Abstract
Fluconazole (FLZ) has emerged as a highly successful agent in the management of systemic infections of Candida. Cure rates for symptomatic candidiasis following single 150-mg FLZ dose therapy exceed 90%. In vitro, however, FLZ is fungistatic only in a narrow pH range and is not effective at vaginal pH, 4.2. This study evaluated the effect of FLZ on Candida albicans under in vitro conditions resembling the vaginal microenvironment, using vagina-simulative medium (VS). We found that FLZ was fungicidal for C. albicans in VS, but not in other media at the same pH, 4.2. In VS, FLZ was fungicidal at concentrations of ≥8 μg/ml and reduced viability by greater than 99.9%. Analysis of the components of VS indicated that 17 mM acetic acid, a concentration achieved in the vagina, was responsible for the synergistic, fungicidal effect. This effect was not seen at neutral pH. Other substrates were not effective substitutes for acetic acid; however, short-chained carboxylic acids, glyoxylate and malonate, were effective. Most strains of C. albicans that were resistant to FLZ under standard conditions were killed by FLZ plus acetate. Other species of Candida were also killed, except C. krusei and C. glabrata. This study shows that FLZ has fungicidal activity for Candida species under in vitro conditions that mimic the vaginal microenvironment. This raises the possibility that FLZ may also have fungicidal effects during treatment of vaginal candidiasis. Elucidating the mechanism by which FLZ and acetate interact may disclose vulnerable pathways that could be exploited in drug development.
Over the last two decades, there has been a dramatic increase in the rate of superficial and invasive fungal infections (4, 6, 12, 13, 34, 52, 53). Since its introduction in the early 1990s, fluconazole (FLZ) has emerged as the primary treatment option for virtually all forms of susceptible Candida infections in both immunocompetent and immunocompromised hosts (27, 28, 34, 35, 37, 38). FLZ inhibits 14α-lanosterol demethylase in the ergosterol biosynthetic pathway, resulting in the accumulation of lanosterol and toxic 14α-methylated sterols in the fungal membrane (5, 19-21).
Under NCCLS standardized conditions, FLZ consistently demonstrates in vitro inhibition of Candida microorganisms and is considered fungistatic only (30). Fungistatic effects are narrowly defined, requiring a low initial cell density and heavily buffered media at neutral pH. NCCLS conditions attempt to reflect in vivo conditions resembling systemic infections. Accordingly, MICs generated with these tests show reasonable correlation with patient outcome in FLZ-treated systemic infections (1, 2, 11, 22, 39, 48, 51). It is generally assumed that FLZ is fungistatic for Candida species in vivo, inhibiting growth and allowing or aiding the body's defenses that eliminate the infection (10, 14, 17, 29, 49, 50). However, treatment of vaginal candidiasis with FLZ is even more effective than for other sites of infection. Typically, a single dose suffices to rapidly eliminate infection (43, 44). Paradoxically, even at very high concentrations, at vaginal pH of 4.2 FLZ is ineffective in vitro.
Thus, the main objective of this paper was to determine whether factors present in the vaginal microenvironment are synergistic with FLZ. In particular we wanted to determine if FLZ, based on its clinical efficacy, was fungicidal under these conditions. To approach this, we used a synthetic medium with physical and chemical properties closely resembling those of vaginal secretions from healthy nonpregnant premenopausal women (32). We show that FLZ is fungicidal under these conditions and that acetic acid, present at concentrations found in the vagina, is responsible for this effect.
MATERIALS AND METHODS
Organisms.
Candida albicans strains used in these studies are listed in Table 1. Our Saccharomyces cerevisiae isolate was ATCC 22244. Organisms were stored in 25% glycerol in 0.5% yeast extract (Difco), 2% Proteose Peptone (Difco), and 1% glucose (YPD) at −20°C, or at −70°C for long-term storage. Cells were streaked for single-colony isolation on YPD plates, and a fresh colony was inoculated into YPD broth and incubated at 37°C overnight with shaking at 200 rpm. These overnight cultures were the source of cells used to inoculate various media to assess cell growth and death under various growth conditions at 37°C.
TABLE 1.
MICs to FLZ and viabilities of C. albicans strains in YNB plus acetic acid plus FLZ
| Strain | MIC (μg/ml)c | Viability ratiod |
|---|---|---|
| ATCC 32354a | 0.5 | <0.0005 |
| 116b | 0.5 | <0.0005 |
| 28P | 0.5 | <0.0005 |
| 132 | >64 | <0.0005 |
| 368 | >64 | <0.0005 |
| 370 | >64 | <0.0005 |
| 372 | >64 | <0.0005 |
| 453 | >64 | 4 |
| 943 | >64 | <0.0005 |
Standard laboratory strain (B311).
Isolate from a patient with recurrent vulvovaginal candidiasis. All others are clinical isolates from our repository.
Determined by standard NCCLS method (45, 46).
Ratio of viable cells on day 6 to initial inoculum. Initial inoculum of 2 × 104 cells/ml cells/ml into YNB plus 20 mM acetic acid, 64 μg of FLZ/ml and pH adjusted to 4.2. Since the viability assay cannot detect less than 10 cells/ml, 0 colonies is formally a ratio of <0.0005. Controls without FLZ grew to turbidity (data not shown).
Drugs and reagents.
FLZ (Pfizer-Roerig, Inc., New York, N.Y.) was prepared from 100-mg tablets suspended in distilled water to a final stock concentration of 2 mg/ml and filter sterilized.
Culture media, MICs, and viability assays.
Vagina-simulative medium (VS) consists of 3.5 g of NaCl/liter, 1.4 g of KOH/liter, 0.22 g of Ca(OH)2/liter, 18 mg of bovine serum albumin/liter, 2.2 g of 90% lactic acid/liter, 1 g of glacial acetic acid/liter, 0.32 g of 50% glycerol/liter, 0.4 g of urea/liter, and 5 g of glucose/liter, adjusted to pH 4.2 using concentrated HCl or 40 mM NaOH, as described elsewhere (32). Yeast nitrogen broth (YNB) was made up of 6.7 g of yeast nitrogen base/liter without amino acids (Difco, Detroit, Mich.), 10 g of glucose/liter, and pH adjusted to 4.2 using concentrated HCl or 40 mM NaOH. Roswell Park Memorial Institute-1640 medium (RPMI 1640) with l-glutamine, without bicarbonate (Invitrogen, Carlsbad, Calif.) was buffered and adjusted to pH 7.0 with 165 mM morpholinepropanesulfonic acid (MOPS).
MIC assays were performed according to the NCCLS M27A guidelines (30) in microtiter plates, using visual estimates of 80% reduction in turbidity of resuspended cultures.
Viability assays were performed using overnight cultures of indicated Candida isolates grown in yeast extract (Difco)-Bacto peptone (Becton Dickinson and Co., Sparks, Md.)-glucose agar (YPD). Cultures were counted in a hemocytometer and diluted in test media, typically to 2 × 104 cells/ml. These cultures were sampled at intervals and plated onto YPD plates, diluting to achieve about 100 CFU. Cultures with low viability were plated without dilution (100 μl), so that the limit of detection of viable cells was about 10 cells per 2 × 104 initial cells, or <0.0005 viable cells per initial cell (viability ratio). Colony counts were verified to represent the number of live cells, despite clumping of cells in FLZ. Clumping altered colony counts by at most twofold. Vital dye staining with methylene blue followed a standard protocol (16).
RESULTS
FLZ is fungicidal in VS.
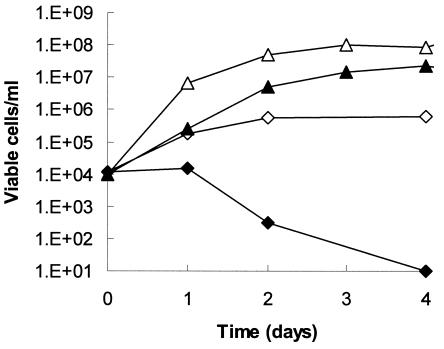
C. albicans isolates responded to FLZ differently when grown in VS compared to YNB. Figure 1 shows an example of our standard laboratory strain, with a low MIC for FLZ (0.5 μg/ml). In VS plus FLZ, viability decreased 4 orders of magnitude by day 4, after an initial 1-day lag. In contrast, C. albicans cells in YNB were not killed by FLZ but instead continued to multiply, albeit at a lower rate and level of saturation than without FLZ (Fig. 1).
FIG. 1.
Effect of medium composition on the fungicidal activity of FLZ in VS. An overnight colony of C. albicans ATCC 32354 (B311) was inoculated into duplicate cultures, in either 1 ml of YNB (squares) or 1 ml of VS (diamonds), to a density of 104 cells/ml. One of each was supplemented with 64 μg of FLZ/ml (closed symbols). Cultures were incubated at 37°C without shaking. The number of viable organisms in each culture was determined as described in Materials and Methods.
Cells subjected to VS plus FLZ were evaluated with vital stain methylene blue and were found to be intact (excluding dye), even though they were not viable by plating assay. In contrast, heat-killed cells (50°C for 1 h) were unable to exclude the dye. Even though intact, the cells were unable to replicate, since the plating assay diluted the cell suspension by 250-fold, well below effective concentrations for FLZ and acetate.
VS can be considered a growth medium for C. albicans, since cell densities reached as much as 107 cells/ml in 24 h from starting densities of 104 cells/ml. Growth rates and final cell densities were lower in VS than in YNB. In YNB, cell densities of 1 × 108 to 5 × 108 cells/ml were typically achieved in the same time interval.
Acetic acid is required for the fungicidal effect of FLZ.
We attempted various manipulations of VS to determine which component(s) of VS might contribute to the fungicidal activity of FLZ. Data in Table 2 suggest that acetic acid is the critical component. In VS medium lacking acetic acid but containing 64 μg of FLZ/ml, cell proliferation continued over 6 days, while in the presence of acetic acid viabilities decreased by 94 to 99%. Components found to be unimportant included lactic acid and urea.
TABLE 2.
Effects of alterations in VS on the fungicidal effect of FLZ
| Alterationa | Viability ratio (day 6/day 0)d
|
||||
|---|---|---|---|---|---|
| Minus lactic acid | Altered Na/Kc | Minus urea | Minus acetic acid | No FLZ | FLZe |
| 187 | 0.01 | ||||
| *b | 181 | 0.04 | |||
| * | 87 | 0.3 | |||
| * | * | 109 | 0.06 | ||
| * | 150 | 0.02 | |||
| * | * | 833 | 168 | ||
| * | 465 | 174 | |||
C. albicans ATCC 32354 was used in all media at 2 × 104 cells/ml. All media were adjusted to an initial pH of 4.2.
An asterisk indicates the item was removed or altered from the base VS medium in line 1.
The Na and K concentrations were inverted to achieve levels found in normal serum (140 mM Na, 4 mM K).
The viable cell ratio on day 6 was chosen as a convenient representation of the net effect.
64 μg/ml.
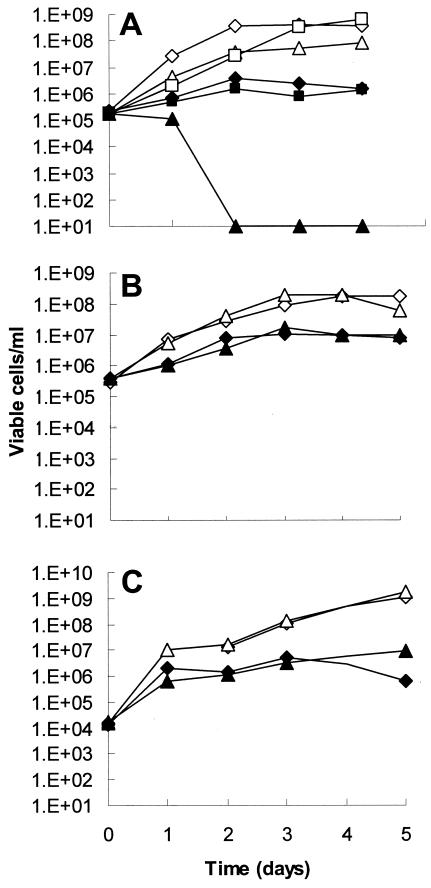
The critical role of acetic acid in this fungicidal effect was determined independently of VS by using YNB medium. Addition of 20 mM acetic acid to YNB, pH 4.2, resulted in marked FLZ-dependent fungicidal activity (Fig. 2A). Lactic acid had no effect. However, no fungicidal interaction between FLZ and acetic acid was seen in YNB buffered to maintain a pH above 7 (Fig. 2B). Similarly, no fungicidal interaction was seen in RPMI 1640, the NCCLS standard medium, at pH 7 (Fig. 2C). FLZ (64 μg/ml) reduced the rate and extent of growth compared to controls without FLZ, clearly demonstrating that FLZ inhibited but certainly did not halt growth, which would be expected at this high initial cell density. The effect of acetic acid was not mediated by pH alone, since control cultures without acetic acid were adjusted to a starting pH of 4.2. These data indicate that acetic acid is sufficient to account for the fungicidal effects of FLZ in VS or YNB and that the interaction does not occur at neutral pH.
FIG. 2.
pH-dependent interaction of acetic acid with FLZ. (A) YNB was adjusted to pH 4.2, without supplement (diamonds), with acetic acid (20 mM; triangles), or with lactic acid (20 mM; squares). FLZ at 64 μg/ml was added to some cultures (closed symbols). (B) YNB was buffered with 165 mM MOPS, pH 7.4. Cultures are represented as for panel A. (C) RPMI 1640, pH 7, cultures are represented as described for panel A. An overnight culture of C. albicans ATCC 32354 was inoculated into each medium to a final cell density of ∼2 × 105 cells/ml. Viability was determined as described in the legend for Fig. 1.
Consistent with the kill curves in Fig. 2, MIC tests also showed a dramatic synergy of FLZ with acetic acid. Strains of C. albicans which are susceptible under NCCLS methods had MICs of 32 to 64 μg/ml in YNB at pH 4.2. However, these MICs were reduced to 1 to 2 μg/ml with 20 mM acetic acid. MICs were not altered by acetic acid when using the highly buffered, neutral medium under NCCLS conditions, consistent with the kill curves in Fig. 2B and C.
The fungicidal effect of FLZ and acetate in YNB was seen in other Candida strains. Table 1 shows data for eight clinical isolates of C. albicans, including six isolates highly resistant to FLZ under standard conditions. All but one were killed in FLZ-acetate-YNB. The single surviving isolate, Ca453, was moderately inhibited (two doublings) but was not killed. Thus, most but not all FLZ-resistant strains were susceptible to FLZ-acetate.
FLZ-acetate killing in YNB was observed in some but not all non-C. albicans Candida species. Clinical isolates of C. lusitaniae, C. dubliniensis, C. parapsilosis, and C. tropicalis were killed by the combination. However, neither of two independent clinical isolates each of C. glabrata and C. krusei and only one of two clinical isolates of C. dubliniensis were killed under the conditions described in Table 1. S. cerevisiae was also killed by FLZ-acetic acid in YNB at pH 4.2; at this pH, 20 mM acetic acid without FLZ was not inhibitory.
We tested a short list of other nonfermentable carbon sources to see if any mimicked acetic acid's interaction with FLZ, using YNB adjusted to a final pH 4.2. No interactions were seen with 20 mM citrate, succinate, fumarate, pyruvate, or ethanol. However, malonic acid and glyoxylic acid were effective and were not inhibitory in the absence of FLZ.
Minimal fungicidal concentration of FLZ in VS medium.
Using an initial cell density of 2 × 104 cells/ml, we tested the minimum concentration of FLZ required for the fungicidal effect. Concentrations of FLZ of ≥8 μg/ml resulted in complete loss of viability by day 4 (Table 3). FLZ at a concentration of 4 μg/ml inhibited growth by day 4 but then over the next 2 days growth resumed, so that viability counts reached those of the no-FLZ control. There was no difference in the growth of cells in VS containing less than 4 μg of FLZ/ml versus cultures without FLZ (data not shown).
TABLE 3.
Minimum fungicidal concentration of FLZ in VS for C. albicansa
| FLZ (μg/ml) | Ratio of viable cellsb |
|---|---|
| 64 | <0.0005 |
| 32 | <0.0005 |
| 16 | <0.0005 |
| 8 | <0.0005 |
| 4 | 3 |
| 2 | 58 |
| 1 | 35 |
| 0.5 | 32 |
| 0.25 | 18 |
| 0.125 | 41 |
| 0 | 87 |
Strain ATCC 32354 was used in all media.
Data from survival curves are represented here as the ratio of viable cells on day 4 relative to the initial density of 2 × 104 cells/ml.
In vitro conditions influencing fungicidal activity of FLZ.
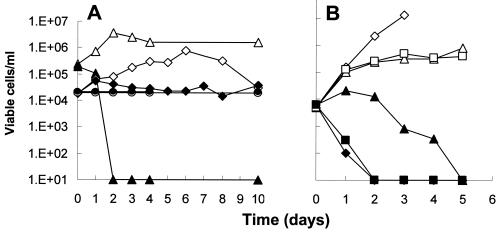
It is possible that FLZ is fungicidal under any conditions which do not support growth (46). To test this possibility, we incubated cells in three growth-limiting environments: YNB medium lacking glucose, distilled water, and complete YNB at 4°C. Figure 3A shows that these conditions limited growth. However, none of these conditions acted in synergy with FLZ to mimic the fungicidal effects observed in VS or YNB supplemented with acetic acid. Thus, Fig. 2 and 3A together show that growth inhibition is neither sufficient nor necessary as a precondition allowing FLZ to mediate fungicidal effects.
FIG. 3.
Effect of growth-limiting conditions on activity of FLZ. (A) An overnight colony of C. albicans ATCC 32354 (B311) was suspended in sterile water and inoculated to an initial cell density of 104 to 105 cells/ml into 1 ml of the following media: YNB (triangles); YNB without glucose (diamonds); distilled water (dH2O; circles with dashed lines). Open symbols, no FLZ; closed symbols, FLZ at 64 μg/ml. Curves for dH2O with and without FLZ are nearly superimposed. (B) Similar 1-ml cultures were divided into three sets, all with FLZ at 64 μg/ml, as follows: static cultures in 1.5-ml tubes (diamonds), shaking cultures in 50-ml tubes (triangles), and shaking cultures contained in a candle jar to exclude oxygen (squares). Duplicates of each were supplemented with 20 mM acetic acid (closed symbols). Initial and subsequent cell viabilities were determined as described in the legend for Fig. 1.
Since the vaginal environment is microaerobic, assays performed to this point used static cultures to mimic this condition. To test whether this was important for the fungicidal effect, we compared static cultures with shaking cultures. Figure 3B shows that the fungicidal effect of FLZ-acetic acid was greatly reduced by shaking, although killing was observed after an extended incubation time. The effect of shaking was negated in a shaking culture that was maintained in a microaerobic environment of a candle jar. This shows that the effect was due to available oxygen rather than to other possible differences between shaking and static cultures.
Effect of initial cell density on the time of killing by FLZ-acetic acid.
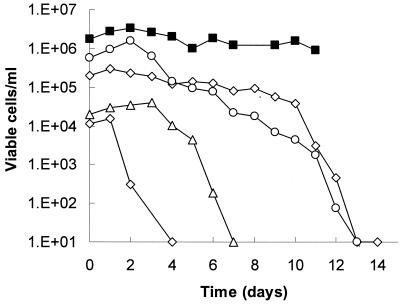
The fungicidal effects seen to this point were slow, occurring over days, not hours. We tested whether this time was dependent on the initial cell density. Figure 4 shows that killing began earlier at lower initial cell densities. This ranged from 1 day for an inoculum of 104 cells/ml up to about 10 days for an inoculum of 2 × 105 cells/ml. Increasing densities imposed an increasing lag time for killing to begin; once begun, the rate of decrease in viability was about the same for all cultures. Cultures started at >106 cells/ml showed no decrease in viability over the time course of this experiment.
FIG. 4.
Effect of initial cell density on fungicidal activity of FLZ. An overnight colony of C. albicans ATCC 32354 (B311) was suspended in sterile water and inoculated into a series of VS cultures (1 ml) at 104 to 106 cells/ml, each indicated by a different symbol. FLZ was added to all cultures to a final concentration of 64 μg/ml. Cultures were incubated at 37°C without shaking. Viability was determined as described in the legend for Fig. 1.
DISCUSSION
This study evaluated the effect of FLZ on C. albicans in medium closely resembling the vaginal microenvironment. The effect of FLZ on C. albicans in this medium was found to be radically different from that seen in conventional yeast media (YNB). In VS, FLZ was fungicidal at or above 8 μg/ml and with cell densities up to 5 × 105 cells/ml. In YNB at the same initial pH, FLZ alone was not even fungistatic and its only effect was to reduce the saturation cell density. The component in VS responsible for fungicidal activity is acetate. This effect did not occur at neutral pH, suggesting that only the undissociated acid, predominant below pH 4.7, is effective. Adding other agents that could perturb the normal balance of metabolic pathways was generally not effective. The effectiveness of two agents, glyoxylate and malonate, in addition to acetic acid, suggests that the short-chain carboxylic acids are directly responsible for the fungicidal interaction with FLZ.
Most tested C. albicans isolates that were resistant to FLZ, by conventional NCCLS testing, were nevertheless killed by FLZ-acetate. Only one resistant strain was moderately inhibited but not killed. This suggests that vaginal therapy is potentially effective even for many strains that have high MICs for FLZ. We speculate that the single isolate that survived did so because its resistance mechanism differs from that of the other isolates.
The present study suggests that FLZ kills C. albicans under conditions easily created in vitro. This killing is dependent on a variety of factors including cell density, pH, and presence of acetate. The vagina, a microaerobic, low pH microenvironment, contains adequate concentrations of acetate (10 to 20 mM) to produce the fungicidal effect seen in our in vitro assay. Densities of C. albicans in symptomatic vulvovaginal candidiasis are typically less than 106 cells/total cells in the vaginal fluid. Comparisons of static to shaking cultures indicate that FLZ-acetate synergy is more effective in a microaerobic environment.
The similarities in the in vitro conditions needed for acetate-FLZ synergy in vitro and the vaginal microenvironment are consistent with the clinical and mycological success observed with FLZ in clinical practice. Vaginal yeast cultures predictably become negative within 72 to 96 h of a single dose of FLZ in more than 90% of women with Candida vaginitis (42, 43). However, 40 to 60% of women with Candida vaginitis who experience mycological cure with FLZ become vaginal culture positive with the identical strain within 30 days of treatment (44). This implies that killing in vivo in some women is inefficient, allowing for persistence of a small number of FLZ-susceptible organisms during treatment and regrowth in the days following drug withdrawal. Based on our observations, it makes sense now to ask whether these women have reduced levels of acetate compared to women who achieve long-term cures after FLZ treatment.
Our data indicate that acetic acid promotes a fungicidal effect with FLZ under conditions in which FLZ is minimally effective alone: acidic pH and high initial cell densities. The relationship between pH and FLZ efficacy is controversial. Most studies show that FLZ or other azoles are not effective at acidic pH (7, 26, 33), similar to our experience. In contrast, one group found that MICs only increased marginally at pH 5.5 compared to 7.4 (15). This study, however, used modifications of the NCCLS MIC method that compromised the effect of the initial pH: they inoculated with 100-fold-higher cell numbers and under-buffered the medium with 1/15 the buffer concentration used in RPMI 1640. In our experience, these two changes would result in a rapid decline in pH during incubation with or without FLZ, so that C. albicans would respond in pH 7.4 medium as it would in the pH 5.5 medium. The most contradictory study showed that pH 4.5 medium reduces “trailing” effects, residual growth above the MIC that compromises endpoint determination, so that FLZ appeared more effective than at neutral pH (25). It will be interesting to see if these differences are due to strain variations or to subtle differences in the media, e.g., 165 mM MOPS adjusted to pH 4.5. However, we have shown that under conditions in which FLZ alone is only marginally inhibitory, FLZ and acetic acid are fungicidal.
Other work has addressed whether FLZ can be fungicidal under special in vitro circumstances. Sohnle et al. have shown that FLZ increases the rate at which C. albicans loses viability during prolonged incubations (more than 7 days) in distilled water (46, 47). Our results extend this observation, since both VS and YNB with acetate were capable of sustaining numerous cell divisions. This shows that FLZ can be fungicidal under conditions that permit growth. Others have shown that FLZ can become fungicidal in combination with calcineurin inhibitors such as cyclosporine A (31); whether the mechanism behind this interaction is related to FLZ-acetate is yet to be determined.
The antifungal effects and interactions of acetate and FLZ on C. albicans has been previously studied (40, 41). Those investigators demonstrated that acetate is inhibitory at 240 mM for cells that are deficient in 14α-lanosterol demethylase, due either to mutation or to the presence of FLZ. They cited, but did not show, data suggesting the inhibition was not fungicidal (40). In contrast, we report that acetate converts a fungistatic response into a fungicidal response. Further, our observation documents an effect with acetate concentrations about 10-fold lower than they used (40, 41). These contrasting observations are most likely due to key differences in the methods. They used shaking cultures while we used static cultures. We observed that the acetic acid-FLZ fungicidal effect in VS fluid was greatly reduced if the cultures were incubated with shaking. Another difference, which may account for the disparity in acetate concentrations, is that our medium was synthetic and adjusted to vaginal pH 4.2, whereas they used complex YPD medium.
Shimokawa and Nakayama suggested that, since wild-type cells were inhibited by 240 mM acetate alone below pH 5 whereas demethylase-deficient cells were inhibited even above pH 7, 14α-demethylase deficiency permeabilizes the cell membrane to dissociated acetate above pH 5 (40). Below this pH, the predominant, undissociated acid is permeable even to intact membranes and thus inhibits untreated wild-type cells. The premise of this suggestion is that acetate alone is directly inhibitory, assisted by FLZ or 14α-demethylase deficiency when above pH 5.
Our data do not determine whether FLZ potentiates killing by acetate or acetate potentiates killing by FLZ. Fungicidal effects of acetate are not a new topic to food microbiologists, who use acetate and other monocarboxylic acids as preservatives (9). Its effects on S. cerevisiae have been studied increasingly of late. Acetate enters S. cerevisiae in the anionic form via proton symporters that are glucose repressed. In the presence of glucose, acetate enters via simple diffusion of the undissociated acid, prevalent only below pH 4.75 (8, 9). Once inside, the acid dissociates, but enzymes for its metabolism are subject to negative regulation by glucose. Under these conditions, acetate accumulates and becomes toxic. One mechanism for its toxicity may be that the increase in protons from the dissociating acid must be pumped out by the H+-ATPase, effectively uncoupling the proton motive force at the plasma membrane. Another is the inhibition of metabolic pathways by the reduced intracellular pH. Perfusion studies in S. cerevisiae show that exposure to 24 mM undissociated acetic acid in an acidic medium causes a dramatic drop in intracellular pH (1 to 2 units) that persists for several minutes and activates the H+-ATPase (3, 18). A flow cytometry study documented a rapid loss in cell membrane integrity following exposure to higher concentrations of acetic acid (36).
These observations suggest that acetate may increase intracellular concentrations of FLZ by increasing membrane permeability or by decreasing the ability of the cell to efflux FLZ. Conversely, energy required to efflux FLZ may impair the cell's ability to restore intracellular pH via the H+-ATPase. In C. albicans acetate is ineffective under aerobic conditions, presumably the result of depletion of acetate by respiration.
A specific mechanism for acetate toxicity in S. cerevisiae is emerging. Recent data show that acetate as low as 20 mM kills S. cerevisiae at pH 3 in a manner that suggests apoptosis: cycloheximide-sensitive chromosome condensation, DNA breaks, and exposure of phosphtidylserine on the outer layer of the plasma membrane (24). This process is accompanied by a release of cytochrome c from the mitochondria, loss of cytochrome c oxidase, and an increase in reactive oxygen species. Most importantly, several mitochondrial mutants did not show this response, suggesting that, as in mammalian cells, the death response requires mitochondrial involvement (23). C. albicans cells killed by FLZ plus acetate are intriguingly intact, not necrotic. It remains to be seen whether apoptotic-like processes are induced by the combined effects of FLZ and acetate in C. albicans.
We have used the term fungicidal to indicate a significant decline in viability over the time course of several days, clearly different than the result seen with FLZ alone. Purists may demand that the term should be restricted to conditions in which 99.9% reduction in viability is achieved within 24 h. Without disputing this, recognizing and acknowledging that killing is occurring both in vivo and in vitro is crucial in evaluating the therapeutic effects of any antimicrobial drug. Clinical responses to single-dose oral FLZ in Candida vaginitis, achieving a negative 10% KOH examination within 24 h and negative cultures within 48 h, strongly support the concept of in vivo, vaginal fungicidal activity, especially in the absence of a host polymorphonuclear leukocyte response.
The synergistic in vitro fungicidal activity of FLZ plus acetate against isolates defined as resistant by NCCLS M27A criteria is significant. Recently, Sobel et al. demonstrated that so-called highly FLZ-resistant strains of C. albicans were susceptible in patients with Candida vaginitis, indicating a unique in vivo activity of FLZ (45). It is also noteworthy that vaginal activity of FLZ is considerably enhanced compared to therapeutic activity in oral candidiasis. The latter site is composed of different bacterial flora and organic acids and a higher pH.
In conclusion, although FLZ is considered a prototypic fungistatic agent, it has a potent fungicidal activity in the presence of acetate under in vitro conditions that closely simulate the human vaginal environment. This fungicidal activity against C. albicans is concentration dependent, pH dependent, and cell density dependent. Of interest and reflecting clinical experience, no fungicidal activity is evident for C. krusei and C. glabrata. Understanding the synergistic interaction may be valuable in identifying new antifungal drug targets.
REFERENCES
- 1.Anaissie, E. J., N. C. Karyotakis, R. Hachem, M. C. Dignani, J. H. Rex, and V. Paetznick. 1994. Correlation between in vitro and in vivo activity of antifungal agents against Candida species. J. Infect. Dis. 170:384-389. (Erratum, 170:1641.) [DOI] [PubMed] [Google Scholar]
- 2.Arikan, S., M. Akova, M. Hayran, O. Ozdemir, M. Erman, D. Gur, and S. Unal. 1998. Correlation of in vitro fluconazole susceptibility with clinical outcome for severely ill patients with oropharyngeal candidiasis. Clin. Infect. Dis. 26:903-908. [DOI] [PubMed] [Google Scholar]
- 3.Arneborg, N., L. Jespersen, and M. Jakobsen. 2000. Individual cells of Saccharomyces cerevisiae and Zygosaccharomyces bailii exhibit different short-term intracellular pH responses to acetic acid. Arch. Microbiol. 174:125-128. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee, S. N., T. G. Emori, D. H. Culver, R. P. Gaynes, W. R. Jarvis, T. Horan, J. R. Edwards, J. Tolson, T. Henderson, and W. J. Martone. 1991. Secular trends in nosocomial primary bloodstream infections in the United States, 1980-1989. National Nosocomial Infections Surveillance System. Am. J. Med. 91:86S-89S. [DOI] [PubMed] [Google Scholar]
- 5.Bard, M., N. D. Lees, T. Turi, D. Craft, L. Cofrin, R. Barbuch, C. Koegel, and J. C. Loper. 1993. Sterol synthesis and viability of erg11 (cytochrome P450 lanosterol demethylase) mutations in Saccharomyces cerevisiae and Candida albicans. Lipids 28:963-967. [DOI] [PubMed] [Google Scholar]
- 6.Beck-Sague, C. M., and W. R. Jarvis. 1993. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. J. Infect. Dis. 167:1247-1251. [DOI] [PubMed] [Google Scholar]
- 7.Beggs, W. H., and C. E. Hughes. 1986. Role of pH in the lethal direct cell damaging action of miconazole. Res. Commun. Chem. Pathol. Pharmacol. 53:407-410. [PubMed] [Google Scholar]
- 8.Casal, M., H. Cardoso, and C. Leao. 1998. Effects of ethanol and other alkanols on transport of acetic acid in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 64:665-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casal, M., H. Cardoso, and C. Leao. 1996. Mechanisms regulating the transport of acetic acid in Saccharomyces cerevisiae. Microbiology 142:1385-1390. [DOI] [PubMed] [Google Scholar]
- 10.Clemons, K. V., and D. A. Stevens. 2000. Treatment of orogastrointestinal candidosis in SCID mice with fluconazole alone or in combination with recombinant granulocyte colony-stimulating factor or interferon-gamma. Med. Mycol. 38:213-219. [DOI] [PubMed] [Google Scholar]
- 11.Dannaoui, E., V. Lacoste, C. Prat, and M. A. Piens. 1997. Fluconazole susceptibility of Candida isolates from oropharyngeal candidosis. Mycoses 40:279-282. [DOI] [PubMed] [Google Scholar]
- 12.Diekema, D. J., S. A. Messer, A. B. Brueggemann, S. L. Coffman, G. V. Doern, L. A. Herwaldt, and M. A. Pfaller. 2002. Epidemiology of candidemia: three-year results from the emerging infections and the epidemiology of Iowa organisms study. J. Clin. Microbiol. 40:1298-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fridkin, S. K., and W. R. Jarvis. 1996. Epidemiology of nosocomial fungal infections. Clin. Microbiol. Rev. 9:499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcha, U. K., E. Brummer, and D. A. Stevens. 1995. Synergy of fluconazole with macrophages for antifungal activity against Candida albicans. Mycopathologia 132:123-128. [DOI] [PubMed] [Google Scholar]
- 15.Garcia, M. T., F. Minguez, M. T. Llorente, and J. Prieto. 2000. Influence of pH and concentration on the postantifungal effect and on the effects of sub-MIC concentrations on the of 4 antifungal agents on previously treated Candida spp. Scand. J. Infect. Dis. 32:669-673. [DOI] [PubMed] [Google Scholar]
- 16.Goihman-Yahr, M., L. Pine, M. C. Albornoz, L. Yarzabal, M. H. de Gomez, B. San Martin, A. Ocanto, T. Molina, and J. Convit. 1980. Studies on plating efficiency and estimation of viability of suspensions of Paracoccidioides brasiliensis yeast cells. Mycopathologia 71:73-83. [DOI] [PubMed] [Google Scholar]
- 17.Gujral, S., E. Brummer, and D. A. Stevens. 1996. Role of extended culture time on synergy of fluconazole and human monocyte-derived macrophages in clearing Candida albicans. J. Infect. Dis. 174:888-890. [DOI] [PubMed] [Google Scholar]
- 18.Guldfeldt, L. U., and N. Arneborg. 1998. Measurement of the effects of acetic acid and extracellular pH on intracellular pH of nonfermenting, individual Saccharomyces cerevisiae cells by fluorescence microscopy. Appl. Environ. Microbiol. 64:530-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly, S. L., D. C. Lamb, A. J. Corran, B. C. Baldwin, and D. E. Kelly. 1995. Mode of action and resistance to azole antifungals associated with the formation of 14α-methylergosta-8,24(28)-dien-3β,6α-diol. Biochem. Biophys. Res. Commun. 207:910-915. [DOI] [PubMed] [Google Scholar]
- 20.Kelly, S. L., D. C. Lamb, D. E. Kelly, N. J. Manning, J. Loeffler, H. Hebart, U. Schumacher, and H. Einsele. 1997. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol delta5,6-desaturation. FEBS Lett. 400:80-82. [DOI] [PubMed] [Google Scholar]
- 21.Kontoyiannis, D. P. 2000. Modulation of fluconazole sensitivity by the interaction of mitochondria and erg3p in Saccharomyces cerevisiae. J. Antimicrob. Chemother. 46:191-197. [DOI] [PubMed] [Google Scholar]
- 22.Krcmery, V., M. Huttova, F. Mateicka, L. Laho, L. Jurga, A. Ondrusova, Z. Tarekova, K. Kralinsky, J. Hanzen, A. Liskova, M. Mrazova, A. Sabo, M. Pisarcikova, G. Kovacicova, D. Chovancova, and Z. Szovenyiova. 2001. Breakthrough fungaemia in neonates and infants caused by Candida albicans and Candida parapsilosis susceptible to fluconazole in vitro. J. Antimicrob. Chemother. 48:521-525. [DOI] [PubMed] [Google Scholar]
- 23.Ludovico, P., F. Rodrigues, A. Almeida, M. T. Silva, A. Barrientos, and M. Corte-Real. 2002. Cytochrome c release and mitochondria involvement in programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Mol. Biol. Cell 13:2598-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludovico, P., M. J. Sousa, M. T. Silva, C. Leao, and M. Corte-Real. 2001. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology 147:2409-2415. [DOI] [PubMed] [Google Scholar]
- 25.Marr, K. A., T. R. Rustad, J. H. Rex, and T. C. White. 1999. The trailing end point phenotype in antifungal susceptibility testing is pH dependent. Antimicrob. Agents Chemother. 43:1383-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIntyre, K. A., and J. N. Galgiani. 1989. In vitro susceptibilities of yeasts to a new antifungal triazole, SCH 39304: effects of test conditions and relation to in vivo efficacy. Antimicrob. Agents Chemother. 33:1095-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meis, J., M. Petrou, J. Bille, D. Ellis, D. Gibbs, et al. 2000. A global evaluation of the susceptibility of Candida species to fluconazole by disk diffusion. Ugeskr. Laeger. 162:1907-1908. [DOI] [PubMed] [Google Scholar]
- 28.Mian, U. K., M. Mayers, Y. Garg, Q. F. Liu, G. Newcomer, C. Madu, W. Liu, A. Louie, and M. H. Miller. 1998. Comparison of fluconazole pharmacokinetics in serum, aqueous humor, vitreous humor, and cerebrospinal fluid following a single dose and at steady state. J. Ocul. Pharmacol. Ther. 14:459-471. [DOI] [PubMed] [Google Scholar]
- 29.Natarajan, U., N. Randhawa, E. Brummer, and D. A. Stevens. 1998. Effect of granulocyte-macrophage colony-stimulating factor on candidacidal activity of neutrophils, monocytes or monocyte-derived macrophages and synergy with fluconazole. J. Med. Microbiol. 47:359-363. [DOI] [PubMed] [Google Scholar]
- 30.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard NCCLS document M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 31.Onyewu, C., J. R. Blankenship, M. Del Poeta, and J. Heitman. 2003. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 47:956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owen, D. H., and D. F. Katz. 1999. A vaginal fluid simulant. Contraception 59:91-95. [DOI] [PubMed] [Google Scholar]
- 33.Peng, T., and J. N. Galgiani. 1993. In vitro studies of a new antifungal triazole, D0870, against Candida albicans, Cryptococcus neoformans, and other pathogenic yeasts. Antimicrob. Agents Chemother. 37:2126-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, and S. A. Messer. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J. Clin. Microbiol. 39:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, G. V. Doern, M. E. Brandt, and R. A. Hajjeh. 1999. Trends in species distribution and susceptibility to fluconazole among blood stream isolates of Candida species in the United States. Diagn. Microbiol. Infect. Dis. 33:217-222. [DOI] [PubMed] [Google Scholar]
- 36.Prudencio, C., F. Sansonetty, and M. Corte-Real. 1998. Flow cytometric assessment of cell structural and functional changes induced by acetic acid in the yeasts Zygosaccharomyces bailii and Saccharomyces cerevisiae. Cytometry 31:307-313. [DOI] [PubMed] [Google Scholar]
- 37.Rex, J. H., T. J. Walsh, J. D. Sobel, S. G. Filler, P. G. Pappas, W. E. Dismukes, and J. E. Edwards. 2000. Practice guidelines for the treatment of candidiasis, Infectious Diseases Society of America. J. Parenter. Enteral Nutr. 24:119-125. [DOI] [PubMed] [Google Scholar]
- 38.Richardson, K., K. Cooper, M. S. Marriott, M. H. Tarbit, P. F. Troke, and P. J. Whittle. 1990. Discovery of fluconazole, a novel antifungal agent. Rev. Infect. Dis. 12:S267-S271. [DOI] [PubMed] [Google Scholar]
- 39.Ruhnke, M., A. Eigler, E. Engelmann, B. Geiseler, and M. Trautmann. 1994. Correlation between antifungal susceptibility testing of Candida isolates from patients with HIV infection and clinical results after treatment with fluconazole. Infection 22:132-136. [DOI] [PubMed] [Google Scholar]
- 40.Shimokawa, O., and H. Nakayama. 1999. Acetate-mediated growth inhibition in sterol 14α-demethylation-deficient cells of Candida albicans. Antimicrob. Agents Chemother. 43:100-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimokawa, O., and H. Nakayama. 2000. Estimation of minimum sterol 14α-demethylation-inhibitory concentration of azoles in Candida yeasts using acetate-mediated growth inhibition: potential utility in susceptibility testing. J. Clin. Microbiol. 38:2893-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sobel, J. D. 1999. Limitations of antifungal agents in the treatment of Candida vaginitis: future challenges. Drug Resist. Update 2:148-152. [DOI] [PubMed] [Google Scholar]
- 43.Sobel, J. D. 2002. Treatment of vaginal Candida infections. Expert Opin. Pharmacother. 3:1059-1065. [DOI] [PubMed] [Google Scholar]
- 44.Sobel, J. D., P. S. Kapernick, M. Zervos, B. D. Reed, T. Hooton, D. Soper, P. Nyirjesy, M. W. Heine, J. Willems, H. Panzer, and H. Wittes. 2001. Treatment of complicated Candida vaginitis: comparison of single and sequential doses of fluconazole. Am. J. Obstet. Gynecol. 185:363-369. [DOI] [PubMed] [Google Scholar]
- 45.Sobel, J. D., M. Zervos, B. D. Reed, T. Hooton, D. Soper, P. Nyirjesy, M. W. Heine, J. Willems, and H. Panzer. 2003. Fluconazole susceptibility of vaginal isolates obtained from women with complicated Candida vaginitis: clinical implications. Antimicrob. Agents Chemother. 47:34-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sohnle, P. G., B. L. Hahn, and M. D. Erdmann. 1996. Effect of fluconazole on viability of Candida albicans over extended periods of time. Antimicrob. Agents Chemother. 40:2622-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sohnle, P. G., B. L. Hahn, T. A. Fassel, and V. M. Kushnaryov. 1998. Analysis of fluconazole effect on Candida albicans viability during extended incubations. Med. Mycol. 36:29-36. [PubMed] [Google Scholar]
- 48.Troillet, N., C. Durussel, J. Bille, M. P. Glauser, and J. P. Chave. 1993. Correlation between in vitro susceptibility of Candida albicans and fluconazole-resistant oropharyngeal candidiasis in HIV-infected patients. Eur. J. Clin. Microbiol. Infect. Dis. 12:911-915. [DOI] [PubMed] [Google Scholar]
- 49.Troke, P. F., R. J. Andrews, K. W. Brammer, M. S. Marriott, and K. Richardson. 1985. Efficacy of UK-49,858 (fluconazole) against Candida albicans experimental infections in mice. Antimicrob. Agents Chemother. 28:815-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tullio, V., A. M. Cuffini, C. De Leo, F. Perrone, and N. A. Carlone. 1996. Interaction of Candida albicans, macrophages and fluconazole: in vitro and ex vivo observations. J. Chemother. 8:438-444. [DOI] [PubMed] [Google Scholar]
- 51.Walsh, T. J., C. E. Gonzalez, S. Piscitelli, J. D. Bacher, J. Peter, R. Torres, D. Shetti, V. Katsov, K. Kligys, and C. A. Lyman. 2000. Correlation between in vitro and in vivo antifungal activities in experimental fluconazole-resistant oropharyngeal and esophageal candidiasis. J. Clin. Microbiol. 38:2369-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wenzel, R. P. 1995. Nosocomial candidemia: risk factors and attributable mortality. Clin. Infect. Dis. 20:1531-1534. [DOI] [PubMed] [Google Scholar]
- 53.Wingard, J. R. 1995. Importance of Candida species other than C. albicans as pathogens in oncology patients. Clin. Infect. Dis. 20:115-125. [DOI] [PubMed] [Google Scholar]