Survival of integrated cells decreases with time after transplantation but can be significantly increased with XIAP antiapoptotic therapy. Preventing programmed cell death through XIAP therapy may be an important component of future therapeutic retinal cell transplantation strategies.
Abstract
Purpose.
To assess the survival of rod precursor cells transplanted into the Rd9 mouse, a model of X-linked retinal degeneration, and the effect of antiapoptotic therapy with X-linked inhibitor of apoptosis (XIAP) on preventing cell loss.
Methods.
Dissociated retinal cells from P4 Nrlp-GFP mice were transplanted into the subretinal space of 2-, 5-, and 8-month-old Rd9 mice. Histology, immunohistochemistry, and quantification of integrated cells were performed every month for up to 3 months after transplantation. XIAP delivery to donor cells was accomplished by transfection with adenoassociated virus (AAV-XIAP). Intraretinal activation of immune modulators was assessed using a quantitative real-time polymerase chain reaction-based immune response array.
Results.
GFP-positive rod precursors were able to integrate into the outer nuclear layer (ONL) of the Rd9 retina. Transplanted cells underwent morphologic differentiation with the formation of inner and outer segments and synaptic projections to bipolar cells. Integration of donor cells into the ONL increased as a function of host age at the time of transplantation. The number of integrated cells was maximal at 1 month after transplantation and then decreased with time. Survival of integrated cells was significantly increased when donor cells were pretreated with AAV-XIAP. We did not detect any donor cell-specific activation of inflammation within the host retina.
Conclusions.
Survival of integrated cells decreases with time after transplantation but can be significantly increased with XIAP antiapoptotic therapy. Preventing programmed cell death through XIAP therapy may be an important component of future therapeutic retinal cell transplantation strategies.
Hereditary, age-related, and acquired retinal degenerative diseases are a significant cause of irreversible vision loss around the world. These diseases represent a heterogeneous group of conditions caused by diverse genetic and environmental factors. Regardless of etiology, the primary reason for vision loss is the death of photoreceptors, the cells that are responsible for transducing a visual stimulus into an electrical signal.
Photoreceptor cell transplantation has been proposed as a novel method for repopulating a retina that is losing its normal complement of these sensory cells.1 This strategy has a unique appeal in that it does not depend on the cause of the retinal degeneration but only seeks to replace the dying photoreceptors with new cells harvested from healthy donor retinas or a cultivated cell line. Earlier studies demonstrated the feasibility of transplanting cells from mildly dissociated retinas into the subretinal space, forming grafts that integrated with the host retina and provided some degree of visual rescue.2 Although the grafts, and photoreceptor cells within the grafts, could be visualized by light microscopy, the integration and survival of individual cells within the host retina could not be evaluated.
Criteria that can be used to select a cell type to use for transplantation, at least in an experimental system, include a commitment to photoreceptor fate (i.e., a cell that will become a photoreceptor and not another type of cell), ease of harvesting, and availability of a marker to allow for tracking after transplantation. One system that satisfies all these criteria is the Nrlp-GFP mouse, in which any cell with activation of the promoter for the rod and pineal-specific gene Nrl (neural retina leucine zipper) will express the green fluorescent protein (GFP) marker.3–5 By definition, cells that have undergone their final mitotic division in the retina and have committed to becoming a rod cell will also express GFP, making them easily identifiable by their fluorescence. Using this mouse strain as the source of donor cells, it was possible to demonstrate that photoreceptor precursors injected into the subretinal space of host mice can migrate, differentiate, and integrate into an existing retinal cell framework.6 However, many questions remain regarding the ability of the transplanted cell to integrate and survive, especially as the host retina degenerates.
Further advances of this technology will require identification of the factors that affect the efficiency of photoreceptor cell integration and determination of whether the transplanted cells can functionally integrate and survive in a degenerating retina. To begin to address these issues, we evaluated the results of transplanting rod precursor cells harvested from young Nrlp-GFP mice into the eyes of aging Rd9 (retinal degeneration 9) mice, which is a model of X-linked retinitis pigmentosa (Khan NW, et al. IOVS 2010;51:ARVO E-Abstract 4068).7 We found that the degree of integration of transplanted rod precursor cells into degenerating retinas increases with the age of the host but that the number of surviving cells decreases with time after transplantation. The rate of integrated cell loss was significantly reduced by transfecting the transplanted cells with the gene coding for X-linked inhibitor of apoptosis (XIAP) before transplantation. XIAP is a potent inhibitor of caspases 3, 7, and 9—all of which are downstream components of the apoptosis cascade—and has been shown to be a photoreceptor-protective molecule in various models of retinal disease, including retinal degeneration and retinal detachment.8–10 This suggests that antiapoptotic therapy is a potentially viable adjunctive mechanism for enhancing the efficiency of cellular integration and survival of photoreceptors after transplantation.
Methods
Animals
Four strains of mice were used in the experiments. Donor cells were obtained from the Nrlp-GFP transgenic mouse strain.6 Host animals were of the Rd9 strain, a murine model of X-linked retinitis pigmentosa (Khan NW, et al. IOVS 2010;51:ARVO E-Abstract 4068).7 C57BL/6 mice served as wild-type controls. We also performed transplantation of rod precursors into the Nrl-knockout (Nrl−/−) mouse, a strain that lacks rod cells and its photopigment rhodopsin.3 The mice were bred and housed in the University of Michigan, Kellogg Eye Center animal facility. All animal experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and after approval was received from the University Committee on the Use and Care of Animals of the University of Michigan.
Donor Cells
Donor cells were prepared from Nrlp-GFP mice at postnatal day (P) 4. Mice were killed by cervical dislocation, eyes were enucleated, and neural retinas were dissected free from surrounding tissues. Cells were dissociated with a papain dissociation kit (Worthington Biochemical, Lorne Laboratories, UK) according to manufacturer's directions. Cells were washed once with phosphate-buffered saline (PBS) and diluted to a final concentration of approximately 2 × 105 cells/μL DMEM/F12 solution (Invitrogen, Carlsbad, CA). The dissociated cell suspension contains all retinal cell types, not just GFP-positive cells.
AAV-XIAP Transfection of Donor Cells
AAV5-CBA-XIAP-wPRE was produced and purified as described previously.8–10 The AAV serotype used has been shown to induce transgene expression in an explant system from as early as 24 hours.11 Donor Nrlp-GFP mice at age P2 were anesthetized on crushed wet ice for 2 minutes. Eyelids were not yet open at this age, thus requiring their surgical separation. The lids were cleaned with 70% ethanol using a cotton-tipped applicator, and the interface of the upper and lower lids was cut with a sterile scalpel. A sclerotomy was made in the inferior sclera just posterior to the cornea using the tip of a 30-gauge needle. A tapered pulled glass pipette was inserted into the sclerotomy across the vitreous to the opposite wall of the eye until resistance was encountered. The pipette was slightly retracted from that point to position it in the subretinal space. The glass pipette was loaded with 1 μL AAV-XIAP (1.87 × 1013 physical particles/mL) connected to a nanoinjector (Nanoinjector II; Drummond Scientific Company, Broomall, PA), which allowed for a slow injection of the AAV-XIAP into the subretinal space. Donor cells were prepared from injected animals at P4, as described.
Transplantation Procedure
Each host (Rd9) mouse was anesthetized by intraperitoneal injection of an anesthetic cocktail that consisted of ketamine (100 mg/mL; Fort Dodge Animal Health, Fort Dodge, IA), xylazine (Lloyd, Inc., Shenandoah, IA) and sterile PBS in the ratio of 8:8:34. The dose of cocktail injected was 0.1 mL/20 g body weight. Two microliters of freshly dissociated Nrlp-GFP retinal cell suspension (2.0 × 105 cells/μL) was drawn into a tapered pulled glass pipette connected to a nanoinjector (Nanoject II; Drummond Scientific Company). The subretinal injection was performed as described with direct visualization through a dissecting microscope. Donor cells were introduced into host mice of three different ages: 2 months, 5 months, and 8 months. Nrl−/− mice were approximately 2 to 3 months old at the time they were injected with rod precursor cells.
Tissue Processing
The mice were killed and the globes were removed at 1, 2, and 3 months after transplantation according to the following procedure. Animals were deeply anesthetized and then perfused transcardially with 4% paraformaldehyde in PBS. Eyes were removed and immersion fixed with 4% paraformaldehyde at room temperature for 30 minutes. The cornea and lens were dissected away, and the eyecup was rinsed three times in PBS, transferred to 10% and then 20% sucrose in PBS for 2 hours each, before embedding in OCT (Tissue Tek; Sakura Finetek, Torrance, CA) mixed in a ratio of 1:1 with 20% sucrose. A cryostat was used to obtain serial 16-μm sections.
Histology
Sections were washed in PBS, incubated with blocking solution containing 5% goat serum and 0.1% Triton X-100 for 1 hour, and incubated with primary antibodies diluted with 0.1% Triton X-100 in PBS overnight at 4°C. The primary antibodies and working dilutions were as follows: mouse and rabbit anti-GFP (1:1000, Molecular Probes, Eugene, OR), rabbit polyclonal protein kinase C (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal rho1D2 (1:1000; a kind gift from Robert Molday). XIAP-transfected cells were visualized by immunostaining with an antibody against a hemagglutinin (HA) tag that was introduced along with the protein, as described.8–10 After three 10-minute rinses with 0.1% Triton X-100, sections were incubated for 90 minutes with secondary antibodies diluted 1:1000 in PBS containing 0.1% Triton X-100. Secondary antibodies used in the study were as follows: goat anti-mouse IgG, AlexaFluor 647, and goat anti-rabbit IgG (H+L) AlexaFluor 546. Sections were counterstained with antifade reagent (ProLong Gold with DAPI; Invitrogen) to reveal cell nuclei.
Microscopy and Cell Counts
Retinal sections were examined using a fluorescence microscope (Eclipse E800, Nikon Corp., Tokyo, Japan) or a confocal microscope (Olympus FluoView 500; Olympus Corp., Tokyo, Japan). Eyes were processed, serially sectioned through the total area of the transplantation, and labeled for the GFP protein as described. The number of integrated donor cells was quantified by counting the total number of GFP-positive cells in all the serial sections. Four host animals at each age of transplantation were evaluated at each time after transplantation. Cells were considered integrated into the host retina if the whole cell body was visible in the outer nuclear layer (ONL), together with at least one of the following: axonal projection with a spherule synapse at the junction with the bipolar cell or the presence of a clearly defined inner and outer segment connecting to the cell nucleus.
Quantitative Real-Time Polymerase Chain Reaction Analysis
Intraretinal transcript levels for an array of immune modulatory genes were measured in four groups of animals: wild-type untreated C57BL/6 mice, untreated Rd9 mice, Rd9 mice receiving a sham transplantation procedure (i.e., whole surgical procedure with injection of buffer into the subretinal space), and Rd9 mice in which transplanted cells were injected into the subretinal space. The age of the mice in all groups was 8 months, and 3 mice per group were used. Total ribonucleic acid (RNA) was isolated from retinas using the RNeasy mini kit (Qiagen, Valencia, CA). First-strand complementary deoxyribonucleic acid was generated from 500 ng total RNA, and qRT-PCR was performed using the mouse inflammatory response and autoimmunity RT2 (Profiler PCR Array system; SABiosciences, Valencia, CA), which contains primers for 84 genes involved in autoimmunity and inflammation and 12 housekeeping and quality control genes. Data were acquired using PCR master mix (SYBR Green/Fluorescein; Thermo Scientific, Pittsburgh, PA) on a real-time PCR detection system (iCycler; Bio-Rad, Hercules, CA) as follows: 95°C for 10 minutes, 95°C for 15 seconds, and 60°C for 1 minute, with steps 2 to 3 repeated 39 times. Fold change (ΔΔCt method) and quality control assessment were calculated and analyzed (PCR Array Data Analysis Web Portal software; SABiosciences).
Statistical Analysis
Comparison of transplanted cell survival as a function of both host age and time after transplantation was performed using analysis of variance. Pairwise comparison at each time point (i.e., 2-month-old host vs. 5 month-old host at 1 month after transplantation) was performed using the least squares difference and Duncan procedures. To compare survival of transplanted cells in the 8-month-old host as a function of XIAP transfection, Student's t-test was used. Significance was defined as a P < 0.05.
Results
Donor Cell Integration and Maturation
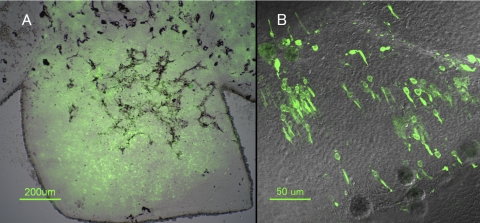
For all the experiments described here, we harvested retinas from the eyes of donor Nrlp-GFP mice at P4. Approximately 50% of the cells in the dissociated retinal suspension injected into the subretinal space were GFP positive. Figure 1 shows a flat mount of a retina 1 month after transplantation. Approximately half the retina was detached by the initial injection of donor cells in suspension buffer. The injection resulted in a slow dissection of the potential space between the retina and retinal pigment epithelium (RPE) by the fluid bleb containing the transplanted cells, and this allowed for the lateral migration of the cells away from the injection site. The fluid reabsorbed within 24 hours after the injection, leaving only the transplanted cells in the subretinal space. Funduscopic examination at 1-week after injection showed that the green fluorescence occupied approximately one-quarter to one-half of the retina.
Figure 1.
Flat mount of the Rd9 retina 1 month after subretinal injection of dissociated retinal cells from P4 Nrlp-GFP donor mouse. The host mouse was 8 months old at the time of transplantation. The retina is separated from the underlying RPE to allow for transmission microscopy. (A) Low-magnification Nomarski image with GFP fluorescence overlay. Approximately 25% of the retina has integrated donor cells. (B) High-power magnification of retina in (A).
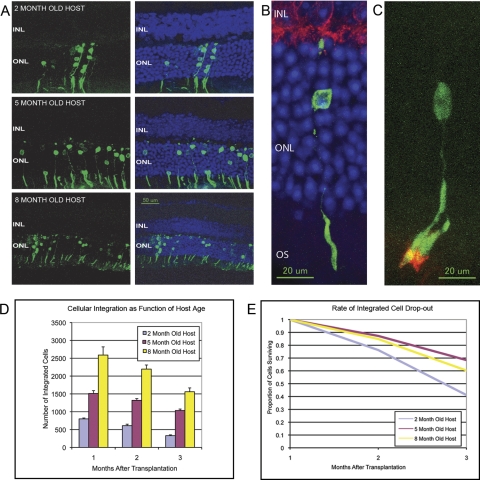
At 1 month after transplantation, the first time point at which we performed histologic assessment of the retina, we observed significant numbers of GFP-positive cells within the ONL (Fig. 2A). These cells were seen only in the ONL, suggesting a true tropism of GFP-positive cells for the photoreceptor layer within the retina. The only means for differentiating donor cells from host cells was the green fluorescence signal or staining with the antibody against the GFP protein. As such, we could not exclude the possibility that non–GFP-labeled cells, such as cone photoreceptors or inner retinal neurons, also integrated into the host retina, either into the ONL or into other layers.
Figure 2.
Integration and survival of transplanted rod precursor cells into the Rd9 mouse eye. (A) Cross-section of Rd9 host retinas after subretinal injection of Nrlp-GFP donor cells showing increased integration of donor cells with increasing age of host animal. Sections were obtained 1 month after transplantation. For each time point the left panel shows the GFP fluorescence (green) from the donor cells, and the right panel shows the GFP fluorescence overlaid on the DAPI staining (blue) of the nuclear layers. Note that the GFP signal is detected only in the ONL. Integrated cells formed outer segments (OS). No green fluorescent signal was detected in the inner nuclear layer (INL) or other retinal layers. (B) High-magnification view of an integrated donor cell (stained for GFP; green), forming a complete OS and morphologic connections with the bipolar cells (stained for protein kinase C; red). (C) High-magnification view of a donor cell (with green fluorescence from the GFP) 1 month after transplantation into a 3-month-old Nrl−/− host. The integrated cell has matured into a rod cell, with an OS staining positively for rhodopsin (red). Note that there is a second OS from another integrated cell whose nucleus is not visible in this section. (D) Number of donor (Nrlp-GFP) cells that integrated into the Rd9 retina as a function of host (Rd9) age and time after transplantation. There was a significant increase in the number of integrated cells in the 5- and 8-month-old host retinas compared with the 2-month-old host (P = 0.05). Regardless of the host age, the number of cells observed at 3 months after transplantation was significantly reduced compared with the number of integrated cells at 1 month after transplantation (P < 0.0001). (E) The rate of integrated donor cell loss decreased significantly in older hosts (P = 0.05).
Only a small number of GFP-expressing cells remained in the subretinal space, localized primarily to the injection site. How the nonintegrated cells were removed is unknown but occurred presumably through the phagocytic properties of the RPE. We detected only minimal loss of ONL cells in the area of the localized detachment created by the subretinal injection of cells compared with areas outside of the injection site.
The GFP-positive cells that integrated into the ONL appeared to be fully mature rod photoreceptors (Fig. 2B). These cells initially had a small, spheroid configuration at the time of transplantation but subsequently developed a normal rod-like morphology with inner and outer segments as well as axonal projections to the outer plexiform layer, suggesting a maturation process toward differentiated rods. When staining Rd9 eyes after transplantation, we detected photopigment in both host and donor cells, making it difficult to discern the production of rhodopsin in the transplanted cells. To show that the transplanted cells were producing photoreceptor-specific protein, we transplanted rod precursors into the Nrl−/− mouse, a strain that contains only cone cells.3 Figure 2C shows that the rod precursor cells integrated and matured in the Nrl−/− host and stained positively for rhodopsin. The production of rod-specific photopigment further supports the conclusion that transplanted cells had matured into functional rods.
Survival of Integrated Cells: Effect of Host Age and Antiapoptotic Therapy
The extent of transplanted cell integration varied as a function of host age, with significantly more cells entering the ONL of older mice (P = 0.05; Fig. 2D). At 1 month after transplantation, the 8-month-old hosts had more than three times as many transplanted cells integrated into the ONL as host mice that were only 2 months old at the time of transplantation. There was a moderate decrease in the ONL cell count in these older host mice, consistent with the baseline degeneration present in the Rd9 mouse strain.
Regardless of host age at the time of transplantation, integrated cells appeared to have a limited survival in the Rd9 host retina (Fig. 2D). Maximal numbers of integrated cells were detected at approximately 1 month after transplantation. Because this was the first time point tested, we cannot exclude that more cells were integrated even earlier. There was a significant reduction in the number of integrated photoreceptors over the time points tested (P < 0.0001). By 3 months after transplantation, the number of integrated cells had decreased significantly to approximately 40% to 60% of those integrated at 1 month after transplantation, with a greater rate of cell loss seen in younger hosts (Fig. 2E). Immunohistochemical staining of the GFP protein confirmed the loss of transplanted cells, not just loss of fluorescence. The presence of GFP is not toxic to the retina,5 and we confirmed that the retinal histology and electroretinograms of Nrlp-GFP mice (donor mice) were essentially equivalent to those of age-matched C57BL/6 control mice (data not shown).
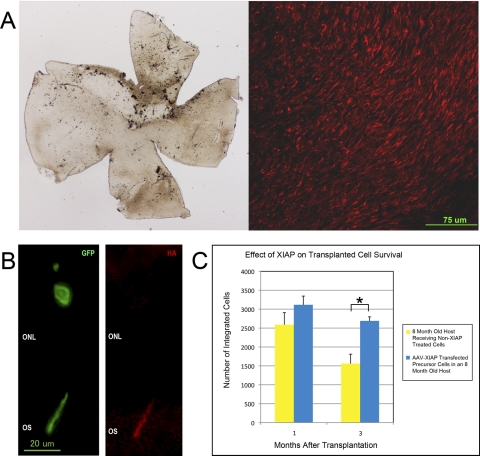
Transfection of the donor cells with AAV-XIAP before transplantation resulted in expression of the XIAP protein, as measured by immunohistochemical staining of an HA tag introduced along with the XIAP protein (Figs. 3A, 3B). The expression of XIAP appeared stable across all time points of this experiment. When transplanting XIAP-transfected cells into an 8-month-old host, there was a slight but not significantly higher number of integrated cells at the 1-month-time point compared with non–XIAP-treated cells (Fig. 3C). The major effect of AAV-XIAP pretreatment, however, was in the survival of the integrated cells. In the XIAP-treated group, 86% of integrated cells remained at 3 months after transplantation compared with only 60% of the non–XIAP-treated group (P = 0.006). Subretinal injection of AAV-GFP or phosphate-buffered saline into the subretinal space of neonatal Rd9 mice (age P4) does not reduce the rate of photoreceptor degeneration as measured by histology or electroretinography (data not shown), suggesting that the surgical procedure itself does not affect the photoreceptors in a way that would increase their survival.
Figure 3.
Transfection of donor cells with AAV-XIAP increases their survival after transplantation. (A) AAV-XIAP injected into the subretinal space of a P4 Nrlp-GFP mouse eye shows highly efficient transfection of photoreceptors. Left: flat mount of the retina 1 month after transplantation. Right: same retina stained for the HA tag on the XIAP protein. (B, left) High-magnification view of an integrated donor cell (stained for GFP; green) forming a complete outer segment (OS). Right: same cell stained for the HA tag on the XIAP protein (red), confirming the production of XIAP within the donor cell after transplantation into the rd9 mouse. The localization of XIAP to the OS is consistent with previous localization of XIAP within photoreceptors.8–10 (C) The number of integrated XIAP-treated donor cells that survive to 3 months after transplantation was significantly higher than non–XIAP-treated donor cells (P = 0.006).
Analysis of Intraretinal Inflammation
Clinical inspection of eyes after transplantation did not reveal any overt evidence of intraocular inflammation, such as vitreous or anterior chamber cells, nor did histologic analysis show intraretinal infiltration of inflammatory cells. To analyze the intrinsic, intraretinal response to the presence of the causative genetic mutation (i.e., the defect present in the Rd9 strain), the surgical procedure, or the presence of the donor cells, we assayed retina transcript levels for an array of immune- and inflammation-related genes for these groups of animals compared with the transcript levels for these genes in age-matched wild-type retinas (Supplementary Table S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5998/-/DCSupplemental). Intrinsic differences did exist between the transcription profile of the examined genes between the wild-type mice and the Rd9 mice. Although both strains were present on similar backgrounds (C57BL/6), nine genes exhibited differential expression >2.5-fold, with six genes showing increased transcript levels and three showing decreased transcript levels. When comparing the transcript levels of inflammatory genes in retinas undergoing sham surgery in the Rd9 mouse versus the wild-type controls, 24 genes showed increased transcription, and three genes showed decreased transcription. This was essentially the same in the eyes receiving actual transplantation (23 genes with increased transcription and four with decreased transcription). When comparing the transcription profiles of the Rd9 mice undergoing sham surgery to Rd9 mice receiving donor cells, minimal differences were detected.
Discussion
Our studies of photoreceptor precursor cell integration and survival after transplantation into a degenerating retina have resulted in several key findings. First, we established that rod precursor cells from the Nrlp-GFP mouse transplanted into the subretinal space of the Rd9 mutant mouse can integrate into the ONL and generate rod photoreceptors. Second, we showed that the extent of cellular integration increases as a direct function of Rd9 host age, with more precursor cells entering the ONL in older and more degenerated host retinas. Third, the transplanted and integrated cells have a limited survival in the Rd9 host eye, and the rate of transplanted cell death appears to be faster with younger host ages. Fourth, we showed that transfected donor cells overexpressing the antiapoptotic gene XIAP exhibit significantly increased transplanted photoreceptor survival in the Rd9 host retina. Finally, we did not detect any donor-cell specific induction of intraretinal inflammation, suggesting that the mechanism of cell death prevented by XIAP therapy is not a specific immune response against the donor cells.
The functional benefit of photoreceptor precursor cell and embryonic stem cell transplantation has been demonstrated in other models,1,6,12,13 yet the mechanism of such effects is poorly understood. One possible explanation is that the transplanted cells themselves are providing increased signal transduction of light stimuli. Our morphologic analysis shows that the transplanted cells appear to make connections with bipolar cells. The small number of integrated cells is consistent with the numbers reported by other groups.6,12,13 This number represents <1% of the normal complement of rods found in a mouse retina and may be below the threshold for measuring improvement in visual acuity. An early report of transplantation of dissociated whole retinas by Kwan et al.2 showed that the transplanted cells formed small patches of integration with the host retinal circuitry, which could mediate simple light responses. These investigators 2 showed that their retinal grafts survived for approximately 6 weeks without the induction of a significant inflammatory response. Further work by the same group has recently shown that injection of human cortical neural progenitor cells into the subretinal space of the Royal College of Surgeons rat, a rodent model of retinal degeneration, prevented host photoreceptor degeneration for prolonged periods of time up to several months.12 There was minimal integration of the donor cells into the host retina. Instead the donor cells appeared to differentiate into sheets of cells located between the host RPE and retina. The exact nature of these cells was unclear, but these data suggest an alternative explanation of how transplanted cells exert a protective effect on the host retina. Perhaps the exogenous cells exert a neurotrophic effect that prevents degeneration of the endogenous cells. This would be expected to provide a more robust benefit to vision than the direct transduction of visual stimuli by these small numbers of cells. If this mechanism indeed proves to be true, it would suggest that in future clinical applications, transplantation earlier in the course of the disease might be needed to provide a greater protective therapeutic effect.
The increased integration of rod precursor cells into older host retinas presents interesting implications with respect to optimal timing of cell-based therapies for future applications in a clinical setting. Previous reports have suggested that the extracellular matrix of the retina may present a barrier to integration. For example, disruption of chondroitin sulfate proteoglycans14,15 or of the outer limiting membrane may allow for improved integration of transplanted cells.16,17 These findings suggest that there may be a component of mechanical obstruction to the successful incorporation of the implanted cells into the host retina. In our experiments, we observed greater number of cells integrated when placed into older, thus more degenerated, eyes. We suggest that the transplanted cells fill the void created by endogenous cell loss or damage in the host retina. It is known, however, that severely degenerated retinas have secondary changes in the inner retina, such as neuronal rewiring, glial cell hypertrophy, and optic nerve degeneration.18–20 Thus, the advantage of age-dependent increased integration of cells may have to be weighed against the possible loss of functional success that may result from the secondary changes to the retina. Strategies for successful cell-based therapies may, therefore, include the use of adjunctive treatments to increase cellular integration at earlier ages, preserve inner retinal function, or both.
In the Rd9 mouse model of XLRP, we demonstrate that regardless of the age or degenerative stage of the host retina, donor cells did not survive for long periods of time after transplantation. The mechanism of transplanted cell death is unknown but is presumed to be apoptotic because of the lack of inflammatory infiltrate or necrotic debris accumulating in the treatment zone. Our screen of intraretinal changes in inflammatory gene transcript levels did not show any significant changes between the Rd9 retina receiving subretinal transplantation of cells and the Rd9 retinas undergoing sham surgery. We interpret this to mean that there was not a “donor-cell” induced inflammatory response but rather that inflammatory changes detected were caused by the surgery itself. Our analysis has several key limitations. First, we were analyzing the intrinsic gene expression profile within the retina for inflammation-associated genes. We were not measuring actual cytokine or chemokine levels present in the retina. Second, we analyzed the transcript levels at only one time point, 1 month after transplantation. This time point was chosen because the highest level of integration was detected then, and we hypothesized that that would be a large stimulus for any inflammatory response. Curiously, even at 1 month after transplantation, there were significant differences in the inflammatory gene transcript levels found even in the eyes receiving sham surgery. This suggests that surgery itself may be a relatively toxic procedure.
The proapoptotic stimulus that ultimately results in the death of the transplanted cells is unknown. One possibility is that the cells are degenerating because of a toxic signal emanating from the degenerating host retina. A second possibility is that the transplantation process itself initiates proapoptotic pathways. This seems less likely because the cells do survive for some period after the transplantation. Regardless of the signal initiating the apoptotic pathways, we find that overexpression of the XIAP gene in the donor cells significantly increases their survival in the degenerating host environment. These surviving cells have all the morphologic features of the non–XIAP-treated integrated cells, suggesting that they are functional photoreceptors. Given that XIAP targets the most downstream components of the apoptotic cascade (caspases 3, 7, and 9), we find it surprising that surviving cells are able to maintain relatively normal photoreceptor morphology. However, previous work with XIAP in photoreceptor degenerations has shown similar results.8–10 This suggests that the ability to prevent apoptosis, regardless of how far downstream in the pathway, can yield therapeutically significant results. It should be noted that the effect of XIAP is not absolute, and some cells do still die. This may suggest the need for additional, complementary methods for increasing cell survival.
In summary, we show successful transplantation of photoreceptor precursors in the degenerating retinas of Rd9 mice. Nonetheless, significant obstacles (such as the identification of a suitable cell line to inject in humans) remain before photoreceptor precursor cell transplantation can become a viable therapeutic option for patients with retinal degenerative diseases. Further investigations are necessary in this exciting field to facilitate cell-based therapies.
Footnotes
Supported by Foundation Fighting Blindness, Inc. (DNZ), National Eye Institute Grant RO1-EY007961 (HK), and National Eye Institute Core Center for Vision Research Grant P30-REY007003.
Disclosure: J. Yao, None; K.L. Feathers, None; H. Khanna, None; D. Thompson, None; C. Tsilfidis, None; W.W. Hauswirth, None; J.R. Heckenlively, None; A. Swaroop, None; D.N. Zacks, None
References
- 1. Lamba D, Karl M, Reh T. Neural regeneration and cell replacement: a view from the eye. Cell Stem Cell. 2008;2:538–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kwan AS, Wang S, Lund RD. Photoreceptor layer reconstruction in a rodent model of retinal degeneration. Exp Neurol. 1999;159:21–33 [DOI] [PubMed] [Google Scholar]
- 3. Mears AJ, Kondo M, Swain PK, et al. Nrl is required for rod photoreceptor development. Nat Genet. 2001;29:447–452 [DOI] [PubMed] [Google Scholar]
- 4. Swain PK, Hicks D, Mears AJ, et al. Multiple phosphorylated isoforms of NRL are expressed in rod photoreceptors. J Biol Chem. 2001;276:36824–36830 [DOI] [PubMed] [Google Scholar]
- 5. Akimoto M, Cheng H, Zhu D, et al. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc Natl Acad Sci U S A. 2006;103:3890–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207 [DOI] [PubMed] [Google Scholar]
- 7. Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vis Res. 2002;42:517–525 [DOI] [PubMed] [Google Scholar]
- 8. Petrin D, Baker A, Coupland SG, et al. Structural and functional protection of photoreceptors from MNU-induced retinal degeneration by the X-linked inhibitor of apoptosis. Invest Ophthalmol Vis Sci. 2003;44:2757–2763 [DOI] [PubMed] [Google Scholar]
- 9. Leonard KC, Petrin D, Coupland SG, et al. XIAP protection of photoreceptors in animal models of retinitis pigmentosa. PLoS One. 2007;2:e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zadro-Lamoureux LA, Zacks DN, Baker AN, Zheng QD, Hauswirth WW, Tsilfidis C. XIAP effects on retinal detachment-induced photoreceptor apoptosis. Invest Ophthalmol Vis Sci. 2009;50:1448–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pang J, Lauramore A, Deng W, et al. Comparative analysis of in vivo and in vitro AAV vector transduction in the neonatal mouse retina: effects of serotype and site of administration. Vis Res. 2008;48:377–385 [DOI] [PubMed] [Google Scholar]
- 12. Wang S, Girman S, Lu B, et al. Long-term vision rescue by human neural progenitors in a rat model of photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2008;49:3201–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suzuki T, Akimoto M, Imai H, et al. Chondroitinase ABC treatment enhances synaptogenesis between transplant and host neurons in model of retinal degeneration. Cell Transplant. 2007;16:493–503 [DOI] [PubMed] [Google Scholar]
- 15. Singhal S, Lawrence JM, Ghatia B, et al. Chondroitin sulfate proteoglycans and microglia prevent migration and integration of grafted Muller stem cells into degenerating retina. Stem Cells. 2008;26:1074–1082 [DOI] [PubMed] [Google Scholar]
- 16. West EL, Pearson RA, Tschernutter M, Sowden JC, Maclaren RE, Ali RR. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp Eye Res. 2008;86:601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pearson RA, Barber AC, West EL, et al. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. Cell Transplant. 2010;19:487–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Ret Eye Res. 2003;22:607–655 [DOI] [PubMed] [Google Scholar]
- 19. Jones BW, Watt CB, Frederick JM, et al. Retinal remodeling triggered by photoreceptor degeneration. J Comp Neurol. 2003;464:1–16 [DOI] [PubMed] [Google Scholar]
- 20. Marc RE, Jones BW, Anderson JR, et al. Neural reprogramming in retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48:3364–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]