Abstract
A number of C57BL/6 (B6) substrains are commonly used by scientists for basic biomedical research. One of several B6 strain specific background diseases is focal alopecia that may resolve or progress to severe, ulcerative dermatitis. Clinical and progressive histologic changes of skin disease commonly observed in C57BL/6J and preliminary studies in other closely related substrains are presented. Lesions develop due to a primary follicular dystrophy with rupture of severely affected follicles leading to formation of secondary foreign body granulomas (trichogranulomas) in affected B6 substrains of mice. Histologically these changes resemble the human disease called central centrifugal cicatrical alopecia (CCCA). Four B6 substrains tested have a polymorphism in alcohol dehydrogenase 4 (Adh4) that reduces its activity and potentially affects removal of excess retinol. Using immunohistochemistry, differential expression of epithelial retinol dehydrogenase (DHRS9) was detected which may partially explain anecdotal reports of frequency differences between B6 substrains. The combination of these two defects have the potential to make high dietary vitamin A levels toxic in some B6 substrains while not affecting most other commonly used inbred strains.
Keywords: alcohol dehydrogenase 4, animal model, B6 dermatitis, chronic ulcerative dermatitis, follicular degeneration syndrome, hair follicle dystrophy, hot comb alopecia, scarring alopecia, vitamin A, wax stripping, hair cycle
One of the most commonly used mouse strains in biomedical research for more than 50 years is the C57BL/6J inbred strain. While this substrain is officially abbreviated as the B6 strain, the B6 symbol is commonly used generically for all C57BL/6 substrains and will be used in that context here. A variety of mouse models were and continue to be placed on this background through creation of congenic strains, thereby reducing experimental variability due to background modifier genes.15 It is critical to identify intrinsic disease processes to separate strain specific phenotypes from experimentally induced effects. Diseases (phenotypes) commonly seen in C57BL/6J and other closely related substrains of mice include internal hydrocephalus,44,49 polyarteritis nodosa,44 spontaneous, asymmetrical microphthalmia,34,50 and a group of poorly defined skin diseases. Skin problems that arise in the B6 substrains include mild focal alopecia, chronic ulcerative dermatitis, and scarring (cicatricial) alopecia that were separated into barbering/trichotillomania for mild alopecia that affected the facial regions21 which is different from focal alopecia on the trunk (often starting on the dorsal skin behind the ears) that can progress to chronic ulcerative dermatitis.40
The pathogenesis of alopecia of the trunk and ulcerative dermatitis in B6 substrains remains poorly understood. One group suggested that immune complex vasculitis underlies the pathogenesis of chronic ulcerative dermatitis in aged C57BL/6Nnia mice;1 however, no form of alopecia or ulcerative dermatitis has been identified concurrently with the immune complex vasculitis of polyarteritis in B6 mice (JP Sundberg, unpublished data). Data from nearly 1000 cases of chronic ulcerative dermatitis submitted to The Jackson Laboratory Diagnostic Pathology Program between 1988 and 1991 identified a marked female predilection in strains with a black coat color.40 When the time of the year and frequency of disease were evaluated, there was a seasonal variation of notably higher frequency of disease in the spring and fall. These variations were interpreted at the time to be related to the difficulty in stabilizing mouse room humidity when heating (fall) or air conditioning (spring) were changing.48 While technologic improvements have since made animal rooms far more stable and environmental control units are on constantly today, there remains a seasonal variation in skin disease frequency. Institutions in other regions of the United States with small colonies report different seasonal variations.16 The severity and frequency of disease within a colony was reported to be dependent upon nutritional and husbandry factors, such as diet type, weaning times, and humidity. Dietary restrictions were reported to effectively reduce the frequency of skin disease in both wild-type and Trp53-null mice on a B6 background.28,51 Despite dietary restrictions, B6 skin diseases are still a serious problem in aging studies (DE Harrison, personal communication).
The occurrence of skin problems in B6 mice is of most concern for investigators who use genetically engineered mouse mutations aimed at inducing skin and hair lesions, although it can often be a background complication when other organ systems are targeted.10,16 B6 alopecia and dermatitis can also be a problem when obtaining mutant mice from a repository only to find that they develop a skin disease not described as part of the phenotype. The spontaneous occurrence of these skin diseases raises concerns with immunological and other types of studies that use B6 substrains since selected cellular molecular markers may reflect an inflammatory response associated with the B6 skin abnormality rather than the experimental manipulation.
The variability of B6 alopecia and dermatitis may affect data interpretation. The onset of alopecia in B6 substrains commences shortly after weaning and presents with various degrees of dorsal hair loss most commonly, but not exclusively, over the dorsal thoracic region.45 The alopecia waxes and wanes. Some cases may progress to acute ulceration, which heals by second intention with a bed of granulation tissue, pseudoepitheliomatous hyperplasia, and scarring. Mice have various degrees of pruritus that may lead to extensive progressive ulcerative disease requiring euthanasia for humane reasons16,21 and greatly affect data in aging studies. Treatments, such as topical chlorohexiderm or corticosteriod-antimicrobial combination ointments, have been palliative at best (JP Sundberg, unpublished observations) although vitamin E may have some beneficial effects.18 It is therefore important to define the pathogenesis of this B6 dermatologic problem.
In this study we describe the general clinical and progressive histologic changes of skin disease commonly observed in C57BL/6J and preliminary studies in closely related substrains. Our findings suggest a primary follicular dystrophy with rupture of severely affected follicles, and subsequent formation of secondary foreign body granulomas occurs in these B6 substrains of mice. Histologically these changes resemble the human disease currently termed central centrifugal cicatrical alopecia (CCCA). Dietary vitamin A levels in B6 substrains of mice with Adh4 polymorphisms and upregulated DHRS9 may play a role in the pathogenesis of this disease.
MATERIALS AND METHODS
Mice
All studies were approved by The Jackson Laboratory Institutional Animal Care and Use Committee (IACUC) and were performed in compliance with stipulations of that body. C57BL/6J (JR# 664) and C3H/HeJ (JR# 659) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). C57BL/6Tac (Taconic Farms, Germantown, NY), C57BL/6Crl (Charles River Laboratories, Willmington, MA), and C57BL/6NCr (National Cancer Institute, Fredrick, MD) mice were imported to The Jackson Laboratory, rederived by cesaerian section, and colonies maintained for 3 generations prior to use on the same diet, water, and caging systems. Mice were maintained in conventional barrier facilities at 24±2°C and 51±7% relative humidity, housed in 333.6 cm2 maxi-miser Duplex II cages (Thoren caging systems, Hazleton, PA) with pine shavings, exposed to a 14 h light/10 h dark cycle, allowed free access to sterilized acidified water (pH 2.8–3.2), and fed autoclaved NIH-31 Rat & Mouse 5K54 Lab Diet® (PMI Nutrition International, St. Louis, MO) ad libitum. The health status of each animal room is evaluated every 13 weeks. Mice were negative for ectromelia, GDVII, Hantaan virus, K virus, lactic dehydrogenase elevating virus, lymphocytic choriomeningitis virus, mouse adenovirus, mouse hepatitis virus, mouse minute virus, mouse papillomavirus, mouse parvovirus, pneumonia virus of mice, polyomavirus, reovirus 3, rotavirus, Sendai virus, Bordetella bronchiseptica, CAR bacillus, Citrobacter rodentium, Clostridium piliforme, Corynebacterium bovis, Corynebacterium kutscheri, Mycoplasma pulmonis, Salmonella spp., Streptobacillus moniliformis, Klebsiella pneumonia, Pseudomonas spp., Staphylococcus aureus, Giardia spp., and Spironucleus spp. Sporatic positive cases of Helicobacter spp., Klebsiella oxytoca, trichomonads, mouse norovirus, and Pasteurella pneumotropica were diagnosed in our conventional mouse room. In addition, microbiological workup of alopecic and ulcerated skin from C57BL/6J mice with skin disease did not yield any known infectious agent.
Histologic evaluation of disease progression
A cross sectional study was performed to evaluate successive histological changes in affected C57BL/6J mice over a 10-week period. A total of 200 C57BL/6J mice, ten units of 20 age-matched females, were used. Mice with clinical disease at the beginning of the study were ear notched in their right ear.3 One unit of 20 mice was submitted each week for gross examination, photography, and tissue harvesting for histological and scanning electron microscopy analysis. Each box contained at least one mouse with alopecia or alopecia and ulcers of various sizes at the start of the investigation.
Synchronization of hair cycle by depilation
To determine if histologic changes seen in C57BL/6J mice with focal dorsal alopecia progressing to scarring was secondary to traumatic hair removal, such as barbering, depilation was performed. Anagen was induced by depilation of hair shafts on the backs of 40 female C57BL/6J and 40 gender-matched control C3H/HeJ mice, all 6 weeks of age, with follicles in telogen, as described previously.27,33 Mice were anesthetized with 2% tribromoethanol solution (IP, 0.2 ml/10g; Sigma-Aldrich, Milwaukee, WI)3 and all dorsal skin follicles in telogen were painted with warm wax (Surgi-wax, Ardell International, Los Angeles, CA) that was peeled off after hardening, thus depilating all dorsal skin hair shafts. Telogen skin was identified by its pink color. Although immediately after depilation the skin may go through a short phase of discrete wound-healing response, this technique predictably induces the development of very homogeneous anagen follicle populations, which are morphologically and functionally indistinguishable from spontaneous developing anagen follicles. Two, 3 cm × 2 cm full-thickness skin samples (one from the dorsocervical and one from the dorsolumbarsacral regions) were harvested from C57BL/6J and C3H/HeJ wax stripped mice at various time points to ensure representative samples of cycling hair from immediately after and every three days thereafter up to 35 days after wax stripping. Two mice were used for each time point. Tissues were processed for histologic evaluation as described below. Hair cycle was estimated as the percentage of hair follicles in each stage in a minimum of 3 skin sections 1 cm long on each of two slides. Epidermal thickness was measured using an ocular micrometer at 400 × magnification calibrated with a ruled slide. Ten measurements were taken from sections on each slide, 2 slides per mouse. Epidermal thickness was measured from the basement membrane to the top of the stratum corneum. Results were entered into an Excel spreadsheet (Microsoft Corp., Redmond, WA) and data graphed.46
Tissue processing, ectoparasite examination, histologic analysis, and immunohistochemistry
Mice were euthanized by CO2 asphyxiation. The pelage of each mouse was examined for ectoparasites. Skin was fixed overnight in Feketes acid-alcohol-formalin solution processed routinely and stained with hematoxylin and eosin (H&E), acidic toluidine blue, periodic acid-Schiff (PAS), or Gomori’s methenamine silver. Immunohistochemistry was performed routinely to evaluate terminal differentiation proteins in the epidermis (mouse specific keratins 1, 5, 6, 10, and 14, filaggrin, loricrin, and involucrin).23 Rabbit anti-mouse eosinophilic major basic protein polyclonal antibody (gift from The Lee Lab, Mayo Clinic, Scottsdale, AZ) was used for analysis of eosinophils. Evaluation of enzymes and binding proteins involved in retinoic acid synthesis was also done (retinol dehydrogenase, DHRS9; retinal dehydrogenase 1, ALDH1A1; retinal dehydrogenase 2, ALDH1A2; retinal dehydrogenase 3, ALDH1A3; cellular retinol binding protein 1, RBP1 formerly CRBP; and cellular retinoic acid binding protein II, CRABP2) as previously described.9
Scanning electron microscopy of the hair shafts and skin
Scanning electron microscopy (SEM) was used to evaluate the skin surface and quality of hair shafts in areas of alopecia and processed routinely.2 The same specimens were analyzed for elemental content using an attached X-ray microanalysis system (EDAX Inc., Mahwah, NJ, USA).22
Comparison with human diseases
Representative slides of skin from C57BL/6J mice in different stages of disease were provided to two human dermatopathologists (LS, LEK). They compared diagnostic features of human skin specimens with scarring and non-scarring forms of human alopecias with different forms of alopecia and dermatitis in C57BL/6J mice and controls. Photomicrographs of the histologic features of scarring and non-scarring human alopecias were provided for a direct comparison of similarities and differences in between the two species.
Gene expression and genotype studies
Five clinically normal female C57BL/6J mice, a second group of C57BL/6J mice 5 with focal alopecia in the dorsal thoracic region that was 1 cm in diameter, and a third group of 5 C57BL/6NCr mice with no alopecia, all age matched, had RNA isolated from skin and brain. RNA extraction and quantitative real-time RT-PCR (TaqMan®, Applied Biosystems, Foster City, CA). Briefly, tissues were stored in RNAlater® (Ambion Austin, TX) per manufacturer’s instructions and homogenized in Trizol® Reagent (Invitrogen, Carlsbad, CA). Total RNA was isolated by standard Trizol methods, and quality was assessed using a 2100 Bioanalyzer instrument. Total RNA was then treated with RNase-free DNase I (Qiagen, Valencia, CA) according to manufacturer’s protocol. Total RNA (1µg) was reverse transcribed employing standard random hexamer priming methods and Superscript III enzyme (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocols. Diluted reaction products were then used in a subsequent PCR reaction containing Taqman Univeral PCR Master Mix (Applied Biosystems, Foster City, CA). Gene-specific primers and probe sets were obtained from the Applied Biosystems Assay on Demand service and used according to manufacturer’s protocols. Real-Time PCR reactions were performed using the ABI PRISM® 7900HT Sequence Detection System (Applied Biosystems) with recommended thermal cycling protocols and 40 cycles of amplification. Threshold cycle (Ct) values were determined using the supplied Sequence Detection System (SDS v2.2) software package. For data analysis the endogenous control used in this study was beta-glucuronidase (Gusb) and the fold change of Adh4 and Hoxb8 gene expression was compared to clinically normal mice.
Allelic variants for Adh4 were determined by PCR amplification and sequencing as previously described.6
RESULTS
Clinical disease presentations in a production colony
When raised in conventional barrier facilities, 6 week old, female C57BL/6J mice were selected from cages with at least one mouse with dorsal thoracic alopecia. These mice were maintained in large group cages containing 20 mice and one box analyzed each week to follow skin changes with age. Individual mice were not followed; rather, groups of 20 mice were euthanized and necropsied so the skin could be evaluated histologically. In this selected group of C57BL/6J female mice, the mice developed multiple clinical presentations of alopecia that varied from extremely small punctate ulcers to extensive areas of alopecia, ulceration, and ultimately scarring to various degrees. Alopecia or grossly evident ulcerations were often on the dorsal aspect of the mouse but, in general, were not limited to a single body region. Other regions involved include the muzzle, rostral aspect of the forehead, periauricular areas, margin of the ear pinnae, caudal ear base, cervical, cervicothoracic, thoracic, thoracolumbar, lumbar, lumbosacral, and sacral skin. The spectrum of dermatological primary and secondary lesions included the following: generalized erythroderma, severe localized erythema, generalized or localized fine white non-adherent scale localized to extensive alopecia, papules, excoriations, erosions, ulcers, and scars. Excorations, erosions, and ulcers exuded serosanguinous exudate or were covered with adherent yellow crusts. Moderate to severe pruritus involving the ears, neck, and back was often observed when the mice had evidence of ulcerative dermatitis and alopecia. At this time, it is difficult to assess the exact onset of pruritus as it may precede clinically evident disease or be a direct result of the disease. However, pruritus is tightly linked to progressive traumatic hair loss as are the progression of papules, erosions, and finally, ulcerations.
One common presentation in 8-week old females commenced as 1–5 mm areas of alopecia on the dorsal cervicothoracic region that over a 14-day period extended caudally to involve the dorsal thoracolumbar and lumbosacral regions. This was the dominant pattern observed in the cross sectional study (Fig. 1). Progression was variable; some mice had complete resolution of their alopecia while others had progressive disease. When 10–13 week old mice were examined, many previous areas of alopecia had evidence of new hair regrowth that appeared clinically normal. Other mice developed scaling and small healing ulcers. Some ulcers coalesced to create areas at least one centimeter or more in diameter. As these large ulcers healed the surrounding skin contracted forming extensive areas of scarring.
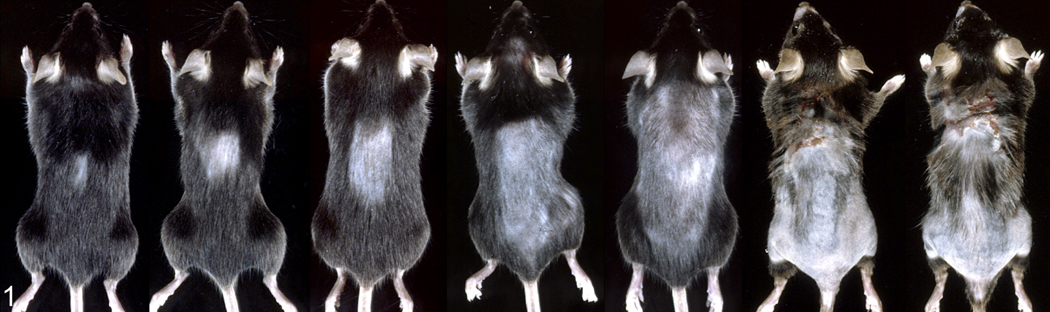
Fig. 1.
A series of C57BL/6J mice with progressive alopecia and dermatitis of the dorsal skin. These mice were progressively older but not all mice with focal alopecia progressed to the severe ulceration that leads to scarring and contracture as seen in the two mice on the right.
Microbiology and ectoparasite analysis
Positive aerobic bacterial cultures were obtained from alopecic and ulcerated skin. Of these only two (of 54, 0.03%) mice, both of which had ulcers, were positive for coagulase negative Staphylococcus spp. and Lactobacillus spp. These isolates are considered to be nonpathogenic in immunocompetent mice. No fungi were isolated or identified in tissue sections. No ectoparasites were identified.
Histology
At the time of necropsy it was common to find hair embedded in the soft tissue of the alveolus adjacent to teeth which was confirmed histologically suggesting some of the alopecia was due to grooming. Histologic examination of full thickness vertical skin sections from the longitudinal study revealed that clinically unaffected mice had changes reflective of normal regenerative cycling that maintains the dense pelage throughout the lifetime of the animal. Late stage anagen hair follicles were of different lengths, reflecting the hair shaft type produced. By contrast, hair follicles found in sections with clinical alopecia were affected only in late anagen and early catagen stages. Hair follicles had normal appearing bulbs but exhibited suprabulbar desquamation of the inner root sheath with associated degenerative changes of the hair shaft (Fig. 2). This was commonly manifested by the presence of twisted hair shafts associated with hyperplasia of adjacent root sheaths in or near the infundibulum (Fig. 3a). Penetration of the shaft through the outer root sheath (Fig. 3b) resulted in hair shaft fragments free in the dermis and hypodermal fat layer (Fig. 3 c, d). Free shaft fragments were surrounded by a mixed inflammatory cell infiltrate consisting of neutrophils, macrophages, multinucleated giant cells, and occasional lymphocytes forming a foreign body granuloma (trichogranuloma, Fig. 3 c, d). Initial resolution of smaller ulcers was characterized by pseudoepitheliomatous hyperplasia of the adjacent epidermis covering a shallow area of granulation tissue (Fig. 4). Complete re-epithelialization left small areas of fibroblasts and collagen bundles that paralleled the epidermis and stained lightly with H&E as compared to adjacent normal dermis. The overlying epidermis exhibited mild to moderate acanthosis, hypergranulosis, and orthokeratotic hyperkeratosis. Large ulcerated areas developed into chronic lesions with extensive areas of granulation tissue. These large areas healed by scarring in some mice. Other mice were not able to heal these large areas so they were euthanized.
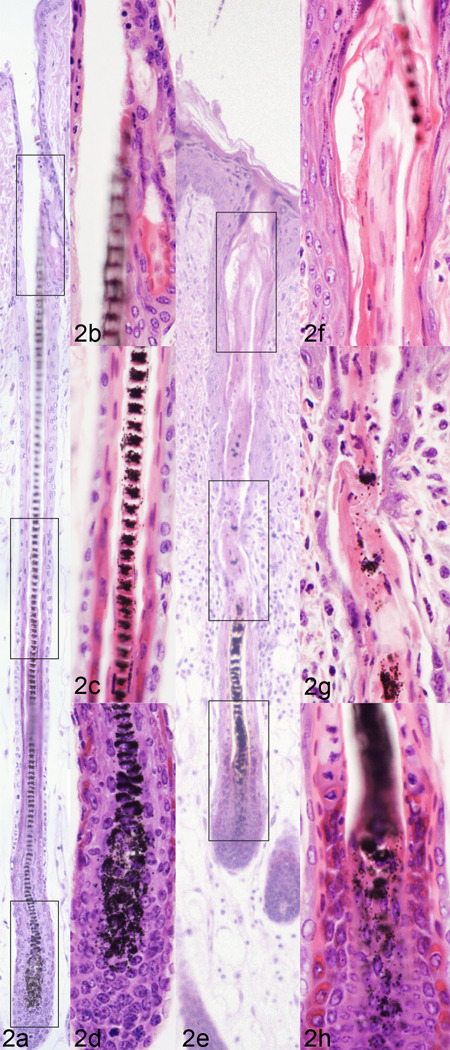
Fig. 2.
Normal mouse anagen hair follicle with a well-formed bulb, mid region, and infundibulum (a, boxed areas enlarged on right, b, c, d) contrasted with an anagen stage follicle from a mouse with alopecia and dermatitis (e, boxed areas enlarged on right, f, g, h). In the mouse with alopecia the hair shaft is misshapen and there is premature loss (desquamation) of the inner root sheath (and to a lesser extent the outer root sheath and hair fiber) in the mid region and infundibulum.
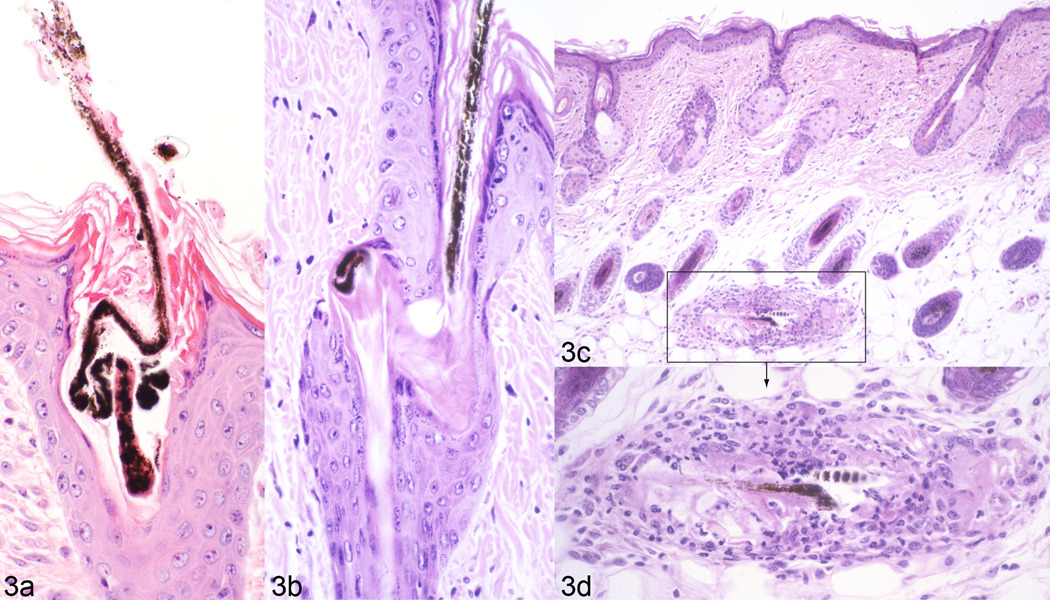
Fig. 3.
In this C57BL/6J mouse with alopecia a hair shaft twists severely in the infundibulum disintegrating as it emerges on the surface (a). In another follicle the shaft penetrates the outer root sheath (b). The “naked” shaft incites a trichogranuloma (c, boxed areas enlarged, d).
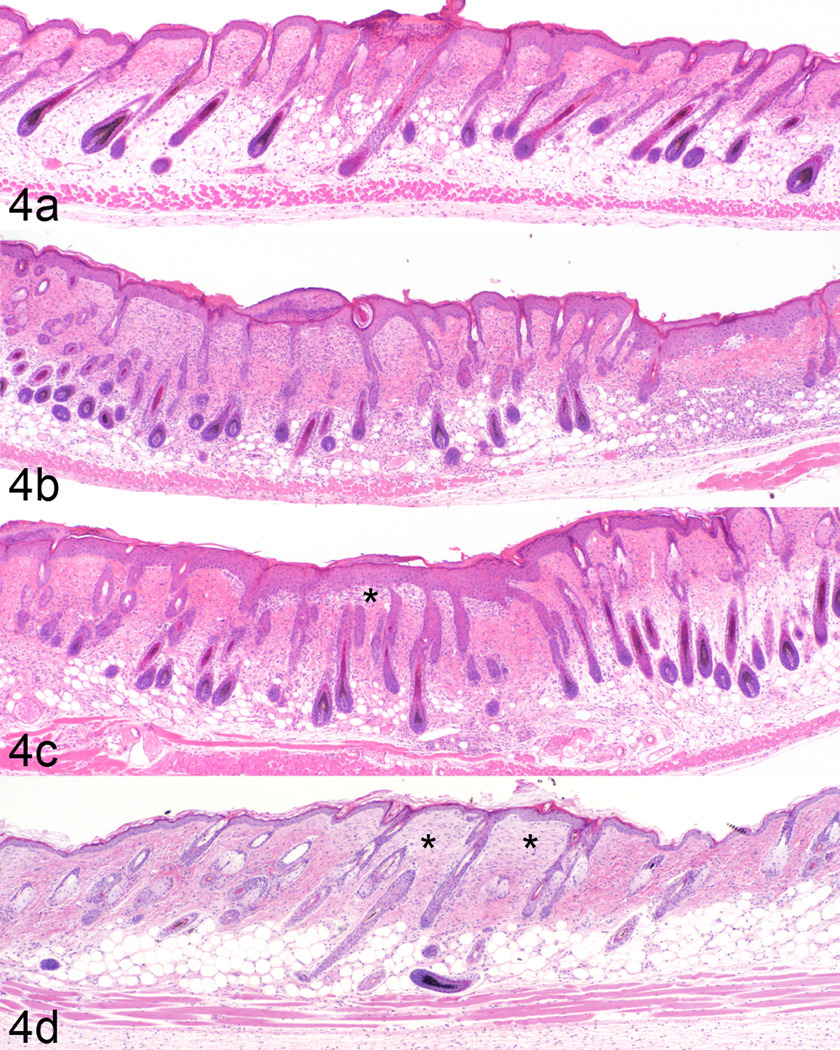
Fig. 4.
Mice with B6 alopecia and dermatitis develop focal ulcers (a). These heal by re-epithelialization, dermal inflammation, and adjacent epidermal hyperplasia (b). Marked epidermal hyperplasia (pseudoepitheliomatous hyperplasia) that involves hair follicles covers areas of dermal fibsosis (c, asterix). Healed foci resolve with dermal fibrosis (asterix) and mild epidermal hyperplasia (d).
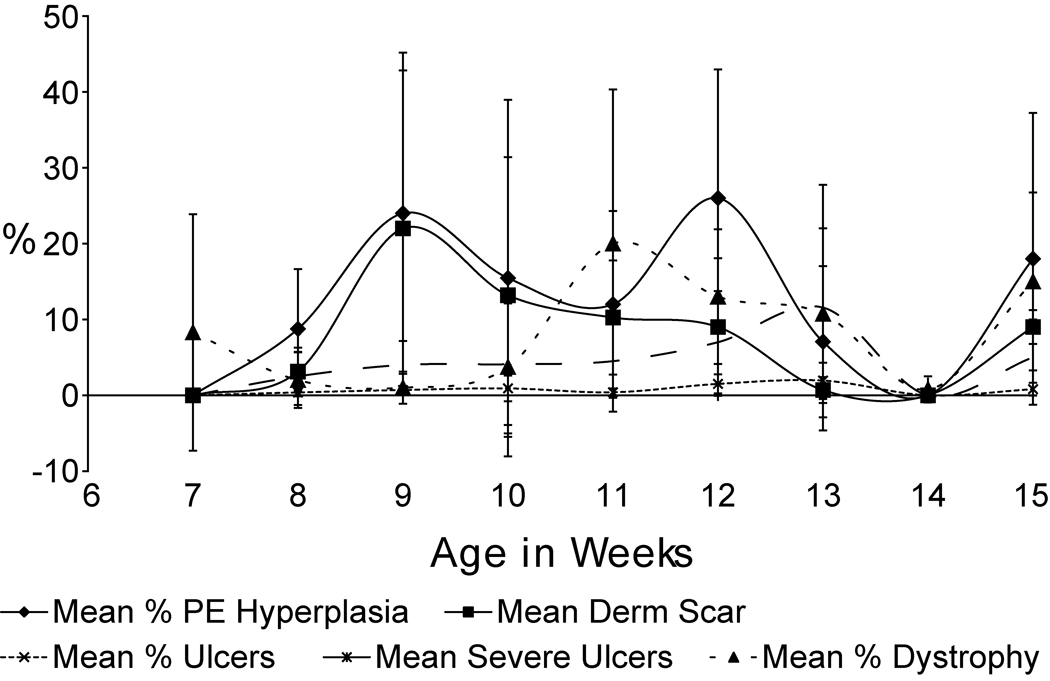
To quantify these sequential changes in follicular dystrophy, hair shaft penetration, acute and chronic inflammation, and scarring, a series of criteria that could be scored were evaluated in a blind manner (only accession numbers available to the pathologist, JPS). Scores were averaged per group and plotted longitudinally (Fig. 5). Results suggest follicular dystrophy precedes other lesions.
Fig. 5.
Semiquantitative evaluation of longitudinal changes in C57BL/6J mice with alopecia and dermatitis. Follicular dystrophy precedes ulceration, pseudoepitheliomatous hyperplasia, and dermal scaring.
Immunohistochemistry and special stains
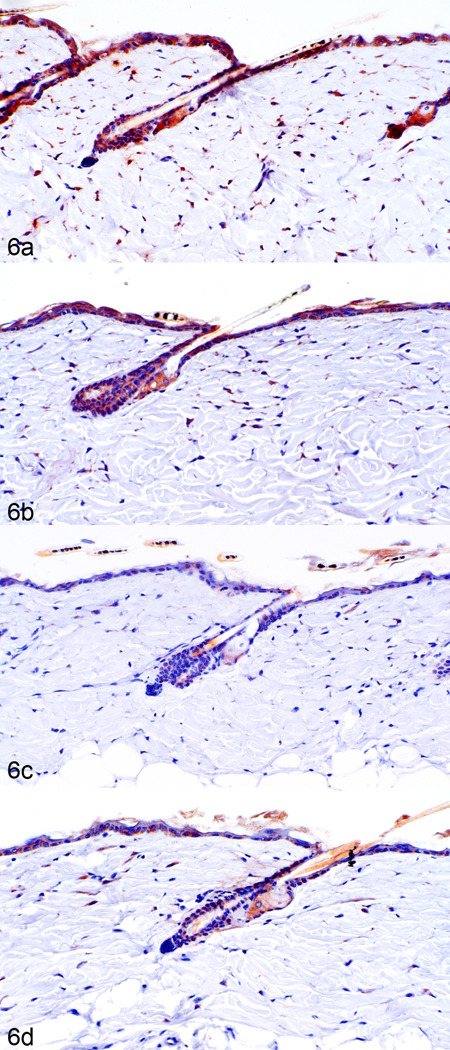
Changes in keratin expression reflected acanthosis and pseudoepitheliomatous hyperplasia.42 Mouse specific keratins 1 and 10, expression markers for normal suprabasilar keratinocytes, were progressively down regulated while keratin 6, normally expressed only in the companion layer, was expressed in the suprabasal keratinocytes in mice with follicular dystrophy and epidermal hyperplasia. Increased thickness of the stratum granulosum layer was detected by increased expression of filaggrin and loricrin expression (data not shown). These data are consistent with progressive epidermal hyperplasia and hypergranulosis observed histologically in these mice.42 DHRS9 was increased during telogen in C57BL/6Tac (Fig. 6 a) and C57BL/6J (Fig. 6 b) but not in the C57BL/NCr or C57BL/Crl substrains (Fig. 6 c, d). No other significant changes in expression of vitamin A metabolism markers were seen in these telogen hair follicles. Eosinophils (anti-eosinophilic major basic protein positive) and mast cells (metachromatic with toluidine blue) were mildly increased in more severely affected acutely ulcerated and healing ulcers.
Fig. 6.
DHRS9 expression by immunohistochemistry in telogen follicles is highest in C57BL/6Tac (a), slightly less in C57BL/6J (b), and reduced in C57BL/6Crl (c), and C57BL/6NCr (d) mice.
Scanning electron microscopy and element analysis of hair shafts and skin
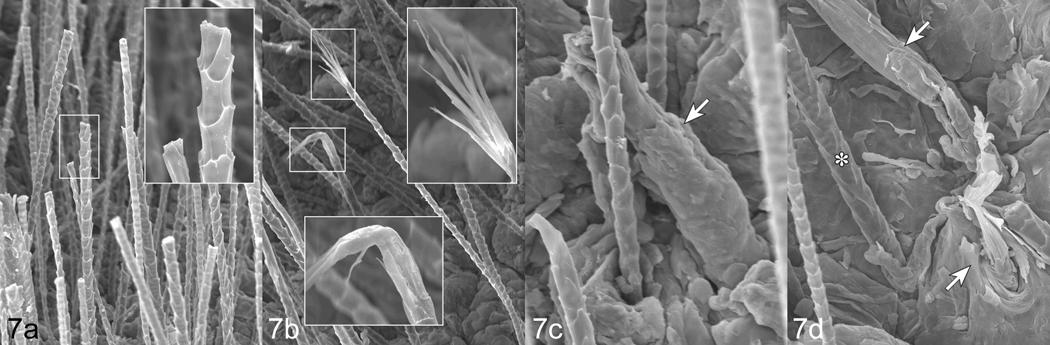
The normal pelage of unaffected C57BL/6J mice is very dense. The long hair shafts were cut with electric clippers resulting in flat ends. Additional normal mice were examined without the hairs clipped to verify no defects were evident at their distal ends (data not shown). The cuticles were distinct and uniform (Fig. 7 a). By contrast, C57BL/6J mice with alopecia had sparse hair coats that did not necessitate clipping and the hairs were relatively uniform in size with deformed tips. The tips were most often bent and tapered to a point while others were split and frayed to various degrees (Fig. 7 b). Some older mice had follicular dystrophy with severe cuticle defects and emerging hair shafts with a sharp point (Fig. 7 c, d).
Fig. 7.
Scanning electron microscopy of normal C57BL/6J mouse skin (a) illustrates the sharp edges from shaving with electric clippers and the well defined cuticular surface scale pattern. Barbered hair from unshaved skin has pointed, bent ends or frayed ends but normal cuticles (b). Cases with histologically confirmed B6 alopecia and dermatitis had both pointed, bent hair fibers with normal cuticles (astrix) but also large, irregular, deformed hair shafts (arrows) with no evidence of normal cuticle formation (c, d).
Structural defects of hair fibers can often be confirmed using x-ray microanalysis for sulfur levels.22 The hair fibers with normal cuticles had no difference in sulfur concentration from the clinically normal mice or controls used from many other mice of different inbred strains (data not shown).12
Depilation analysis
Concerns have been raised that B6 skin lesions are initiated by excessive grooming. The common assumption being that the mouse pulls/plucks the hair fiber from the follicle. To test the hypothesis that manual removal of hair from the follicles would initiate the dystrophy, hairs were mechanically removed by wax stripping. Both the C3H/HeJ and C57BL/6J female mice underwent normal progression through the various stages of the mouse hair cycle after depilation. There was essentially no difference between the two strains in their response to wax stripping (data not shown) suggesting traumatic removal of hair shafts had little to do with B6 alopecia.
Gene expression studies
Alcohol dehydrogenase 4 (Adh4) is involved with detoxification of vitamin A and polymorphisms between the strains are associated with differences in efficiency of this process.6 This may be important since high levels of vitamin A can cause these types of skin lesions and commercial rodent diets are fortified with more vitamins than are needed to deal with degradation during shipping and storage. Similarly, null mutations in Hoxb8 (Hoxb8tm1.1Mrc) develop severe trichotillomania as their primary phenotype,13 which resembles the skin lesions that arise spontaneously in B6 mice. To determine expression levels of these genes, quantitative real-time RT-PCR (Taqman®) analysis of Adh4 and Hoxb8 were done and revealed no significant expression level differences in skin for either skin or brain derived transcripts for Hoxb8 or Adh4 between 5 clinically normal female C57BL/6J mice, another 5 C57BL/6J mice with focal alopecia in the dorsal thoracic region 1 cm in diameter, and 5 C57BL/6NCR with no alopecia. Using the published methods for genotyping Adh4a from Adh4b,6 we confirmed C3H/HeJ mice carried the normal Adh4a while all 4 C57BL/6 substrains carried the hypomorphic Adh4b allele.
Comparison with human disease
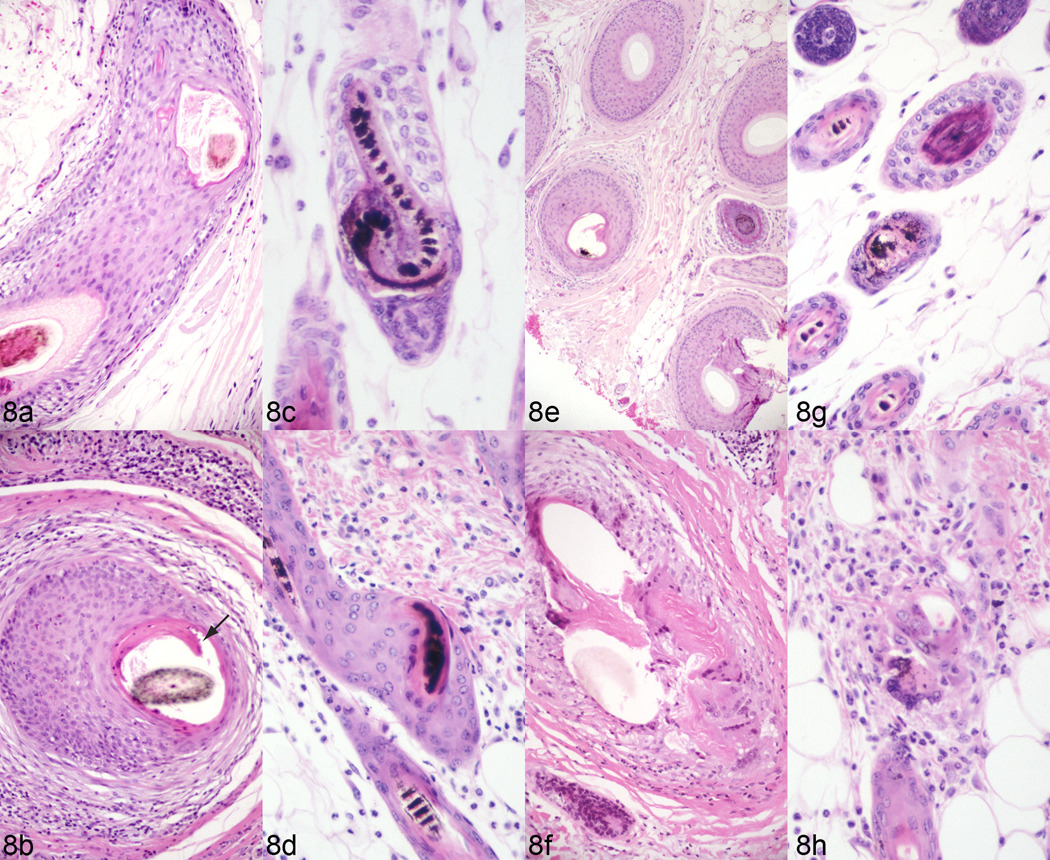
Human central centrifugal cicatricial alopecia (CCCA) was originally thought to be a grooming disorder (“hot comb alopecia”) primarily but not exclusively affecting dark skinned, curly haired women of African descent (e.g. African-Americans).35,37 CCCA is now viewed as a primary form of cicatricial alopecia that occasionally can affect men as well as women, and persons of other races besides persons of color of African descent. Biopsy specimens from both CCCA patients and C57BL/6J mice with alopecia show premature desquamation of the inner root sheath, marked thinning of the outer root sheath with reactive perifollicular inflammation, eventually entry of the hair shaft into the dermis, and resulting destructive chronic and granulomatous inflammation (Fig. 8). Histologic changes are remarkably similar in both the human patients and C57BL/6J mice with alopecia and ulcerative dermatitis suggesting this strain specific disease could be a model for human centrifugal cicatricial alopecia.
Fig. 8.
Human (a, b, e, f) cases of central centrifugal cicatricial alopecia compared to mouse B6 alopecia and dermatitis (c, d,g, h). Premature desquamation of the inner root sheath (arrow) is noted in some follicles on vertical (b) and horizontal (f) sections. Such follicles are prone to marked thinning of the outer root sheaths with reactive perifollicular inflammation (b, f) and eventually entry of the hair fiber into the dermis with resulting destructive chronic granulomatous inflammation (f). Changes are similar if not identical in mouse skin (c, d, g, h).
Discussion
A group of skin lesions, ranging from mild to severe alopecia and ulceration, are commonly encountered in various substrains of B6 mice. Anecdotal reports suggest there are differences in frequency and severity of skin disease among these B6 substrains. These differences have been difficult to substantiate due to variations in diet, water, and husbandry conditions between institutions. Independent studies in progress show that alopecia of the head in B6 mice, the commonly accepted form of barbering and a form of trichotillomania in mice,21 occurs in all 4 of the most common B6 substrains. The frequency is equal when large numbers of mice are maintained under identical husbandry conditions and evaluated concurrently by the same observer (A Nicholson, unpublished data). Earlier studies found no histologic lesions in barbered skin.21 Subsequent studies revealed clean cut hair shafts within the follicular osteum (JP Sundberg, unpublished observations). By contrast, focal, dorsal, thoracic region alopecia showed a marked strain dichotomy: C57BL/6J>C57BL/6Tac≫C57BL/6Crl=C57BL/6NCr (A Nicholson, unpublished data). As C57BL/6J mice age, they may develop, at various frequencies, a mild punctuate ulcerative dermatitis, often overlooked at the gross level, that may heal by scarring or expand to severe ulceration.40 Finding hair shafts between the gingival mucosa and incisors and, as commonly reported, between the molars,19 combined with breakage of normal hair shafts, corresponds with grooming commonly seen in these mice. We and others have observed these mice excessively grooming themselves and associated cage mates11,21 to both remove hair as well as to dig at the ulcers leading to centrifugal ulcer expansion.21
Longitudinal analysis of skin samples did not clearly reveal in what order lesions appeared but follicular dystrophy did precede more severe lesions in a cyclical manner. Wax stripping was an attempt to experimentally reproduce barbering as an initiating cause of the skin disease problems in these mice. There were essentially no differences between C3H/HeJ that get a form of alopecia areata41 and C57BL/6J inbred strains of mice and no follicular dystrophy was found histologically. This is consistent with many published reports using this technique in B6 substrains to initiate anagen in a controlled manner.33 Initial changes after wax stripping resembled those seen in mice with mutations in the desmoglein 3 gene,25,30 indicating hair shafts were physically removed from anagen follicles by disrupting adhesion molecule connections. This is in sharp contrast to the histologic lesions observed in groomed mouse skin where either no changes are evident in hair follicles21 or only broken hair shafts within the follicular infundibulum are seen indicating the shafts were cut or broken (JP Sundberg, unpublished observations).
Scanning electron microscopy revealed defects at the tips of hair shafts; however, below the break, shafts had normal architecture. The sulfur content of normal mouse hair is three times that of the epidermis, reflecting the high sulfur containing amino acid content of the hard keratins and keratin associated proteins that make up the structural proteins of hair and nails.22 No differences were detected in the hair sulfur levels between C57BL/6J mice with various degrees of alopecia or clinically nonaffected mice. These findings suggest these hairs were mechanically broken; however, in cases in which severe follicular dystrophy was identified histologically, there were severely deformed and missing hair shafts. It is likely, based on the histologic evidence, that most of these defective hairs were lost or destroyed within the hair follicle or there may be a transient defect in hair shaft structure causing it to twist in follicles and/or break at or near the surface.
Genetically engineered mice with Hoxb8 null mutations (Hoxb8tm1.1Mrc) develop severe trichotillomania as their primary phenotype, which may be a primary or complicating part of this disease in C57BL/6 mice.13 As Hoxb8 is expressed primarily in the brain, RNA was tested from this organ. QPCR revealed slight increases in Hoxb8 in the brain of C57BL/6J mice with alopecia with moderate reduction in C57BL/6NCr mice and no changes in the skin. This is in marked contrast with the null mutant mice13 suggesting that this gene has no role in barbering or alopecia and ulcerative dermatitis in B6 substrains.
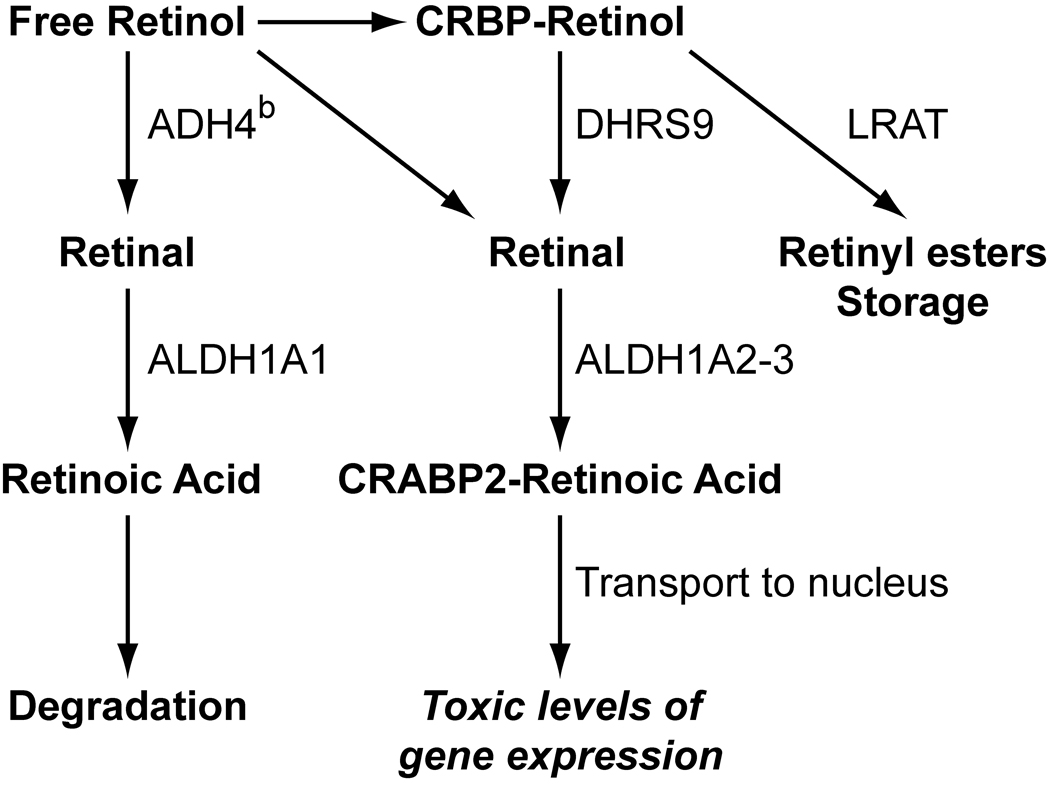
In this study C57BL/6J mice were found to have potential defects in vitamin A metabolism. The skin takes up circulating retinol and can either store it in the form of retinyl esters or metabolize it to retinoic acid (Fig. 9).32 Two enzymes are present in the skin that can oxidize retinol to retinal. These include the medium chain alcohol dehydrogenase type 4 (ADH4)14 and the short chain dehydrogenase/reductases epithelial retinol dehydrogenase (DHRS9; formerly eRoldh in rat and hRODH-E2 in human).9,20,31 The main difference between these two enzymes is that ADH4 is cytoplasmic and oxidizes free, but not CRBP-bound, retinol,26 while DHRS9 is microsomal and can oxidize both free and CRBP-bound retinol.20,31 Because it is believed that CRBP-bound retinol is the physiologically active form that is metabolized to active retinoic acid, DHRS9 and other short chain dehydrogenase/reductases are more likely to be involved in the biosynthesis of transcriptionally active retinoic acid.
Fig. 9.
Free retinol in the blood is metabolized by keratinocytes where it is either degraded, stored, or enters the nucleus and activates its transcription factor. All C57BL/6 substrains tested have a hypofunctional alcohol dehydrogenase 4b allele (ADH4b) which pushes retinol to transcriptionally active RA or it is stored. Upregulation of DHRS9 in C57BL/6 substrains prone to alopecia and dermatitis appears to favor transcriptional activation.
The exact role for ADH4 in the skin has not been firmly established. Mice that lack the Adh4 gene (Adh4tm1Gdu) have no observed defect when fed a standard diet, but have decreased survival on a vitamin A deficient diet4 and have decreased retinol oxidation in the kidney4,5 but not in the liver24 when given a toxic retinol dose. ADH4 may act locally in the skin to clear free retinol from the skin when CRBP is saturated. In addition, both ADH1 and ADH4 can oxidize other alcohols to aldehydes and may play a role in general alcohol detoxification as well. There are two alleles of Adh4. The Adh4a allele is found in most strains of laboratory mice (C3H/He, DBA/2, AKR, SWR, 129, SM, and others) while the Adh4b allele is found in C57BL/6J and a few other strains.6 These polymorphisms in the C57BL/6J substrain result in reduced enzymatic function.6 We confirmed that C3H/HeJ mice carry the Adh4a allele while the 4 B6 substrains tested (J, NCr, Tac, and Crl) all carry the Adh4b allele. This would suggest that all 4 C57BL/6 substrains were equally susceptible to problems with metabolizing excess vitamin A and that the C57BL/6J substrain may have one or more additional genetic changes that make them more susceptible to dermatitis than the other C57BL/6 substrains. Upregulation of DHRS9 in C57BL/6J and C57BL/6Tac but not C57BL/6NCr or C57BL/6Crl mice provides a potential explanation for why the first two strains have a much higher frequency of dorsal skin alopecia. We also observed an increase in DHRS9 in human patients with CCCA and 2 other mouse models for cicatricial alopecias (H Everts et al., manuscript in preparation). As expected, no significant alterations were seen in the expression of other components of vitamin A metabolism in these telogen samples, since these proteins are present more during anagen than telogen.8,9 Telogen may not be the best hair cycle stage to compare these B6 substrains and future studies will examine expression levels during anagen. This study provides preliminary data that an altered genotype of Adh4, combined with elevated DHRS9 and a diet high in vitamin A may play a role in B6 dermatitis. Future studies are needed to prove this using defined diets, analyzing retinoic acid synthesis components in hair follicles during anagen, and measuring the expression of retinoic acid target genes.
Although many believe that humans are distinctly different from mice, both species are mammals and share numerous genetic similarities.47 Often single gene mutations in mice are similar or identical to mutations present in human diseases, especially when the affected gene is present in both species. Identifying genes associated with novel mouse disease phenotypes can lead to elucidating and defining the pathogenesis and molecular mechanisms of homologous defects in humans.17,29 To this end, a common form of cicatricial alopecia of female African-Americans formerly called “hot comb syndrome”, “follicular degeneration syndrome”, and currently referred to as central centrifugal cicatrical alopecia (CCCA) exists.39 The human disease, CCCA, is characterized by progressive development of a chronic, expanding cicatricial alopecia. This alopecia was originally thought to be an effect of styling but is now recognized as a primary hair follicle disorder. Similarly, progressive alopecia in B6 substrains of mice was and by many still is considered to be a grooming problem.11,21 Although this hypothesis is considered by some to be a factor in alopecia and ulcerative dermatitis in B6 substrains of mice, the degenerative changes of the inner root sheath are comparable to CCCA.
The primary follicular dystrophy in B6 substrains of mice appears to differ somewhat from CCCA in humans in the current observations. Normal hair regrowth and resolution occurs in some mice in areas that were previously recognized as diseased. By contrast, CCCA, as currently defined and diagnosed, is a progressively scarring (cicatricial) and permanently alopecic process. It is possible that not all involved follicles will progress to follicular rupture and scarring as observed in some B6 mice. Mice, unlike humans, have 4 hair follicle types on their body which may explain these differences.43 However, foci of alopecia, even in the same mouse, may spread centrifugally with expanding cicatricial foci, very similar to that observed in CCCA.7,36–38 Hair regrowth was not clinically apparent in the residual scars in the mice as expected due to complete hair follicle destruction. The majority of the microscopically identified dystrophic hair follicles were adjacent to the fibrotic areas of the specimens in an area with normal hair regrowth. Since vitamin A metabolism shows similar changes in CCCA in humans as that observed in alopecia and ulcerative dermatitis in B6 substrains, then these mice may become a valuable model for human CCCA. Identifying the underlying genetic changes in both affected mice and human patients is necessary to confirm that these B6 substrains are an important preclinical model for CCCA.
Acknowledgements
This work was supported in part by mentorship grants from the North American Hair Research Society (to HE, DT, and GL), the Wauford Foundation (to HE), and the National Institutes of Health (RR00173, CA34196, AR41931, AR052710 to JPS; AR052009 to HE; and 5P30AR041943 to LEK).
The authors thank L. Bechtold and J. Haywood for their assistance.
Abbreviations
- Adh4 and ADH4
alcohol dehydrogenase 4 gene and protein symbols
- Aldh1a1 and ALDH1a1
retinal dehydrogenase 1 gene and protein symbols
- Aldh1a2 and ALDH1a2
retinal dehydrogenase 2 gene and protein symbols
- Aldh1a3 and ALDH1a3
retinal dehydrogenase 3 gene and protein symbols
- B6
C57BL/6 mouse substrains
- Dhrs9 and DHRS9
Dehydrogenase/reductase (SDR family) member 9, formerly called epithelial retinol dehydrogenase gene and protein symbols
Footnotes
Author contributions. JPS, HE, LEK, LS, DO, and DR conceived and designed the experiments. JPS, HE, DT, GL, JM, KAS, BAS, DR, LS, DO, and LEK performed the experiments. JPS, HE, LS, and LEK analyzed the data and DR and DO contributed reagents/materials/analysis tools. JPS and HE wrote the paper.
References
- 1.Andrews AG, Dysko RC, Spilman SC, Kunkel RG, Brammer DW, Johnson KJ. Immune complex vasculitis with secondary ulcerative dermatitis in aged C57BL/6NNia mice. Vet Pathol. 1994;31:293–300. doi: 10.1177/030098589403100301. [DOI] [PubMed] [Google Scholar]
- 2.Bechtold LS. Ultrastructural evaluation of mouse mutations. In: Sundberg JP, Boggess D, editors. Systematic characterization of mouse mutations. Boca Raton: CRC Press; 2000. pp. 121–129. [Google Scholar]
- 3.Boggess D, Silva KA, Landel C, Mobraaten L, Sundberg JP. Approaches to handling, breeding, strain preservation, genotyping, and drug administration for mouse models of cancer. In: Holland EC, editor. Mouse models of human cancer. Hoboken, NJ: John Wiley & Sons, Inc.; 2004. pp. 3–14. [Google Scholar]
- 4.Deltour L, Foglio MH, Duester G. Impaired retinol utilization in Adh4 alcohol dehydrogenase mutant mice. Dev Genet. 1999;25:1–10. doi: 10.1002/(SICI)1520-6408(1999)25:1<1::AID-DVG1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 5.Deltour L, Foglio MH, Duester G. Metabolic deficiencies in alcohol dehydrogenase Adh1, Adh3, and Adh4 null mutant mice. Overlapping roles of Adh1 and Adh4 in ethanol clearance and metabolism of retinol to retinoic acid. J Biol Chem. 1999;274:16796–16801. doi: 10.1074/jbc.274.24.16796. [DOI] [PubMed] [Google Scholar]
- 6.Dolney DEA, Szalai G, Felder MR. Differences in charge and kinetic properties of alcohol dehydrogenase 4 from C57BL/6 mice compared to other inbred strains are associated with a cysteine 120 to arginine 120 substitution. Biochem Genet. 2001;39:239–250. doi: 10.1023/a:1010278631535. [DOI] [PubMed] [Google Scholar]
- 7.Elston DM, McCollough ML, Warschaw KE, Bergfeld WF. Elastic tissue in scars and alopecia. J Cutan Pathol. 2000;27:147–152. doi: 10.1034/j.1600-0560.2000.027003147.x. [DOI] [PubMed] [Google Scholar]
- 8.Everts HB, King LE, Sundberg JP, Ong DE. Hair cycle-specific immunolocalization of retinoic acid synthesizing enzymes Aldh1a2 and Aldh1a3 indicate complex regulation. J Invest Dermatol. 2004;2004:258–263. doi: 10.1111/j.0022-202X.2004.23223.x. [DOI] [PubMed] [Google Scholar]
- 9.Everts HB, Sundberg JP, L E King J, Ong DE. Immunolocalization of enzymes, binding proteins, and receptors sufficient for retinoic acid synthesis and signaling during the hair cycle. J Invest Dermatol. 2007;127:1593–1604. doi: 10.1038/sj.jid.5700753. [DOI] [PubMed] [Google Scholar]
- 10.Forlow SB, White EJ, Thomas KL, Bagby GJ, Foley PL, Ley K. T cell requirement for development of chronic ulcerative dermatitis in E- and P-selectin-deficient mice. J Immunol. 2003;169:4797–4804. doi: 10.4049/jimmunol.169.9.4797. [DOI] [PubMed] [Google Scholar]
- 11.Garner JP, Weisker SM, Dufour B, Mench JA. Barbering (fur and whisker trimming) by laboratory mice as a model of human trichotillomania and obsessive-compulsive spectrum disorders. Comp Med. 2004;54:216–224. [PubMed] [Google Scholar]
- 12.Giehl KA, Potter CS, Wu B, Silva KA, Rowe L, Awgulewitsch A, Sundberg JP. Hair interior defect (hid) in AKR/J mice maps to mouse Chromosome 1. Clin Exp Dermatol. 2009;34:509–517. doi: 10.1111/j.1365-2230.2008.03135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greer JM, Capecchi MR. Hoxb8 is required for normal grooming behavior in mice. Neuron. 2002;33:23–34. doi: 10.1016/s0896-6273(01)00564-5. [DOI] [PubMed] [Google Scholar]
- 14.Haselbeck RJ, Ang HL, Duester G. Class IV alcohol/retinol dehydrogenase localization in epidermal basal layer: potential site of retinoic acid synthesis during skin development. Development Dynamics. 1997;208:447–453. doi: 10.1002/(SICI)1097-0177(199704)208:4<447::AID-AJA1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 15.Holstein J. The first fifty years at the Jackson Laboratory. Bar Harbor: The Jackson Laboratory; 1979. p. 231. [Google Scholar]
- 16.Kastenmayer RJ, Fain MA, Perdue KA. A retrospective study of idiopathic ulcerative dermatitis in mice with a C57BL/6 background. J Am Assoc Lab Anim Sci. 2006;45:8–12. [PubMed] [Google Scholar]
- 17.Kljuic A, Bazzi H, Sundberg JP, Martinex-Mir A, O'Shaugnessy R, Mahoney MG, Levy M, Montagutelli X, Ahmad W, Alta VM, Gordon D, Uitto J, Whiting D, Ott J, Fisher S, Gilliam TC, Jahoda CAB, Morris RJ, Panteleyev AA, Nguyen VT, Christiano AM. Desmoglein 4 in hair follicle differentiation and epidermal adhesion: evidence from inherited hypotrichosis and acquired pemphigus vulgaris. Cell. 2003;113:249–260. doi: 10.1016/s0092-8674(03)00273-3. [DOI] [PubMed] [Google Scholar]
- 18.Lawson GW, Sato A, Fairbanks LA, Lawson PT. Vitamin E as a treatment for ulcerative dermatitis in C57BL/6 mice and strains with a C57BL/6 background. Contemp Top Lab Anim Sci. 2005;44:18–21. [PubMed] [Google Scholar]
- 19.Mahler M, Rozell B, Mahler JF, Merlino G, Devor-Henneman D, Ward JM, Sundberg JP. Pathology of the gastrointestinal tract of genetically engineered and spontaneous mutant mice. In: Ward JM, Mahler JF, Maronpot RR, Sundberg JP, editors. Pathology of genetically engineered mice. Ames: Iowa State University Press; 2000. pp. 269–297. [Google Scholar]
- 20.Markova NG, Pinkas-Sarafova A, Karaman-Jurukovska N, Simon M. Expression pattern and biochemical characteristics of a major epidermal retinol dehydrogenase. Mol Genet Metabolism. 2003;78:119–135. doi: 10.1016/s1096-7192(02)00226-3. [DOI] [PubMed] [Google Scholar]
- 21.McMahon WM, Sundberg JP. Barbering behavior abnormalities in inbred laboratory mice. In: Sundberg JP, editor. Handbook of mouse mutations with skin and hair abnormalities. Animal models and biomedical tools. Boca Raton: CRC Press; 1994. pp. 493–497. [Google Scholar]
- 22.Mecklenburg L, Paus R, Halata Z, Bechtold LS, Fleckman P, Sundberg JP. FOXN1 is critical for onychocyte terminal differentiation in nude (Foxn1nu) mice. J Invest Dermatol. 2004;123:1001–1011. doi: 10.1111/j.0022-202X.2004.23442.x. [DOI] [PubMed] [Google Scholar]
- 23.Mikaelian I, Nanney LB, Parman KS, Kusewitt D, Ward JM, Naf D, Krupke DM, Eppig JT, Bult CJ, Seymour R, Ichiki T, Sundberg JP. Antibodies that label paraffin-embedded mouse tissues: a collaborative endeavor. Toxicol Pathol. 2004;32:1–11. doi: 10.1080/01926230490274335. [DOI] [PubMed] [Google Scholar]
- 24.Molotkov A, Fan X, Duester G. Excessive vitamin A toxicity in mice genetically deficient in either alcohol dehydrogenase Adh1 or Adh3. Eur J Biochem. 2002;269:2607–2612. doi: 10.1046/j.1432-1033.2002.02935.x. [DOI] [PubMed] [Google Scholar]
- 25.Montagutelli X, Lalouette A, Boulouis HG, Guenet J-L, Sundberg JP. Vesicle formation and follicular root sheath separation in mice homozygous for deleterious alleles at the balding (bal) locus. J Invest Dermatol. 1997;109:324–328. doi: 10.1111/1523-1747.ep12335844. [DOI] [PubMed] [Google Scholar]
- 26.Napoli JL. Interactions of retinoid binding proteins and enzymes in retinoid metabolism. Biochem biophys Acta. 1999;1440:139–162. doi: 10.1016/s1388-1981(99)00117-1. [DOI] [PubMed] [Google Scholar]
- 27.Paus R, Stenn K, Link RE. Telogen skin contains an inhibitor of hair growth. Br J Dermatol. 1990;60:777–784. doi: 10.1111/j.1365-2133.1990.tb06266.x. [DOI] [PubMed] [Google Scholar]
- 28.Perkins SN, Hursting SD, Phang JM, Haines DC. Calorie restriction reduces ulcerative dermatitis and infection-related mortality in p53-deficient and wild-type mice. J Invest Dermatol. 1998;111:292–296. doi: 10.1046/j.1523-1747.1998.00270.x. [DOI] [PubMed] [Google Scholar]
- 29.Peters L, Robledo RF, Bult CJ, Churchill GA, Paigen BJ, Svenson KL. The mouse as a model for human biology; a resource guide for complex trait analysis. Nat Rev Genet. 2007;8:58–69. doi: 10.1038/nrg2025. [DOI] [PubMed] [Google Scholar]
- 30.Pulkkinen L, Choi YW, Simpson A, Montagutelli X, Sundberg J, Uitto J, Mahoney MG. Loss of cell adhesion in Dsg3bal-Pas mice with homozygous deletion mutation (2079del14) in the desmoglein 3 gene. J Invest Dermatol. 2002;119:1237–1243. doi: 10.1046/j.1523-1747.2002.19645.x. [DOI] [PubMed] [Google Scholar]
- 31.Rexer BN, Ong DE. A novel short-chain alcohol dehydrogenase from rats with retinol dehydrogrenase activity, cyclically expressed in uterine epithelium. Biol Reprod. 2002;67:1555–1564. doi: 10.1095/biolreprod.102.007021. [DOI] [PubMed] [Google Scholar]
- 32.Roos TC, Jugert FK, Merk HF, Bickers DR. Retinoid metabolism in the skin. Pharmacol Rev. 1998;50:315–333. [PubMed] [Google Scholar]
- 33.Slominski A, Paus R, Costantino R. Differential expression and activity of melanogenesis-related proteins during induced hair growth in mice. J Invest Dermatol. 1991;96:172–179. doi: 10.1111/1523-1747.ep12460956. [DOI] [PubMed] [Google Scholar]
- 34.Smith RS, Roderick TH, Sundberg JP. Microphthalmia and associated abnormalities in inbred black mice. Lab Anim Sci. 1994;44:551–560. [PubMed] [Google Scholar]
- 35.Sperling LC. Overview of hair shaft disorders. In: Sperling LC, editor. An atlas of hair pathology with clinical correlations. Boca Raton, FL: Parthenon Publishing Group; 2003. pp. 141–151. [Google Scholar]
- 36.Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333–342. doi: 10.1034/j.1600-0560.2001.280701.x. [DOI] [PubMed] [Google Scholar]
- 37.Sperling LC, Sau P. The follicular degeneration syndrome in black patients: `Hot comb alopecia' revisited and revised. ArchDermatol. 1992;128:68–74. [PubMed] [Google Scholar]
- 38.Sperling LC, Skelton HG, Smith KJ, Sau P, Friedman K. Follicular degeneration syndrome in men. Arch Dermatol. 1994;130:763–769. [PubMed] [Google Scholar]
- 39.Sperling LC, Solomon AR, Whiting RA. A new look at scarring alopecia. Arch Derm. 2000;136:235–242. doi: 10.1001/archderm.136.2.235. [DOI] [PubMed] [Google Scholar]
- 40.Sundberg JP, Brown KS, McMahon WM. Chronic ulcerative dermatitis in black mice. In: Sundberg JP, editor. Handbook of mouse mutations with skin and hair abnormalities: animal models and biomedical tools. Boca Raton, FL: CRC Press; 1994. pp. 485–492. [Google Scholar]
- 41.Sundberg JP, Cordy WR, King LE. Alopecia areata in aging C3H/HeJ mice. J Invest Dermatol. 1994;102:847–856. doi: 10.1111/1523-1747.ep12382416. [DOI] [PubMed] [Google Scholar]
- 42.Sundberg JP, Erickson AA, Roop DR, Binder RL. Ornithine decarboxylase expression in cutaneous papillomas in SENCAR mice is associated with altered expression of keratins 1 and 10. Cancer Res. 1994;54:1344–1351. [PubMed] [Google Scholar]
- 43.Sundberg JP, Hogan ME. Hair types and subtypes in the laboratory mouse. In: Sundberg JP, editor. Handbook of mouse mutations with skin and hair abnormalities: animal models and biomedical tools. Boca Raton, FL: CRC Press; 1994. [Google Scholar]
- 44.Sundberg JP, Ichiki T. Genetically engineered mice handbook. Boca Raton: CRC Press; 2005. p. 314. [Google Scholar]
- 45.Sundberg JP, King LE. Skin and its appendages: normal anatomy and pathology of spontaneous, transgenic and targeted mouse mutations. In: Ward JM, Mahler JF, Maronpot RR, Sundberg JP, editors. Pathology of genetically engineered mice. Ames: Iowa State University Press; 2000. pp. 181–213. [Google Scholar]
- 46.Sundberg JP, Rourk M, Boggess D, Hogan ME, Sundberg BA, Bertolino A. Angora mouse mutation: altered hair cycle, follicular dystrophy, phenotypic maintenance of skin grafts, and changes in keratin expression. Vet Pathol. 1997;34:171–179. doi: 10.1177/030098589703400301. [DOI] [PubMed] [Google Scholar]
- 47.Sundberg JP, Schofield PN. One medicine, one pathology, one health concept. J Am Vet Med Assoc. 2009;234:1530–1531. doi: 10.2460/javma.234.12.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sundberg JP, Sundberg BA, King LE. Cutaneous changes in commonly used inbred mouse strains and mutant stocks. In: Mohr U, Dungworth DL, Capen CC, Carlton WW, Sundberg JP, Ward JM, editors. Pathobiology of the aging mouse. Washington D.C.: ILSI Press; 1996. pp. 325–337. [Google Scholar]
- 49.Sundberg JP, Woolcott BL, Cunliffe-Beamer T, Brown KS, Bronson R. Spontaneous hydrocephalus in inbred strains of mice. Jax Notes. 1991:2–3. [Google Scholar]
- 50.Tyndall DA, Cook CS. Spontaneous, asymetrical microphthalmia in C57BL/6J. J Craniofac Genet Dev Biol. 1990;10:353–361. [PubMed] [Google Scholar]
- 51.Witt WM. An idiopathic dermatitis in C57BL/6N mice effectively modulated by dietary restriction. Lab Anim Sci. 1989;39:470. [Google Scholar]