Abstract
The efficacy of BB-83698, a novel potent peptide deformylase inhibitor, was evaluated in a mouse model of acute pneumonia. The Streptococcus pneumoniae isolates tested included four virulent strains (one penicillin-susceptible wild-type strain, one macrolide-resistant strain, and two quinolone-resistant mutants [a mutant carrying mutations in ParC and GyrA and an efflux mutant] isogenic to the wild type) and two poorly virulent penicillin-resistant strains. Pneumonia was induced by intratracheal inoculation of 105 CFU (virulent strains) into immunocompetent mice or 107 CFU (less virulent strains) into leukopenic mice. Animals received three or six subcutaneous injections of antibiotics at 12- or 24-h intervals, with antibiotic treatment initiated at 3, 6, 12, or 18 h postinfection (p.i.). BB-83698 showed potent in vitro activity against all strains (MICs, 0.06 to 0.25 μg/ml). In the in vivo model, all control animals died within 2 to 5 days of infection. BB-83698 (80 mg/kg of body weight twice daily or 160 mg/kg once daily) protected 70 to 100% of the animals, as measured 10 days p.i., regardless of the preexisting resistance mechanisms. In contrast, the survival rates for animals treated with the comparator antibiotics were 30% for animals treated with erythromycin (100 mg/kg) and infected with the macrolide-resistant strain, 34% for animals treated with amoxicillin (200 mg/kg every 8 h) and infected with the penicillin-resistant strain, and 0 and 78% for animals treated with ciprofloxacin (250 mg/kg) and infected with the ParC and GyrA mutant and the efflux mutant, respectively. At 80 mg/kg, BB-83698 generated a peak concentration in lung tissue of 61.9 μg/ml within 1 h and areas under the concentration-times curves of 57.4 and 229.4 μg · h/ml for plasma and lung tissue, respectively. The emergence of S. pneumoniae isolates with reduced susceptibilities to BB-83698 was not observed following treatment with a suboptimal dosing regimen. In conclusion, the potent in vitro activity of BB-83698 against S. pneumoniae, including resistant strains, translates into good in vivo efficacy in a mouse pneumonia model.
Streptococcus pneumoniae remains the leading cause of community-acquired pneumonia and continues to be a significant cause of mortality. The prevalence of drug-resistant S. pneumoniae infections continues to rise at an alarming rate (2, 3). Despite this, the trend in the pharmaceutical industry remains the production of more potent variants of conventional antibiotics to which organisms have numerous preexisting resistance mechanisms, which is a drawback of the variants.
BB-83698 was identified from the initial lead compound BB-3497 following a structure-based design program (9) and represents the first of a potential new class of antibiotics for the treatment of serious respiratory tract infections. Its mechanism of action is through inhibition of the essential bacterial enzyme peptide deformylase (PDF), which deformylates the N-formylmethionine of newly synthesized proteins.
All ribosome-mediated synthesis of proteins starts with a methionine residue. In eubacteria and the organelles of eukaryotes, the amino group of the methionyl moiety carried by the initiator tRNAfMet is N-formylated by formyltransferase prior to its incorporation into a polypeptide. This is required for efficient initiation of protein synthesis. Consequently, N-formylmethionine is always present at the N terminus of a nascent bacterial polypeptide. However, most mature bacterial proteins do not retain the N-formyl group or the terminal methionine residue. Following translation the formyl group is hydrolyzed by PDF, which is a prerequisite for further processing at the N terminus by methionine aminopeptidase. Deformylation is therefore a crucial step in bacterial protein biosynthesis, and PDF is essential for bacterial growth (11). The novel mode of action of BB-83698 confers potent activity against a range of pathogens (14) and offers to significantly strengthen the antibiotic arsenal available to combat drug-resistant S. pneumoniae.
We compared the activity of BB-83698 with those of macrolides (azithromycin and erythromycin), a fluoroquinolone (ciprofloxacin), and a beta-lactam (amoxicillin) in a mouse model of acute experimental pneumonia. The pharmacokinetic, bacterial clearance, and 10-day survival data were used to evaluate the in vivo efficacy of BB-83698. The propensity for the emergence of strains with reduced susceptibility to BB-83698 was also examined by using a suboptimal dosing regimen.
(This work was presented in part at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., December 2001 [E. Azoulay-Dupuis, J. Mohler, V. Rieux, J. P. Bédos, P. Moine, and C. Carbon, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-354, 2001] and the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., September 2002 [E. Azoulay-Dupuis, J. Mohler, and J. P. Bédos, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-707, 2002].)
MATERIALS AND METHODS
Animals.
Female Swiss mice (body weight, 20 to 22 g) were obtained from Iffa-Credo Laboratories.
Challenge organisms.
Pneumococcal pneumonia was induced in immunocompetent mice with four virulent strains. Two of the virulent strains, a serotype 3 strain (P-4241; penicillin MIC, 0.032 μg/ml) and a macrolide-resistant serotype 1 strain (P-6254; erm with constitutive macrolide-lincosamide-streptogramin B resistance; erythromycin MIC, 1,024 μg/ml), were originally isolated from blood cultures and were provided by the Centre de Référence du Pneumocoque (P. Geslin, Créteil, France). The other two virulent strains were isogenic mutants of strain P-4241 selected for quinolone resistance. One isogenic mutant, C42-Sp6 (ParC and GyrA), contained a mutation in both ParC and GyrA and was obtained after subsequent exposure of strain P-4241 to ciprofloxacin and sparfloxacin. Norfloxacin at or slightly above the MIC was used to preferentially select the other isogenic mutant, N42-6R1, an efflux pump mutant.
All strains belonging to serotypes 6, 9, 14, 19, and 23 are naturally less virulent in Swiss mice, independent of their site of isolation in humans (6, 7, 8). Thus, leukopenia was first induced in mice infected with these strains. Leukopenic mice were infected with two poorly virulent penicillin-resistant strains provided by the Laboratoire de Microbiologie, Hôpital Bichat, Paris, France: serotype 23 strain P-12698 (penicillin MIC, 4 μg/ml), which was isolated from a tracheal aspirate, and serotype 19 strain P-15986 (penicillin MIC, 8 μg/ml), which was erythromycin resistant (erythromycin MIC, >256 μg/ml) and which was isolated from middle ear fluid. Both P-12698 and P-15986 are tolerant to penicillin, as they are lysed only by about 1 log10 CFU at 50 times the relevant MIC. Moreover, P-15986 is a nonautolytic strain (5).
In vitro studies.
MICs and minimal bactericidal concentrations (MBCs) were determined by the tube dilution method (13) in Mueller-Hinton infusion broth (Diagnostic Pasteur, Marnes-la-Coquette, France) supplemented with 5% sheep blood. The tubes contained twofold dilutions of antibiotics and a final bacterial density of 105 CFU/ml. The tubes were incubated for 18 h at 37°C in 10% CO2-air. The MIC was defined as the lowest concentration of antibiotic at which no turbidity was visible to the naked eye. For MBC determinations, 0.01-ml aliquots from tubes with no visible growth were plated onto Columbia agar with 5% sheep blood (BioMérieux, Lyon, France) and incubated overnight at 37°C in 10% CO2-air. The MBC was defined as the lowest concentration of antibiotic that killed 99.9% of the original inoculum.
Leukocyte depletion in mice.
Sustained leukopenia was induced in Swiss mice by three daily intraperitoneal injections (150 mg/kg of body weight) of cyclophosphamide (Endoxan; Sarget Laboratories, Mérignac, France) starting 4 days before infection. The circulating leukocyte counts were reduced from 7.362 ± 2.949 to 1.331 ± 0.014/mm3 of blood on the day of infection. The average leukocyte count reached 1.009 ± 0.233/mm3 of blood 1 day after infection and then increased progressively to 4.688 ± 0.263/mm3 of blood 3 days after infection and 7.122 ± 3.906/mm3 of blood 5 days after infection.
Experimental pneumococcal pneumonia.
Pneumococcal pneumonia was induced in mice as described in detail elsewhere (4). Briefly, animals were anesthetized by intraperitoneal injection of sodium pentobarbital and were then infected by intratracheal inoculation with approximately 105 or 107 CFU of log-phase virulent or less virulent strains, respectively. Under these conditions, mice develop acute pneumonia and quickly become bacteremic. Death occurs after 2 to 5 days when the bacterial population exceeds 108 CFU/lung.
Antibiotics.
The study drugs included BB-83698 (British Biotech Pharmaceuticals, Oxford, England); a fluoroquinolone, ciprofloxacin (Ciflox, Bayer Laboratories, Sens, France); two macrolides, azithromycin (Pfizer Laboratories, Groton, Conn.) and erythromycin lactobionate (Abbott Laboratories, North Chicago, Ill.); and a β-lactam, amoxicillin sodium salt (Beecham Laboratories, Paris, France). Each antibiotic was made up as directed on the package insert and diluted to the desired concentration in sterile water.
Survival studies.
Initiation of therapeutic treatments varied according to the immunocompetence of the host and the virulence of the strains. When immunocompetent mice were infected with virulent susceptible strain P-4241, the mice were found to be bacteremic at 8 h postinfection and treatment was initiated at 12 or 18 h postinfection. Pneumonia was found to be more acute with macrolide-resistant strain P-6254. In this case treatment began at 6 h postinfection. Leukopenic mice infected with resistant strains became bacteremic after only 1 h and were thus treated at 3 h postinfection. In all cases, efficacy was evaluated after the infections were allowed to become established and prior to the initiation of therapy. Thus, treatments were initiated 3, 6, 12, or 18 h after bacterial challenge and were given in three, six, or nine subcutaneous (s.c.) injections at 8-, 12-, or 24-h intervals. The treatment regimens are presented with the results. Thirteen to 15 animals were used per treatment group, and all of the animals in the same experiment were infected simultaneously. The experiments were repeated at least twice. When the results were similar, no further experiments were performed. Only the results of one representative experiment are given. The observation period was 10 days. Death rates were recorded daily, and cumulative survival rates were compared.
Bactericidal activity in vivo.
The study drugs were assessed for their abilities to eradicate bacteria from the lungs. s.c. injections of BB-83698 at 40 or 80 mg/kg were given 18 h after infection with strain P-4241. At 6 h after the first treatment and 12 h after the second, fourth, and sixth treatments, the mice were killed by intraperitoneal injection of sodium pentobarbital and were exsanguinated by cardiac puncture; the blood was then cultured. The lungs were removed and homogenized in 1 ml of saline. The total numbers of CFU recovered from whole-lung homogenates were determined from serial 10-fold dilutions plated onto Columbia agar. The results are expressed as the mean number (log10) of CFU per lung for groups of three mice.
Pharmacokinetic studies.
The pharmacokinetic profile of BB-83698 in immunocompetent mice infected with P-4241 was examined. The concentrations in lung tissue and serum were determined after administration of a single s.c. dose of 80 mg/kg at 12 h postinfection. Serum samples and lungs were collected from groups of six mice each at 0.25, 0.5, 1, 2, 3, 4, 6, 8, and 10 h postdosing. Serum plasma (50 μl) was diluted with 450 μl of double-distilled H2O (ddH2O). The lungs were weighed prior to homogenization in 9 volumes of ddH2O with an Ultra-Turrax T25 homogenizer. BB-3497 (0.25 μg) (9) was added to each 500 μl of diluted serum or lung homogenate as an internal standard, and the compounds were extracted with 2 ml of ethyl acetate. The samples were then dried under nitrogen at 40°C and reconstituted with 50 μl of methanol. ddH2O (150 μl) was added, and the concentration of BB-83698 was determined by reverse-phase high-performance liquid chromatography with tandem mass spectrometric detection (Micromass Quattro Ultima). Chromatographic separation was accomplished on a Hichrom RPB column (50 by 2.1 mm) at 45°C with a methanol-water mobile phase containing 0.1% formic acid. The sample was eluted at 0.25 ml/min by using a short gradient, with a total run time of 6 min. BB-83698 levels remained within linearity, as determined by using a dilution series containing known quantities of BB-83698.
Resistance studies.
Immunocompetent mice were infected by intratracheal inoculation of 105 CFU of strain P-4241 into the mice as described above. Two groups of 12 mice each were injected s.c. every 12 h for 3 days, and treatment with BB-83698 at 20 or 40 mg/kg was initiated at 18 h postinfection. These conditions were sufficient to cure approximately 50% of the mice treated with 40 mg/kg and 25% of the mice treated with 20 mg/kg. After establishment of the infection, potentially resistant organisms were isolated from the animal's blood by spreading the blood on Columbia agar plates containing 5% sheep blood. Individual colonies were selected, and their antibiotic susceptibility profiles were determined. Similar dosing regimens have previously been shown to allow recovery of strains with decreased susceptibilities to quinolones when the infecting organism carried a first-step quinolone mutation (E. Azoulay-Dupuis, T. Köhler, R. Isturiz, J. P. Bédos, D. Théron, C. Cherbuliez, P. Moine, V. Rieux, J. Mohler, J. C. Péchère, and C. Carbon, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-1015, 2000).
RESULTS
In vitro data.
BB-83698 demonstrated potent in vitro activity against all of the strains, regardless of preexisting resistance mechanisms (MICs, 0.06 to 0.25 μg/ml) (Table 1).
TABLE 1.
In vitro activities of BB-83698 and comparator agents against susceptible and resistant S. pneumoniae strains
| Resistance mechanism or phenotype | Strain | BB-83698 MIC/ MBC (μg/ml) | MIC (μg/ml)a
|
||
|---|---|---|---|---|---|
| ERY | CIP | PEN | |||
| Wild type | P-4241 | 0.06/0.125 | 0.06 | 1 | 0.032 |
| Macrolide resistant | P-6254 | 0.125/0.125 | 1,024 | 0.016 | |
| ParC and GyrA | C42-Sp6 | 0.125/0.125 | 32 | ||
| Quinolone efflux | N42-6R1 | 0.125/0.25 | 4 | ||
| Penicillin resistant | P-12698 | 0.125/0.25 | 0.06 | 4 | |
| Multiresistant | P-15986 | 0.125/0.125 | >256 | 8 | |
ERY, erythromycin; CIP, ciprofloxacin; PEN, penicillin.
Therapeutic efficacy in experimental pneumonia.
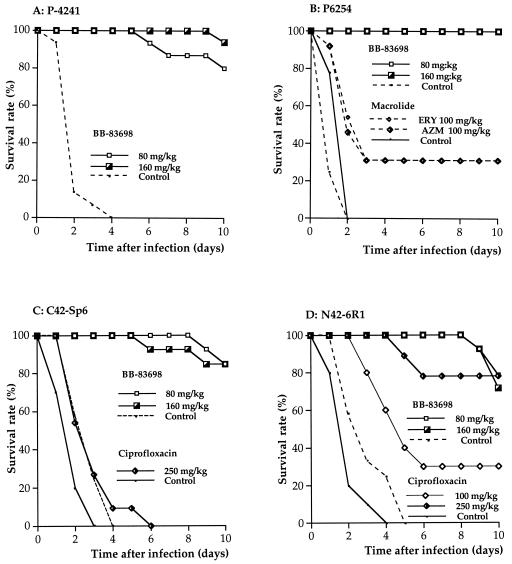
Pneumonia was induced in immunocompetent mice by intratracheal inoculation of 105 CFU of the virulent strains. All untreated control mice infected with penicillin-susceptible strain P-4241 (penicillin MIC, 0.032 μg/ml) died within 2 to 5 days of infection. Animals given s.c. injections of BB-83698 at 80 mg/kg/12 h or 160 mg/kg/24 h at either 12 or 18 h postinfection had 10-day survival rates of 80 and 93%, respectively (Fig. 1A). Treatment with amoxicillin and ceftriaxone, each at 5 mg/kg, was associated with an 87% survival rate. A 100% survival rate was achieved by treatment with ceftriaxone at 10 mg/kg (12). Treatment with the quinolone ciprofloxacin at doses of 100 and 250 mg/kg resulted in survival rates of 50 and 77%, respectively. Newer quinolones were more effective than ciprofloxacin; sparfloxacin (4) and gemifloxacin (Azoulay-Dupuis et al., 40th ICAAC), each at a dose of 50 mg/kg, protected 67 and 100% of mice, respectively.
FIG. 1.
Efficacy of BB-83698 against antibiotic-susceptible and macrolide- and quinolone-resistant strains of S. pneumoniae in a pneumococcal pneumonia model. (A) Survival of mice challenged with wild-type S. pneumoniae strain P-4241 and treated by injection of BB-83698 at 80 mg/kg/12 h or 160 mg/kg/24 h starting 12 h after challenge for 3 days. Groups of 13 to 15 mice were used for each dosage. (B) Survival of mice challenged with macrolide-resistant S. pneumoniae strain P-6254 and treated by injection of BB-83698 at 80 mg/kg/12 h or 160 mg/kg/24 h or a macrolide (ERY, erythromycin; AZM, azithromycin) at 100 mg/kg/12 h starting 6 h after challenge for 3 days. Groups of 13 to 15 mice were used for each dosage. (C) Survival of mice challenged with quinolone-resistant S. pneumoniae strain C42-Sp6 (GyrA and ParC double mutant) treated by injection of BB-83698 at 80 mg/kg/12 h or 160 mg/kg/24 h starting 12 h after challenge or ciprofloxacin at 250 mg/kg/12 h starting 6 h after challenge for 3 days. Groups of 13 to 15 mice were used for each dosage. (D) Survival of mice challenged with quinolone-resistant S. pneumoniae strain N42-6R1 (efflux mutant) treated by injection of BB-83698 at 80 mg/kg/12 h or 160 mg/kg/24 h starting 12 h after challenge or ciprofloxacin at 250 mg/kg/12 h starting 6 h after challenge for 3 days. Groups of 13 to 15 mice were used for each dosage.
Mice infected with penicillin-susceptible and macrolide-resistant strain P-6254 (erythromycin MIC, 1,024 μg/ml) had 10-day survival rates of 100% when they were treated with BB-83698 at 80 mg/kg/12 h or 160 mg/kg/24 h starting at 6 h postinfection. Comparator mice treated with erythromycin or azithromycin at 100 mg/kg/12 h had 10-day survival rates of only 30% (Fig. 1B). Treatment with amoxicillin at 5 mg/kg protected 80% of mice.
Mice infected with quinolone-resistant strain C42-Sp6 (ParC and GyrA mutant; ciprofloxacin MIC, 32 μg/ml) failed to respond to treatment with ciprofloxacin from 6 h postinfection; even when the mice were treated with a very high dose (250 mg/kg/12 h), all mice died after 6 days. In tests with the new quinolones, trovafloxacin provided no protection (0% survival) even when it was used at 200 mg/kg, whereas 50% of animals survived after treatment with gemifloxacin at 75 mg/kg (Azoulay-Dupuis et al., 40th ICAAC). Administration of BB-83698 at both 80 mg/kg/12 h and 160 mg/kg/24 h starting at 12 h postinfection protected the mice and gave 10-day survival rates of 87% (Fig. 1C).
Mice infected with quinolone-resistant strain N42-6R1 (efflux mutant; ciprofloxacin MIC, 4 μg/ml) had a 10-day survival rate of 78% when they were treated with ciprofloxacin at 250 mg/kg/12 h starting at 6 h postinfection. Trovafloxacin and gemifloxacin provided 100% survival when they were administered at 50 mg/kg (Azoulay-Dupuis et al., 40th ICAAC). Dosing with BB-83698 at 80 mg/kg/12 h or 160 mg/kg/24 h starting at 12 h postinfection resulted in 10-day survival rates of 80 and 73%, respectively (Table 2; Fig. 1D).
TABLE 2.
Clearance of S. pneumoniae (P-4241) from lungs of infected mice treated with BB-83698a
| Time p.i. (h) | Time after treatment | Mortality rate (%) for controls | Mean S. pneumoniae titer (log10 CFU/ml of whole-lung homogenate [no. of animals with positive blood cultures/total no. of animals])
|
||
|---|---|---|---|---|---|
| Control | BB-83698
|
||||
| 40 mg/kg | 80 mg/kg | ||||
| 21 | 6 h after first treatment | 0 | 5.4 (3/3) | 3.8 (2/3) | 3.1 (0/3) |
| 40 | 12 h after second treatment | 50 | 7.8 (7/7)b | 2.2 (1/3) | <LD (0/3) |
| 64 | 12 h after fourth treatment | 88 | 7.9 (4/4)c | <LD (0/3) | <LD (0/3) |
| 88 | 12 h after sixth treatment | 100 | 8.0 (1/1)d | <LD (0/3) | <LD (0/3) |
Lungs from three mice were tested. Abbreviations: LD, limit of detection (1 log10 CFU/ml); p.i., postinfection.
Seven mice were taken at this time; four were dead and three were alive.
Four mice were taken at this time; three were dead and one was alive.
One dead mouse was taken at this time.
Leukopenic Swiss mice infected with 107 CFU of poorly virulent, penicillin-resistant strain P-15986 (penicillin MIC, 8 μg/ml) had a 10-day survival rate of only 34% when they were given amoxicillin at 200 mg/kg/8 h beginning at 3 h postinfection. Ciprofloxacin at 200 mg/kg and trovafloxacin at 50 mg/kg provided 100% survival. BB-83698 administered at either 80 mg/kg/12 h or 160 mg/kg/24 h protected the mice, and each dose gave a 10-day survival rate of 100%. Similar results were obtained with BB-83698 when mice were infected with penicillin-resistant strain P-12698.
Bactericidal activity in vivo.
Following the initial inoculation of 105 CFU of strain P-4241 into the tracheas of immunocompetent mice, the burden in the lungs was seen to rise steadily from 5.4 log10 CFU/ml of lung homogenate at 21 h postinfection to 7.8 log10 CFU/ml at 40 h postinfection. All control animals were bacteremic at 21 h postinfection and died within 88 h of infection when no antibiotic was administered. BB-83698 given over 3 days at 40 mg/kg/12 h or 80 mg/kg/12 h rapidly reduced the burdens in the lungs in a dose-dependent manner. Six hours after the first treatment with 40 or 80 mg/kg, the burdens in the lungs had dropped by 1.6 and 2.3 log units, respectively. BB-83698 reduced the burdens in the lungs to below detectable limits (1 log10 CFU/ml) by 12 h after the second treatment with 80 mg/kg and 12 h after treatment with the fourth 40-mg/kg dose. Similarly, blood was also completely cleared of bacteria by these times (Table 2).
Pharmacokinetics in sera and lungs.
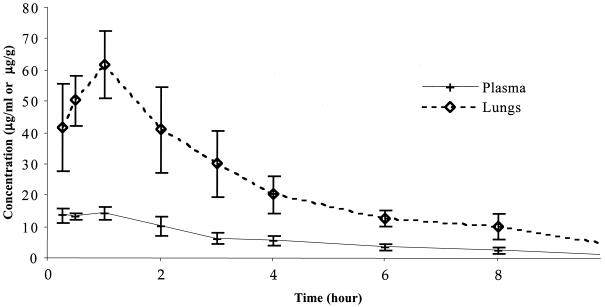
The pharmacokinetic parameter values for BB-83698 in the sera and lungs immunocompetent mice infected with S. pneumoniae P-4241 following injection of a single s.c. dose of 80 mg/kg are presented in Table 3 and Fig. 2. BB-83698 rapidly penetrated the lung tissue, reaching peak concentrations in lung tissue of 61.9 μg/ml within 1 h. This compares with concentrations in serum of 14.3 μg/ml at this time, giving a concentration in lung tissue/concentration in plasma ratio of 4:1. The areas under the concentration-time curves (AUCs) between 0 and 10 h were 57.4 and 229.4 μg · h/ml for plasma and lung tissue, respectively, giving an AUC for lung tissue/AUC for plasma ratio of 4. Moreover, at 10 h posttreatment levels as high as 0.9 μg/ml and 4.9 μg/g were still detectable in plasma and lung tissue, respectively.
TABLE 3.
Pharmacokinetic parameters of BB-83698 following injection of a single subcutaneous dose (80 mg/kg) to mice infected with S. pneumoniae (P-4241)a
| Compartment | Cmax (μg/ml or μg/g) | Cmax/MIC ratio | Tmax (h) | C10 (μg/ml or μg/g) | AUC0-10 (μg · h/ml) | AUC/MIC ratio | t1/2 (h) |
|---|---|---|---|---|---|---|---|
| Serum | 14.3 | 238.3 | 1 | 0.9 | 57.4 | 956.7 | 2.5 |
| Lung tissue | 61.9 | 1,031.7 | 1 | 4.9 | 229.4 | 3,823.3 | 2.6 |
The values were calculated by using data for serum and lung tissue samples taken at 0.25, 0.5, 1, 2, 3, 4, 6, 8, and 10 h postdosing. Groups of six mice were used at each time point. The BB-83698 MIC for P-4241 is 0.06 μg/ml. Abbreviations: Cmax, maximum concentration observed in serum; Tmax, time to maximum concentration in serum; C10, concentration at 10 h; AUC0-10, AUC from time zero to 10 h; t1/2, half-life.
FIG. 2.
Mean concentration-time profiles for BB-83698 in serum and lung tissue following injection of a single s.c. dose (80 mg/kg) to mice infected with S. pneumoniae (P-4241). Groups of six mice were used at each time point.
Resistance studies.
Infected mice were given six suboptimal doses of BB-83698 (20 or 40 mg/kg). On reestablishment of the infection, the potentially resistant organisms were isolated from the animals' blood by spreading the blood on Columbia agar plates containing 5% sheep blood. Individual colonies were selected, and their susceptibility profiles were determined. No changes in susceptibility were observed for any of the 14 isolates recovered from 24 mice treated with the PDF inhibitor BB-83698 at 20 mg/kg (6 mice) and 40 mg/kg (8 mice).
DISCUSSION
PDF represents one of the most promising new targets in the development of novel antimicrobial chemotherapies. PDF is an essential bacterial metalloenzyme that deformylates the N-formylmethionine of newly synthesized bacterial polypeptides. Recent progress in understanding the structure and function of PDF has greatly facilitated the drug discovery process. BB-83698 is one of the most advanced compounds reported, and the in vitro and in vivo efficacies of BB-83698 against susceptible and drug-resistant S. pneumoniae strains were examined in a mouse lung infection model.
BB-83698 showed good in vitro activities against the S. pneumoniae strains investigated, irrespective of their resistance profiles. This confirmed the results of Wise et al. (14) and Clements et al. (9), who showed that a series of PDF inhibitors, including BB-83698, had good in vitro activities against the major gram-positive respiratory pathogens, including quinolone- and penicillin-resistant pneumococci and methicillin-resistant Staphylococcus aureus.
In vivo, the survival rates of animals treated with BB-83698 (80 mg/kg/12 h or 160 mg/kg/24 h) were 70 to 100% at 10 days postinfection, regardless of the preexisting resistance mechanisms of the infecting strains. The activity of BB-83698 compared well with those of existing therapeutic agents against penicillin-susceptible strains. A β-lactam such as amoxicillin given at 5 mg/kg twice a day for 3 days protected animals against pneumonia. Macrolides such as azithromycin were effective at 50 mg/kg. Newer fluoroquinolones (sparfloxacin, trovafloxcin, and gemifloxacin) (4; Azoulay-Dupuis et al., 40th ICAAC) also eradicate pneumococci at a dosage of 50 mg/kg. However, none of the fluoroquinolones studied (trovafloxacin, gemifloxacin) (Azoulay-Dupuis et al., 40th ICAAC) was effective against clinical isolates carrying a double mutation (in ParC and GyrA) or a triple mutation (in ParC, GyrA, and ParE). The corresponding survival rates were 30% for mice infected with macrolide-resistant strains and treated with the comparator agents azithromycin and erythromycin (100 mg/kg/12 h). Amoxicillin (200 mg/kg/8 h) was poorly effective (survival rate, 34%) against a multiresistant (Penr Macr) strain, whereas a cephalosporin such as ceftriaxone at a dosage of 50 mg/kg had better efficacy than amoxicillin against a multiresistant strain (12). Although the expanded-spectrum cephalosporins, macrolides, and fluoroquinolones that have been developed have proven to be highly effective in combating pneumococcal infections, their widespread use by outpatients invariably compromises their long-term utility by promoting the emergence of resistance. BB-83698 remained effective regardless of any preexisting resistance mechanism.
BB-83698 was bactericidal in immunocompetent mice infected with penicillin-susceptible S. pneumoniae, reducing the burden in the lungs to below detectable limits (1 log10 CFU/ml) 12 h after treatment with the second 80-mg/kg dose and 12 h after treatment with the fourth 40-mg/kg dose. At those times the bacterial burdens in the blood of infected mice were reduced to below detectable limits. The rapid killing observed in vivo is promising for the potential therapeutic use of PDF inhibitors in the clinic.
Resistance to BB-83698 and other PDF inhibitors has been documented to occur in vitro at a low frequency (<10−8) in S. pneumoniae (10; A. Noel, K. E. Bowker, R. A. Howe, and A. P. MacGowan, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1680, 2002), and the rates of resistance are similar to those of the quinolone class of antibiotics. We used the lung infection model to determine the potential for the development of resistance to BB-83698 in vivo. By using a suboptimal 3-day dosing regimen designed to promote resistance development, no S. pneumoniae isolates with reduced susceptibility to BB-83698 were recovered. Similar quinolone dosing regimens with this model have previously allowed the recovery of strains with decreased susceptibilities to quinolones when the infecting organism carried a first-step quinolone mutation (Azoulay-Dupuis et al., 40th ICAAC). Therefore, resistance to BB-83698 in S. pneumoniae does not occur at a high frequency in the lung infection model and confirms the published in vitro data. BB-83698 has been examined for its potency against strains with some defB mutations (I. D. Johnson, A. Waller, and J. M. Clements, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother. abstr. F-1681, 2002). A spontaneous mutation frequency of <10−9 was observed in strains treated with BB-83698 at four and eight times the MIC. Although the mutants did not have the same mutations as the mutants generated by Margolis et al. (10), these mutants showed 2- to 8-fold decreased sensitivities (MICs, ≤1 μg/ml), whereas the mutants had 16- to 32-fold decreased sensitivities to actinonin and 64-fold decreased sensitivities to ciprofloxacin.
Animal models of infection are an invaluable tool with which to predict the effectiveness of an antibiotic in the clinical setting. Numerous studies have shown that the pharmacokinetic and pharmacodynamic indices predictive of efficacy are similar for drugs within the same class and for most pathogens and different sites of infection (1). The efficacy of BB-83698 in the pneumonia model is consistent with the pharmacokinetic and pharmacodynamic properties of BB-83698 determined in a neutropenic mouse thigh S. pneumoniae infection model (W. Craig and D. Andes, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-355, 2001). Those studies demonstrated that BB-83698 is rapidly bactericidal when free drug concentrations exceeded the MIC and had a prolonged postantibiotic effect (based on 80% protein binding in mouse plasma). The activity of the drug was shown to be concentration dependent, with the 24-h AUC/MIC being the pharmacodynamic parameter that best predicted in vivo activity. That parameter was found to be similar for the different strains of S. pneumoniae tested, including those resistant to penicillin and macrolides. Once- and twice-daily dosings were equally effective in the pneumonia model; and efficacy was not affected by resistance to penicillin, quinolones, or macrolides. We believe that the efficacy of BB-83698 in this stringent model of pneumococcal pneumonia is conferred by this compound's good pharmacokinetic and pharmacodynamic properties, which generate high AUCs for mouse serum and lung tissue (Table 3).
In conclusion, the efficacy of BB-83698 against sensitive and drug-resistant S. pneumoniae isolates in a mouse model of pneumonia presents an outstanding therapeutic opportunity. The progression of the first PDF inhibitor, BB-83698, into clinical evaluation in 2003 has allowed its safety and efficacy to be fully assessed and the true contribution of PDF inhibitors toward combating antimicrobial resistance to be realized.
REFERENCES
- 1.Andes, D., and W. Craig. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19:261-268. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum, P. C. 1992. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin. Infect. Dis. 15:77-83. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum, P. C. 1996. Emergence of resistance to antimicrobial agents in gram-positive bacteria. Pneumococci. Drugs 51(Suppl. 1):1-5. [DOI] [PubMed] [Google Scholar]
- 4.Azoulay-Dupuis, E., E. Vallée, B. Veber, J. P. Bédos, J. Bauchet, and J. J. Pocidalo. 1992. In vivo efficacy of a new fluoroquinolone, sparfloxacin, against penicillin-susceptible and -resistant and multiresistant strains of Streptococcus pneumoniae in a mouse model of pneumonia. Antimicrob. Agents Chemother. 36:2698-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azoulay-Dupuis, E., P. Moine, J. P. Bédos, V. Rieux, and E. Vallée. 1996. Amoxicillin dose-effect relationship with Streptococcus pneumoniae in a mouse pneumonia model and roles of in vitro penicillin susceptibilities, autolysis, and tolerance properties of the strains. Antimicrob. Agents Chemother. 40:941-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azoulay-Dupuis, E., V. Rieux, M. Muffat-Joly, J. P. Bédos, E. Vallée, C. Rivier, R. Isturiz, C. Carbon, and P. Moine. 2000. Relationship between capsular type, penicillin susceptibility, and virulence of human Streptococcus pneumoniae isolates in mice. Antimicrob. Agents Chemother. 44:1575-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bédos, J. P., O. Rollin, D. H. Bouanchaud, and J. J. Pocidalo. 1991. Relation entre virulence et résistance aux antibiotiques des pneumocoques. Pathol. Biol. 39:984-990. [PubMed] [Google Scholar]
- 8.Briles, D. E., M. J. Crain, B. M. Gray, C. Forman, and J. Yother. 1992. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect. Immun. 60:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clements, J. M., R. P. Beckett, A. Brown, G. Catlin, M. Lobell, S. Palan, W. Thomas, M. Whittaker, S. Wood, S. Salama, P. J. Baker, H. F. Rodgers, V. Barynin, D. W. Rice, and M. G. Hunter. 2001. Antibiotic activity and characterisation of BB-3497, a novel peptide deformylase inhibitor. Antimicrob. Agents Chemother. 45:563-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margolis, P., C. Hackbarth, S. Lopez, M. Maniar, W. Wang, Z. Yuan, R. White, and J. Trias. 2001. Resistance of Streptococcus pneumoniae to deformylase inhibitors is due to mutations in defB. Antimicrob. Agents Chemother. 45:2432-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazel, D., S. Pochet, and P. Marliere. 1994. Genetic characterisation of polypeptide deformylase, a distinctive enzyme of eubacterial translation. EMBO J. 13:914-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moine, P., E. Vallée, E. Azoulay-Dupuis, P. Bourget, J. P. Bédos, J. Bauchet, and J. J. Pocidalo. 1994. In vivo efficacy of a broad-spectrum cephalosporin, ceftriaxone, against penicillin-susceptible and -resistant strains of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob. Agents Chemother. 38:1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial disk susceptibility tests; approved standard, 7th ed. M2-A7, vol. 20, no. 1. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Wise, R., J. M. Andrews, and J. Ashby. 2002. In vitro activities of peptide deformylase inhibitors against gram-positive pathogens. Antimicrob. Agents Chemother. 46:1117-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]