Abstract
Accurate diagnosis and staging are essential for the optimal management of cancer patients. Positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro- D-glucose integrated with computed tomography (18F-FDG PET/CT) has emerged as a powerful imaging tool for the detection of various cancers. The combined acquisition of PET and CT has synergistic advantages over PET or CT alone and minimizes their individual limitations. It is a valuable tool for staging and restaging of some tumors and has an important role in the detection of recurrence in asymptomatic patients with rising tumor marker levels and patients with negative or equivocal findings on conventional imaging techniques. It also allows for monitoring response to therapy and permitting timely modification of therapeutic regimens. In about 27% of the patients, the course of managment is changed. This review provides guidance for oncologists/ radiotherapists and clinical and surgical specialists on the use of 18F-FDG PET/CT in oncology.
Cancer is one of the leading causes of death worldwide. Accurate diagnosis, staging and restaging are essential for the optimal therapeutic management of cancer patients. Positron emission tomography (PET) with 2-deoxy-2-[fluorine-18]fluoro-D-glucose (18F-FDG), an analogue of glucose, provides valuable functional information based on the increased glucose uptake and glycolysis of cancer cells and depicts metabolic abnormalities before morphological alterations occur. 18F-FDG PET/CT acquires PET and CT data in the same imaging session and allows accurate anatomical localization of the lesions detected on the 18F-FDG PET scan (Figure 1). Following its introduction, integrated PET/CT rapidly gained clinical acceptance, and in the last decade it has become an important imaging tool in routine clinical oncology (Table 1).
Figure 1.
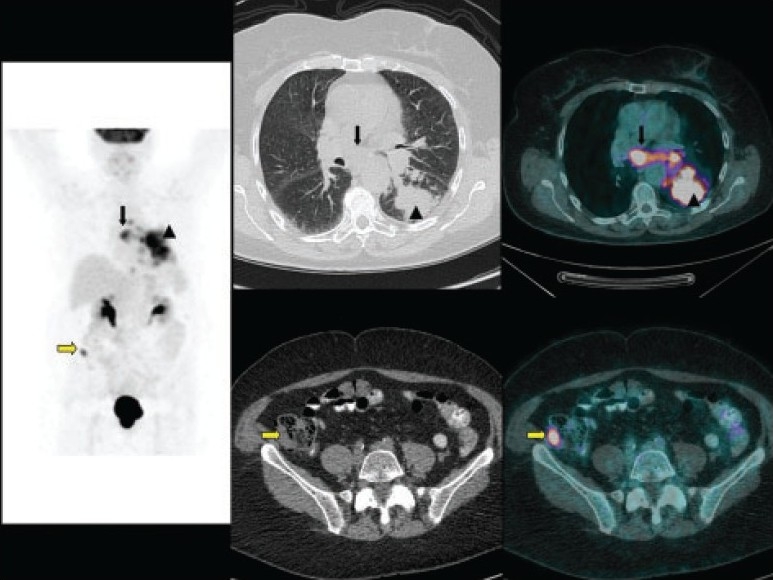
A 73-year-old woman who came for initial staging of non-small cell lung cancer. Maximum-intensity-projection (MIP) image (left panel), CT images (middle panels) and fused images (right panels) of 18F-FDG PET/CT show the primary tumor (arrow head) with mediastinal nodal metastases (black arrow). Incidental right iliac fossa small focal uptake (yellow arrow) is noted, which cross-correlated to a small soft tissue lesion in the cecum and turned out to be a synchronous primary adenocarcinoma.
Table 1.
Clinical indications of 18F-FDG PET/CT in oncology (include but are not limited to the following).
|
Modified from the European Association of Nuclear Medicine (EANM) and Society of Nuclear Medicine (SNM).
18F-FDG PET/CT is more sensitive and specific in certain cancers and has been applied primarily as a staging and restaging tool that can guide patient care. It has also been used to distinguish responders from nonresponders before any reduction in tumor size occurs. In some tumors, e.g., lymphoma, non-small cell lung cancer and esophageal cancer, reduction in the 18F-FDG PET activity within days or weeks of initiating therapy correlates significantly with prolonged survival and other clinical endpoints now used in drug approvals.
There is evidence that 18F-FDG PET/CT is particularly useful for detecting recurrence, especially in asymptomatic patients with rising tumor marker levels and those with negative or equivocal conventional imaging findings. Yet there are some limitations and areas of uncertainty, mainly regarding the lack of specificity of 18F-FDG uptake and the variable avidity of some cancers for this tracer. This article reviews the main applications, advantages and limitations of 18F-FDG PET/CT in oncology.
METHODS
A search was performed to identify mainly all published randomized controlled trials and systematic reviews in the English language literature. An additional search was performed to identify relevant unpublished systematic reviews. These publications comprised both retrospective and prospective studies of variable methodological quality. The consequences of false-positive and false-negative test results when evaluating the clinical usefulness of tests, as well as the impact of 18F-FDG PET/CT on the management of cancer patients, were also reviewed.
Breast Cancer
18F-FDG PET/CT has no role in the diagnosis of primary breast cancer as its ability to detect small and/ or noninvasive carcinomas is poor, with an overall sensitivity of only 68% for tumors of size <2 cm.1,2 For axillary nodal staging, 18F-FDG PET/CT has variable sensitivity (79%-94%) and specificity (86%-92%),3,4 and therefore the predictive accuracy is insufficient to recommend this modality for routine use.5
The most important current clinical applications of 18F-FDG PET/CT in breast cancer patients are for the detection and evaluation of recurrent or metastatic disease (Figure 2) and for monitoring response to therapy.6 In a patient-based analysis, it was shown that 18F-FDG PET/CT has a high overall sensitivity, specificity and accuracy for the detection of locoregional recurrence (89%, 84% and 87%, respectively) and distant metastases (100%, 97% and 98%, respectively) (Table 2) and is also more sensitive than the serum tumor marker CA 15-3 in detecting relapsed disease.7.
Figure 2.
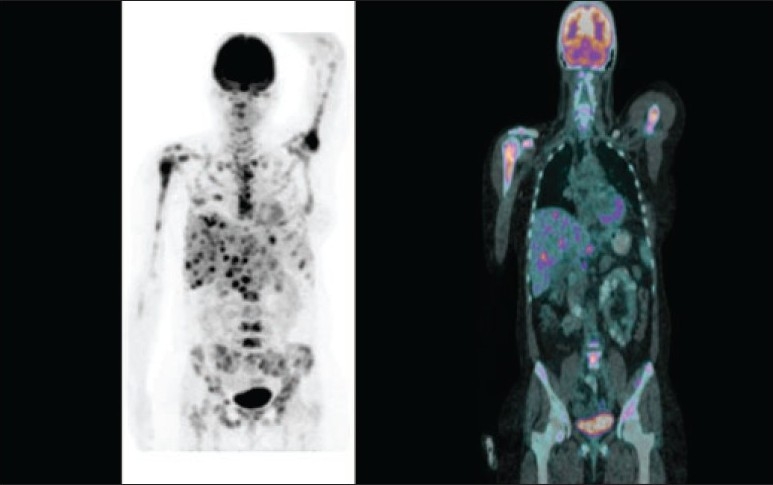
A 66-year-old woman who came for restaging of breast cancer. MIP image (left panel) and coronal fused images (right panel) of 18F-FDG PET/CT showed extensive hepatic and bony metastases.
Table 2.
Sensitivity, specificity and accuracy of 18F-FDG PET/CT in different tumor types and settings.
| Sensitivity (%) | Specificity (%) | Accuracy (%) | References | |
|---|---|---|---|---|
| Breast cancer | ||||
| Locoregional recurrence | 89 | 84 | 87 | 7 |
| Distant metastasis | 100 | 97 | 98 | 7 |
| Early response assessment | 83-100 | 85-94 | 88-91 | 8 |
| Colorectal cancer | ||||
| Recurrence | 89 | 92 | 90 | 12 |
| Intra-abdominal/extrahepatic recurrence | 88 | 94 | 92 | 12 |
| Extra-abdominal and/or hepatic recurrence | 95 | 100 | 99 | 12 |
| Oesophageal cancer | ||||
| Metastases (M-staging) | 43-78 | 93-99 | 62-86 | 30-34 |
| Recurrence (locoregional and distant) | 94 | 82 | 87 | 42 |
| Head and neck cancer | ||||
| Initial staging (nodal) | 94 | 84 | 90 | 46, 47 |
| Restaging/recurrence | 88 | 78 | 86 | 55 |
| Lung cancer | ||||
| Solitary pulmonary nodule | 81-100 | 63-100 | 90-92 | |
| Mediastinal staging (N2/N3) | 67-92 | 82-99 | 84-96 | 65-68 |
| Recurrence | 93-100 | 89-92 | 86-88 | |
| Response to treatment (≥80% threshold) | 90 | 100 | 96 | 89 |
| Lymphoma | ||||
| Initial staging and restaging (HL) | 86 | 96 | 95 | |
| Nodal involvement in HL or high-grade NHL | 94 | 100 | 96 | |
| Organ involvement in HL or high-grade NHL | 88 | 100 | 96 |
Detection of a decrease in the standardized uptake value (SUV) to a level below 55% of the baseline study is a powerful tool in monitoring histopathological response to chemotherapy for locally advanced breast cancers. Using this criterion, 18F-FDG PET/CT was found to have a sensitivity of 100%, a specificity of 85% and an accuracy of 88% in identifying responders after the first cycle, while corresponding values after the second cycle were 83%, 94% and 91%.8 After a single pulse of chemotherapy, 18F-FDG PET was able to predict complete pathological response with a sensitivity of 90% and a specificity of 74%.9 The reported overall survival in 18F-FDG PET/CT nonresponders is 8.8 months, compared with 19.2 months in responders.10 In the case of bone metastases, the responding bony lesion may become more sclerotic on the CT component of 18F-FDG PET/CT while its 18F-FDG activity reduces, which is a sign of bone healing.
Colorectal Cancer
In colorectal cancer, 18F-FDG PET/CT plays a pivotal role in the detection of recurrent disease, the assessment of residual post-therapy masses, the localization of recurrence in patients with an unexplained rise in serum carcinoembryonic antigen (CEA) and the staging of patients before surgical resection of local recurrence and distant metastatic disease.11 For the detection of intra-abdominal but extrahepatic colorectal recurrence, the sensitivity of 18F-FDG PET/CT is 88%; the specificity, 94%; and accuracy, 92%. For extra-abdominal and/ or hepatic recurrence, the sensitivity is 95%; specificity, 100%; and accuracy, 99%. The overall reported average sensitivity, specificity and accuracy for detecting recurrent disease are 89%, 92% and 90%, respectively (Table 2).12
The residual pelvic soft tissue abnormalities frequently seen in the tumor bed region after therapy usually complicate the detection of local recurrence by the conventional imaging techniques.13,14 Abnormal 18F-FDG activity in a residual pelvic soft tissue lesion after 6 months from the completion of radiotherapy most likely represents tumor recurrence, and accuracy and positive predictive value (PPV) are even higher after 12 months.11,15,16 Elevated CEA levels are seen in two-thirds of patients with recurrent colorectal cancer.17–20 18F-FDG PET/CT is recommended for patients with an unexplained increase in serum CEA level after primary curative treatment of colorectal cancer, since it changes the course of management in 59% to 68% of the patients (Figure 3).11,21–23
Figure 3.
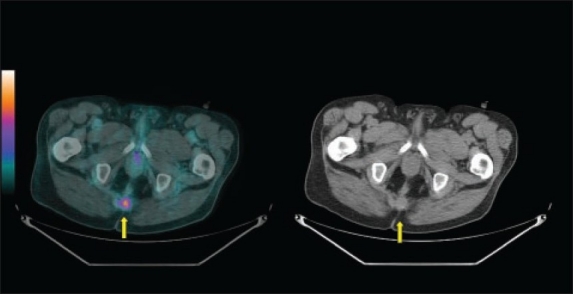
A 72-year-old man who had unexplained elevation of CEA during his follow-up after treatment of anorectal cancer. Fused (left panel) and CT (right panel) images of 18F-FDG PET/CT scan showed recurrent avid disease at the residual surgical bed soft tissue density (yellow arrow).
18F-FDG PET/CT is emerging as a potentially valuable technique in radiotherapy planning, as well as in the prediction and evaluation of response to therapy.11,24 The use of 18F-FDG PET/CT for preoperative radiotherapy planning in rectal cancer significantly alters both the gross tumor volume and the clinical target volume, with a mean increase in size of 25% and 4%, respectively.25
Esophageal Cancer
Endoscopic ultrasound (EUS) provides more accurate and cost-effective T-staging and N-staging than 18F-FDG PET/CT and conventional CT26–28 and remains the standard for local tumor evaluation.29 The most important role of 18F-FDG PET/CT in the initial staging of esophageal cancer lies in M-staging (Figure 4) through its ability to identify unexpected metastases (i.e., metastases not visible on conventional imaging), which are present in up to 30% of the patients. 18F-FDG PET/CT has better sensitivity, specificity and accuracy (43%-78%, 93%-99% and 62%-86%, respectively) than CT and EUS for the detection of distant metastases (Table 2).30–34 In M-staging, the addition of 18F-FDG PET/CT results in up-staging of 15% to 20% and down-staging of 5% to 7% of the patients.34,35 In addition, synchronous primary tumors are identified in 5.5% of patients, of which 75% are not identified by conventional imaging.36
Figure 4.
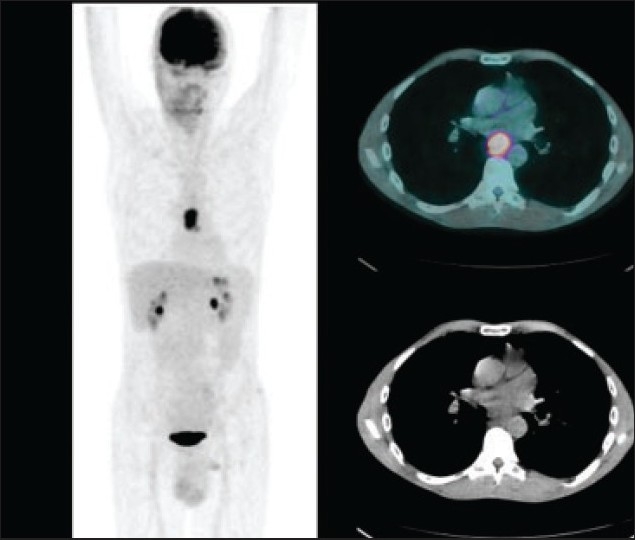
A 56-year-old man who came for initial staging of esophageal cancer. The MIP (left panel) and axial fused (right upper panel) and axial CT (right lower panel) images of 18F-FDG PET/CT showed the primary mid-esophageal tumor with no evidence of FDG-avid distant metastases.
Assessment of tumor response to neoadjuvant therapy by 18F-FDG PET/CT has been found to be an important prognostic factor,37 with a reported diagnostic accuracy of 85%; this is similar to the diagnostic accuracy of EUS (86%) and significantly higher than that of conventional CT (54%).38
In patients with squamous cell carcinoma of the esophagus and some inoperable cases, 18F-FDG PET/CT plays an important role in radiotherapy planning,39–41 with a reported modification of gross tumor volume in 56% of the patients and alteration of the planning treatment volume in 53%.41
18F-FDG PET is a highly sensitive tool for the detection of regional and distant recurrences, with a reported sensitivity, specificity and accuracy of 94%, 82% and 87%, respectively, in comparison to 81%, 82% and 81% for conventional imaging. Furthermore, 18F-FDG PET depicted recurrences in 12% of the patients with negative or equivocal findings on conventional imaging.42
Head and Neck Cancer
18F-FDG PET/CT has an impact on the assessment of both newly diagnosed and previously treated patients with head and neck cancer.43 18F-FDG PET/CT alters the initial clinical staging and TNM category of the tumor in 14% to 57% of the patients when compared with CT-based evaluation alone,44,45 and has an accuracy of approximately 90% compared with 86% for conventional CT.46,47
The reported sensitivity and specificity of standard 18F-FDG PET/CT for the detection of lymph node metastases in a per-patient analysis were 94% and 84%, respectively (Table 2), in comparison to 78% and 84% for conventional CT.48
18F-FDG PET/CT has been found to identify synchronous primaries in 8.1%, distant metastases in 15.4% and the site of an unknown primary in 73% of the patients with head and neck cancer.46 In addition, it alters the initial management in 18% to 37% of the patients.46,49 The impact of 18F-FDG PET/CT on radiotherapy planning is especially important: planning is changed in 29% of the patients,50 with an alteration in the gross tumor volume in 57% of the patients.45 It has been reported that the gross tumor volume is statistically significantly larger with 18F-FDG PET/CT–based assessment than with CT-based assessment.51,52 There is still a high risk of locoregional recurrence (18%-31%) and distant metastasis (20%-25%) despite aggressive treatment.53,54 The sensitivity, specificity and accuracy of 18F-FDG PET/CT in restaging patients with head and neck cancer are 88%, 78% and 86%, respectively.55
Postoperative, but pre-radiotherapy, 18F-FDG PET/CT evaluation within a median of 4 weeks after surgery has been found to alter the course of management in 15% of the patients.56 In addition, it has a higher accuracy than conventional CT when used at 4 to 8 weeks following the end of chemoradiotherapy, with an even higher sensitivity and specificity after 8 weeks.57
Lung Cancer
Correct initial staging of non-small cell lung cancer (NSCLC) is important in distinguishing operable patients from those who are inoperable, but can benefit from neoadjuvant treatment.58 The American College of Chest Physicians guidelines59 recommend 18F-FDG PET for noninvasive staging owing to the low sensitivity and specificity of the commonly used conventional CT in mediastinal nodal staging. 18F-FDG PET/CT is a more accurate method and is the emerging standard test for preoperative diagnosis and staging of NSCLC; it changes the course of management in up to 52% of cases and has a major role in reducing the number of futile thoracotomies.60–63
Diagnostic accuracy and sensitivity of 18F-FDG PET/CT staging of lung cancer in terms of operability have recently been reported to be 79% and 64%, respectively, in comparison to 60% and 32% for conventional staging64 The initial reported sensitivity and specificity for 18F-FDG PET in mediastinal nodal assessment are 67% to 92% and 82% to 99%, respectively (Table 2), in comparison to 25% to 71% and 66% to 98% for CT alone. Overall, the correct stage is assessed by 18F-FDG PET in 85% to 96% of the cases as compared with 58% to 59% by conventional CT alone, and 18F-FDG PET has a negative predictive value (NPV) of 97% (CT, 87%).65–67 Sensitivity, specificity and accuracy of 18F-FDG PET/CT for the depiction of malignant nodes are 85%, 84% and 84%, respectively, in comparison to 70%, 69% and 69% for CT alone.68
The high NPV of 18F-FDG PET/CT (up to 97%) for mediastinal disease69–71 has led to the recommendation to omit mediastinoscopy in patients with negative mediastinal 18F-FDG PET/CT.70,72,73 However, special attention should be paid to central tumors, which have a high incidence of occult N2 disease.74 If 18F-FDG PET/CT is positive, then mediastinoscopy is necessary to exclude a false-positive result.59 18F-FDG PET/CT detects unexpected extrathoracic metastases (Figure 5) in 11% to 15% of asymptomatic patients, avoiding futile surgical intervention.70,75,76
Figure 5.
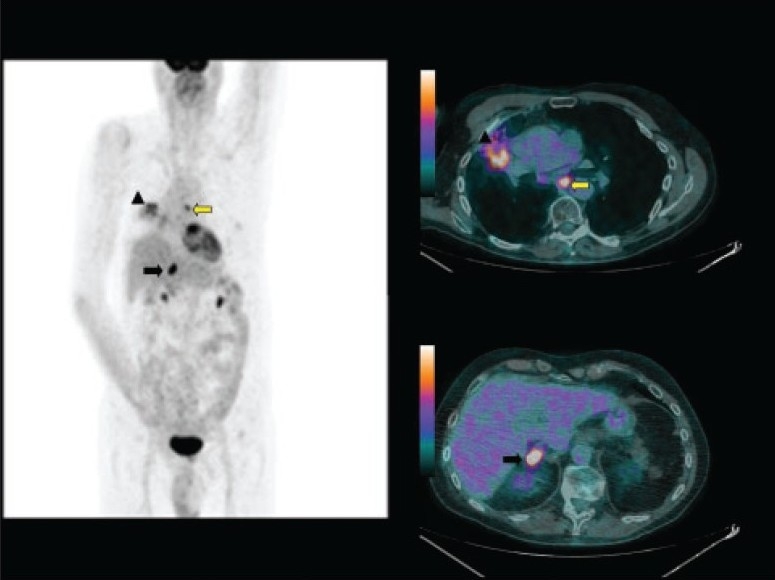
A 68-year-old man who came for initial staging of non-small cell lung cancer. MIP image (left panel) and fused images (right panel) of 18F-FDG PET/CT showed the primary tumor (arrow head) with mediastinal nodal involvement (yellow arrow) and extra-thoracic right adrenal metastasis (black arrow).
18F-FDG PET/CT is useful for radiation therapy planning since it provides more accurate initial staging, allowing omission of elective radiation of clinically uninvolved nodal stations.77 In addition, its CT data may be used for radiation therapy planning if properly acquired.78 This modality can be successfully applied to patients with limited-disease small cell lung cancer for whom the treatment is concurrent chemoradiotherapy, with a reported 24% change in the treatment field.79
Post-treatment fibrosis and scarring are common, and 18F-FDG PET/CT is more accurate than conventional CT in detecting residual and recurrent disease, which allows more reliable treatment planning decisions.80–83 In addition, conventional CT alone has been shown to be suboptimal in mediastinal restaging after treatment.84,85 18F-FDG PET has sensitivity of 93% to 100% and a specificity of 89% to 92% for detecting recurrent NSCLC.86–88 Patients with residual 18F-FDG uptake after treatment have a poor prognosis when compared to those without residual 18F-FDG uptake, taking into consideration the expected post-therapeutic inflammatory changes to avoid false-positive interpretation.84
Reduction in the baseline maximum SUV on 18F-FDG PET is predictive of a complete pathologic response with a sensitivity of 90%, a specificity of 100% and an accuracy of 96%, irrespective of the cell type or neoadjuvant treatment.89 Indeterminate solitary pulmonary nodules (SPNs) remain a clinical dilemma. 18F-FDG PET/CT currently should be reserved for cases where CT-guided fine-needle biopsy either is technically difficult or has been non-diagnostic.80 Compared with CT scan, 18F-FDG PET has similar sensitivity but better specificity in depicting malignancy in SPNs, the reported values ranging from 81% to 100% and from 63% to 100%, respectively.90–92
Lymphoma
18F-FDG PET/CT is now an established standard in the initial staging, monitoring of response to therapy and restaging after treatment of patients with Hodgkin lymphoma (HL) and high-grade non-Hodgkin lymphoma (NHL).93 The clinical utility of 18F-FDG PET/CT depends on the pathological subtype but not necessarily on the tumor grade.94 18F-FDG PET/CT shows a sensitivity of 86% and a specificity of 96%, in comparison to 81% and 41% with conventional CT alone, in disease assessment (presence or absence) of HL during both initial staging and restaging.95 In patients with HL or high-grade NHL, the sensitivity and specificity of 18F-FDG PET/CT for lymph node involvement are 94% and 100%, respectively, while for organ involvement they are 88% and 100% (Table 2).96
False-negative scans are noted in MALT (mucosal-associated lymphoid tissue) lymphomas, which are not highly metabolically active.97 Aggressive (high-grade) NHL typically shows more intense 18F-FDG activity in comparison to lower-grade NHL, although there is significant overlap between them.96 Detection of an FDG-avid lesion in a documented low-grade NHL should raise the suspicion of transformation to a higher-grade lymphoma.97,98 Infectious and/or inflammatory diseases are known causes of false-positive 18F-FDG PET/CT scans, and the possibility of their presence should be entertained at interpretation.99–102
Residual post-therapy masses are seen in up to 85% of the cases of HL and up to 40% of the cases of NHL.103,104 Early interim 18F-FDG PET/CT results (after two to four cycles) correlate well with event-free survival in HL (Figure 6)105–107 and high-grade NHL.108,109 In high-grade NHL, the event-free survival at 2 years and 5 years has been reported to be 82% and 88.8%, respectively, for negative interim PET patients in compassion to 43% and 16.2%, respectively, for positive interim PET patients.108,109 In another study, the 2-year event-free survival in HL patients with negative interim 18F-FDG PET was 95% in comparison to 12.8% in those with positive interim 18F-FDG PET.107
Figure 6.
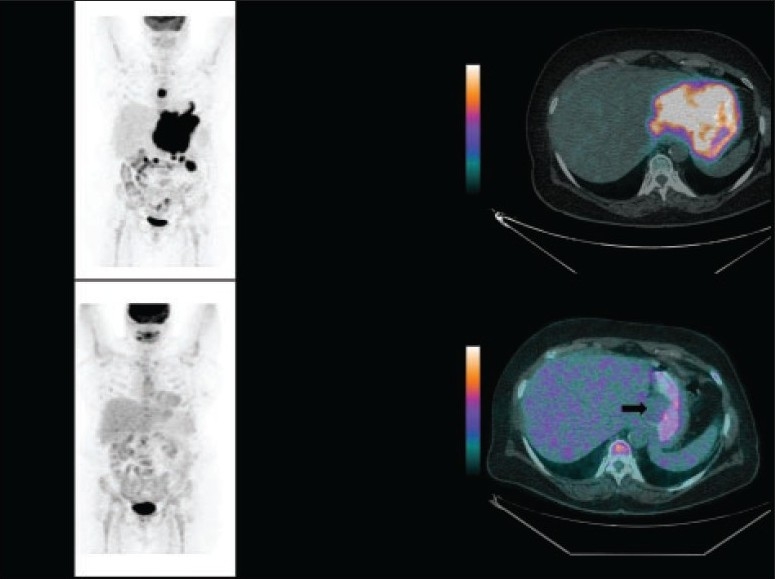
A 66-year-old woman diagnosed with Hodgkin lymphoma. The 18F-FDG PET/CT study (left and right upper panels) for initial staging showed nodal involvement above and below the diaphragm. 18F-FDG PET/CT after four cycles of chemotherapy (left and right lower panels) showed complete metabolic resolution of the disease with small non–FDG-avid residual soft tissue (black arrow on the fused image).
Thyroid Cancer
More than 90% of thyroid cancers are differentiated, comprising papillary and follicular carcinoma.110 In de-differentiated thyroid cancer, recurrent or metastatic tumor cells may lose the expression of sodium iodide symporter and have a decreased ability to concentrate radioiodine.111 A multicenter trial showed that the sensitivity of 18F-FDG PET is 85% in patients with raised thyroglobulin and negative 131I whole-body scans.112 In this subgroup of patients, 18F-FDG PET/CT alters clinical management in 23% to 51% of the pateints.113–118
Urological Cancer
Renal cell carcinoma
18F-FDG PET/CT has limited sensitivity in the evaluation of metastatic Renal cell carcinoma (RCC), particularly for small metastatic lesions. However, a positive 18F-FDG PET/CT scan should be considered strongly suspicious for local recurrence or metastasis because of the high specificity and PPV of this test. A combined test (PET/contrast-enhanced CT) may be necessary if important management decisions are to be based on the test result.119
Prostate cancer
Currently there is no established role for 18F-FDG PET/CT in the assessment of prostatic cancer, since it has a low accuracy owing to the relatively low metabolic rate of the tumor as well as the interfering adjacent urinary excretion of the tracer. However, other new PET radiotracers such as 11C-choline and 18F-fluorocholine have shown promising results in the management of prostate cancer.119
Bladder cancer
Currently there is no established role for 18F-FDG PET/CT in the assessment of bladder cancer, since the high adjacent physiological urinary excretion of the tracer renders the signal-to-noise ratio unfavorable for lesion detection.
Gynecological Cancers
Cervical cancer
18F-FDG PET(/CT) has a major role in preoperative staging of advanced cervical cancer and restaging after treatment.120,121 18F-FDG PET has a sensitivity of 86%, a specificity of 94% and an accuracy of 92% for detection of para-aortic nodal metastases in patients with advanced cervical cancer and negative abdominal CT.122 Furthermore, preoperative evaluation with 18F-FDG PET influences patient management in 18% of patients; while in the case of recurrent cervical cancer, 18F-FDG PET shows an overall sensitivity of 86% to 94% and specificity of 76% to 100%.123 The 2-year progression-free survival rate is 86% for patients with a negative post-treatment scan in comparison to 40% for those with persistent abnormal 18F-FDG uptake.124
Ovarian cancer
18F-FDG PET(/CT) has a major role in the evaluation of recurrent ovarian cancer when there is an increase in serum CA-125 and inconclusive or negative conventional (CT/MRI) imaging.121 The reported sensitivity and PPV of 18F-FDG PET/CT for detection of recurrent disease at least 1 cm in size are 83.3% and 93.8%, respectively.125
Cutaneous Melanoma
There is no role for 18F-FDG PET/CT in early cutaneous melanoma (American Joint Committee on Cancer stages I and II).126,127 In advanced (AJCC stages III and IV) and recurrent cutaneous melanoma, 18F-FDG PET shows 100% sensitivity for visceral and abdominal nodal metastases and 100% accuracy for superficial lymph node metastases, but lower sensitivity for pulmonary metastases.128 However, the CT component of a combined PET/CT scan would allow better evaluation of pulmonary metastases. The reported rate of synchronous tumor on 18F-FDG PET was 4.3%.129 18F-FDG PET results in changes in staging in 12% to 34% of the patients130,131 and changes in overall management in 8% to 61% of the patients.132,133
Brain Tumors
Sensitivity and specificity of 18F-FDG PET/CT in evaluating low-grade and recurrent tumors and treatment-induced changes are relatively low, mainly owing to the adjacent high physiological brain 18F-FDG activity; however, this can be improved significantly by co-registration with magnetic resonance imaging and potentially by delayed imaging. 18F-FDG PET/CT is capable of identifying anaplastic transformation of a documented low-grade tumor and has a prognostic value.134
Pitfalls
It is extremely important to consider some pitfalls of 18F-FDG PET/CT imaging during scan interpretation. The ability to detect tumors depends on various factors, such as their size, metabolic activity, the surrounding background activity and the serum glucose level. False-negative results may be obtained in small lesions (<7 mm), in tumors with a low metabolic rate (e.g., differentiated neuroendocrine tumors, prostate cancer, hepatocellular carcinoma, MALT and mucinous adenocarcinoma), in the presence of interfering cytostatic treatments that may decrease the tumor 18F-FDG uptake and when there is suboptimal preparation of patients with glucose intolerance or diabetes (since elevated serum glucose levels result in decreased FDG uptake in tumors owing to competitive inhibition). In addition, local high physiological FDG activity (as in the brain and the genitourinary tract) can render the signal-to-noise ratio unfavorable for lesion detection (Figure 7), and may give rise to a false-negative result by masking a malignant lesion.
Figure 7.
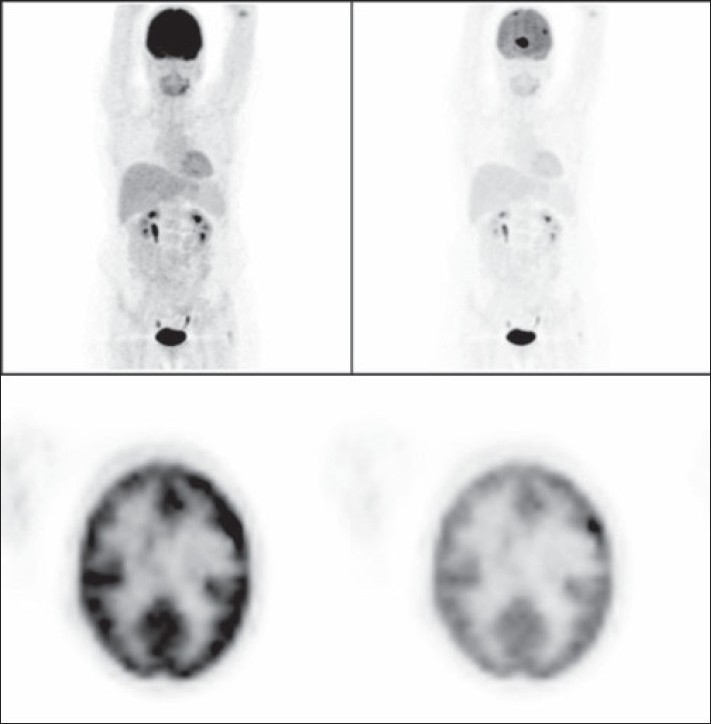
A 62-year-old woman with history of breast cancer. The left column images (MIP and axial PET images) show the normal-intensity images, which could hide metastatic deposits and give a false-negative result due to the physiological high background intensity of the brain. The same images after reducing their intensity on the right column show the metastatic deposits.
On the other hand, activated macrophages, neutrophils, fibroblasts and granulation tissue show increased 18F-FDG activity; therefore, infectious/ inflammatory processes (e.g., granulomatous diseases, abscesses, active thyroiditis), post-surgical changes (healing surgical wounds, scars, stoma, tube placement) and post-radiation changes (active fibrosis, radiation pneumonitis) may demonstrate increased 18F-FDG activity and cause a false-positive result.
The Future
As to the evolving role of 18F-FDG PET/CT and possible future directions for PET/CT, the need to evaluate early response to therapy remains, and there are no good imaging tools at present. Data shows that 18F-FDG PET/CT predicts not only response to therapy, but also further hard endpoints, such as time to progression. It is likely that more well-designed and large clinical studies on 18F-FDG PET/CT will expand its approved clinical indications in this context. Currently the majority of PET/CT investigations in oncology use 18F-FDG (glucose metabolic marker) as a tracer. However, the changing demand to evaluate tumor angiogenesis, tumor hypoxia, tumor cell proliferation and tumor receptors, has led to the development of other specific tracers, which will get greater clinical acceptance with time.
REFERENCES
- 1.Yang SK, Cho N, Moon WK. The role of PET/CT for evaluating breast cancer. Korean J Radiol. 2007;8:429–37. doi: 10.3348/kjr.2007.8.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avril N, Rosé CA, Schelling M, Dose J, Kuhn W, Bense S, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: Use and limitations. J Clin Oncol. 2000;18:3495–502. doi: 10.1200/JCO.2000.18.20.3495. [DOI] [PubMed] [Google Scholar]
- 3.Schirrmeister H, Kühn T, Guhlmann A, Santjohanser C, Hörster T, Nüssle K, et al. Fluorine-18 2-deoxy-2-fluoro-D-glucose PET in the preoperative staging of breast cancer: Comparison with the standard staging procedures. Eur J Nucl Med. 2001;28:351–8. doi: 10.1007/s002590000448. [DOI] [PubMed] [Google Scholar]
- 4.Greco M, Crippa F, Agresti R, Seregni E, Gerali A, Giovanazzi R, et al. Axillary lymph node staging in breast cancer by 2-fluoro-2-deoxy-D-glucose-positron emission tomography: Clinical evaluation and alternative management. J Natl Cancer Inst. 2001;93:630–5. doi: 10.1093/jnci/93.8.630. [DOI] [PubMed] [Google Scholar]
- 5.Benard F, Turcotte E. Imaging in breast cancer: Single-photon computed tomography and positron-emission tomography. Breast Cancer Res. 2005;7:153–62. doi: 10.1186/bcr1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eubank WB, Mankoff DA. Evolving role of positron emission tomography in breast cancer imaging. Semin Nucl Med. 2005;35:84–99. doi: 10.1053/j.semnuclmed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Kamel EM, Wyss MT, Fehr MK, von Schulthess GK, Goerres GW. [18F]-Fluorodeoxyglucose positron emission tomography in patients with suspected recurrence of breast cancer. J cancer Res Clin Oncol. 2003;129:147–53. doi: 10.1007/s00432-003-0424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schelling M, Avril N, Nährig J, Kuhn W, Römer W, Sattler D, et al. Positron emission tomography using [(18)F]Fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol. 2000;18:1689–95. doi: 10.1200/JCO.2000.18.8.1689. [DOI] [PubMed] [Google Scholar]
- 9.Smith IC, Welch AE, Hutcheon AW, Miller ID, Payne S, Chilcott F, et al. Positron emission tomography using [(18)F]-fluorodeoxy-D-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol. 2000;18:1676–88. doi: 10.1200/JCO.2000.18.8.1676. [DOI] [PubMed] [Google Scholar]
- 10.Dose Schwarz J, Bader M, Jenicke L, Hemminger G, Janicke F, Avril N. Early prediction of response to chemotherapy in metastatic breast cancer using sequential 18F-FDG PET. J Nucl Med. 2005;46:1144–50. [PubMed] [Google Scholar]
- 11.De Geus-Oei LF, Ruers TJ, Punt CJ, Leer JW, Corstens FH, Oyen WJ. FDG-PET in colorectal cancer. Cancer Imaging. 2006;6:S71–81. doi: 10.1102/1470-7330.2006.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Votrubova J, Belohlavek O, Jaruskova M, Oliverius M, Lohynska R, Trskova K, et al. The role of FDG-PET/CT in the detection of recurrent colorectal cancer. Eur J Nucl Med Mol Imaging. 2006;33:779–84. doi: 10.1007/s00259-006-0072-z. [DOI] [PubMed] [Google Scholar]
- 13.Chessin DB, Kiran RP, Akhurst T, Guillem JG. The emerging role of 18F-fluorodeoxyglucose positron emission tomography in the management of primary and recurrent rectal cancer. J Am Coll Surg. 2005;201:948–56. doi: 10.1016/j.jamcollsurg.2005.06.277. [DOI] [PubMed] [Google Scholar]
- 14.Ogunbiyi OA, Flanagan FL, Dehdashti F, Siegel BA, Trask DD, Birnbaum EH, et al. Detection of recurrent and metastatic colorectal cancer: Comparison of positron emission tomography and computed tomography. Ann Surg Oncol. 1997;4:613–20. doi: 10.1007/BF02303744. [DOI] [PubMed] [Google Scholar]
- 15.Moore HG, Akhurst T, Larson SM, Minsky BD, Mazumdar M, Guillem JG. A case-controlled study of 18-fluorodeoxyglucose positron emission tomography in the detection of pelvic recurrence in previously irradiated rectal cancer patients. J Am Coll Surg. 2003;197:22–8. doi: 10.1016/S1072-7515(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 16.Even-Sapir E, Parag Y, Lerman H, Gutman M, Levine C, Rabau M, et al. Detection of recurrence in patients with rectal cancer: PET/CT after abdominoperineal or anterior resection. Radiology. 2004;232:815–22. doi: 10.1148/radiol.2323031065. [DOI] [PubMed] [Google Scholar]
- 17.Esteves FP, Schuster DM, Halkar RK. Gastrointestinal tract malignancies and positron emission tomography: An overview. Semin Nucl Med. 2006;36:169–81. doi: 10.1053/j.semnuclmed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Liu FY, Chen JS, Changchien CR, Yeh CY, Liu SH, Ho KC, et al. Utility of 2-fluoro-2-deoxy-D-glucose positron emission tomography in managing patients of colorectal cancer with unexplained carcinoembryonic antigen elevation at different levels. Dis Colon Rectum. 2005;48:1900–12. doi: 10.1007/s10350-005-0097-6. [DOI] [PubMed] [Google Scholar]
- 19.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen C. An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer. JAMA. 1993;270:943–7. [PubMed] [Google Scholar]
- 20.Engaras B. Individual cutoff levels of carcinoembryonic antigen and CA 242 indicate recurrence of colorectal cancer with high sensitivity. Dis Colon Rectum. 2003;46:313–21. doi: 10.1007/s10350-004-6548-7. [DOI] [PubMed] [Google Scholar]
- 21.Flamen P, Hoekstra OS, Homans F, Van Cutsem E, Maes A, Stroobants S, et al. Unexplained rising carcinoembryonic antigen (CEA) in the postoperative surveillance of colorectal cancer: The utility of positron emission tomography (PET) Eur J Cancer. 2001;37:862–9. doi: 10.1016/s0959-8049(01)00049-1. [DOI] [PubMed] [Google Scholar]
- 22.Blokhuis TJ, van der Schaaf MC, van den Tol MP, Comans EF, Manoliu RA, van der Sijp JR. Results of radio frequency ablation of primary and secondary liver tumors: Long-term follow-up with computed tomography and positron emission tomography-18F-deoxyfluoroglucose scanning. Scand J Gastroenterol Suppl. 2004;241:93–7. doi: 10.1080/00855920410014623. [DOI] [PubMed] [Google Scholar]
- 23.Simó M, Lomeña F, Setoain J, Pérez G, Castellucci P, Costansa JM, et al. FDG-PET improves the management of patients with suspected recurrence of colorectal cancer. Nucl Med Commun. 2002;23:975–82. doi: 10.1097/00006231-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Ciernik IF, Dizendorf E, Baumert BG, Reiner B, Burger C, Davis JB, et al. Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): A feasibility study. Int J Radiat Oncol Biol Phys. 2003;57:853–63. doi: 10.1016/s0360-3016(03)00346-8. [DOI] [PubMed] [Google Scholar]
- 25.Bassi MC, Turri L, Sacchetti G, Loi G, Cannillo B, La Mattina P, et al. FDG-PET/CT imaging for staging and target volume delineation in preoperative conformal radiotherapy of rectal cancer. Int J Radiat Oncol Biol Phys. 2008;70:1423–6. doi: 10.1016/j.ijrobp.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 26.Pfau PR, Perlman SB, Stanko P, Frick TJ, Gopal DV, Said A, et al. The role and clinical value of EUS in a multimodality esophageal carcinoma staging program with CT and positron emission tomography. Gastrointest Endosc. 2007;65:377–84. doi: 10.1016/j.gie.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Watson DI, Lally C, Bessell JR. Endoscopic ultrasound for preoperative staging of esophageal carcinoma. Surg Endosc. 2005;19:1618–21. doi: 10.1007/s00464-005-0250-2. [DOI] [PubMed] [Google Scholar]
- 28.Gan SI, Rajan E, Adler DG, Baron TH, Anderson MA, et al. ASGE Standards of Practice Committee. Role of EUS. Gastrointest Endosc. 2007;66:425–34. doi: 10.1016/j.gie.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Yang GY, Wagner TD, Jobe BA, Thomas CR. The role of positron emission tomography in esophageal cancer. Gastrointest Cancer Res. 2008;2:3–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Kato H, Miyazaki T, Nakajima M, Takita J, Kimura H, Faried A, et al. The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer. 2005;103:148–56. doi: 10.1002/cncr.20724. [DOI] [PubMed] [Google Scholar]
- 31.Choi JY, Lee KH, Shim YM, Lee KS, Kim JJ, Kim SE, et al. Improved detection of individual nodal involvement in squamous cell carcinoma of the esophagus by FDG PET. J Nucl Med. 2000;41:808–15. [PubMed] [Google Scholar]
- 32.Yoon YC, Lee KS, Shim YM, Kim BT, Kim K, Kim TS. Metastasis to regional lymph nodes in patients with esophageal squamous cell carcinoma: CT versus FDG PET for presurgical detection prospective study. Radiology. 2003;227:764–70. doi: 10.1148/radiol.2281020423. [DOI] [PubMed] [Google Scholar]
- 33.Luketich JD, Friedman DM, Weigel TL, Meehan MA, Keenan RJ, Townsend DW, et al. Evaluation of distant metastases in esophageal cancer: 100 consecutive positron emission tomography scans. Ann Thorac Surg. 1999;68:1133–6. doi: 10.1016/s0003-4975(99)00974-1. [DOI] [PubMed] [Google Scholar]
- 34.Heeren PA, Jager PL, Bongaerts F, van Dullemen H, Sluiter W, Plukker JT. Detection of distant metastases in esophageal cancer with (18)F-FDG PET. J Nucl Med. 2004;45:980–7. [PubMed] [Google Scholar]
- 35.Flamen P, Lerut A, Van Cutsem E, De Wever W, Peeters M, Stroobants S, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol. 2000;18:3202–10. doi: 10.1200/JCO.2000.18.18.3202. [DOI] [PubMed] [Google Scholar]
- 36.van Westreenen HL, Westerterp M, Jager PL, van Dullemen HM, Sloof GW, Comans EF, et al. Synchronous primary neoplasms detected on 18F-FDG PET in staging of patients with esophageal cancer. J Nucl Med. 2005;46:1321–5. [PubMed] [Google Scholar]
- 37.Brücher BL, Weber W, Bauer M, Fink U, Avril N, Stein HJ, et al. Neoadjuvant therapy of esophageal squamous cell carcinoma: Response evaluation by positron emission tomography. Ann Surg. 2001;233:300–9. doi: 10.1097/00000658-200103000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westerterp M, van Westreenen HL, Reitsma JB, Hoekstra OS, Stoker J, Fockens P, et al. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy--systematic review. Radiology. 2005;326:841–51. doi: 10.1148/radiol.2363041042. [DOI] [PubMed] [Google Scholar]
- 39.Konski A, Doss M, Milestone B, Haluszka O, Hanlon A, Freedman G, et al. The integration of 18-fluoro-deoxy-glucose positron emission tomography and endoscopic ultrasound in the treatment-planning process for esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1123–8. doi: 10.1016/j.ijrobp.2004.07.717. [DOI] [PubMed] [Google Scholar]
- 40.Vrieze O, Haustermans K, De Wever W, Lerut T, Van Cutsem E, Ectors N, et al. Is there a role for FDG-PET in radiotherapy planning in esophageal carcinoma? Radiother Oncol. 2004;73:269–75. doi: 10.1016/j.radonc.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Moureau-Zabotto L, Touboul E, Lerouge D, Deniaud-Alexandre E, Grahek D, Foulquier JN, et al. Impact of CT and 18F-deoxyglucose positron emission tomography image fusion for conformal radiotherapy in esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2005;63:340–5. doi: 10.1016/j.ijrobp.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 42.Flamen P, Lerut A, Van Cutsem E, Cambier JP, Maes A, De Wever W, et al. The utility of positron emission tomography for the diagnosis and staging of recurrent esophageal cancer. J Thorac Cardiovasc Surg. 2000;120:1085–92. doi: 10.1067/mtc.2000.110464. [DOI] [PubMed] [Google Scholar]
- 43.Fleming AJ, Jr, Johansen ME. The clinician’s expectations from the use of positron emission tomography/computed tomography scanning in untreated and treated head and neck cancer patients. Curr Opin Otolaryngol Head Neck Surg. 2008;16:127–34. doi: 10.1097/MOO.0b013e3282f4939a. [DOI] [PubMed] [Google Scholar]
- 44.Koshy M, Paulino AC, Howell R, Schuster D, Halkar R, Davis LW. F-18 FDG PET-CT fusion in radiotherapy treatment planning for head and neck cancer. Head Neck. 2005;27:494–502. doi: 10.1002/hed.20179. [DOI] [PubMed] [Google Scholar]
- 45.Wang D, Schultz CJ, Jursinic PA, Bialkowski M, Zhu XR, Brown WD, et al. Initial experience of FDG-PET/CT guided IMRT of head-and-neck carcinoma. Int J Radiat Oncol Biol Phys. 2006;65:143–51. doi: 10.1016/j.ijrobp.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 46.Fleming AJ, Jr, Smith SP, Jr, Paul CM, Hall NC, Daly BT, Agrawal A, et al. Impact of [18F]-2-fluorodeoxyglucose-positron emission tomography/computed tomography on previously untreated head and neck cancer patients. Laryngoscope. 2007;117:1173–9. doi: 10.1097/MLG.0b013e31805d017b. [DOI] [PubMed] [Google Scholar]
- 47.Gordin A, Golz A, Keidar Z, Daitzchman M, Bar-Shalom R, Israel O. The role of FDG-PET/CT imaging in head and neck malignant conditions: Impact on diagnostic accuracy and patient care. Otolaryngol Head Neck Surg. 2007;137:130–7. doi: 10.1016/j.otohns.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto Y, Wong T, Turkington T, Hawk T, Coleman R. Head and neck cancer: Dedicated FDG PET/CT protocol for detection – phantom and initial clinical studies. Radiology. 2007;244:263–72. doi: 10.1148/radiol.2433060043. [DOI] [PubMed] [Google Scholar]
- 49.Zanation AM, Sutton DK, Couch ME, Weissler MC, Shockley WW, Shores CG. Use, accuracy, and implications for patient management of [18F]-2-fluorodeoxyglucose-positron emission/computerized tomography for head and neck tumors. Laryngoscope. 2005;115:1186–90. doi: 10.1097/01.MLG.0000163763.89647.9F. [DOI] [PubMed] [Google Scholar]
- 50.Connell CA, Corry J, Milner AD, Hogg A, Hicks RJ, Rischin D, et al. Clinical impact of, and prognostic stratification by, F-18 FDG PET/CT in head and neck mucosal squamous cell carcinoma. Head Neck. 2007;29:986–95. doi: 10.1002/hed.20629. [DOI] [PubMed] [Google Scholar]
- 51.Deantonio L, Beldì D, Gambaro G, Loi G, Brambilla M, Inglese E, et al. FDG-PET/CT imaging for staging and radiotherapy treatment planning of head and neck carcinoma. Radiat Oncol. 2008;3:29. doi: 10.1186/1748-717X-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz DL, Ford E, Rajendran J, Yueh B, Coltrera MD, Virgin J, et al. FDG-PET/CT imaging for preradiotherapy staging of head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:129–36. doi: 10.1016/j.ijrobp.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 53.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–52. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 54.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–44. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 55.Halpern BS, Yeom K, Fueger BJ, Lufkin RB, Czernin J, Allen-Auerbach M. Evaluation of suspected local recurrence in head and neck cancer: A comparison between PET and PET/CT for biopsy proven lesions. Eur J Radiol. 2007;62:199–204. doi: 10.1016/j.ejrad.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 56.Shintani SA, Foote RL, Lowe VJ, Brown PD, Garces YI, Kasperbauer JL. Utility of PET/CT imaging performed early after surgical resection in the adjuvant treatment planning for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70:322–9. doi: 10.1016/j.ijrobp.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 57.Andrade RS, Heron DE, Degirmenci B, Filho PA, Branstetter BF, Seethala RR, et al. Posttreatment assessment of response using FDG-PET/CT for patients treated with definitive radiation therapy for head and neck cancers. Int J Radiat Oncol Biol Phys. 2006;65:1315–22. doi: 10.1016/j.ijrobp.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 58.Miele E, Spinelli GP, Tomao F, Zullo A, De Marinis F, Pasciuti G, et al. Positron emission tomography (PET) radiotracers in oncology--utility of 18F-fluoro-deoxy-glucose (FDG)-PET in the management of patients with non-small-cell lung cancer (NSCLC) J Exp Clin Cancer Res. 2008;27:52. doi: 10.1186/1756-9966-27-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silvestri GA, Tanoue LT, Margolis ML, Barker J, Detterbeck F. The noninvasive staging of non-small cell lung cancer: The guidelines. Chest. 2003;123:147S–56. doi: 10.1378/chest.123.1_suppl.147s. [DOI] [PubMed] [Google Scholar]
- 60.Diva F, Oliveira A, Barroso A, Conde S, Parente B. Positron emission tomography: Indications in lung cancer - a prospective experience. Rev Port Pneumol. 2005;11:12–3. [PubMed] [Google Scholar]
- 61.Gupta NC, Graeber GM, Bishop HA. Comparative efficacy of positron emission tomography with fluorodeoxyglucose in evaluation of small (<1 cm), intermediate (1 to 3 cm), and large (>3 cm) lymph node lesions. Chest. 2000;117:773–8. doi: 10.1378/chest.117.3.773. [DOI] [PubMed] [Google Scholar]
- 62.Weber WA, Petersen V, Schmidt B, Tyndale-Hines L, Link T, Peschel C, et al. Positron emission tomography in non-small cell lung cancer: Prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol. 2003;21:2651–7. doi: 10.1200/JCO.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Eschmann SM, Friedel G, Paulsen F, Reimold M, Hehr T, Budach W, et al. 18F-18F-FDG PET for assessment of therapy response and preoperative re-evaluation after neoadjuvant radiochemotherapy in stage III non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2007;34:463–71. doi: 10.1007/s00259-006-0273-5. [DOI] [PubMed] [Google Scholar]
- 64.Fischer B, Lassen U, Mortensen J, Larsen S, Loft A, Bertelsen A, et al. Preoperative staging of lung cancer with PET-CT. N Eng J Med. 2009;361:32–9. doi: 10.1056/NEJMoa0900043. [DOI] [PubMed] [Google Scholar]
- 65.Steinert HC, Hauser M, Allemann F, Engel H, Berthold T, von Schulthess GK, et al. Non-small cell lung cancer: Nodal staging with FDG PET versus CT with correlative lymph node mapping and sampling. Radiology. 1997;202:441–6. doi: 10.1148/radiology.202.2.9015071. [DOI] [PubMed] [Google Scholar]
- 66.Marom EM, McAdams HP, Erasmus JJ, Goodman PC, Culhane DK, Coleman RE, et al. Staging non-small cell lung cancer with whole-body PET. Radiology. 1999;212:803–9. doi: 10.1148/radiology.212.3.r99se21803. [DOI] [PubMed] [Google Scholar]
- 67.Gould MK, Kuschner WG, Rydzak CE, Maclean CC, Demas AN, Shigemitsu H, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: A metaanalysis. Ann Intern Med. 2003;139:879–92. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- 68.Shim SS, Lee KS, Kim BT, Chung MJ, Lee EJ, Han J, et al. Non-small cell lung cancer: Prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005;236:1011–9. doi: 10.1148/radiol.2363041310. [DOI] [PubMed] [Google Scholar]
- 69.Pieterman RM, van Putten JW, Meuzelaar JJ, Mooyaart EL, Vaalburg W, Koèter GH, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343:254–61. doi: 10.1056/NEJM200007273430404. [DOI] [PubMed] [Google Scholar]
- 70.Verhagen AF, Bootsma GP, Tjan-Heijnen VC, van der Wilt GJ, Cox AL, Brouwer MH, et al. FDG-PET in staging lung cancer: How does it change the algorithm? Lung cancer. 2004;44:175–81. doi: 10.1016/j.lungcan.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 71.Vansteenkiste JF, Stroobants SG, De Leyn PR, Dupont PJ, Bogaert J, Maes A, et al. Lymph node staging in non-small-cell lung cancer with FDG-PET scan: A prospective study on 690 lymph node stations from 68 patients. J Clin Oncol. 1998;16:2142–9. doi: 10.1200/JCO.1998.16.6.2142. [DOI] [PubMed] [Google Scholar]
- 72.Jett JR. How to optimize staging in early non-small cell lung cancer. Lung cancer. 2002;38:S13–6. doi: 10.1016/s0169-5002(02)00246-5. [DOI] [PubMed] [Google Scholar]
- 73.Kramer H, Groen HJ. Current concepts in the mediastinal lymph node staging of nonsmall cell lung cancer. Ann Surg. 2003;238:180–8. doi: 10.1097/01.SLA.0000081086.37779.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al-Sarraf N, Aziz R, Gately K, Lucey J, Wilson L, McGovern E, et al. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardiothorac Surg. 2008;33:104–9. doi: 10.1016/j.ejcts.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 75.Weder W, Schmid RA, Bruchhaus H, Hillinger S, von Schulthess GK, Steinert HC. Detection of extrathoracic metastases by positron emission tomography in lung cancer. Ann Thorac Surg. 1998;66:886–92. doi: 10.1016/s0003-4975(98)00675-4. [DOI] [PubMed] [Google Scholar]
- 76.Valk PE, Pounds TR, Hopkins DM, Haseman MK, Hofer GA, Greiss HB, et al. Staging non-small cell lung cancer by whole-body positron emission tomographic imaging. Ann Thorac Surg. 1995;60:1573–81. doi: 10.1016/0003-4975(95)00752-0. [DOI] [PubMed] [Google Scholar]
- 77.De Ruysscher D, Wanders S, van Haren E, Hochstenbag M, Geeraedts W, Utama I, Simons J, et al. Selective mediastinal node irradiation based on FDG-PET scan data in patients with non-small-cell lung cancer: A prospective clinical study. Int J Radiat Oncol Biol Phys. 2005;62:988–94. doi: 10.1016/j.ijrobp.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 78.Bradley J, Thorstad WL, Mutic S, Miller TR, Dehdashti F, Siegel BA, et al. Impact of FDG-PET on radiation therapy volume delineation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;59:78–86. doi: 10.1016/j.ijrobp.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 79.van Loon J, Offermann C, Bosmans G, Wanders R, Dekker A, Borger J, et al. 18FDG-PET based radiation planning of mediastinal lymph nodes in limited disease small cell lung cancer changes radiotherapy fields: A planning study. Radiother Oncol. 2008;87:49–54. doi: 10.1016/j.radonc.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 80.Joshi SC, Pant I, Hamzah F, Kumar G, Shukla AN. Integrated positron emission tomography/computed tomography fusion imaging: An emerging gold standard in lung cancer. Indian J Cancer. 2008;45:137–41. doi: 10.4103/0019-509x.44660. [DOI] [PubMed] [Google Scholar]
- 81.Kiffer JD, Berlangieri SU, Scott AM, Quong G, Feigen M, Schumer W, et al. The contribution of 18F-fluoro-2-deoxy-glucose positron emission tomographic imaging to radiotherapy planning in lung cancer. Lung cancer. 1998;19:167–77. doi: 10.1016/s0169-5002(97)00086-x. [DOI] [PubMed] [Google Scholar]
- 82.Mac Manus MP, Hicks RJ. PET scanning in lung cancer: Current status and future directions. Semin Surg Oncol. 2003;21:149–55. doi: 10.1002/ssu.10032. [DOI] [PubMed] [Google Scholar]
- 83.Hellwig D, Gröschel A, Graeter TP, Hellwig AP, Nestle U, Schäfers HJ, et al. Diagnostic performance and prognostic impact of FDG-PET in suspected recurrence of surgically treated non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2006;33:13–21. doi: 10.1007/s00259-005-1919-4. [DOI] [PubMed] [Google Scholar]
- 84.Rohren EM, Lowe VJ. Update in PET imaging of nonsmall cell lung cancer. Semin Nucl Med. 2004;34:134–53. doi: 10.1053/j.semnuclmed.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 85.Stroobants S, Verschakelen J, Vansteenkiste J. Value of FDG-PET in the management of non-small cell lung cancer. Eur J radiol. 2003;45:49–59. doi: 10.1016/s0720-048x(02)00282-6. [DOI] [PubMed] [Google Scholar]
- 86.Bury T, Corhay JL, Duysinx B, Daenen F, Ghaye B, Barthelemy N, et al. Value of FDG-PET in detecting residual or recurrent nonsmall cell lung cancer. Eur Respir J. 1999;14:1376–80. doi: 10.1183/09031936.99.14613769. [DOI] [PubMed] [Google Scholar]
- 87.Hellwig D, Gröschel A, Graeter TP, Hellwig AP, Nestle U, Schäfers HJ, et al. Diagnostic performance and prognostic impact of FDG-PET in suspected recurrence of surgically treated non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2006;33:13–21. doi: 10.1007/s00259-005-1919-4. [DOI] [PubMed] [Google Scholar]
- 88.Hicks RJ, Kalff V, MacManus MP, Ware RE, McKenzie AF, Matthews JP, et al. The utility of (18)F-FDG PET for suspected recurrent non-small cell lung cancer after potentially curative therapy: Impact on management and prognostic stratification. J Nucl Med. 2001;42:1605–13. [PubMed] [Google Scholar]
- 89.Cerfolio RJ, Bryant AS, Winokur TS, Ohja B, Bartolucci AA. Repeat FDG-PET after neoadjuvant therapy is a predictor of pathologic response in patients with non-small cell lung cancer. Ann Thorac Surg. 2004;78:1903–9. doi: 10.1016/j.athoracsur.2004.06.102. [DOI] [PubMed] [Google Scholar]
- 90.Lowe V, Naunheim K. Positron emission tomography in lung cancer. Ann Thorac Surg. 1998;65:1821–9. doi: 10.1016/s0003-4975(98)00106-4. [DOI] [PubMed] [Google Scholar]
- 91.Behzadi A, Ung Y, Lowe V, Deschamps C. The role of positron emission tomography in the management of non-small cell lung cancer. Can J Surg. 2009;52:235–42. [PMC free article] [PubMed] [Google Scholar]
- 92.Fletcher JW, Kymes SM, Gould M, Alazraki N, Coleman RE, Lowe VJ. et al.A comparison of the diagnostic accuracy of 18F-FDG PET and CT in the characterization of solitary pulmonary nodules. J Nucl Med. 2008;49:179–85. doi: 10.2967/jnumed.107.044990. [DOI] [PubMed] [Google Scholar]
- 93.Dhanapathi H, Kumar R. F-18 FDG PET/PET-CT in the management of lymphoma. Indian J Med Pediatr Oncol. 2007;28:17–23. [Google Scholar]
- 94.Elstrom R, Guan L, Baker G, Nakhoda K, Vergilio JA, Zhuang H, et al. Utility of FDG-PET scanning in lymphoma by WHO classification. Blood. 2003;101:3875–6. doi: 10.1182/blood-2002-09-2778. [DOI] [PubMed] [Google Scholar]
- 95.Stumpe KD, Urbinelli M, Steinert HC, Glanzmann C, Buck A, von Schulthess GK. Whole-body positron emission tomography using fluorodeoxyglucose for staging of lymphoma: Effectiveness and comparison with computed tomography. Eur J Nucl Med. 1998;25:721–8. doi: 10.1007/s002590050275. [DOI] [PubMed] [Google Scholar]
- 96.Schaefer NG, Hany TF, Taverna C, Seifert B, Stumpe KD, von Schulthess GK, et al. Non-Hodgkin lymphoma and Hodgkin disease: Coregistered FDG PET and CT at staging and restaging: Do we need contrast-enhanced CT? Radiology. 2004;232:823–89. doi: 10.1148/radiol.2323030985. [DOI] [PubMed] [Google Scholar]
- 97.Hoffmann M, Kletter K, Diemling M, Becherer A, Pfeffel F, Petkov V, et al. Positron emission tomography with fluorine-18-2-fluoro-2-deoxy-D-glucose (F18-FDG) does not visualize extranodal B-cell lymphoma of the mucosa-associated lymphoid tissue (MALT)-type. Ann Oncol. 1999;10:1185–9. doi: 10.1023/a:1008312726163. [DOI] [PubMed] [Google Scholar]
- 98.Schöder H, Noy A, Gönen M, Weng L, Green D, Erdi YE, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:4643–51. doi: 10.1200/JCO.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 99.MacManus MP, Seymour JF, Hicks RJ. Overview of early response assessment in lymphoma with FDG-PET. Cancer Imaging. 2007;7:10–8. doi: 10.1102/1470-7330.2007.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Joe A, Hoegerle S, Moser E. Cervical lymph node sarcoidosis as a pitfall in F-18 FDG positron emission tomography. Clin Nucl Med. 2001;26:542–3. doi: 10.1097/00003072-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 101.Taaleb K, Kaiser KP, Wieler H. Elevated uptake of F-18 FDG in PET scans in nonmalignant disease. Clin Nucl Med. 2000;25:939–40. doi: 10.1097/00003072-200011000-00026. [DOI] [PubMed] [Google Scholar]
- 102.Sandherr M, von Schilling C, Link T, Stock K, von Bubnoff N, Peschel C, et al. Pitfalls in imaging Hodgkin’s disease with computed tomography and positron emission tomography using fluorine-18-fluorodeoxyglucose. Ann Oncol. 2001;12:719–22. doi: 10.1023/a:1011136324038. [DOI] [PubMed] [Google Scholar]
- 103.Cremerius U, Fabry U, Neuerburg J, Zimny M, Osieka R, Buell U. Positron emission tomography with 18F-FDG to detect residual disease after therapy for malignant lymphoma. Nucl Med Commun. 1998;19:1055–63. doi: 10.1097/00006231-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 104.Talbot JN, Haioun C, Rain JD, Meignan M, Wioland M, Misset JL, et al. [18F]-FDG positron imaging in clinical management of lymphoma patients. Crit Rev Oncol Hematol. 2001;38:193–221. doi: 10.1016/s1040-8428(01)00127-5. [DOI] [PubMed] [Google Scholar]
- 105.Hutchings M, Loft A, Hansen M, Pedersen LM, Buhl T, Jurlander J, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107:52–9. doi: 10.1182/blood-2005-06-2252. [DOI] [PubMed] [Google Scholar]
- 106.Hutchings M, Mikhaeel NG, Fields PA, Nunan T, Timothy AR. Prognostic value of interim FDG-PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann Oncol. 2005;16:1160–8. doi: 10.1093/annonc/mdi200. [DOI] [PubMed] [Google Scholar]
- 107.Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: A report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–52. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- 108.Mikhaeel NG, Hutchings M, Fields PA, O’Doherty MJ, Timothy AR. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol. 2005;16:1514–23. doi: 10.1093/annonc/mdi272. [DOI] [PubMed] [Google Scholar]
- 109.Haioun C, Itti E, Rahmouni A, Brice P, Rain JD, Belhadj K, et al. [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: An early prognostic tool for predicting patient outcome. Blood. 2005;106:1376–81. doi: 10.1182/blood-2005-01-0272. [DOI] [PubMed] [Google Scholar]
- 110.Sherman SI. Thyroid carcinoma. Lancet. 2003;361:501–11. doi: 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 111.Min JJ, Chung JK, Lee YJ, Jeong JM, Lee DS, Jang JJ, et al. Relationship between expression of the sodium/iodide symporter and 131I uptake in recurrent lesions of differentiated thyroid carcinoma. Eur J Nucl Med. 2001;28:639–45. [PubMed] [Google Scholar]
- 112.Horn J, Lock-Andersen J, Sjostrand H, Loft A. Routine use of FDG-PET scans in melanoma patients with positive sentinel node biopsy. Eur J Nucl Med Mol Imaging. 2006;33:887–92. doi: 10.1007/s00259-006-0077-7. [DOI] [PubMed] [Google Scholar]
- 113.Grünwald F, Kälicke T, Feine U, Lietzenmayer R, Scheidhauer K, Dietlein M, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography in thyroid cancer: Results of a multicentre study. Eur J Nucl Med. 1999;26:1547–52. doi: 10.1007/s002590050493. [DOI] [PubMed] [Google Scholar]
- 114.Nahas Z, Goldenberg D, Fakhry C, Ewertz M, Zeiger M, Ladenson PW, et al. The role of positron emission tomography/computed tomography in the management of recurrent papillary thyroid carcinoma. Laryngoscope. 2005;115:237–43. doi: 10.1097/01.mlg.0000154725.00787.00. [DOI] [PubMed] [Google Scholar]
- 115.Palmedo H, Bucerius J, Joe A, Strunk H, Hortling N, Meyka S, et al. Integrated PET/CT in differentiated thyroid cancer: Diagnostic accuracy and impact on patient management. J Nucl Med. 2006;47:616–24. [PubMed] [Google Scholar]
- 116.Zoller M, Kohlfuerst S, Igerc I, Kresnik E, Gallowitsch HJ, Gomez I, et al. Combined PET/CT in the follow-up of differentiated thyroid carcinoma: What is the impact of each modality.? Eur J Nucl Med Mol Imaging. 2007;34:487–95. doi: 10.1007/s00259-006-0276-2. [DOI] [PubMed] [Google Scholar]
- 117.Iagaru A, Kalinyak JE, McDougall IR. F-18 FDG PET/CT in the management of thyroid cancer. Clin Nucl Med. 2007;32:690–5. doi: 10.1097/RLU.0b013e318125037a. [DOI] [PubMed] [Google Scholar]
- 118.Shammas A, Degirmenci B, Mountz JM, McCook BM, Branstetter B, Bencherif B, et al. 18F-FDG PET/CT in patients with suspected recurrent or metastatic well-differentiated thyroid cancer. J Nucl Med. 2007;48:221–6. [PubMed] [Google Scholar]
- 119.Zuijdwijk MD, Vogel WV, Corstens FH, Oyen WJ. Utility of fluorodeoxyglucose-PET in patients with differentiated thyroid carcinoma. Nucl Med Commun. 2008;29:636–41. doi: 10.1097/MNM.0b013e3282f813e1. [DOI] [PubMed] [Google Scholar]
- 120.Tsakiris P, De la Rosette J. Imaging in genitourinary cancer from the urologists’ perspective. Cancer Imaging. 2007;7:84–92. doi: 10.1102/1470-7330.2007.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakamoto Y, Saga T, Fujii S. Positron emission tomography application for gynecologic tumors. Int J Gynecol Cancer. 2005;15:701–9. doi: 10.1111/j.1525-1438.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 122.Basu S, Li G, Alavi A. PET and PET-CT imaging of gynecological malignancies: Present role and future promise. Expert Rev Anticancer Ther. 2009;9:75–96. doi: 10.1586/14737140.9.1.75. [DOI] [PubMed] [Google Scholar]
- 123.Lin WC, Hung YC, Yeh LS, Kao CH, Yen RF, Shen YY. Usefulness of (18)F-fluorodeoxyglucose positron emission tomography to detect para-aortic lymph nodal metastasis in advanced cervical cancer with negative computed tomography findings. Gynecol Oncol. 2003;89:73–6. doi: 10.1016/s0090-8258(03)00058-1. [DOI] [PubMed] [Google Scholar]
- 124.Belhocine T, Thille A, Fridman V, Albert A, Seidel L, Nickers P, et al. Contribution of whole-body 18FDG PET imaging in the management of cervical cancer. Gynecol Oncol. 2002;87:90–7. doi: 10.1006/gyno.2002.6769. [DOI] [PubMed] [Google Scholar]
- 125.Grigsby PW, Siegel BA, Dehdashti F, Mutch DG. Posttherapy surveillance monitoring of cervical cancer by FDG-PET. Int J Radiat Oncol Biol Phys. 2003;55:907–13. doi: 10.1016/s0360-3016(02)04287-6. [DOI] [PubMed] [Google Scholar]
- 126.Bristow RE, del Carmen MG, Pannu HK, Cohade C, Zahurak ML, Fishman EK, et al. Clinically occult ovarian cancer: Patient selection for secondary cytoreductive surgery using combined PET/CT. Gynecol Oncol. 2003;90:519–28. doi: 10.1016/s0090-8258(03)00336-6. [DOI] [PubMed] [Google Scholar]
- 127.Ho Shona I, Chungc D, Sawd R, Thompsond J. Imaging in cutaneous melanoma. Nucl Med Commun. 2008;29:847–76. doi: 10.1097/MNM.0b013e32830439fb. [DOI] [PubMed] [Google Scholar]
- 128.Havenga K, Cobben DC, Oyen WJ, Nienhuijs S, Hoekstra HJ, Ruers TJ, et al. Fluorodeoxyglucose-positron emission tomography and sentinel lymph node biopsy in staging primary cutaneous melanoma. Eur J Surg Oncol. 2003;29:662–4. doi: 10.1016/s0748-7983(03)00147-1. [DOI] [PubMed] [Google Scholar]
- 129.Gritters LS, Francis IR, Zasadny KR, Wahl RL. Initial assessment of positron emission tomography using 2-fluorine-18-fluoro-2-deoxy-Dglucose in the imaging of malignant melanoma. J Nucl Med. 1993;34:1420–7. [PubMed] [Google Scholar]
- 130.Bastiaannet E, Oyen WJ, Meijer S, Hoekstra OS, Wobbes T, Jager PL, et al. Impact of [18F]fluorodeoxyglucose positron emission tomography on surgical management of melanoma patients. Br J Surg. 2006;93:243–9. doi: 10.1002/bjs.5174. [DOI] [PubMed] [Google Scholar]
- 131.Eigtved A, Andersson AP, Dahlstrom K, Rabol A, Jensen M, Holm S, et al. Use of fluorine-18 fluorodeoxyglucose positron emission tomography in the detection of silent metastases from malignant melanoma. Eur J Nucl Med. 2000;27:70–5. doi: 10.1007/pl00006666. [DOI] [PubMed] [Google Scholar]
- 132.Jadvar H, Johnson DL, Segall GM. The effect of fluorine-18 fluorodeoxyglucose positron emission tomography on the management of cutaneous malignant melanoma. Clin Nucl Med. 2000;25:48–51. doi: 10.1097/00003072-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 133.Fulham M, Kelley B, Ramshaw J, Scott A. Impact of FDG PET on the management of patients with suspected or proven metastatic melanoma prior to surgery: A prospective, multi-centre study as part of the Australian PET Data Collection Project. Paper presented at: 54th Annual Meeting of the Society of Nuclear Medicine, Washington, DC. 2007. [Google Scholar]
- 134.Chen W, Silverman D. Advances in evaluation of primary brain tumors. Semin Nucl Med. 2008;38:240–50. doi: 10.1053/j.semnuclmed.2008.02.005. [DOI] [PubMed] [Google Scholar]


