The absence of TNFα protects against diabetes-associated blood–retinal barrier breakdown, leukostasis, and apoptosis in the retina, making it a good therapeutic target for the prevention of the progressive BRB breakdown, inflammation, and apoptosis associated with diabetic retinopathy.
Abstract
Purpose.
Blood–retinal barrier [BRB] breakdown, characteristic of diabetic retinopathy (DR), is believed to depend on inflammation and apoptosis. Retinal inflammation is almost completely suppressed in the absence of TNFα, which is also associated with apoptosis. This study was conducted to determine the role of TNFα in these diabetic complications.
Methods.
Diabetes was induced with streptozotocin in Tnfa knockout (KO) mice, to provide a chemical model of diabetes, and Tnfa (KO) mice were crossed with Ins2Akita mice to generate a genetic model, with both models being devoid of TNFα. The BRB was assessed at 1, 1.5, 3, and 6 months. Leukostasis was assessed using FITC-conjugated ConA to label leukocytes. Apoptosis was assessed with TUNEL and activated caspase-3 staining. PECAM1 identified endothelial cells, and SMA identified pericytes.
Results.
At 1 month of diabetes, the absence of TNFα had no effect on DR-associated BRB breakdown, even though it prevented retinal leukostasis, demonstrating that neither TNFα nor inflammation is essential for early BRB breakdown in DR in either model of diabetes. At 3 months of diabetes, BRB breakdown was significantly suppressed and at 6 months, it was completely prevented in the absence of TNFα in both models, showing that TNFα is essential for progressive BRB breakdown. DR-mediated apoptosis in the retina, which appears to involve endothelial cells, pericytes, and neurons, was inhibited in the absence of TNFα in both models.
Conclusions.
Although neither TNFα nor inflammation is necessary for early BRB breakdown in DR, TNFα is critical for later complications and would be a good therapeutic target for the prevention of the progressive BRB breakdown, retinal leukostasis, and apoptosis associated with DR.
The blood–retinal barrier [BRB], the analog of the blood–brain barrier, consists of an outer component, the retinal pigment epithelium, and an inner component, the retinal vascular endothelium.1,2 Tight junctional protein complexes, a paucity of transport vesicles, and the maintenance of ionic and metabolic gradients through the interactions of endothelial cells with their regulatory pericytes and glial cells are essential for maintaining BRB integrity and normal function.3–5 By controlling the access of fluids and solutes from the blood to the retina, the BRB plays a central role in maintaining the homeostasis of the retinal microenvironment, and its breakdown occurs in diabetic retinopathy (DR) and other ischemic retinopathies. BRB breakdown due to increased vascular permeability leads to macular edema, which is a major complication of DR and is the most common cause of visual loss, but current treatments to prevent or decrease macular edema have only limited efficacy. Understanding the mechanisms underlying BRB breakdown in diabetes is fundamental in designing better therapeutic approaches to prevent BRB breakdown leading to macular edema in DR.
Several cellular mechanisms and molecular pathways activated by hyperglycemia and/or hypoxia have been demonstrated to be involved in promoting BRB breakdown in diabetes. First, upregulation of vascular endothelial growth factor (VEGF) is a major contributor to BRB breakdown in diabetes, and the antagonists against VEGF or its receptors are potential therapeutic agents for suppressing the diabetic macular edema (DME) caused by BRB breakdown.6–8 Second, evidence has increasingly suggested that leukocyte adhesion to the retinal vasculature or retinal leukostasis results in BRB breakdown in early DR,9–11 and this effect may be critical in the development of DME.12–14 Increased retinal leukostasis in DR is mediated, at least in part, by VEGF,15,16 and VEGF-mediated vascular permeability is largely dependent on tumor necrosis factor (TNF)-α.17,18 Third, the inhibitors of receptor tyrosine kinase, particularly protein kinase C (PKC), can effectively inhibit VEGF-induced BRB breakdown, revealing the roles of activation of PKC in promoting BRB breakdown.6 One recent study demonstrated that TNFα mediates the death/apoptosis of endothelial cells in diabetes and is implicated in the progression of DR.19 Finally, other vasoactive and/or vascular permeability factors, such as inducible nitric oxide synthase,20 intercellular adhesion molecule-1, nuclear factor-κB, and angiotensin-converting enzyme21 may contribute to BRB dysfunction directly or by interacting with VEGF.22
The proinflammatory cytokine TNFα is a potent mediator of the leukostasis induced by VEGF, interleukin (IL)-1β, and platelet-activating factor (PAF) in the retinal vasculature,18 and it also mediates the cell death/apoptosis of retinal neurons and vascular endothelial cells in DR.19 Furthermore, the expression of TNFα is elevated in diabetic animals23,24 and patients,25–30 and its inhibition prevents the pathologic events of early DR including BRB breakdown.23 These studies suggest that the retinal leukostasis and apoptosis mediated by TNFα contribute to BRB breakdown in DR; however, definitive evidence for these conclusions is lacking. The present study was conducted to see whether TNFα is essential for mediating BRB breakdown in DR, in which case it would be a potential therapeutic target. We also determined whether TNFα plays an important role throughout the entire progression of DR or at which stage it is critical. In the present study, we tested the influence of TNFα on BRB breakdown and factors that influence it, such as retinal leukostasis and apoptosis, through the use of Tnfa knockout (KO) mice in the presence of diabetes, which was induced chemically by streptozotocin (STZ) or genetically by crossing Tnfa (KO) mice with Ins2Akita diabetic mice, developed to serve as a genetic model of diabetes,31 to generate a line of diabetic mice devoid of TNFα. The hypothesis was that the retinal leukostasis and apoptosis mediated by TNFα are direct contributors to BRB breakdown in murine models of diabetes.
Materials and Methods
Mice
Animal use was in accordance with the approved protocols by the Institutional Animal Care and Use Committee of The Johns Hopkins University School of Medicine and the guidelines of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The Ins2Akita mice obtained from The Jackson Laboratory (Bar Harbor, ME) are heterozygous for the insulin2 gene with a transition from cystine to tyrosine at the seventh amino acid of the gene, leading to the disruption of the disulfide bond between chains A and B of insulin, the accumulation of misfolded insulin protein inside the cells, and consequently the death of pancreatic β-cells. These spontaneously mutant mice serve as an excellent animal model of diabetes with the phenotypes of hypoinsulinemia and hyperglycemia, with symptoms similar to those observed in diabetic patients. Retinal complications have been well characterized in these diabetic mice.31 Only male Ins2Akita mice were used for experimentation. Ins2Akita mice were crossed with TNFα−/− mice in a C57BL/6J background for two generations to give birth to the mice with the genotype of Tnfa −/−/Ins2Akita. Additional mating of Tnfa−/−/Ins2Akita and Tnfa−/−/Ins2+/+ was performed to fix the Tnfa mutation to homozygosity while maintaining the Ins2Akita mutation in the heterozygous state. The diabetic phenotype was confirmed at 4 to 5 weeks after birth with a blood glucose monitor (Accu-Check Active GlucoMeter; Roche Diagnostics, Indianapolis, IN) testing a drop of blood from a tail puncture. The genotype was confirmed by PCR.
PCR Genotyping
The tail genomic DNA was prepared with the lysis reagent (DirectPCR; Wiagen Biotech, Los Angeles, CA). The PCR protocol for the genotyping of the insulin2 gene was performed according to the instructions provided by The Jackson Laboratory (http://jaxmice.jax.org/strain/003548.html). In brief, PCR amplification was performed with the pair of primers TGCTGATGCCCTGGCCTGCT and TGGTCCCACATATGCACATG, followed by restriction enzyme digestion with Fnur4H1. The digested PCR products were dissolved in a 2% agarose gel containing 0.5 μg/mL ethidium bromide. To confirm the genotype of the Tnfa−/−/ Ins2Akita mice, we performed the protocol for PCR genotyping of Tnfa homozygous KO mice, as described by the Jackson Laboratories (http://jaxmice.jax.org/strain/003008.html) and in a prior study.18 Briefly, PCR amplification was performed with three primers: TAGCCAGGAGGGAGAACAGA (common), AGT GCCTCTTCTGCCAGTTC (wild-type reverse), and CGTTGGCTACCCGTGATATT (mutant reverse). The PCR products were separated by gel electrophoresis on a 1.5% agarose gel. The mice with only 318-bp amplicons were the homozygous Tnfa KO mice. The diabetic phenotype of the Tnfa−/−/Ins2Akita mice was confirmed by blood glucose monitoring with the glucometer.
Induction of Experimental Diabetes in Mice
Diabetes was chemically induced in 4-week-old mice, according to the method of Phelan et al.32 The mice received an intraperitoneal (IP) injection of 75 mg/kg STZ dissolved in sodium citrate buffer (0.01 M; pH 4.5) for three successive days. They were screened for diabetes beginning 3 days after the initial dose of STZ by testing for glucose in the urine with a urine strip test. If the test was positive, the precise blood glucose level was measured with a glucometer. Mice that did not develop diabetes after this dose of STZ receive a second treatment with STZ. At the time of the experiments, the diabetic state of the animal was confirmed by measuring blood glucose levels with the glucometer. Fasting blood glucose levels higher than 250 mg/dL are considered to be diabetic. Insulin was not administered to the animals. Age-matched, nondiabetic mice with the same genetic background were used for controls.
BRB Assay
The quantitative BRB assay was performed according to a previously described technique.33 The mice were sedated and given an IP injection of 1 μCi/g body weight of [3H]mannitol. One hour after injection, the mice were sedated, and the retinas from the experimental and control eyes were rapidly removed. The posterior portion of the globe was firmly grasped with forceps, and a razor blade was used to cut across the cornea and extrude the lens, vitreous, and retina. The retinas were dissected from the lens, vitreous, and any RPE that was extruded, and were placed within preweighed scintillation vials within 30 seconds of death. The thoracic cavity was opened and the left superior lobe of the lung was removed, blotted free of excess blood, and placed in another preweighed scintillation vial. A left dorsal incision was made, and the retroperitoneal space was entered without entering the peritoneal cavity. The renal vessels were clamped with a forceps and the left kidney was removed, cleaned of fat, blotted, and placed into a preweighed scintillation vial. Superficial liquid was allowed to evaporate over 20 minutes in the open vials. The vials containing the tissue were weighed, and the tissue weights were calculated and recorded. One milliliter of solubilizing solution (NCSII; GE Healthcare, Piscataway, NJ) was added to each vial, and the vials were incubated overnight in a 50°C water bath. Solubilized tissue was brought to room temperature (RT) and decolorized with 20% benzoyl peroxide in toluene in a 50°C water bath. The vials were brought to RT, and 5 mL of scintillation fluid (Cytoscint ES; MP Biomedicals, Solon, OH) and 30 μL of glacial acetic acid were added. The vials were stored for several hours in darkness at 4°C to eliminate chemiluminescence. Radioactivity was counted with a scintillation counter (LS 6500; Beckman, Brea, CA). The counts per minute per milligram tissue were measured for lung, kidney, and experimental and control retina. Retina/lung, retina/kidney, and lung/kidney ratios were calculated and compared.
Retinal Leukostasis
Mice were anesthetized with ether, the descending aorta was clamped, and the right atrium was cut. The mice were perfused with 50 mL PBS to remove erythrocytes and nonadherent leukocytes, followed by perfusion with fluorescein-conjugated concanavalin A to label adherent leukocytes.10,18 Another PBS perfusion was used to flush out unbound fluorescein. Retinal flat mounts were prepared as previously described, to assess leukostasis.34 The eyes were removed and fixed for more than 1 hour with phosphate-buffered formalin. The cornea and lens were removed and, under a stereomicroscope (Stemi 2000C; Carl Zeiss Meditec, Inc., Thornwood, NY), the entire retina was carefully dissected from the eye cup, rapidly cut from the edge to the equator in all four quadrants, and flat-mounted with the photoreceptors facing upward. Leukocytes adherent to the vessel walls were labeled with fluorescein, and the total number of leukocytes within the vessels of each retina was counted under an epifluorescence microscope (Axiopan2; Carl Zeiss Meditec, Inc.) by an investigator masked to the nature of the specimen. The counting began at the optic disc. The vessel nearest the 12 o'clock position and its branches were followed all the way to the periphery, with the focus changed as necessary to include all arteries, veins, and capillaries in the field. This process was repeated in a clockwise direction for each vessel radiating from the optic disc, so the total number of adherent leukocytes in all the vessels of the retina was counted.
TUNEL Assay for Detection of Apoptotic Nuclei
The terminal dUTP nick-end labeling (TUNEL) assay was performed with a cell viability kit (ApopTAG Red In Situ Apoptosis Detection Kit; Millipore, Temecula, CA), by procedures performed according to the manufacturer's manual. In brief, eye sections were fixed in 1% paraformaldehyde for 10 minutes at RT and in ethanol:acetic acid (2:1) for 5 minutes at −20°C and then washed twice for 5 minutes in PBS (pH7.4). After the tailing of digoxigenin-dNTP catalyzed by the TdT enzyme, the sections were incubated with the anti-digoxigenin-rhodamine antibody for 30 minutes at RT. For negative controls, deionized water was substituted for the TdT enzyme. Processed sections were mounted with antifade mounting medium for fluorescence-containing DAPI (Vectashield; Vector, Burlingame, CA) and viewed with a fluorescence microscope (Axiopan2; Carl Zeiss Meditec, Inc.). The number and locations of TUNEL-positive cells were recorded.
Immunofluorescent Staining of Activated Caspase-3, PECAM1, and SMA
Cryosections of eyes were fixed in ice-cold methanol:acetone (1:1) for 10 minutes at −20°C, washed with 0.01 M PBS (pH 7.4), and blocked with 4% normal goat serum for 90 minutes. They were incubated overnight at 4°C with polyclonal antibodies against activated caspase-3 (1:200; Cell Signaling Technology, Boston, MA) and/or FITC-conjugated rat anti-mouse CD31 or PECAM1 antibody (1:100; BD Biosciences, San Diego, CA) to label vascular endothelial cells or smooth muscle actin (SMA; 1:50; Biogenix, San Ramon, CA) to label pericytes. Negative control sections were similarly treated, but the primary antibodies were omitted. Sections were rinsed and incubated for 1 hour with Alexa Fluor 594-conjugated goat anti-rabbit IgG (1:1000; Invitrogen, Carlsbad, CA). Fluorescence microphotography was performed on the epifluorescence microscope (Axiopan2; Carl Zeiss Meditec, Inc.). Each section was scanned systematically from the temporal to the nasal side for fluorescent cells indicative of cells undergoing apoptosis, by an investigator masked to the nature of the specimens. The number and locations of positive cells were counted and photographed.
Real-time PCR Analysis
Total RNA from retinas was isolated (RNeasy kit; Qiagen, Valencia, CA), then treated with DNase (Qiagen). Single-strand cDNA was synthesized from 0.5 μg total RNA using oligo (dT)12-18 primer (Invitrogen) and MMLV reverse transcriptase (Invitrogen) in a final reaction volume of 25 μL. Real-time PCR was performed using SYBR master mix (Premix Ex Taq; Takara, Dalian, China) with a quantitative (q)PCR system (Mx3005P; Agilent Technologies, Palo Alto, CA). mTNF-α primer sequences were sense (5′-GACAAGGCTGCCCCGACTA-3′) and antisense (5′-AGGGCTCTTGATGGCAGAGA-3′). mGAPDH (sense: 5′-AACGACCCCTTCATTGAC-3′; antisense: 5′-TCCACGACATACTCAGCAC-3′) was used as the reference for normalization. Each cDNA sample was run in duplicate.
Enzyme-Linked Immunosorbent Assay for TNFα
Each retina was homogenized in 0.1% Triton X-100 in PBS supplemented with a cocktail of protease inhibitors (Invitrogen, Carlsbad, CA). The samples were cleared by centrifugation and then assessed for protein concentration with a protein assay (DC Protein Assay; Bio-Rad, Hercules, CA). TNFα levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The reaction was stopped and the absorption measured by a microplate reader at 450 nm, with subtracted reading at 540 nm. All measurements were performed in duplicate. The retina sample TNFα concentration was calculated from a standard curve and corrected for total protein concentration. Four retinas were used for each condition.
Statistical Analysis
Statistical comparisons were made using analysis of variance (ANOVA) or a linear mixed model.35 P-values for comparison of treatments were adjusted for multiple comparisons by the Dunnett method. For data sets with two groups, statistical analyses were performed with the unpaired t-test (Excel 2003; Microsoft, Redmond, WA).
Results
Chemically Induced and Genetically Mutant Mouse Models of Diabetes
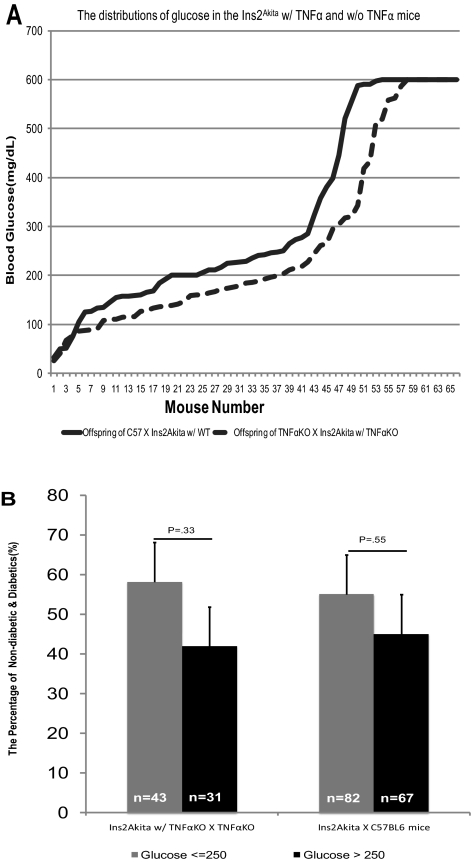
Diabetes was induced in Tnfa (KO) and C57BL/6J control mice by STZ, and they served as the chemically induced mouse model of diabetes. Tnfa (KO) mice were crossed with Ins2Akita mice to generate the genetic model of diabetes. The deletions of the Tnfa gene and the insulin2 gene alleles in the genetic model were characterized as described earlier. To see whether the genetic deletion of the Tnfa gene affects the development of hyperglycemia due to the mutation of the insulin2 gene, we compared the level of glucose in the progeny from Ins2Akita diabetic mouse lines, with and without Tnfa: Tnfa+/+/Ins2Akita and Tnfa−/−/Ins2Akita. The concentration and distribution of blood glucose levels in the presence or absence of TNFα were not significantly different (Fig. 1A). Because 250 mg/dL of glucose was used as the baseline of diabetes in the chemically induced diabetic models,9,10,23 we compared the frequency of mice with glucose greater or less than 250 mg/dL in the two genetic diabetic mouse lines, with or without TNFα, and found that the percentage of mice with glucose greater than 250 mg/dL was not significantly different in the two groups at 4 to 5 weeks: 58% versus 42% (P = 0.33) for Tnfa−/−/Ins2Akita versus Tnfa (KO) and 55% versus 45% (P = 0.55) for C57/Ins2Akita versus wild-type (Fig. 1B).
Figure 1.
Genetic animal model of diabetes. (A) Distribution of glucose in the offspring of a breeding pair TNFα+/+/Ins2Akita and TNFα−/−/Ins2Akita mice. The glucose was determined in the 4- to 5-week-old mice with a glucose monitor. The HI reading, which shows that the level of glucose is beyond the range of the meter, was designated 600 mg/dL. (B) The percentage of mice with blood glucose ≤250 mg/dL versus >250 mg/dL in the two lines is shown.
Suppression of BRB Breakdown in Tnfa (KO) Mice with Diabetes
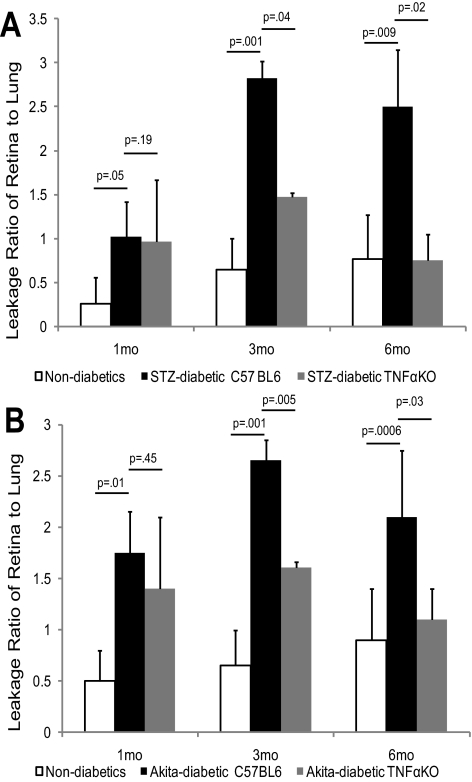
BRB breakdown occurs in the early stages of DR and progresses into the later stages. The mechanisms of early hyperglycemia-induced changes in DR, which takes years to develop in humans, are not clear. We hypothesized that TNFα may be a molecule that mediates the progression of the disease. To test the hypothesis, we measured retinal vascular leakage at different stages (1 month, 6 weeks, 3 months, and 6 months) in diabetic mice, with or without TNFα, and in their nondiabetic counterparts. At 1 month and 6 weeks of diabetes, the retina-to-lung leakage ratio (RLLR) significantly increased, but the increase was not prevented by the absence of TNFα (RLLR at 1 month is 1.02 ± 0.3 vs. 0.96 ± 0.2, P = 0.19 in STZ-induced diabetic TNFα [KO] mice compared to diabetic C57BL/6J mice; Fig. 2A). The retina-to-renal leakage ratio (RRLR), however, was not increased at 6 weeks, and TNFα had no effect. Realizing that the kidney is adversely affected in diabetes, we found the vascular permeability in the kidneys of 6-week diabetic mice had increased comparable to that in the retina, leaving no difference in the ratio. Based on this finding, further BRB assessments in diabetic mice compared vascular leakage in the retina to that in the lung. After 3 months of diabetes, vascular leakage was significantly suppressed in the absence of TNFα (RLLR was 2.82 ± 0.7 vs. 1.47 ± 0.32, P = 0.04), and at 6 months of STZ-induced diabetes, retinal vascular permeability increased more than twofold, compared with that in nondiabetic controls, and this increase was prevented by the absence of TNFα (Fig. 2B). The data showed this finding regardless of whether retinal vascular leakage was compared to that of lung (RLLR) or kidney (RRLR). In the Ins2Akita genetic model of diabetes, which may be more relevant to human DR, similar results were obtained. The absence of TNFα did not result in a significant reduction of vascular leakage at 1 month or 6 weeks of diabetes (RLLR, 1.75 ± 0.4 vs. 1.4 ± 0.7, P = 0.49 for 1 month), whereas the vascular leakage was significantly suppressed at 3 months (RLLR, 2.65 ± 0.2 vs. 1.61 ± 0.05, P = 0.005) and totally prevented at 6 months (RLLR, 2.1 ± 0.65 vs. 1.1 ± 0.3, P = 0.04) in the absence of TNFα. These results show that in both the chemical and genetic models of DR, TNFα was critical for BRB breakdown at 3 and 6 months, but not at 1 month and 6 weeks.
Figure 2.
Suppression of BRB breakdown in TNFα (KO) mice with diabetes at the late stage of DR. (A) The RLLR of the nondiabetic controls, Ins2Akita diabetic TNFα+/+, and TNFα−/− mice at 1, 3, and 6 months of diabetes. (B) The RLLR of nondiabetic controls, STZ-induced diabetic TNFα+/+ and TNFα−/− mice at 1, 3 and 6 months of diabetes. The results are expressed as the mean ± SE of 6 to 12 independent experiments.
Suppression of Retinal Leukostasis in Tnfa (KO) Mice with Diabetes
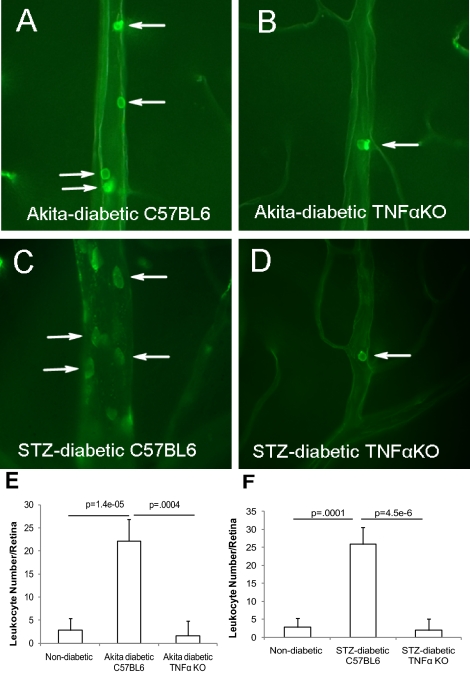
Several lines of evidence suggest that retinal leukostasis is increased in diabetes, which results in vascular injury that contributes to BRB breakdown in DR.10,16 In agreement with previous reports, our results demonstrated that adherent leukocytes in the retinal vasculatures were significantly elevated in Ins2Akita diabetic mice (22 ± 5 leukocytes per retina, P < 0.0001, compared with 2 ± 3 in nondiabetic controls of the same genetic background at 1 to 2 weeks of diabetes; Figs. 3A, 3E) and the STZ-induced diabetic C57BL/6J mice (26 ± 4 leukocytes per retina, P = 0.0001 compared to 3 ± 2 in nondiabetic controls (Figs. 3C, 3F). Surprisingly, the number of leukocytes in both diabetic mouse models without TNFα was equivalent to that observed in nondiabetic control mice (2 ± 3 leukocytes per retina for both STZ- and Ins2Akita-diabetic Tnfa [KO] mice at 1 to 2 weeks of diabetes; Figs. 3B, 3D, 3E, 3F), implying that TNFα is a potent mediator of retinal leukostasis in the context of chemically induced and genetically mutant DR, as it is in the case of intravitreal administration of VEGF, IL-1β, and PAF18 and in oxygen-induced ischemic retinopathy (Supplementary Fig. S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5768/-/DCSupplemental). The number of adherent leukocytes in diabetic mouse retina, although significantly greater than in control retina, may seem too low to cause vascular complications, but DR is associated with a chronic low-grade, subclinical inflammation that is responsible for the vascular lesions characteristic of DR12 and the number of adherent leukocytes that we observed in diabetic retina was somewhat greater, but similar to that reported in another study.36 Although adherent leukocytes were illustrated in larger vessels in Figures 3A to 3D, to show comparable fields, all adherent leukocytes within the retinal vasculature, including capillaries, were counted and the results included in Figures 3E and 3F. Diabetes-induced retinal leukostasis was also completely eliminated at 5 weeks in Ins2Akita-diabetic Tnfa (KO) mice (2.83 ± 1.01 leukocytes/retina for nondiabetics, 22.17 ± 1.90 for diabetic Ins2Akita mice and 1.67 ± 1.28 for Ins2Akita-diabetic Tnfa [KO] mice).
Figure 3.
Suppression of retinal leukostasis in TNFα (KO) mice with diabetes. (A–D) The representative images show the leukocytes adherent to the retinal vasculatures in the Ins2Akita diabetic TNFα+/+ (A), TNFα−/− mice (B), and STZ-induced diabetic TNFα+/+ (C) and TNFα−/− mice (D) at 1 to 2 weeks of diabetes. (E, F) The quantification of retinal leukostasis for Ins2Akita (E) and STZ-induced (F) diabetic mice. The results are expressed as the mean ± SE of five independent experiments. Arrows: leukocytes.
Reduction of Apoptotic Cells in Tnfa (KO) Mice with Diabetes
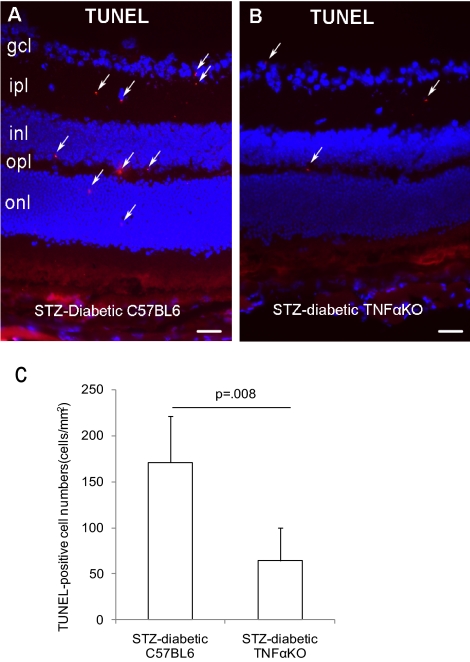
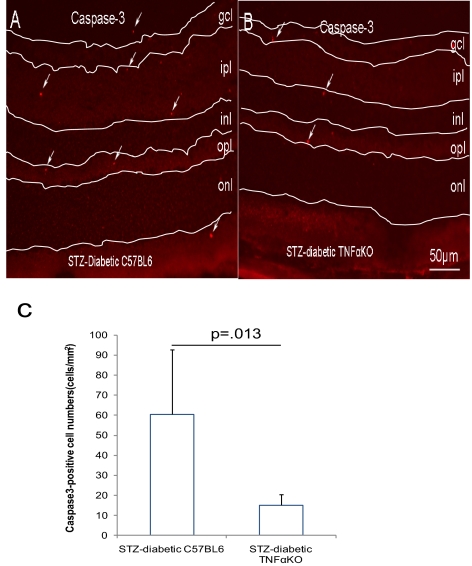
In addition to its proinflammatory activity, TNFα-mediated cell death and apoptosis have been suggested to contribute to the pathogenesis of DR.19 To investigate the association of cell apoptosis and BRB malfunction contributed by TNFα, apoptotic cells were identified by TUNEL and activated caspase-3 staining in diabetic Tnfa+/+ and Tnfa−/− mice to confirm apoptosis using two distinct markers. In the STZ-induced diabetics, both TUNEL-positive (171 ± 50 cells/mm2 for Tnfa+/+ vs. 64 ± 35 cells/mm2 for Tnfa−/−, P = 0.008) and activated caspase-3–positive cells (60 ± 32 cells/mm2 for Tnfa+/+ vs. 15 ± 5 cells/mm2 for Tnfa−/−, P = 0.01) were significantly reduced at 3 months of diabetes in the Tnfa−/− mice compared with the Tnfa+/+ mice (Figs. 4C, 5C). These apoptotic cells were largely localized in the inner and outer plexiform layers and a few cells were situated on the ventral surface of the retina close to the ganglion cell layer and in the outer nuclear layer (Figs. 4A, 4B, 5A, 5B).
Figure 4.
Reduction of TUNEL-positive cells in STZ-induced diabetic TNFα (KO) mice[b]. Mice with diabetes of 3 month's duration were used. The apoptotic cells were identified by TUNEL staining in STZ-induced diabetic TNFα+/+ (A) or TNFα−/− mice (B). (C) The quantification of TUNEL-positive cells. The data are expressed as the mean ± SE of results in five independent experiments. Arrows: apoptotic cells. gcl, ganglion cell layer; ipl, inner plexiform layer; inl, inner nuclear layer; opl, outer plexiform layer; onl, outer nuclear layer.
Figure 5.
Reduction of activated caspase-3–positive cells in STZ-induced diabetic TNFα (KO) mice. Mice with diabetes of 3 month's duration were used. The apoptotic cells were indicated by activated caspase-3 staining in STZ-induced diabetic TNFα+/+ (A) or TNFα−/− (B) mice. (C) The quantification of activated caspase-3–positive cells. The results are expressed as the mean ± SE of five independent experiments. Arrows: apoptotic cells. gcl, ganglion cell layer; ipl, inner plexiform layer; inl, inner nuclear layer; opl, outer plexiform layer; onl, outer nuclear layer.
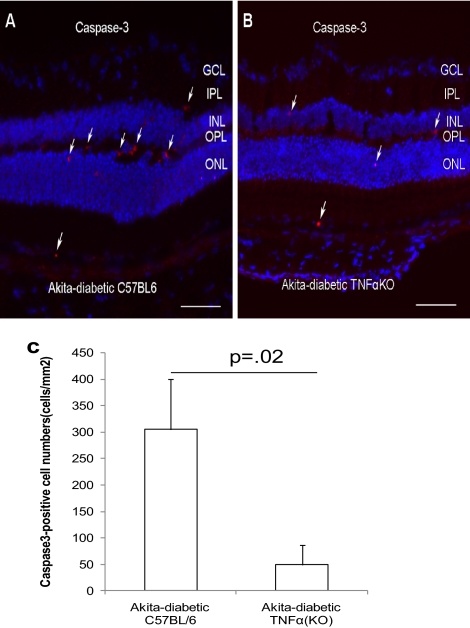
In the Ins2Akita diabetic mice, there were significantly fewer activated caspase-3–positive cells in the Tnfa−/− than in the Tnfa+/+ mice (304 ± 94 cells/mm2 for Tnfa+/+ vs. 49 ± 37 cells/mm2 for Tnfa−/−; P = 0.02) at 3 months of diabetes (Fig. 6C). Activated caspase-3–positive cells appeared to be largely localized in the outer and inner plexiform layers, and a few positive cells were found in the outer nuclear layer and choroid (Figs. 6A, 6B). Furthermore, double-labeling of activated caspase-3 and platelet/endothelial cell adhesion molecule (PECAM)-1, a marker of vascular endothelial cells, and SMA, a marker of pericytes, demonstrated that the activated caspase-3-positve cells include both PECAM1-positive and SMA-positive cells (Supplementary Fig. S2, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5768/-/DCSupplemental).
Figure 6.
Reduction of apoptotic cells in the Ins2Akita diabetic TNFα (KO) mice. Ins2Akita mice with diabetes of 3 month's duration were used. The apoptotic cells were indicated by activated caspase-3 in the diabetic TNFα+/+ (A) and TNFα−/− mice (B). (C) The quantification of activated caspase-3–positive cells. The results are expressed as the mean ± SE of five independent experiments. Arrows: apoptotic cells. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer.
TNFα Expression in Diabetic Mice
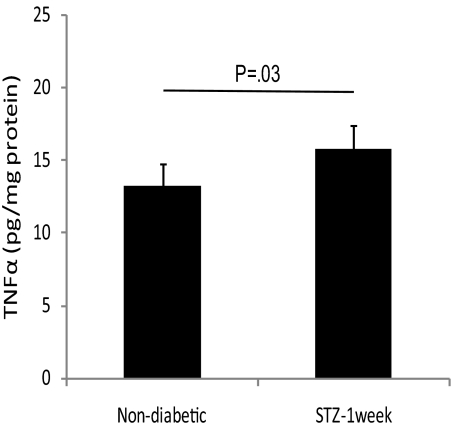
Although the observed effects on retinal vascular permeability and apoptosis at 3 months of diabetes and beyond were clearly TNFα-dependent, the expression of TNFα did not correlate with these events. Real-time PCR showed a significant decrease in Tnfa expression in the retina after 3 months of STZ-induced diabetes and no change in 3-month diabetic Ins2Akita mice, compared with that in their nondiabetic counterparts (data not shown). ELISA results showed a modest but significant increase in the level of TNFα protein in the retinas of 1-week STZ diabetic mice (Fig. 7), but this increase was not observed in 1- or 6-month STZ diabetic mice or in 2-week diabetic Ins2Akita mice.
Figure 7.
Expression of TNFα in the diabetic mice. ELISA results for TNFα protein levels in the mice with STZ-induced diabetes of 1-week's duration were compared with that in the nondiabetic controls.
The Proposed Mechanisms by Which TNFα Regulates BRB Breakdown in Diabetes
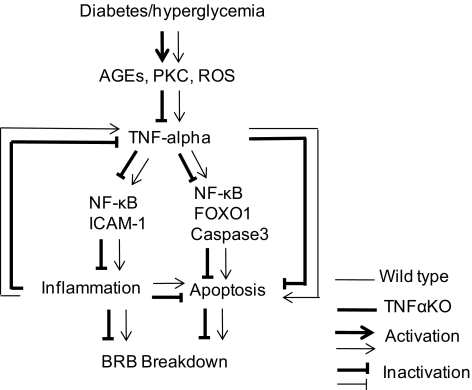
The evidence reveals that the absence of TNFα suppresses the pathogenesis of DR including retinal leukostasis, apoptosis, and BRB breakdown, and previous reports demonstrated that retinal leukostasis and apoptosis contribute to the breakdown of the BRB in DR.19,23,37 Therefore, we postulate that TNFα-mediated retinal leukostasis and apoptosis are mechanisms that regulate BRB breakdown in the later stages of diabetes. A pathway whereby TNFα regulates BRB breakdown is proposed in Figure 8, which is potentially applicable to later time points of DR (i.e., 3 and 6 months).
Figure 8.
The proposed mechanisms by which TNFα regulates BRB breakdown in diabetes. TNFα is activated in diabetic mice and regulates the BRB breakdown in the later stages of DR. The proinflammatory and proapoptotic activities of TNFα are associated with the breakdown of the BRB at the later time points of DR. AGEs, advanced glycation end products; PKC, protein kinase C; ROS, reactive oxygen species; NF-κB, nuclear factor-κB; ICAM-1, intercellular adhesion molecule-1; FOXO1, foxhead box O1.
Discussion
The main findings of this study were that (1) TNFα is critical for BRB breakdown at the later time points of DR, but not in the early stage. The observation that TNFα was not necessary for BRB breakdown in early DR, but was essential for BRB breakdown at the later time points, as the disease progressed, illustrates the complexity of the process and that multiple and distinct factors play a role in mediating BRB breakdown at different stages of the disease. (2) TNFα plays a role in the development of diabetes-related leukostasis and apoptosis in the retina. (3) The observations with respect to retinal leukostasis, apoptosis, and BRB breakdown were confirmed in two distinct models of diabetes: a chemically induced diabetic (STZ-induced) model and genetically mutant Ins2Akita diabetic mice.
The apparent contradiction between our findings that neither TNFα nor inflammation was necessary for early (1-month and 6-week) BRB breakdown and previous reports showing that TNFα and VEGF are upregulated as early as 1 to 2 weeks after the onset of diabetes in rats and that the inhibition of TNFα attenuated BRB breakdown in rats,23,24 could be due to species differences between rats and mice. A recent study, using TNFα receptor KO mice and a TNFα inhibitor, confirmed the role of TNFα in the pathogenesis of DR, particularly related to apoptosis.19 If TNFα is also upregulated at that time in mice, a delay in the induction of TNFα cannot account for the lack of an effect of TNFα on the BRB in the early stages of DR. The molecular mechanisms that regulate diabetes-induced BRB breakdown are varied (see the introduction and the review by Ehrlich et al.38). Little is known about the relationships or interactions of these molecules or signaling pathways in the pathogenesis of DR. For instance, do TNFα and VEGF, both of which are upregulated in diabetes and implicated in the pathogenesis of DR,39–43 interact to promote the diabetes-induced BRB breakdown? If so, how do they interact with each other? An initial step in addressing these questions could be to perform a study to determine the level of VEGF expression in diabetic Tnfa−/− and wild-type mice at the stage of DR when TNFα is known to regulate BRB breakdown. If VEGF expression is elevated to the same degree in both diabetic mice strains with and without TNFα, compared with nondiabetic control mice, it is most likely that TNFα at least partially acts as a downstream mediator of BRB breakdown in DR, since BRB breakdown is blocked as a result of the absence of TNFα. Although the expression of VEGF is elevated by diabetes, if VEGF is not increased by hyperglycemia in Tnfa−/− mice or the level of elevation is lower in Tnfa−/− than in wild-type mice and the blood glucose levels are not significantly different in diabetic Ins2Akita mice with or without TNFα, it is likely that TNFα regulates BRB breakdown indirectly through VEGF. Conversely, if the expression of VEGF is elevated more in diabetic Tnfa−/− mice than in control mice, it is possible that there is a regulatory loop in which the lack of one factor is dependent on compensation by the other factor or that they are dependent on each other to promote BRB breakdown in DR.
The dependence of BRB breakdown and apoptosis at 3 months or more of diabetes on TNFα and the lack of correlation of these events with TNFα expression at these time points suggests that either the changes in TNFα expression necessary to mediate physiological activity at these time points are too subtle to be detected by the assays used or that these activities are indirect or downstream events initiated by TNFα early in diabetes. We have demonstrated a significant diabetes-associated increase in TNFα in the retina at 1 week, but this increase was transient and did not persist at later time points. Wang et al.44 showed an increase in TNFα in 2-month STZ diabetic mice, but in that study, the strain was not specified and the difference could have been strain related. This early effect of TNFα could generate a cascade of events that would result in increased vasopermeability and apoptosis at a much later time point. An increase in TNFα expression has been reported in diabetic rats,23,24 but only at 16 or fewer days' duration of diabetes and not at later time points, which is consistent with our findings. It is quite possible that TNFα expression in diabetic rats is also not increased at 3 and 6 months. It is not known what indirect or downstream events are responsible for TNFα-mediated BRB breakdown and apoptosis at later time points in diabetic mice, but it may be related to factors released by inflammatory cells recruited by the proinflammatory activity of TNFα; regulation of TNFα receptors, which could be enhancing or inhibitory45; or other unknown events. The fact that TNFα-dependent events occur later in the course of DR may be more relevant to the clinic, but the results suggest that TNFα may need to be targeted early in the course of the disease to be effective.
Retinal leukostasis occurs at the early stage and is a causative pathologic factor for vascular cell apoptosis/death and BRB breakdown in DR.9,11,12 Our study demonstrated that TNFα-mediated retinal leukostasis and apoptosis are associated with progressive BRB breakdown in both chemically induced and genetically mutant animal models of diabetes, but TNFα-mediated retinal leukostasis is not necessary for BRB breakdown in the early stages of DR. We hypothesized a possible pathway, in which TNFα-mediated retinal leukostasis and apoptosis could promote BRB breakdown in DR (Fig. 8). Occurrence of retinal leukostasis is dependent on adhesion molecules such as ICAM-1, and the integrity of the BRB is maintained by tight junctional proteins such as occludin and ZO-1,46–49 as well as by an absence of transendothelial vesicular transport.50–55 Whether TNFα regulates these executive molecules was not determined in this study, but it would be interesting to investigate, because the elucidation of the molecular mechanism by which TNFα regulates retinal leukostasis and BRB breakdown is of significance in designing therapeutic strategies to treat DME, which is the leading cause of vision loss in developed countries, but effective treatments are very limited. It is possible that TNFα does not act directly on the retinal vascular endothelium or indirectly through an induction of VEGF, but through its proinflammatory activity and by factors released by the recruited inflammatory cells. Other mechanisms must be operative, however, at the early stages of DR.
Diabetes-induced TNFα upregulation may promote loss of retinal microvascular cells associated with early pathogenesis of DR, and a TNFα inhibitor may block retinal vascular cell loss in diabetes.56 A potential mechanism by which TNFα may induce apoptosis in retinal vascular cells in DR may be through the recently reported diabetes-associated enhancement of FOXO1 DNA binding activity and nuclear translocation in diabetic retinas through a process that is mediated by TNFα. Inhibition of FOXO1 activation reduces microvascular cell apoptosis and microvascular cell loss in diabetic retinas.57
Finally, TNFα is a multifunctional cytokine that transmits signaling via two receptors, tumor necrosis factor receptor-1 and -2.58,59 In addition to the pathogenesis of DR, these molecules are implicated in the pathogenesis of other inflammatory disorders such as rheumatoid arthritis (RA)60 and mediate cell death or apoptosis in neuronal degenerative disorders such as Alzheimer's disease (AD).61 TNFα inhibitors are widely used to treat RA,62,63 and they are being assessed for the treatment of AD.64,65 The data obtained from the present study and those of other investigators16,19,23,66 provide evidence that the TNFα signaling pathway is a promising target for suppressing retinal complications of diabetes and its inhibitors have potential for use as agents to treat diabetic patients.
Footnotes
Supported by National Institutes of Health Grants EY017164 (SAV) and EY18138 (EJD) from the National Eye Institute and an unrestricted grant from Research to Prevent Blindness. EJD is a recipient of an RPB Career Development Award.
Disclosure: H. Huang, None; J.K. Gandhi, None; X. Zhong, None; Y. Wei, None; J. Gong, None; E.J. Duh, None; S.A. Vinores, None
References
- 1. Cunha-Vaz JG. The blood-retinal barriers. Doc Ophthalmol. 1976;41:287–327 [DOI] [PubMed] [Google Scholar]
- 2. Cunha-Vaz JG. Blood-retinal barriers in health and disease. Trans Ophthalmol Soc U K. 1980;100:337–340 [PubMed] [Google Scholar]
- 3. Vinores SA, Kuchle M, Derevjanik NL, et al. Blood-retinal barrier breakdown in retinitis pigmentosa: light and electron microscopic immunolocalization. Histol Histopathol. 1995;10:913–923 [PubMed] [Google Scholar]
- 4. Vinores SA, Derevjanik NL, Ozaki H, et al. Cellular mechanisms of blood-retinal barrier dysfunction in macular edema. Doc Ophthalmol. 1999;97:217–228 [DOI] [PubMed] [Google Scholar]
- 5. Barber AJ, Antonetti DA, Gardner TW. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. The Penn State Retina Research Group. Invest Ophthalmol Vis Sci. 2000;41:3561–3568 [PubMed] [Google Scholar]
- 6. Saishin Y, Takahashi K, Melia M, et al. Inhibition of protein kinase C decreases prostaglandin-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195:210–219 [DOI] [PubMed] [Google Scholar]
- 7. Vinores SA, Derevjanik NL, Vinores MA, et al. Sensitivity of different vascular beds in the eye to neovascularization and blood-retinal barrier breakdown in VEGF transgenic mice. Adv Exp Med Biol. 2000;476:129–138 [DOI] [PubMed] [Google Scholar]
- 8. Vinores SA, Xiao WH, Aslam S, et al. Implication of the hypoxia response element of the Vegf promoter in mouse models of retinal and choroidal neovascularization, but not retinal vascular development. J Cell Physiol. 2006;206:749–758 [DOI] [PubMed] [Google Scholar]
- 9. Miyamoto K, Khosrof S, Bursell SE, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96:10836–10841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joussen AM, Murata T, Tsujikawa A, et al. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158:147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adamis AP, Berman AJ. Immunological mechanisms in the pathogenesis of diabetic retinopathy. Semin Immunopathol. 2008;30:65–84 [DOI] [PubMed] [Google Scholar]
- 12. Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452 [DOI] [PubMed] [Google Scholar]
- 13. Chibber R, Ben-Mahmud BM, Chibber S, Kohner EM. Leukocytes in diabetic retinopathy. Curr Diabetes Rev. 2007;3:3–14 [DOI] [PubMed] [Google Scholar]
- 14. Patel N. Targeting leukostasis for the treatment of early diabetic retinopathy. Cardiovasc Hematol Disord Drug Targets. 2009;9:222–229 [DOI] [PubMed] [Google Scholar]
- 15. Lu M, Perez VL, Ma N, et al. VEGF increases retinal vascular ICAM-1 expression in vivo. Invest Ophthalmol Vis Sci. 1999;40:1808–1812 [PubMed] [Google Scholar]
- 16. Joussen AM, Poulaki V, Qin W, et al. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol. 2002;160:501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clauss M, Sunderkotter C, Sveinbjornsson B, et al. A permissive role for tumor necrosis factor in vascular endothelial growth factor-induced vascular permeability. Blood. 2001;97:1321–1329 [DOI] [PubMed] [Google Scholar]
- 18. Vinores SA, Xiao WH, Shen J, Campochiaro PA. TNF-alpha is critical for ischemia-induced leukostasis, but not retinal neovascularization nor VEGF-induced leakage. J Neuroimmunol. 2007;182:73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joussen AM, Doehmen S, Le ML, et al. TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vis. 2009;15:1418–1428 [PMC free article] [PubMed] [Google Scholar]
- 20. Leal EC, Manivannan A, Hosoya K, et al. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2007;48:5257–5265 [DOI] [PubMed] [Google Scholar]
- 21. Kim JH, Yu YS, Cho CS, Kim KW. Blockade of angiotensin II attenuates VEGF-mediated blood-retinal barrier breakdown in diabetic retinopathy. J Cereb Blood Flow Metab. 2009;29:621–628 [DOI] [PubMed] [Google Scholar]
- 22. Wilkinson-Berka JL. Diabetes and retinal vascular disorders: role of the renin-angiotensin system. Expert Rev Mol Med. 2004;6:1–18 [DOI] [PubMed] [Google Scholar]
- 23. Joussen AM, Poulaki V, Mitsiades N, et al. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002b;16:438–440 [DOI] [PubMed] [Google Scholar]
- 24. Zhang SX, Wang JJ, Gao G, et al. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006;20:323–325 [DOI] [PubMed] [Google Scholar]
- 25. Franks WA, Limb GA, Stanford MR, et al. Cytokines in human intraocular inflammation. Curr Eye Res. 1992;11(suppl):187–191 [DOI] [PubMed] [Google Scholar]
- 26. Limb GA, Chignell AH, Green W, et al. Distribution of TNF alpha and its reactive vascular adhesion molecules in fibrovascular membranes of proliferative diabetic retinopathy. Br J Ophthalmol. 1996;80:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Limb GA, Webster L, Soomro H, et al. Platelet expression of tumour necrosis factor-alpha (TNF-alpha), TNF receptors and intercellular adhesion molecule-1 (ICAM-1) in patients with proliferative diabetic retinopathy. Clin Exp Immunol. 1999;118:213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spranger J, Meyer-Schwickerath R, Klein M, et al. TNF-alpha level in the vitreous body: increase in neovascular eye diseases and proliferative diabetic retinopathy (in German). Med Klin (Munich) 1995;90:134–137 [PubMed] [Google Scholar]
- 29. Lechleitner M, Koch T, Herold M, et al. Tumour necrosis factor-alpha plasma level in patients with type 1 diabetes mellitus and its association with glycaemic control and cardiovascular risk factors. J Intern Med. 2000;248:67–76 [DOI] [PubMed] [Google Scholar]
- 30. Yuuki T, Kanda T, Kimura Y, et al. Inflammatory cytokines in vitreous fluid and serum of patients with diabetic vitreoretinopathy. J Diabetes Complications. 2001;15:257–259 [DOI] [PubMed] [Google Scholar]
- 31. Barber AJ, Antonetti DA, Kern TS, et al. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–2218 [DOI] [PubMed] [Google Scholar]
- 32. Phelan SA, Ito M, Loeken MR. Neural tube defects in embryos of diabetic mice: role of the Pax-3 gene and apoptosis. Diabetes. 1997;46:1189–1197 [DOI] [PubMed] [Google Scholar]
- 33. Derevjanik NL, Vinores SA, Xiao WH, et al. Quantitative assessment of the integrity of the blood–retinal barrier in mice. Invest Ophthalmol Vis Sci. 2002;43:2462–2467 [PubMed] [Google Scholar]
- 34. Tobe T, Okamoto N, Vinores MA, et al. Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Invest Ophthalmol Vis Sci. 1998;39:180–188 [PubMed] [Google Scholar]
- 35. Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer-Verlag, Inc.; 2000 [Google Scholar]
- 36. Kern TS, Du Y, Miller CM, et al. Overexpression of Bcl-2 in vascular endothelium inhibits the microvascular lesions of diabetic retinopathy. Am J Pathol. 2010;176:2550–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kociok N, Radetzky S, Krohne TU, et al. Pathological but not physiological retinal neovascularization is altered in TNF-Rp55-receptor-deficient mice. Invest Ophthalmol Vis Sci. 2006;47:5057–5065 [DOI] [PubMed] [Google Scholar]
- 38. Ehrlich R, Harris A, Ciulla TA, et al. Diabetic macular oedema: physical, physiological and molecular factors contribute to this pathological process. Acta Ophthalmol. 2010;88:279–291 [DOI] [PubMed] [Google Scholar]
- 39. Duh E, Aiello LP. Vascular endothelial growth factor and diabetes: the agonist versus antagonist paradox. Diabetes. 1999;48:1899–1906 [DOI] [PubMed] [Google Scholar]
- 40. Tanaka Y, Katoh S, Hori S, et al. Vascular endothelial growth factor in diabetic retinopathy. Lancet. 1997;349:1520. [DOI] [PubMed] [Google Scholar]
- 41. de Gooyer TE, Stevenson KA, Humphries P, et al. Rod photoreceptor loss in Rho−/− mice reduces retinal hypoxia and hypoxia-regulated gene expression. Invest Ophthalmol Vis Sci. 2006;47:5553–5560 [DOI] [PubMed] [Google Scholar]
- 42. Gustavsson M, Wilson MA, Mallard C, et al. Global gene expression in the developing rat brain after hypoxic preconditioning: involvement of apoptotic mechanisms? Pediatr Res. 2007;61:444–450 [DOI] [PubMed] [Google Scholar]
- 43. Mysliwiec M, Balcerska A, Zorena K, et al. The role of vascular endothelial growth factor, tumor necrosis factor alpha and interleukin-6 in pathogenesis of diabetic retinopathy. Diabetes Res Clin Pract. 2008;79:141–146 [DOI] [PubMed] [Google Scholar]
- 44. Wang J, Xu X, Elliott MH, Zhu M, Le Y-Z. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jasielska M, Semkova I, Shi X, et al. Differential role of tumor necrosis factor (TNF)-α receptors in the development of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2010;51:3874–3883 [DOI] [PubMed] [Google Scholar]
- 46. Vinores SA, Sen H, Campochiaro PA. An adenosine agonist and prostaglandin E1 cause breakdown of the blood–retinal barrier by opening tight junctions between vascular endothelial cells. Invest Ophthalmol Vis Sci. 1992;33:1870–1878 [PubMed] [Google Scholar]
- 47. Antonetti DA, Barber AJ, Khin S, et al. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes. 1998;47:1953–1959 [DOI] [PubMed] [Google Scholar]
- 48. Antonetti DA, Barber AJ, Hollinger LA, et al. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1: a potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274:23463–23467 [DOI] [PubMed] [Google Scholar]
- 49. Barber AJ, Antonetti DA. Mapping the blood vessels with paracellular permeability in the retinas of diabetic rats. Invest Ophthalmol Vis Sci. 2003;44:5410–5416 [DOI] [PubMed] [Google Scholar]
- 50. Vinores SA, Van Niel E, Swerdloff JL, Campochiaro PA. Electron microscopic immunocytochemical evidence for the mechanism of blood-retinal barrier breakdown in galactosemic rats and its association with aldose reductase expression and inhibition. Exp Eye Res. 1993;57:723–735 [DOI] [PubMed] [Google Scholar]
- 51. Vinores SA, Van Niel E, Swerdloff JL, Campochiaro PA. Electron microscopic immunocytochemical demonstration of blood-retinal barrier breakdown in human diabetics and its association with aldose reductase in retinal vascular endothelium and retinal pigment epithelium. Histochem J. 1993b;25:648–663 [DOI] [PubMed] [Google Scholar]
- 52. Vinores SA, Derevjanik NL, Mahlow J, et al. Electron microscopic evidence for the mechanism of blood-retinal barrier breakdown in diabetic rabbits: comparison with magnetic resonance imaging. Pathol Res Pract. 1998;194:497–505 [DOI] [PubMed] [Google Scholar]
- 53. Luna JD, Chan CC, Derevjanik NL, et al. Blood-retinal barrier (BRB) breakdown in experimental autoimmune uveoretinitis: comparison with vascular endothelial growth factor, tumor necrosis factor alpha, and interleukin-1beta-mediated breakdown. J Neurosci Res. 1997;49:268–280 [DOI] [PubMed] [Google Scholar]
- 54. Hofman P, Blaauwgeers HG, Tolentino MJ, et al. VEGF-A induced hyperpermeability of blood-retinal barrier endothelium in vivo is predominantly associated with pinocytotic vesicular transport and not with formation of fenestrations: vascular endothelial growth factor-A. Curr Eye Res. 2000;21:637–645 [PubMed] [Google Scholar]
- 55. Klaassen I, Hughes JM, Vogels IM, et al. Altered expression of genes related to blood-retina barrier disruption in streptozotocin-induced diabetes. Exp Eye Res. 2009;89:4–15 [DOI] [PubMed] [Google Scholar]
- 56. Behl Y, Krothapalli P, Desta T, et al. Diabetes-enhanced tumor necrosis factor-α production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Amer J Pathol. 2008;172:1411–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Behl Y, Krothapalli P, Desta T, et al. FOXO1 plays an important role in enhanced microvascular cell apoptosis and microvascular cell loss in type 1 and type 2 diabetic rats. Diabetes. 2009;58:917–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65 [DOI] [PubMed] [Google Scholar]
- 59. Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635 [DOI] [PubMed] [Google Scholar]
- 60. Charles P, Elliott MJ, Davis D, et al. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J Immunol. 1999;163:1521–1528 [PubMed] [Google Scholar]
- 61. Perry RT, Collins JS, Wiener H, et al. The role of TNF and its receptors in Alzheimer's disease. Neurobiol Aging. 2001;22:873–883 [DOI] [PubMed] [Google Scholar]
- 62. Bradley JR. TN. F-mediated inflammatory disease. J Pathol. 2008;214:149–160 [DOI] [PubMed] [Google Scholar]
- 63. Sekut L, Connolly K. AntiTNF-alpha agents in the treatment of inflammation. Expert Opin Investig Drugs. 1998;7:1825–1839 [DOI] [PubMed] [Google Scholar]
- 64. Tobinick E, Gross H, Weinberger A, Cohen H. TNF-alpha modulation for treatment of Alzheimer's disease: a 6-month pilot study. Med Gen Med. 2006;8:25. [PMC free article] [PubMed] [Google Scholar]
- 65. Tobinick E. Tumour necrosis factor modulation for treatment of Alzheimer's disease: rationale and current evidence. CNS Drugs. 2009;23:713–725 [DOI] [PubMed] [Google Scholar]
- 66. Mohamed Q, Wong TY. Emerging drugs for diabetic retinopathy. Expert Opin Emerg Drugs. 2008;13:675–694 [DOI] [PubMed] [Google Scholar]