B–D).
Figure 8.
Immunohistochemical analyses of identified proteins. Cornea sections were derived from a human cadaveric eye (77-year-old Caucasian male) with no known eye disease. Confocal images were taken under a microscope at 10× magnification. (A) DAPI-stained anterior human cadaver eye segment. (B–E) Same sections stained with anti-plexin D1, anti-dynein, GAPDH, and no antibody (control), respectively. Secondary antibodies were coupled with FITC and Alexa 594. Boxed region: subjected to imaging at a higher magnification. Epi, epithelial layer; Endo, endothelial layer.
Discussion
The objective of the current investigation was to determine whether changes in a soluble protein yield normalized to the amount of tissue taken together with protein profile can be a predictor of severity and duration of exposure to alkali (and whether specific proteins can be identified in post-alkali–exposed corneal proteins). Although the strength of the alkali is expected to contribute to acute severity, the duration of exposure is expected to result in cumulative damage. We selected representative strong, weak/moderate alkali: sodium, ammonium, and calcium hydroxide. Our selection of alkali was based on the fact that several alkaline materials are valuable industrial chemicals, often stored in large amounts in areas adjacent to and transported through populated areas.2 Sodium and ammonium hydroxide are used in a number of industrial processes,1 and calcium hydroxide is used as quicklime for white wash and painting and as an edible paste that has been known to cause accidental corneal burns.28,29 Alkali damages are usually more penetrating and more severe than acid damages. We used different time intervals, as outlined in the experimental procedure (see Methods) representing different real-life scenarios, and we used bovine and porcine eyes for initial experiments. We reasoned that the use of two types of mammalian eyes of different sizes would enable the identification of common features that could be applicable to human eyes and that would allow performance of a limited subset of experiments on human eyes. We carefully examined the eyes for any damaged caused by enucleation, transportation, or handling, and a subset of eyes in every batch was subjected to staining and destaining with fluorescein that revealed lack of any damage to the corneal epithelial layer.
When exposed to sodium hydroxide the bovine eyes showed aggregation within 30 seconds (for 11 M; Fig. 2B) or 12 minutes (for 0.25 M; Fig. 2D) and an intermediate time point for 6 M (Fig. 2C). The protein yield for exposure tissue was always lower than the control water-exposed cornea (Fig. 2A; Supplementary Figs. S3A, S7A, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental).
The lowering of the protein yield at each time interval was a function of the strength of the alkali. The higher the strength of the alkali, the lower the normalized protein yield at the selected time of exposure (Figs. 2A, 4A, 5A, 7B). The protein profiles after alkali exposure were different compared with controls. Although some protein bands in the lower molecular range (30–98 kDa) showed a significant decrease in all exposed tissue, new bands in the higher molecular range (>98 kDa) and aggregates were clearly observed as a function of exposure to alkali (Figs. 2B–D, 4B–D, 5B, 7B). Similar changes in yield as well as protein profile were found in alkali-exposed porcine tissue (Supplementary Figs. S3–5, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental), suggesting similarities in mammalian eyes irrespective of size and species differences. For example, the bovine cornea subjected to 11 M NaOH exposure showed a distinct decrease in intensity of two bands in the molecular weight range 36 to 50 kDa (arrowheads), the presence of a high molecular weight band (∼98 kDa), and aggregates giving the appearance of a smear in the molecular range 148 to 250 kDa (Figs. 3B, 3D). Together this profile, with distinct bands (disappearance/decreased intensity of lower and appearance of higher molecular weight) and aggregate, can potentially serve as a readout of alkali exposure. Differences in protein profile as a function of time for a particular concentration of alkali, such as 11 M NaOH on bovine cornea (Fig. 2B), in contrast to that for 0.25 M, shows the shift in protein aggregate from 30 seconds to approximately 12 minutes, suggesting that protein profiles may also provide clues to the extent of exposure compared with controls. Protein modification thus appears to be the cumulative multiplicative effect of exposure to the active chemical ingredient and time (Figs. 2B, 2D).
In contrast to the clear shift in the time point of aggregate formation (range, 250–148 kDa) from 30 seconds for 11 M to 12 minutes for 0.25 M in response to strong alkali (sodium hydroxide; Figs. 2B–D), exposure to ammonium hydroxide, a weak alkali, did not show such a clear shift (Figs. 4B–D). However, even the weak alkali (ammonium hydroxide) showed a similar general trend—that is, the reduction in protein yield normalized to tissue amount (Fig. 4A) compared with controls and alteration of protein profile due to alkali exposure. A similar trend was observed for calcium hydroxide (Fig. 5A). When handling tissue exposed to calcium hydroxide, we noted that after homogenization, the samples were extremely viscous. Protein extraction necessitated the addition of relatively larger volumes of buffer compared with other alkali exposures to reduce the viscosity of the solution. Calcium hydroxide–exposed tissue also showed a persistent presence of aggregated proteins across more exposure time points compared with other sodium-exposed or ammonium hydroxide–exposed tissue after gel separation.
The alkali-exposed cornea, even at 30 seconds, shows a smear (Figs. 2B, 2D, 7B) that is consistent with the presence of nonhomogeneous entities of nearly similar (but not identical) mass-to-charge ratio and is consistent with aggregates of proteins or proteins with nonprotein entities. Quantitative mass spectrometry identified peptides of different proteins from these locations from gel-excised smears (Table 1). In Figure 7C, two bands corresponding to two isoforms of plexin D1 (212 and 195 kDa) were identified by Western blot analysis in the control lane, whereas less immunoreactivity was found in the alkali-treated lane (Fig. 7C). This is inconsistent with quantitative mass spectrometry, which suggested more area for the identified plexin D1 peptide in 117- to 121-plex than in 113- to 116-plex. To determine whether this discrepancy was due to hydrolysis followed by aggregation that will distribute the plexin D1 peptide all over the gel location but will show only diminished immunoreactivity in the Western blot, we performed dot blot analysis (Fig. 7E). Dot blot analysis clearly showed more immunoreactivity for plexin D1 and dynein in the alkali-exposed lanes compared with control (Fig. 7E) and was consistent with alkali-induced hydrolysis and aggregation of peptides from these proteins. These findings are further corroborated by ELISA analysis (Fig. 7F).
Exposure of alkaline chemicals to the cornea usually results in opacification, vascularization, and recruitment of healing processes.30–32 Although the alkali-burned corneas seldom heal properly to restore complete transparency, healing processes result in the upregulation of several mRNAs and in the synthesis of new proteins and the clearance of damaged protein.33,34 Thus damaged proteins are likely to be best understood in corneas in which the repair and synthesis process is either absent or minimal. In the present investigation, we used eyes 72 hours after enucleation. Comparison of the BrdU incorporation immediately after and 72 hours after enucleation revealed substantial reduction of DNA synthesis and repair. Although DNA synthesis does not necessarily mean the reduction of protein synthesis, a subset of control experiments also revealed minimal or no protein synthesis (Fig. 1B), suggesting the observed protein changes are largely a function of alkali exposure. Contributions due to repair and synthesis were greatly diminished in the eyes used. A previous study reported protein profile changes caused by acetic acid and ammonium hydroxide exposure using isoelectric focusing for rabbit and human corneal proteins.35 However, the previous study design was very different from the present study. For example, the thin corneal sections (100 μm) were immersed in solution for a brief 15 seconds, which does not resemble an accidental splash situation. The investigation was also not designed to use repair and protein synthesis–deficient cornea and encompassed only a relatively low (2%) of ammonium hydroxide.35 The present study used several different techniques in addition to gel electrophoretic separation, such as dynamic light scattering and mass spectrometry followed by Western blot confirmation and immunohistochemical localization of select identified proteins.
Fundamentally, proteins undergo changes when exposed to alkaline conditions. These changes range from aggregation to changes in solubility, elimination reactions involving the amino acid side chains, cross-linking with lipids (or other nonprotein constituents), and hydrolysis.36 Proteins with amino acid side chains that undergo posttranslational modifications are amenable to chemical modification and cross-linking when exposed to alkaline chemicals.36,37 Thus hydrolytic truncation and aggregation of proteins remain key features of alkali exposure to tissues, which contain protein and nonprotein entities.36,38 We would like to emphasize that the models used here have the limitation of being static, in contrast to dynamic in vivo models. The corneal protein composition of individual animals may show variation, and the possibility of slight variation of protein composition may be expected because of nonhomogeneous physical interaction of alkali with the corneal surface among individual repeat experiments. As noted before, the enucleated eye models were chosen to capture very initial modifications of the protein profile after the onset of burn. The protein profile in in vivo models is expected to undergo rapid changes because the onset of the secondary immune reaction and the enzymatic remodeling of the cornea. The aqueous humor and the endothelial pump play key roles in the pH homeostasis of the corneal tissue. It must be noted that the experimental corneas used here had nonfunctional endothelial pumps39; these are known to become nonfunctional after several hours of incubation at 4°C.
In our current analysis a number of proteins were identified. For example, plexin D1 (Table 1; Supplementary Table S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5472/-/DCSupplemental) was identified for the first time at the protein level in corneal tissue. A database (Unigene) search revealed that identified proteins showed the presence of mRNA for proteins in the eye. The cornea is not specifically investigated in the Unigene database, but RT-PCR analysis corroborates the presence of mRNA in the cornea for the identified proteins (data not shown). It remains unclear whether the identified proteins are from the epithelium or from other layers of the cornea because the analysis was performed on whole corneal extract. We performed immunohistochemical analysis in part to determine whether select identified proteins were from the corneal epithelium, and we found both plexin D1 and dynein to be present in the epithelium (Fig. 8). Their presence in the epithelium raises the possibility that plexin D1 and dynein may be associated with alkali exposure–induced changes. Further investigation along these lines may provide better understanding of the involvement of plexin D1, dynein, and other proteins and will suggest the feasibility/potential of such proteins serving as biomarkers for alkali-induced damage.
In summary, we used normalized protein yields and protein profile changes to determine the effects caused by alkali exposure on the cornea. Results broadly show that changes from alkali exposure are consistent and, thus, suggest that these methods may possibly be used as indicators of alkali exposure. However, similar procedures, methods, and subsequent identification of protein entities provide alternative and, in some instances, advanced assay systems for testing other chemicals used in cosmetics, food, and pharmaceuticals.40–42 Perfection of such methods as outcome measures may reduce the number of animals used for evaluation. Protein separation is also essential for subsequent mass spectrometric identification and quantitative estimation of proteins. The identification of proteins poises assignment and evaluation of exposure-specific biomarkers. Such identification and subsequent investigations may provide insight into the damage caused by alkali exposure. The ability to separate exposure-induced damage from processes of repair and new protein synthesis (and clearance of damaged proteins) will expand our understanding of the protein changes with greater clarity; the current investigations are a step in that direction.
Acknowledgments
The authors thank Ebrahem Quteba, Leonard Real, and Tom Straub for their assistance with tissue procurement; Shari Seidman, Patricia Garcia, Tom Pitts, and Elizabeth Zapata for their assistance; and Henry Edelhauser, Sonia Yoo, and Reza Dana for their comments on the manuscript.
Footnotes
Supported by Department of Defense Grant W81XWH-09-1-0674 (Project 2.2), a career award and unrestricted funds from Research to Prevent Blindness and National Institutes of Health Core Grant P30-EY14801.
Disclosure: T. Parikh, None; N. Eisner, None; P. Venugopalan, None; Q. Yang, None; B.L. Lam, None; S.K. Bhattacharya, None
References
- 1. Hom GG. Chemical, biological, and radiological weapons: implications for optometry and public health. Optometry. 2003;74:81–98 [PubMed] [Google Scholar]
- 2. Bhattacharya SK, Hom GG, Fernandez C, Hom LG. Ocular effects of exposure to industrial chemicals: clinical management and proteomic approaches to damage assessment. Cutan Ocul Toxicol. 2007;26:203–225 [DOI] [PubMed] [Google Scholar]
- 3. Patel N, Solanki E, Picciani R, Cavett V, Caldwell-Busby JA, Bhattacharya SK. Strategies to recover proteins from ocular tissues for proteomics. Proteomics. 2008;8:1055–1070 [DOI] [PubMed] [Google Scholar]
- 4. Kales SN, Christiani DC. Acute chemical emergencies. N Engl J Med. 2004;350:800–808 [DOI] [PubMed] [Google Scholar]
- 5. Khaw PT, Shah P, Elkington AR. Injury to the eye. BMJ. 2004;328:36–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paterson CA, Williams RN, Parker AV. Characteristics of polymorphonuclear leukocyte infiltration into the alkali burned eye and the influence of sodium citrate. Exp Eye Res. 1984;39:701–708 [DOI] [PubMed] [Google Scholar]
- 7. Panda A, Mohan M, Gupta AK, Chawdhary S. Keratoplasty in alkali burned corneas. Indian J Ophthalmol. 1984;32:441–446 [PubMed] [Google Scholar]
- 8. Pahlitzsch T, Sinha P. The alkali burned cornea: electron microscopical, enzyme histochemical, and biochemical observations. Graefes Arch Clin Exp Ophthalmol. 1985;223:278–286 [DOI] [PubMed] [Google Scholar]
- 9. O'Driscoll AM, Aggarwal RK, Shah P, Chell PB, Hope-Ross MW, McDonnell PJ. Ocular injuries due to alkaline substances. BMJ. 1995;310:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Obenberger J, Babicky A. Alkali and acid burns of the rabbit eye: uptake of intravenously injected Na125I and Na131I into the cornea and iris. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1975;194:65–72 [DOI] [PubMed] [Google Scholar]
- 11. Obenberger J, Babicky A. Alkali and acid burns of the rabbit eye: measurement of aqueous flow by means of intravenously injected Na-125 I and Na-131I. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1975;193:253–257 [DOI] [PubMed] [Google Scholar]
- 12. Obenberger J, Dobiasova M, Babicky A. [Burns of the rabbit's cornea by NaOH and HCl. Hydration and uptake of 45 Ca in vitro]. Cesk Oftalmol. 1974;30:163–166 [PubMed] [Google Scholar]
- 13. Ormerod LD, Garsd A, Reddy CV, Gomes SA, Abelson MB, Kenyon KR. Dynamics of corneal epithelial healing after an alkali burn: a statistical analysis. Invest Ophthalmol Vis Sci. 1989;30:1784–1793 [PubMed] [Google Scholar]
- 14. Tuft SJ, Shortt AJ. Surgical rehabilitation following severe ocular burns. Eye. 2009;23:1966–1971 [DOI] [PubMed] [Google Scholar]
- 15. Meller D, Pires RT, Mack RJ, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107:980–989, discussion 990 [DOI] [PubMed] [Google Scholar]
- 16. Pal-Ghosh S, Pajoohesh-Ganji A, Brown M, Stepp MA. A mouse model for the study of recurrent corneal epithelial erosions: α9β1 integrin implicated in progression of the disease. Invest Ophthalmol Vis Sci. 2004;45:1775–1788 [DOI] [PubMed] [Google Scholar]
- 17. Pal-Ghosh S, Tadvalkar G, Jurjus RA, Zieske JD, Stepp MA. BALB/c and C57BL6 mouse strains vary in their ability to heal corneal epithelial debridement wounds. Exp Eye Res. 2008;87:478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254 [DOI] [PubMed] [Google Scholar]
- 19. Zor T, Selinger Z. Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal Biochem. 1996;236:302–308 [DOI] [PubMed] [Google Scholar]
- 20. Stoscheck CM. Quantitation of protein. Methods Enzymol. 1990;182:50–68 [DOI] [PubMed] [Google Scholar]
- 21. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275 [PubMed] [Google Scholar]
- 22. Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85 [DOI] [PubMed] [Google Scholar]
- 23. Olson BJ, Markwell J. Assays for determination of protein concentration. Curr Protoc Protein Sci. 2007; Chapter 3: Unit 3 4 [DOI] [PubMed] [Google Scholar]
- 24. Noble JE, Bailey MJ. Quantitation of protein. Methods Enzymol. 2009;463:73–95 [DOI] [PubMed] [Google Scholar]
- 25. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685 [DOI] [PubMed] [Google Scholar]
- 26. Tang WH, Shilov IV, Seymour SL. Nonlinear fitting method for determining local false discovery rates from decoy database searches. J Proteome Res. 2008;7:3661–3667 [DOI] [PubMed] [Google Scholar]
- 27. Menegay M, Lee D, Tabbara KF, et al. Proteomic analysis of climatic keratopathy droplets. Invest Ophthalmol Vis Sci. 2008;49:2829–2837 [DOI] [PubMed] [Google Scholar]
- 28. Diedler JL, Benoit A. [Prevention of symblepharon in severe ocular burns: apropos of 3 cases]. J Fr Ophtalmol. 1984;7:31–33 [PubMed] [Google Scholar]
- 29. Agarwal T, Vajpayee R. A warning about the dangers of chuna packets. Lancet. 2003;361:2247. [DOI] [PubMed] [Google Scholar]
- 30. Reim M, Redbrake C, Schrage N. Chemical and thermal injuries of the eyes: surgical and medical treatment based on clinical and pathophysiological findings. Arch Soc Esp Oftalmol. 2001;76:79–124 [PubMed] [Google Scholar]
- 31. Brodovsky SC, McCarty CA, Snibson G, et al. Management of alkali burns: an 11-year retrospective review. Ophthalmology. 2000;107:1829–1835 [DOI] [PubMed] [Google Scholar]
- 32. Wagoner MD, Kenyon KR. Chemical injuries: clinical course and management. In: Kuhn F, Pieramici DJ. eds. Ocular Trauma: Principles and Practice. New York: Thieme; 2002:335–349 [Google Scholar]
- 33. Ishizaki M, Zhu G, Haseba T, Shafer SS, Kao WW. Expression of collagen I, smooth muscle alpha-actin, and vimentin during the healing of alkali-burned and lacerated corneas. Invest Ophthalmol Vis Sci. 1993;34:3320–3328 [PubMed] [Google Scholar]
- 34. Ishizaki M, Wakamatsu K, Matsunami T, et al. Dynamics of the expression of cytoskeleton components and adherens molecules by fibroblastic cells in alkali-burned and lacerated corneas. Exp Eye Res. 1994;59:537–549 [DOI] [PubMed] [Google Scholar]
- 35. Eurell TE, Sinn JM, Gerding PA, Alden CL. In vitro evaluation of ocular irritants using corneal protein profiles. Toxicol Appl Pharmacol. 1991;108:374–378 [DOI] [PubMed] [Google Scholar]
- 36. Whitaker JR, Feeney RE. Chemical and physical modification of proteins by the hydroxide ion. Crit Rev Food Sci Nutr. 1983;19:173–212 [DOI] [PubMed] [Google Scholar]
- 37. Gould DH, MacGregor JT. Biological effects of alkali-treated protein and lysinoalanine: an overview. Adv Exp Med Biol. 1977;86B:29–48 [DOI] [PubMed] [Google Scholar]
- 38. Hameed M, Ahmad B, Khan RH, Andrabi KI, Fazili KM. Tertiary butanol induced amyloidogenesis of hen egg white lysozyme (HEWL) is facilitated by aggregation-prone alkali-induced molten globule like conformational state. Protein Pept Lett. 2009;16:56–60 [DOI] [PubMed] [Google Scholar]
- 39. Hatou S, Yamada M, Akune Y, et al. Role of insulin in regulation of Na+-/K+-dependent ATPase activity and pump function in corneal endothelial cells. Invest Ophthalmol Vis Sci. 51:3935–3942 [DOI] [PubMed] [Google Scholar]
- 40. Gautheron P, Dukic M, Alix D, Sina JF. Bovine corneal opacity and permeability test: an in vitro assay of ocular irritancy. Fundam Appl Toxicol. 1992;18:442–449 [DOI] [PubMed] [Google Scholar]
- 41. Sina JF, Galer DM, Sussman RG, et al. A collaborative evaluation of seven alternatives to the Draize eye irritation test using pharmaceutical intermediates. Fundam Appl Toxicol. 1995;26:20–31 [DOI] [PubMed] [Google Scholar]
- 42. Muir CK. Opacity of bovine cornea in vitro induced by surfactants and industrial chemicals compared with ocular irritancy in vivo. Toxicol Lett. 1985;24:157–162 [DOI] [PubMed] [Google Scholar]