Morphologic studies of human eyes reveal that vascular loss occurs early in the pathogenesis of AMD and is related to the cross-sectional area of drusen, supporting the concept that vascular changes may lead to drusen formation in AMD.
Abstract
Purpose.
Age-related macular degeneration (AMD) is a common, potentially blinding disease characterized by the presence of extracellular deposits beneath the retinal pigment epithelium (RPE). Choroidal vascular changes have also been noted in AMD. This study examined the relationship between the choroidal vasculature and extent of drusen and other sub-RPE deposits, the key pathologic landmarks of AMD.
Methods.
Sections of the maculas of 45 human eyes (21 early AMD and 24 age-matched control) were evaluated morphometrically. The cross-sectional area of sub-RPE deposits, vascular density, number of CD45+ leukocytes, and number of “ghost vessels” were determined in a masked fashion and evaluated by regression analysis. In addition, the extramacular vascular density either directly beneath drusen or adjacent to drusen was evaluated in a separate set of donor eyes.
Results.
The vascular density of the choriocapillaris showed a trend toward decreasing in association with AMD status. By linear regression analysis, vascular density was inversely associated with sub-RPE deposit density (r2 = 0.22, P < 0.01). The number of ghost vessels was negatively correlated with vascular density (r2 = 0.55, P < 0.001) and positively correlated with sub-RPE deposit density (r2 = 0.57, P < 0.001). In morphologic studies of extramacular solitary drusen, vascular density beneath drusen was found to be 45% lower than adjacent to drusen (P < 0.01).
Conclusions.
These findings support the concept that microvascular changes are related to the pathogenesis of AMD and suggest that vascular endothelial cell loss occurs in association with sub-RPE deposit formation. Whether microvascular events are a cause or consequence of drusen or other deposit formation remains to be determined.
Age-related macular degeneration (AMD) is a common, potentially blinding disorder affecting the aged. In advanced stages of the disease, vision in the macular region can be severely compromised by either atrophic changes in the retinal pigment epithelium (RPE), overlying retina, and underlying choriocapillaris or compromise of Bruch's membrane and subsequent invasion of choroidal endothelial cells into the subretinal space, with severe consequences for vision.1,2 Although outcomes are more favorable in patients with neovascular disease than was previously possible,3 a better understanding of cellular events that occur in the earliest stages of disease will lead to improved future therapies.
Clinically, the earliest stages of AMD are characterized by changes in the evenness of the RPE pigmentation and the observation of deposits in Bruch's membrane, termed drusen.4–6 Because drusen and other sub-RPE deposits (basal laminar deposit and basal linear deposit, also referred to as diffuse drusen) are diagnostic and predictive of AMD,7 there has been considerable interest in defining the composition of sub-RPE deposits as well as understanding their origin. Immunohistochemical8–11 and biochemical12,13 studies performed on human donor eyes with drusen have identified an array of molecular species associated with these deposits, including inflammatory mediators and lipids of retinal and choroidal origin.
Interestingly, the formation of drusen does not appear to be spatially random but is influenced by the anatomy of the underlying choroid. Friedman noted in whole mounts that drusen were likely to develop over what were interpreted as collecting venules of the choroid.14 It was further noted by Sarks et al.15 that the smallest, earliest drusen were likely to accumulate over choriocapillary pillars at what were called “entrapment sites.” The relationship of drusen to vessel walls, rather than lumens, was confirmed in a series of whole mounted choroids by Lengyel et al.,16 which is further supported by findings of RPE protein deposition in Bruch's membrane at pillar sites.17
The mechanism(s) driving sub-RPE deposit formation, and the basis for the predilection of these deposits to form in areas over intercapillary pillars or devoid of capillary lumens, is unknown. One might speculate that, if drusen preferentially develop over regions of capillary lumen-depleted choroid, reduced vascular perfusion may contribute to drusen formation. To further understand the relationship between drusen and other sub-RPE deposits with the choriocapillaris, we performed a series of morphometric experiments in which we assessed the relationship of the vasculature with sub-RPE deposits in the human macula. The density of pathologic deposits was strongly linked to the density of the choroidal vasculature, with eyes having the most drusen showing the lowest vascular density. We also found that choroidal “ghost” vessels, remnants of previously healthy capillaries, increased in abundance with increasing drusen density. We discuss these findings in the context of drusen biogenesis and the pathophysiology of AMD.
Methods and Materials
Human Donor Eyes and Histologic Methods
Human donor eyes were obtained from the Iowa Lions Eye Bank (Iowa City, IA) and were preserved within 8 hours of death after informed consent of the donor families. The use of human donor eyes conformed to the Declaration of Helsinki. Eyes were assigned to either early AMD or age-matched control donors based on chart review. Eyes with advanced AMD (geographic atrophy or choroidal neovascularization), identified in the chart, on gross examination of the eyes, or histologically, were omitted from the study. Eyes were designated as early AMD if they had been diagnosed as being notable for macular drusen and/or RPE changes; that is, they were AREDS grades 2 or 3.1 The age of donors ranged from 60 to 100 years. The mean age of AMD donors was 84.8 ± 7.9 years, and the mean age of controls was 81 ± 8.9 years.
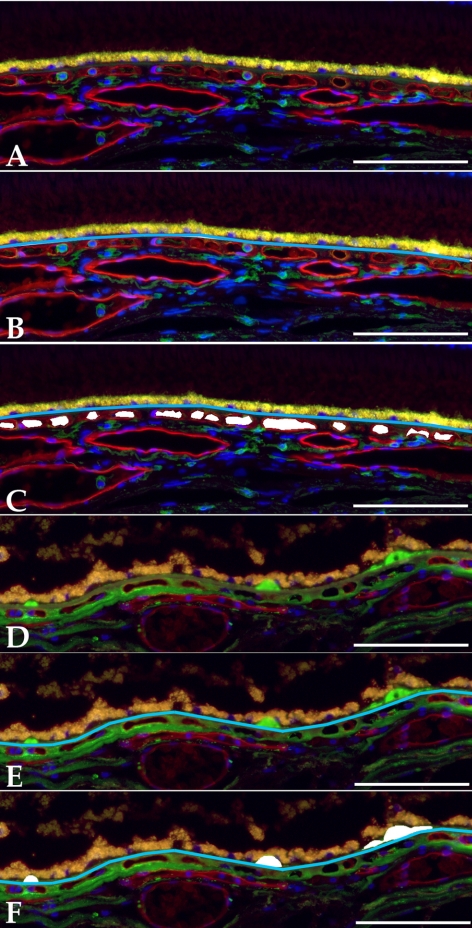
Either a 6-mm or bisected 8-mm punch centered on the fovea of each donor was collected and fixed in 4% paraformaldehyde in 10 mM phosphate-buffered saline, before sucrose cryoprotection and cryostat sectioning, using methods described previously.18 Sections of 45 maculas from 42 donors (n = 24 unaffected, n = 21 clinically diagnosed with early-stage AMD) were collected. Lectin histochemistry using Ulex europaeus agglutinin-I (UEA-I; Vector Laboratories, Burlingame, CA) was performed as described previously,19 and sections were also labeled with either anti-CD45 (2.5 μg/mL; BD Pharmingen, San Diego, CA) to assess leukocytes or anti–C5b-9 terminal complement complex (1.5 μg/mL; Dako, Carpinteria, CA) to image sub-RPE deposits as described previously.20 Sections were counterstained with diamidino-phenol-indole (DAPI; Molecular Probes, Eugene, OR), and triple-labeled sections were photographed using the ×20 objective of a fluorescence microscope (BX41; Olympus, Center Valley, PA) and a digital camera (SPOT RT; Diagnostic Instruments, Inc., Sterling Heights, MI). Nonoverlapping photomicrographs spanning the section (over a mean length of 549 μm/donor) were collected. Examples of this labeling are depicted in Figures 1A and 1B. All images were collected with identical camera settings.
Figure 1.
Examples of measured quantitative features of human maculas used in this study. Images were obtained from a 79-year-old female without known AMD (A–C) and an 86-year-old male with dry AMD (D–F). Raw (A, D) and measured (B, C, E, F) images for choriocapillaris density (A–C) and drusen density (D–F). Depicted sections were labeled with UEA-I lectin (red fluorescence), and antibodies directed against either CD45 (A–C) or terminal complement complex C5b-9 (D–F) (green fluorescence). Sections were counterstained with DAPI. Measured Bruch's membrane lengths are indicated by a blue line, and vascular or drusen areas are shown in white. Raw and measured images are depicted. Scale bars, 100 μm.
Morphometric Analysis
Photomicrographs were evaluated using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) calibrated with a stage micrometer under identical conditions. For each micrograph, the length of Bruch's membrane under which choroidal capillaries were measurable was first measured using the broken line tool in ImageJ (see Fig. 1B). This length varied due to curvature of Bruch's membrane in some sections and/or artifactual damage to some regions of the sections such that the choriocapillaris or drusen were unmeasurable. This length measurement was subsequently used to standardize measurements of choriocapillaris area, number of extravascular CD45+ leukocytes, number of ghost vessels, and drusen/sub-RPE deposit cross-sectional area across all donors. All measurements were performed masked with respect to donor information including AMD diagnosis.
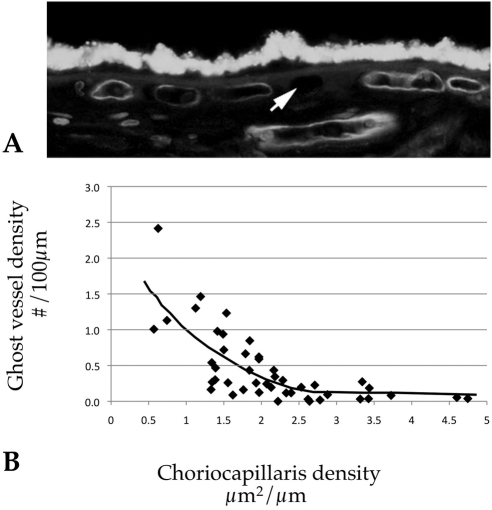
Cross-sectional areas of capillaries were measured based on lumens visualized within the UEA-I reactive vessel walls (see Fig. 1B). In cases in which a venule or arteriole exited or entered the choriocapillaris layer, the lumen was measured only to the depth of the average capillary in the field. Only lumens were scored; collapsed vessels or vessels sectioned obliquely in the image were not measured. Cross-sectional areas of sub-RPE deposits were similarly measured. Drusen, basal laminar deposit, and basal linear deposit were not distinguished for these experiments but were scored only if they had visible thickness and material that was detectable with anti–C5b-9 antibody and/or by UV autofluorescence; serous RPE detachments were not included as drusen area. For the purposes of the statistical analysis, the pooled areas of all deposits are referred to as “drusen.” The number of CD45+ leukocytes was also measured. Leukocytes were counted only if they were CD45 reactive, were extravascular (i.e., were not contained within the UEA-I reactive vasculature), were within 40 μm of Bruch's membrane, and showed a nucleus in the plane of the section. Examples of CD45+ choroidal leukocytes are shown in Figures 1A and 1B. Finally, “ghost” vessel numbers were collected. Choriocapillaris ghosts were scored as regions between two choriocapillary pillars without any UEA-I–positive endothelium. Ghost vessel numbers/length, rather than area/length, were recorded, since the area of “missing” endothelium could not be assessed objectively. An example of a ghost capillary is depicted in Figure 5A (arrow).
Figure 5.
Ghost vessels in relation to capillary density. (A) Example of a ghost vessel unlabeled by UEA-I (arrow). (B) Graph depicting vascular density compared to capillary ghost vessels. Increasing numbers of ghost vessels were strongly correlated with decreasing vascular density (r2 = 0.55, P < 0.001), consistent with lower vascular density in some donors being due to progressive dropout of capillaries rather than congenital differences.
Drusen-Vascular Spatial Relationship
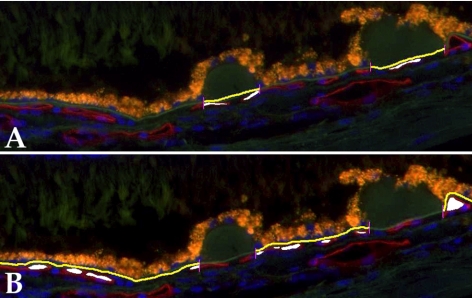
A second set of morphometric analyses was performed in superotemporal sagittal sections of the peripheral RPE-choroid. For these experiments, photomicrographs were collected using a ×20 objective centered on solitary, extramacular drusen from 11 eyes. Only fields with >2 drusen were included in the analysis. For each micrograph, Bruch's membrane length and the vascular density were separately measured either under drusen or under the RPE with no drusen. The vascular density was determined in drusen-containing and drusen-free areas of choriocapillaris in a total of 42 micrographs from 11 donors. The average ratio of non-drusen vascular density to drusen-associated vascular density was determined for each donor and used for statistical analysis. Examples of these measurements are depicted in Figure 2.
Figure 2.
Examples of determination of vascular density in relation to drusen, either beneath drusen (A) or adjacent to drusen (B).
Statistical Analysis
For each measured eye, all values for each measured feature were pooled and divided by the pooled length of Bruch's membrane over which measurements were collected. In this way, each measured feature received a single quantitative score for each eye. For drusen and choriocapillaris lumens, these measurements were in units of μm2 per μm of Bruch's membrane, and for leukocytes and ghost vessels the values were in the form of raw number/μm of Bruch's membrane.
After unmasking of early AMD and control samples, the following parameters were assessed statistically. First, the effect of AMD on drusen/sub-RPE deposit area, number of leukocytes, vascular density, and ghost numbers was assessed using a two-tailed Student's t-test. Second, linear regression analysis was performed for each of these quantitative factors using an online statistics package21 (available at http://www.wessa.net/rwasp_linear_regression.wasp). For each of the slopes, the correlation coefficient and the P value of the slope representing the statistical strength of the association was determined. Drusen cross-sectional areas, which showed a very wide range of values even in AMD eyes, were transformed, and the square root of the drusen area was used in regression analysis. Because four distinct morphologic measurements were performed, features with an uncorrected P value of <0.0125 were considered significant.
Results
AMD Status Related to Sub-RPE Deposits, Leukocytes, and Vascular Density
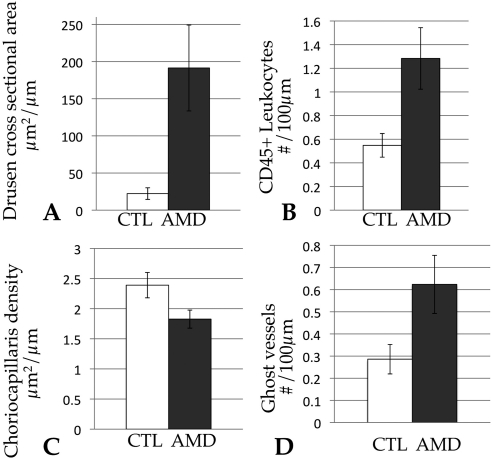
Comparing drusen/sub-RPE deposit area in early AMD donors to controls showed a greater than eightfold difference in these samples (AMD mean, 191 μm2/μm; control mean, 22.3 μm2/μm; P < 0.01). As expected, clinical definitions of early AMD and morphologic measurements of sub-RPE deposits correlate well (Fig. 3A). Vascular density in AMD eyes showed a trend toward decreasing area in AMD eyes (2.4 μm2 in control eyes and 1.8 μm2 in AMD eyes), which was significant to an uncorrected P value of P = 0.037 (Fig. 3B). This trend was not significant after Bonferroni correction, however.
Figure 3.
Graphs depicting morphologic measurements of drusen area (A), CD45+ leukocyte density (B), choriocapillaris density (C), and ghost vessel density (D) as a function of AMD status. The cross-sectional drusen area was significantly associated with AMD status (P < 0.01). The number of extravascular leukocytes (B) showed a trend toward being increased in AMD, and the choriocapillaris vascular density (C) showed a trend toward decreasing in AMD (uncorrected P = 0.016 and 0.037, respectively), but neither was significant after correction for multiple measurements. The total number of ghost vessels per length of Bruch's membrane also showed a trend toward increasing in AMD eyes (uncorrected P = 0.03) (D), but this finding was not statistically significant after correction. CTL, age-matched control eyes without AMD (white bars). Graphs depict the mean values ± SEM.
The number of extravascular CD45 immunoreactive leukocytes within 40 μm of Bruch's membrane was quantified. The average value for AMD eyes was 1.31, and the average value in control eyes was 0.55 cell/100 μm (Fig. 3B). The uncorrected P value for affection status was 0.016. After correction for multiple measures this finding was no longer considered significant (P corrected = 0.064).
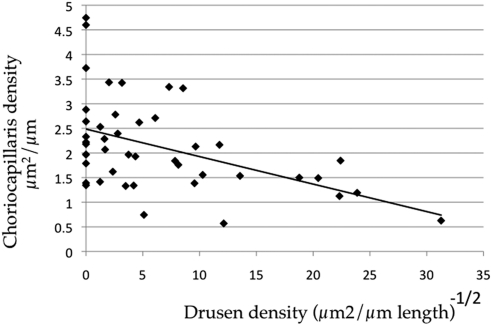
A striking pattern was observed when sub-RPE deposit area was used rather than affection status to assess the relationship between features of AMD and vascular changes. Linear regression analysis of transformed drusen/sub-RPE deposit area versus choriocapillaris density reveals that drusen area is negatively correlated with choriocapillaris density (Fig. 4; r2 = 0.22, corrected P < 0.01); that is, decreased choriocapillaris area is associated with increasing number and/or size of drusen and other sub-RPE deposits. Decreased choriocapillaris density associated with drusen was observed independent of AMD diagnosis based on analyzing two variables in regression.
Figure 4.
Linear regression of vascular density versus the square root of drusen cross-sectional area. Increasing size and/or number of drusen was negatively correlated with vascular density (r2 = 0.22, P < 0.01).
Choriocapillaris Ghosts
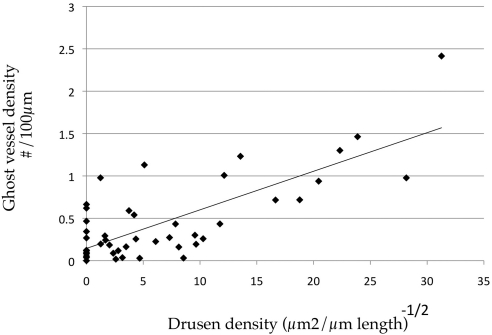
In addition, the number of ghost vessels was determined in aging human eyes. These were scored as empty zones between intact choriocapillary pillars (Fig. 5A). Eyes with early AMD showed an increase in the number of ghost vessels (Fig. 3D), but this difference was not statistically significant after correction (uncorrected P = 0.03). Linear regression analysis comparing ghost vessels to choriocapillaris vessel density showed a strong correlation (Fig. 5B; r2 = 0.55, corrected P < 0.001). The number of ghost vessels also increased with increasing sub-RPE deposit density (Fig. 6; r2 = 0.57, corrected P < 0.001).
Figure 6.
Linear regression analysis of numbers of ghost vessels versus the square root of drusen density. Drusen and ghost vessels showed a strong positive correlation (r2 = 0.57, corrected P <0.001).
Peripheral Drusen and Choriocapillaris Density
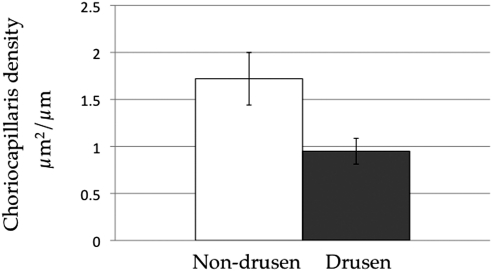
In a separate set of experiments we sought to determine the spatial relationship between solitary extramacular drusen and the choriocapillaris. In a separate set of samples (n = 11 eyes) the vascular density was determined either directly beneath drusen or in areas adjacent to drusen in the same micrographs (Fig. 2). Values were determined per field, rather than per donor, since vascular density varies regionally in the periphery (data not shown). The per field vascular density beneath drusen was 45% lower than in areas between drusen (paired t-test P < 0.01; Fig. 7), consistent with the concept that drusen do not form uniformly with respect to the anatomy of the choroid,16 but rather form in regions depleted of vascular elements.
Figure 7.
Extramacular vascular density beneath drusen, compared with adjacent regions of the choriocapillaris (“non-drusen”), from the same micrographs. The mean vascular density was significantly lower beneath drusen than adjacent to drusen (P < 0.01).
Discussion
AMD is a complex disease with a multifactorial etiology. Although the inciting cause(s) for AMD are poorly understood, there is evidence for contribution of genetic,22,23 inflammatory,11,24 oxidative,25,26 lipidic,27 mitochondrial,28,29 and ischemic30 factors (for additional reviews see Refs. 31–34).
Data from a number of studies using distinct approaches indicate that choroidal vascular changes are associated with aging and early AMD. Evidence for choroidal microvascular dysfunction in AMD includes the following: localization of terminal complement complexes with the choriocapillaris in aging eyes,35–38 suggesting a possible mechanism for vascular injury in AMD; the clinical association of vascular changes in both the choroid39–43 and peripheral vasculature44–48 that implicate endothelial dysfunction as a contributing factor to the disease; morphometric changes in choroidal vascular density in aging and AMD5,49–52; and the observation that the major cause of vision loss in AMD, choroidal neovascularization, arises from capillaries in the choroid.53
Earlier studies have shown AMD-associated increased or decreased vascular density using light microscopy. Ramrattam et al.49 studied a large series of donor eyes and determined that capillary density decreases with age, although after age 50 there did not appear to be further loss as the vasculature was highly variable in the older donors. AMD status, assessed histologically by the presence of basal laminar deposits, geographic atrophy, and/or CNV, was related to decreased vascular density. These findings were later challenged by Spraul and Grossniklaus,50,51 who noted in a series of donor eyes that the vascular density modestly increased in association with AMD. Both of these studies relied on hematoxylin-eosin prepared sections, in which discriminating between a viable vessel and an empty ghost vessel may be challenging, particularly if other cell types (such as pericytes) are still present. These difficulties were addressed in studies by McLeod et al.,52 who evaluated the vasculature at the margins of advanced AMD lesions using alkaline phosphatase histochemistry, which discriminates between living and absent endothelial cells.54 In addition, when these authors assessed RPE density in the same fields, they found that RPE loss occurs preferentially in areas of atrophy in geographic atrophy eyes, whereas choroidal loss occurs preferentially (and paradoxically) in advance of choroidal neovascularization without loss of RPE in wet AMD.52
In the present study, we focused on early AMD eyes and similarly relied on a marker of viable endothelial cells, UEA-I. Fucose-containing glycoconjugates that react with UEA-I are expressed on both normal55 and neovascular19 choroidal endothelial cells, but not ghost capillaries, enabling us to assess the vascular density in a set of eyes either with early macular degeneration (i.e., macular drusen and/or pigmentary changes) or without macular changes. Eyes with advanced disease were excluded since both geographic atrophy and choroidal neovascularization have profound effects on the choriocapillaris, and we wished to evaluate early changes in AMD.
The present study found that, in the absence of advanced degenerative changes, vascular loss was associated with extent of drusen and other sub-RPE deposits. Although we observed a trend toward decreasing vascular density in association with early AMD (uncorrected P = 0.037), this difference did not reach statistical significance after correction for multiple measures. In contrast, ghost vessels and choriocapillaris density were both strongly associated with the cross-sectional area of sub-RPE deposits. In fact, adding AMD status as a covariate in linear regression analysis actually decreased the statistical significance of the association, suggesting that sub-RPE deposit density is a much better predictor of vascular density than AMD diagnosis. These experiments suggest a relationship between the choriocapillaris and drusen that is not widely appreciated.
Moreover, we observed a strong inverse relationship between numbers of ghost vessels and choriocapillaris density. This intriguing finding suggests that a major difference between eyes with a dense vasculature and those with a sparse vasculature is the loss of endothelium, rather than a congenital variation in the distribution of vascular elements. This is encouraging in that it suggests that agents that preserve—while not activating—the failing endothelium could have benefit in early AMD patients.
In addition to assessing the vasculature in early AMD, we also sought to examine leukocyte trafficking into the choroid in AMD and control eyes using a marker for all classes of leukocytes, CD45/leukocyte common antigen. Dual labeling with UEA-I and anti-CD45 antibodies allowed us to discern whether leukocytes in the choroid are extravascular or contained within the vasculature (the latter of which could be attributed to stochastic effects such as body position of the donor after death). The observation that CD45 reactive leukocytes showed increased numbers in association with AMD status is consistent with previous results by Penfold and colleagues56 in which all nonvascular nucleated cells in the choroid were counted and found to increase with AMD severity. In our study, leukocytes that reacted with anti-CD45 antibody were over twofold more abundant in early AMD eyes compared with unaffected controls (uncorrected P = 0.02). There are a number of intriguing questions regarding the role of macrophages and other white cells in AMD. One important question concerning leukocytes in AMD is the mechanism through which these cells are recruited to the choroid. We recently showed that the complement fragment C5a can activate choroidal endothelial cells in vitro to increase ICAM-1 expression38; if similar events occur in vivo, this could drive leukocyte recruitment into eyes with early AMD through a complement-mediated mechanism.
There are limitations to this study. In contrast to in vivo approaches such as laser Doppler flowmetry,43,57 we were limited to a single time point for each donor. Longitudinal changes in vascularity and drusen could not be assessed. In addition, our approach in which all macular sub-RPE deposits were grouped may dilute differences in choroidal responses to different classes of lesions. We made this choice largely on the necessity of transmission electron microscopy to reliably distinguish between categories of deposit, although macular soft drusen may be associated with basal laminar deposits that lead to detachment of the RPE.58 We also did not attempt to discriminate between different classes of leukocytes, as the sample preparation was not compatible with many of the reagents used for phenotyping leukocytes. Based on nuclear morphology, the majority of choroidal cells were not granulocytes and were likely a mixture of monocytes and lymphocytes, perhaps of the CD8+ lineage as suggested previously.59 Labeling of a subset of samples with high and low numbers of CD45+ cells suggests that the elevated choroidal leukocytes react with antibodies directed against the microglia/macrophage marker IBA-1 (data not shown).
Most importantly, these experiments are not capable of distinguishing between cause and effect. It is possible that the observed choroidal microvascular changes are a secondary event that follows the formation of drusen and interference of trophic signaling by the RPE. Since the choriocapillaris relies on VEGF secretion by the RPE,60 the presence of deposits between the RPE and choroid that are rich in lipid species13,27 could impair this signaling and lead to endothelial cell loss. Alternatively, it is possible that vascular loss, due to complement mediated injury or other genetic and nongenetic factors, leads to impaired removal of debris from Bruch's membrane, RPE ischemic injury, and other sequelae of early AMD.
It is intriguing in this context that drusen coalesce between capillary lumens rather than randomly or preferentially over the lumens.16 In our study, the vascular density of the choriocapillaris beneath drusen was 45% lower than the capillary density adjacent to drusen (P < 0.01). This phenomenon may be related to what Sarks and colleagues15 described as entrapment sites, or zones within intercapillary pillars that tend to accumulate debris, leading to drusen coalescence.
In summary, we have demonstrated that choriocapillaris loss is associated with increasing sub-RPE deposit density, and we provide additional evidence for the supposition that drusen develop preferentially over areas of the choroid without vascular lumens. It is feasible that the loss of endothelial cells promotes the formation of drusen during the pathogenesis of AMD. A deeper understanding of the relationship between the vasculature and drusen will provide further insight into the interrelationship between sub-RPE deposits, the choriocapillaris, and the pathogenesis of AMD.
Acknowledgments
The authors thank Elizabeth Nerad for technical assistance; Michael Anderson, John Fingert, and Val Sheffield for helpful comments on the manuscript; and the Iowa Lions Eye Bank for its partnership in acquiring tissue for biological research.
Footnotes
Supported in part by National Eye Institute Grant EY017451, a center grant from the Foundation Fighting Blindness, and the Macula Vision Research Foundation. RFM holds the Hansjoerg E. J. W. Kolder Professorship for Best Disease Research.
Disclosure: R.F. Mullins, None; M.N. Johnson, None; E.A. Faidley, None; J.M. Skeie, None; J. Huang, None
References
- 1. Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. Arch Ophthalmol. 2001;119:1417–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zarbin M. Age-related macular degeneration: review of pathogenesis. [Review] [65 refs]. Eur J Ophthalmol. 1998;8:199–206 [DOI] [PubMed] [Google Scholar]
- 3. Stone EM. A very effective treatment for neovascular macular degeneration. N Engl J Med. 2006;355:1493–1495 [DOI] [PubMed] [Google Scholar]
- 4. Gass J. Drusen and disciform macular detachment and degeneration. Arch. Ophthalmol. 1973;90:206–217 [DOI] [PubMed] [Google Scholar]
- 5. Sarks S. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976;60:324–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pauleikhoff D, Barondes M, Minassian D, Chisholm I, Bird A. Drusen as risk factors in age-related macular disease. Am J Ophthalmol. 1990;109:38–43 [DOI] [PubMed] [Google Scholar]
- 7. Curcio C, Millican C. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999;117:329–339 [DOI] [PubMed] [Google Scholar]
- 8. van der Schaft T, Mooy C, de Bruijn W, de Jong P. Early stages of age-related macular degeneration: an immunofluorescence and electron microscopy study. Br J Ophthalmol. 1993;77:657–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mullins R, Anderson D, Russell S, Hageman G. Ocular drusen contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846 [PubMed] [Google Scholar]
- 10. Johnson L, Ozaki S, Staples M, Erickson P, Anderson D. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res. 2000;70:441–449 [DOI] [PubMed] [Google Scholar]
- 11. Anderson D, Mullins R, Hageman G, Johnson L. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–431 [DOI] [PubMed] [Google Scholar]
- 12. Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang L, Clark ME, Crossman DK, et al. Abundant lipid and protein components of drusen. PLoS ONE. 5:e10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Friedman E, Smith T, Kuwabara T. Senile choroidal vascular patterns and drusen. Arch Ophthalmol. 1963;69:220–230 [DOI] [PubMed] [Google Scholar]
- 15. Sarks S, Arnold J, Killingsworth M, Sarks J. Early drusen formation in the normal and aging eye and their relation to age-related maculopathy: a clinicopathological study. Br J Ophthalmol. 1999;83:358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lengyel I, Tufail A, Hosaini HA, Luthert P, Bird AC, Jeffery G. Association of drusen deposition with choroidal intercapillary pillars in the aging human eye. Invest Ophthalmol Vis Sci. 2004;45:2886–2892 [DOI] [PubMed] [Google Scholar]
- 17. Kochounian H, Johnson LV, Fong HK. Accumulation of extracellular RGR-d in Bruch's membrane and close association with drusen at intercapillary regions. Exp Eye Res. 2009;88:1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barthel L, Raymond P. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J Histochem Cytochem. 1990;38:1383–1388 [DOI] [PubMed] [Google Scholar]
- 19. Mullins R, Grassi M, Skeie J. Glycoconjugates of choroidal neovascular membranes in age-related macular degeneration. Mol Vis. 2005;11:509–517 [PubMed] [Google Scholar]
- 20. Mullins R, Skeie J, Malone E, Kuehn M. Macular and peripheral distribution of ICAM-1 in the human choriocapillaris and retina. Mol Vis. 2006;12:224–235 [PubMed] [Google Scholar]
- 21. Wessa P. Linear Regression Graphical Model Validation (v1.0.6) in Free Statistics Software (v1.1.23-r6). Office for Research Development and Education; 2008 [Google Scholar]
- 22. Haddad S, Chen CA, Santangelo SL, Seddon JM. The genetics of age-related macular degeneration: a review of progress to date. Surv Ophthalmol. 2006;51:316–363 [DOI] [PubMed] [Google Scholar]
- 23. Mullins RF. Genetic insights into the pathobiology of age-related macular degeneration. Int Ophthalmol Clin. 2007;47:1–14 [DOI] [PubMed] [Google Scholar]
- 24. Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Winkler B, Boulton M, Gottsch J, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- 26. Wong RW, Richa DC, Hahn P, Green WR, Dunaief JL. Iron toxicity as a potential factor in AMD. Retina. 2007;27:997–1003 [DOI] [PubMed] [Google Scholar]
- 27. Malek G, Li C, Guidry C, Medeiros N, Curcio C. Apolipoprotein B in cholesterol-containing drusen and basal deposits of human eyes with age-related maculopathy. Am J Pathol. 2003;162:413–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nordgaard CL, Berg KM, Kapphahn RJ, et al. Proteomics of the retinal pigment epithelium reveals altered protein expression at progressive stages of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:815–822 [DOI] [PubMed] [Google Scholar]
- 29. Jarrett SG, Lin H, Godley BF, Boulton ME. Mitochondrial DNA damage and its potential role in retinal degeneration. Prog Retin Eye Res. 2008;27:596–607 [DOI] [PubMed] [Google Scholar]
- 30. Feigl B. Age-related maculopathy - linking aetiology and pathophysiological changes to the ischaemia hypothesis. Prog Retin Eye Res. 2009;28:63–86 [DOI] [PubMed] [Google Scholar]
- 31. Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614 [DOI] [PubMed] [Google Scholar]
- 32. Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293 [DOI] [PubMed] [Google Scholar]
- 33. Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006;51:137–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zarbin MA, Rosenfeld PJ. Pathway-based therapies for age-related macular degeneration: an integrated survey of emerging treatment alternatives. Retina. 30:1350–1367 [DOI] [PubMed] [Google Scholar]
- 35. Hageman G, Anderson D, Johnson L, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seth A, Cui J, To E, Kwee M, Matsubara J. Complement-associated deposits in the human retina. Invest Ophthalmol Vis Sci. 2008;49:743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abrera-Abeleda M, Nishimura C, Smith J, et al. Variations in the complement regulatory genes factor H (CFH) and factor H related 5 (CFHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease). J Med Genet. 2006;43:582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Skeie JM, Fingert J, Russell S, Stone EM, Mullins RF. Complement component C5a activates ICAM-1 expression on human choroidal endothelial cells. Invest Ophthalmol Vis Sci. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Friedman E, Krupsky S, Lane A, et al. Ocular blood flow velocity in age-related macular degeneration. Ophthalmology. 1995;102:640–646 [DOI] [PubMed] [Google Scholar]
- 40. Pauleikhoff D, Spital G, Radermacher M, Brumm G, Lommatzsch A, Bird A. A fluorescein and indocyanine green angiographic study of choriocapillaris in age-related macular disease. Arch Ophthalmol. 1999;117:1353–1358 [DOI] [PubMed] [Google Scholar]
- 41. Chen S, Cheng C, Lee A, et al. Pulsatile ocular blood flow in asymmetric exudative age-related macular degeneration. Br J Opthtalmol. 2001;85:1411–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mori F, Konno S, Hikichi T, Yamaguchi Y, Ishiko S, Yoshida A. Pulsatile ocular blood flow study: decreases in exudative age related macular degeneration. Br J Ophthalmol. 2001;85:531–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grunwald J, Hariprasad S, DuPont J, et al. Foveolar choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1998;39:385–390 [PubMed] [Google Scholar]
- 44. Vingerling J, Dielemans I, Bots M, Hofman A, Grobbee D, de Jong P. Age-related macular degeneration is associated with atherosclerosis. The Rotterdam Study. Am J Epidemiol. 1995;142:404–409 [DOI] [PubMed] [Google Scholar]
- 45. Klein R, Klein B, Jensen S. The relation of cardiovascular disease and its risk factors to the 5-year incidence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:1804–1812 [DOI] [PubMed] [Google Scholar]
- 46. Hyman L, Schachat A, He Q, Leske M. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol. 2000;118:351–358 [DOI] [PubMed] [Google Scholar]
- 47. Anand R, Bressler S, Davis M, et al. Risk factors associated with age-related macular degeneration: a case-control study in the Age-Related Eye Disease Study: Age-Related Eye Disease Study report number 3. Ophthalmology. 2000;107:2224–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lip P, Blann A, Hope-Ross M, Gibson J, Lip G. Age-related macular degeneration is associated with increased vascular endothelial growth factor, hemorheology and endothelial dysfunction. Ophthalmology. 2001;108:705–710 [DOI] [PubMed] [Google Scholar]
- 49. Ramrattan R, van der Schaft T, Mooy C, de Bruijn W, Mulder P, de Jong P. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–2864 [PubMed] [Google Scholar]
- 50. Spraul C, Lang G, Grossniklaus H. Morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:2724–2735 [PubMed] [Google Scholar]
- 51. Spraul C, Lang G, Grossniklaus H, Lang G. Histologic and morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv Ophthalmol. 1999;44:S10–32 [DOI] [PubMed] [Google Scholar]
- 52. McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:4982–4991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kuroki A, Bhutto I, Kitaoka T, Amemiya T. Natural course of experimental choroidal neovascularization: three-dimensional study with corrosion cast and scanning electron microscope. Ophthalmic Res. 2002;34:200–205 [DOI] [PubMed] [Google Scholar]
- 54. Lutty GA, McLeod DS. Phosphatase enzyme histochemistry for studying vascular hierarchy, pathology, and endothelial cell dysfunction in retina and choroid. Vision Res. 2005;45:3504–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Miettinen M, Holthofer H, Lehto VP, Miettinen A, Virtanen I. Ulex europaeus I lectin as a marker for tumors derived from endothelial cells. Am J Clin Pathol. 1983;79:32–36 [DOI] [PubMed] [Google Scholar]
- 56. Penfold P, Killingsworth M, Sarks S. Senile macular degeneration: the involvement of immunocompetent cells. Graefe's Arch Clin Exp Ophthalmol. 1985;223:69–76 [DOI] [PubMed] [Google Scholar]
- 57. Xu W, Grunwald JE, Metelitsina TI, et al. Association of risk factors for choroidal neovascularization in age-related macular degeneration with decreased foveolar choroidal circulation. Am J Ophthalmol. 150:40–47 e42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bressler N, Silva J, Bressler S, Fine S, Green W. Clinicopathologic correlation of drusen and retinal pigment epithelial abnormalities in age-related macular degeneration. Retina. 1994;14:130–142 [PubMed] [Google Scholar]
- 59. Ezzat MK, Hann CR, Vuk-Pavlovic S, Pulido JS. Immune cells in the human choroid. Br J Ophthalmol. 2008;92:976–980 [DOI] [PubMed] [Google Scholar]
- 60. Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D'Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A. 2009;106:18751–18756 [DOI] [PMC free article] [PubMed] [Google Scholar]