ABSTRACT
A dynamic homeostasis is maintained between the host and native bacteria of the gastrointestinal tract in animals, but migration of bacteria from the gut to other organs can lead to disease or death. Enterococcus faecalis is a commensal of the gastrointestinal tract; however, Enterococcus spp. are increasingly frequent causes of nosocomial infections with a high mortality rate. We investigated the commensal-to-pathogen switch undergone by E. faecalis OG1RF in the lepidopteran model host Manduca sexta associated with its location in the host. E. faecalis persists in the harsh midgut environment of M. sexta larvae without causing apparent illness, but injection of E. faecalis directly into the larval hemocoel is followed by rapid death. Additionally, oral ingestion of E. faecalis in the presence of Bacillus thuringiensis insecticidal toxin, a pore-forming toxin that targets the midgut epithelium, induces an elevated mortality rate. We show that the loss of gut integrity due to B. thuringiensis toxin correlates with the translocation of E. faecalis from the gastrointestinal tract into the hemolymph. Upon gaining access to the hemolymph, E. faecalis induces an innate immune response, illustrated by hemocyte aggregation, in larvae prior to death. The degree of hemocyte aggregation is dependent upon the route of E. faecalis entry. Our data demonstrate the efficacy of the M. sexta larval model system in investigating E. faecalis-induced sepsis and clarifies controversies in the field regarding the events leading to larval death following B. thuringiensis toxin exposure.
IMPORTANCE
This study advances our knowledge of Enterococcus faecalis-induced sepsis following translocation from the gut and provides a model for mammalian diseases in which the spatial distribution of bacteria determines disease outcomes. We demonstrate that E. faecalis is a commensal in the gut of Manduca sexta and a pathogen in the hemocoel, resulting in a robust immune response and rapid death, a process we refer to as the “commensal-to-pathogen” switch. While controversy remains regarding Bacillus thuringiensis toxin-induced killing, our laboratory previously found that under some conditions, the midgut microbiota is essential for B. thuringiensis toxin killing of Lymantria dispar (N. A. Broderick, K. F. Raffa, and J. Handelsman, Proc. Natl. Acad. Sci. U. S. A. 103:15196–15199, 2006; B. Raymond, et al., Environ. Microbiol. 11:2556–2563, 2009; P. R. Johnston, and N. Crickmore, Appl. Environ. Microbiol. 75:5094–5099, 2009). We and others have demonstrated that the role of the midgut microbiota in B. thuringiensis toxin killing is dependent upon the lepidopteran species and formulation of B. thuringiensis toxin (N. A. Broderick, K. F. Raffa, and J. Handelsman, Proc. Natl. Acad. Sci. U. S. A. 103:15196–15199, 2006; N. A. Broderick, et al., BMC Biol. 7:11, 2009). This work reconciles much of the apparently contradictory previous data and reveals that the M. sexta-E. faecalis system provides a model for mammalian sepsis.
Introduction
Enterococcus faecalis is a ubiquitous member of the normal gut microbiota in diverse species, including vertebrates and insects. However, Enterococcus species also frequently cause nosocomial infections with a high mortality rate (1–3). Recently, the gastrointestinal tract has been implicated as a reservoir of bacteria that cause serious diseases, including sepsis (4). Therefore, the normal gut microbiota is a significant source of bacteria that have the potential to translocate to the bloodstream and cause septic death. The complexity and diversity of the mammalian indigenous microbiota, coupled with the rapid progress and lethality of the mammalian disease, have hindered previous studies of sepsis and the mechanisms of bacterial translocation, emphasizing the need for a simple model to advance our understanding of this opportunistic pathogen. We utilized an invertebrate model organism, Manduca sexta, to investigate E. faecalis OG1RF-induced sepsis and bacterial translocation from the gut.
M. sexta represents a desirable model system for studying E. faecalis pathogenicity due to the simple gastrointestinal microbiota community, normal presence of E. faecalis in the microbiota, rapid larval life cycle, ease of rearing, and absence of adaptive immunity (allowing specific investigation of the innate immune system during the commensal-to-pathogen switch) (5, 6). Although shown to cause sepsis in humans, E. faecalis is a normal member of the healthy human gastrointestinal tract. The mechanism of E. faecalis translocation from the gut to the bloodstream remains unknown. To investigate bacterial translocation from the midgut, we utilized Bacillus thuringiensis toxin (MVPII formulation) to promote loss of gut integrity, which may contribute to sepsis.
B. thuringiensis toxins are insecticidal crystal proteins used against lepidopteran pests that bind receptors on the gut epithelium, leading to pore formation and lysis of the midgut epithelial cells (7, 8). A previous study by Broderick et al. demonstrated that following the formation of these pores, native midgut bacteria contribute to the death of Lymantria dispar larvae, which respond to infection with activation of the innate immune response (9). However, controversy remains regarding the direct cause of larval death. Some proposed mechanisms attribute death to direct toxin toxicity or B. thuringiensis sepsis (7), translocation of indigenous midgut bacteria into the hemocoel (9–11), or developmental arrest and larval starvation (12, 13).
We report here that E. faecalis is a commensal in the midguts of M. sexta larvae, but when E. faecalis is present in the hemolymph, it causes sepsis and rapid death. We present evidence indicating that B. thuringiensis toxin (Cry1Ac) mediates the translocation of E. faecalis from the gut to the hemolymph, resulting in a commensal-to-pathogen switch and stimulation of the innate immune response.
RESULTS
E. faecalis is a commensal in the gut but a pathogen in the hemocoel.
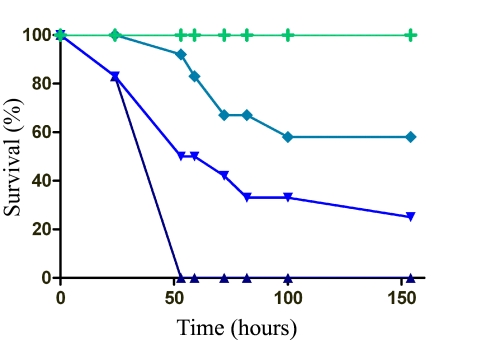
Although it is found in the gastrointestinal tracts of diverse, healthy animal species, E. faecalis has been implicated in translocation from the gut to the bloodstream, causing sepsis (2). Larvae were reared on antibiotic food to clear the midgut microbiota prior to all experiments. E. faecalis induced no morbidity or death when early-5th-instar larvae were force fed E. faecalis (108 CFU), but when injected into the hemocoel, it induced death in a dose-dependent manner (Fig. 1). Prior to death, melanization was observed at the site of injection (first proleg), and over time, melanization progressed through the entire caterpillar in most larvae. E. faecalis does not cause morbidity or death when restricted to the gut environment of M. sexta, but upon access to the hemocoel, E. faecalis leads to rapid, sepsis-like death.
FIG 1 .
E. faecalis is a commensal in the gut and a pathogen in the hemolymph. Early-5th-instar larvae were fed or injected with PBS or E. faecalis, and deaths were recorded over time. The green line (+) represents the following experimental groups, where no deaths were observed: PBS fed, PBS injected, 108 CFU E. faecalis fed, 103 CFU E. faecalis injected, and 104 CFU E. faecalis injected. Symbols: ♦, 105 CFU E. faecalis injected; ▼, 106 CFU E. faecalis injected; ▲, 107 CFU E. faecalis injected (n = 20/group, 1 representative experiment of 12).
B. thuringiensis toxin fed with E. faecalis promotes larval death.
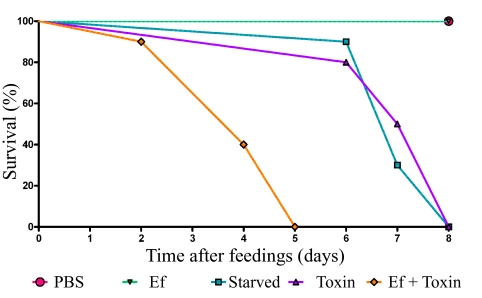
Ingested B. thuringiensis toxin promotes larval death (14), but the mechanism of killing following pore formation remains under debate. To test the effect of E. faecalis in the presence of B. thuringiensis toxin, we force fed or injected early-5th-instar larvae. E. faecalis and E. faecalis plus toxin induced rapid larval death at similar rates when injected (Fig. 2). Phosphate-buffered saline (PBS) induced no death when fed or injected, and E. faecalis induced no death when fed to larvae alone. Larvae fed B. thuringiensis toxin alone refused all food and died slowly over time from apparent starvation. Larvae fed toxin alone or PBS did not melanize. However, larvae fed E. faecalis plus toxin succumbed to a sepsis-like infection that resulted in rapid death. These data support the hypothesis that in the presence of B. thuringiensis toxin, E. faecalis translocates from the gut to the hemocoel and causes septic death.
FIG 2 .
The presence of B. thuringiensis toxin during E. faecalis feeding promotes larval death. Early-5th-instar larvae were force fed PBS, E. faecalis alone (Ef), B. thuringiensis toxin alone (Toxin), or E. faecalis and B. thuringiensis toxin together (Ef + Toxin). Larvae were given unmodified food ad libitum for the duration of the experiment. One experimental group was force fed PBS, and food was subsequently removed for the duration of the experiment (Starved). Larval deaths were recorded over time (n = 12/group, 1 representative experiment of 12).
B. thuringiensis toxin promotes M. sexta larval starvation.
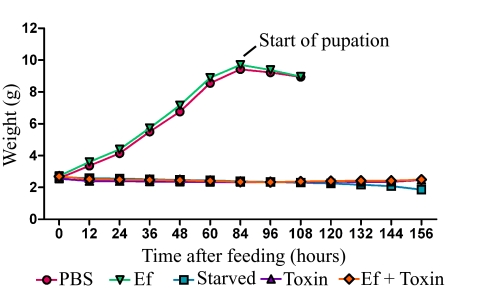
To determine whether toxin ingestion promoted weight loss, similar to starvation, the weights of individual 5th-instar larvae were measured every 12 h after feeding (Fig. 3). All larvae except those in the starvation group were fed an unmodified diet ad libitum for the duration of the experiment. Larvae force fed E. faecalis or PBS continued to gain weight until wandering began at approximately 3 to 4 days. At this point, larvae expelled liquid as they entered the natural pupation phase and were therefore eliminated from the experiment. Larvae fed toxin or E. faecalis plus toxin or not fed at all failed to gain weight (Fig. 2). Furthermore, the times to death for toxin-fed and starved larvae were indistinguishable (see Fig. S1 in the supplemental material). Our data suggest that toxin ingestion alone promotes slow death by larval starvation.
FIG 3 .
B. thuringiensis toxin alone causes larval starvation. Early-5th-instar larvae were force fed PBS, E. faecalis (Ef), B. thuringiensis toxin (Toxin), or E. faecalis and B. thuringiensis toxin (Ef + Toxin). Larvae were given unmodified food ad libitum for the duration of the experiment. Another experimental group was force fed PBS, and food was subsequently removed for the duration of the experiment (Starved). Larval weight was documented every 12 h, and the mean larval weight over time is depicted (n = 20/group, 1 representative experiment of 2).
B. thuringiensis toxin results in E. faecalis translocation from the gut to the hemolymph.
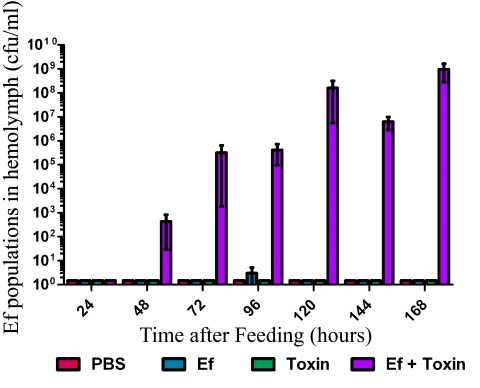
Larvae fed E. faecalis plus toxin died more rapidly than larvae fed toxin alone. To determine whether rapid death was due to E. faecalis translocation to the hemolymph, we cultured larval hemolymph for E. faecalis. At 24 h after feeding, no E. faecalis was detected in the hemolymph of larvae fed PBS, toxin alone, E. faecalis alone, or E. faecalis plus toxin (Fig. 4). At 48 h after feeding, E. faecalis was found in the hemolymph of larvae fed E. faecalis plus toxin, and the populations continued to increase over time until larval death (Fig. 4), suggesting that E. faecalis escaped from the gut into the hemocoel to cause sepsis-like death. The hemolymph collected from larvae fed PBS, toxin alone, or E. faecalis alone contained no detectable E. faecalis at any time during the experiment.
FIG 4 .
B. thuringiensis toxin promotes E. faecalis translocation from the gut to the hemolymph. Early-5th-instar larvae were force fed, and hemolymph was collected over time. Hemolymph was serially diluted and selectively cultured on BHI agar supplemented with rifampin to look for E. faecalis (Ef) translocation to the hemolymph (n = 10/group/time point, 1 representative experiment of 3).
E. faecalis persists in the hemolymph of M. sexta.
To determine whether E. faecalis persisted in the hemolymph of M. sexta, early-5th-instar larvae were injected with 104 CFU E. faecalis and hemolymph was cultured for E. faecalis over time. At 6 h after injection, E. faecalis was culturable in the hemolymph of larvae (see Fig. S2 in the supplemental material). Interestingly, we consistently observed a slight decrease in E. faecalis populations in the hemolymph 24 h after injection. However, E. faecalis populations recovered by 48 h after injection and appear to persist over time until larval death.
E. faecalis in the hemolymph activates the insect innate immune response.
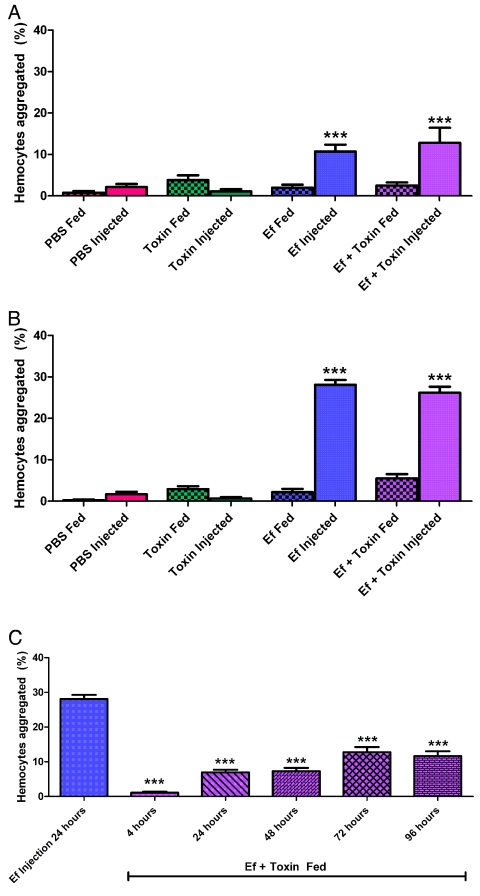
M. sexta larvae succumb to sepsis-like death when E. faecalis is present in the hemolymph. To determine whether death was associated with induction of the host innate immune response, hemocyte aggregation was determined after larval feeding or injections. Previous studies have demonstrated that hemocyte aggregation is a reliable indicator of innate immune activation (15, 16). At 4 h after feeding, hemocyte aggregation was at background levels in larvae fed PBS, toxin, E. faecalis, or E. faecalis plus toxin (Fig. 5A). Furthermore, larvae injected with PBS or toxin alone had minimal hemocyte aggregation, whereas those injected with E. faecalis or E. faecalis plus toxin had significantly elevated levels of hemocyte aggregation (P < 0.001). Hemocyte aggregation did not differ significantly between larvae injected with E. faecalis and those injected with E. faecalis plus toxin. These data suggest that injection of E. faecalis or E. faecalis plus toxin results in rapid innate immune recognition and activation.
FIG 5 .
E. faecalis in the hemolymph activates the insect innate immune response. Early-5th-instar larvae were force fed or injected with PBS, E. faecalis alone (Ef), B. thuringiensis toxin (Toxin), or E. faecalis and B. thuringiensis toxin together (Ef + Toxin). At each time point, larvae were bled and hemocytes were immediately counted on a hemocytometer. Data depict the percentage of hemocytes that are aggregated out of the total hemocytes. Percentages of hemocytes aggregated were determined at 4 h (A), at 24 h (B), and at later times (C) posttreatment. ***, P value of <0.001 between compared experimental groups. All injections at a single time point were compared to PBS injections at the same time point. Similarly, all feedings at a single time point were compared to PBS feedings at the same time point (top and middle). Long-term aggregation was statistically significantly different (P < 0.0001, F = 72.50, df = 5) from aggregation for E. faecalis injections after 24 h (bottom). Statistical analysis was performed with a one-way ANOVA with a Bonferroni correction for all experimental groups (n = 6 larvae/group/time point, 1 representative experiment of 4).
At 24 h after feeding, hemocyte aggregation remained at background levels in larvae fed or injected with PBS or toxin alone, whereas it was elevated in larvae injected with E. faecalis or fed E. faecalis plus toxin (Fig. 5B). These data indicate that a rapid innate immune response to E. faecalis in the hemolymph increases between 4 and 24 h after injection. To determine whether larvae fed E. faecalis plus toxin mount a similar innate immune response but do so more slowly, hemocyte aggregation was determined every day until larval death. Interestingly, despite larval melanization and eventual death, throughout the entire experiment, hemocyte aggregation in larvae fed E. faecalis plus toxin did not reach the level found in those injected with E. faecalis (Fig. 5C), suggesting that the innate immune response to E. faecalis is affected by the route of bacterial entry. Larval hemocytes became activated and aggregated rapidly after E. faecalis injection into the hemolymph, regardless of the presence of toxin. However, translocation of E. faecalis from the gut to the hemocoel does not activate hemocyte aggregation to a similar level, suggesting an altered response.
FIG 6 .
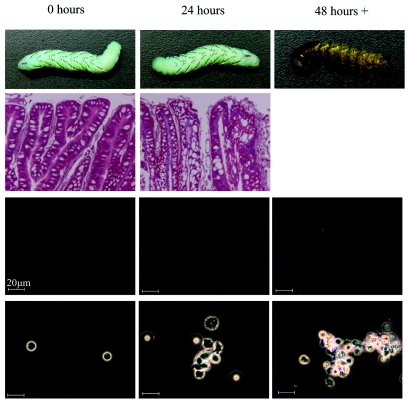
Time course of the commensal-to-pathogen switch of E. faecalis. The commensal-to-pathogen switch of E. faecalis is shown in this representative schematic. Following ingestion of E. faecalis and B. thuringiensis toxin, there is a loss of gut integrity (second row from top) that allows the translocation of E. faecalis into the hemocoel of M. sexta (third row from top). The presence of E. faecalis in the hemolymph results in hemocyte aggregation/innate immune activation; however, the innate immune response (indicated by hemocyte aggregation; bottom row) is not potent enough to prevent melanization (top row) and sepsis-like death of the larvae.
DISCUSSION
The data presented here demonstrate the value of M. sexta as a model system for studying the commensal-to-pathogen switch of the opportunistic pathogen E. faecalis. We show that E. faecalis introduced into the aseptic midgut of early-5th-instar M. sexta larvae persisted without apparent harm to the host, but E. faecalis induced death when it reached the hemocoel by direct injection or by translocation from the gut, which is enabled by feeding B. thuringiensis toxin with E. faecalis. B. thuringiensis toxin fed alone caused larvae to stop eating; they died of starvation much more slowly than from B. thuringiensis and E. faecalis cofeeding. Our work establishes conditions that contribute to the commensal-to-pathogen switch of E. faecalis and resolves some of the conflicting results about B. thuringiensis toxin (Cry1Ac)-mediated killing.
We propose that B. thuringiensis toxin kills M. sexta by starvation when fed alone, whereas the insects died of acute infection when B. thuringiensis toxin was ingested with E. faecalis. These modes of killing are consistent with the three types of killing by B. thuringiensis proposed by Heimpel and Angus (14). Type I species are paralyzed by toxin intake and die within hours, type II species suffer a gut-restricted paralysis that includes feeding cessation and death within 2 to 4 days, and type III species require the toxin and B. thuringiensis spore for death. This original characterization of toxin mode of action was subsequently developed further by the discovery that many factors altered toxin-induced death, including toxin concentration, host genetics, and larval age (8, 17–20). These factors, which often vary among laboratories, produce conflicting results and controversy in the field. In this study, we standardized many factors. We used the MVPII cell-free formulation of B. thuringiensis toxin, which consists of the Cry1Ac protoxin encapsulated in NaCl-killed Pseudomonas fluorescens. Previously published work used MVPII, DiPel, or formulations of various toxins, such as Cry1Aa, Cry1Ab, and Cry2A (21–27). MVPII is useful because it circumvents the possible interaction of the host with B. thuringiensis cells. Furthermore, to avoid the variation among previous experiments regarding the length of time to host death, we continued all experiments involving larval death for at least 1 week or until death. This time course (Fig. 6) enabled us to determine whether host death was due simply to starvation or to acute bacterial infection and to visualize larval melanization. Our data demonstrate that MVPII B. thuringiensis toxin induced larval starvation and eventual death in M. sexta and that the indigenous gut microbiota increased the rate of death dramatically.
The effect of B. thuringiensis toxin on larval death was previously investigated by our laboratory in six larval species. In L. dispar, the indigenous gut microbiota plays a role in B. thuringiensis toxin lethality, although this phenomenon varies among labs. The present study highlights the effect of variation in experimental conditions on the mortality rate, including larval species, larval age, experimental duration, rearing conditions, and route of toxin introduction (21, 28). We chose M. sexta as our model organism as an alternative to L. dispar due to its ease of rearing, numerous previous studies, and ease of altering many experimental variables that may contribute to the conflicting results obtained. To ensure that a consistent dose of toxin or E. faecalis was delivered to each larva, we adopted force feeding and injection protocols. We standardized larval size and developmental stage by using exclusively early-5th-instar larvae, which were large enough to be force fed with a gavage needle. While previous studies relied upon the larvae to eat food containing the toxin or bacteria in it, force feeding standardizes the dose and time of ingestion for each larva. In contrast to our previous finding with L. dispar, in M. sexta, toxin ingestion induced immediate food refusal by M. sexta and resulted in slow death from starvation in the absence of gut microbiota (21). M. sexta starvation to death takes approximately 1 week, which is the same time to death for larvae fed toxin only. In contrast, M. sexta larvae that ingested E. faecalis plus toxin died much more rapidly. We propose that E. faecalis-induced sepsis was the cause of death of M. sexta resulting from B. thuringiensis toxin ingestion.
Previously published work suggested that antibiotics alone may confound experimental results. We studied the effect of antibiotic use on larval killing through the use of larvae reared continuously on an unamended diet and larvae reared on an antibiotic diet. We found that the use of antibiotics did not alter the morbidity and mortality associated with any experimental group (see Fig. S2 in the supplemental material), including those fed E. faecalis plus toxin. We concluded that the choice of antibiotics did not appreciably alter the host immune response to systemic infection with E. faecalis in M. sexta.
Innate immune activation differed between larvae fed or injected with E. faecalis. Feeding E. faecalis plus toxin heightened the immune response more than feeding of E. faecalis alone, but injection of E. faecalis directly into the hemolymph resulted in the most hemocyte aggregation, which might be due to the route of entry into the hemolymph. Persistence in the harsh environment of the lepidopteran gut may alter E. faecalis gene expression, thereby altering bacterial behavior or initial host immune recognition. During translocation from the midgut, E. faecalis levels were lower than those observed during direct injection into the hemocoel, perhaps because it induced a milder host immune response. Although ingestion and injection of E. faecalis plus toxin and E. faecalis, respectively, activated the host’s immune response to different degrees, both treatments induced larval death, suggesting that E. faecalis might kill by slightly different mechanisms, depending on whether it undergoes translocation from the gut. Further studies are required to identify the specific genes altered during the M. sexta innate immune response and the mechanism behind bacterial translocation-induced innate immune alterations.
In this study, we investigated the mode of action of B. thuringiensis toxin (Cry1Ac) and resolved some of the contradictions in the literature by standardizing methodologies. Researchers have investigated B. thuringiensis toxin toxicity with various protocols that differed in lepidopteran species age, antibiotics in the larval diet, and toxin formulations (14, 27, 29–35), all of which we found to affect results. van Frankenhuyzen et al. also found that small differences in methodology led to different mortality responses in larvae (27). Here we standardized conditions (antibiotics, toxin, diet, and developmental stage) and report that E. faecalis is a commensal in the midguts of M. sexta larvae and a pathogen in the hemocoel. B. thuringiensis toxin mediates the translocation of E. faecalis from the gut to the hemolymph, resulting in a commensal-to-pathogen switch and stimulation of the innate immune response.
MATERIALS AND METHODS
M. sexta larva rearing.
M. sexta eggs were obtained from the Carolina Biological Supply Company in Burlington, NC. Eggs were surface sterilized with a solution of Tween 80 (polyoxyethylene sorbitan monooleate), bleach, and distilled water as described previously (36). Eggs were hatched in 100-cm petri plates, and individual larvae were transferred to sterile 2-ounce clear plastic cups with holes for ventilation (SOLO, Lake Forest, IL). Larvae were reared on a sterilized artificial diet (USDA, Hamden Formula) amended with two antibiotics and an antifungal agent (250 mg/liter rifampin, 250 mg/liter gentamicin, or 20 mg/liter nystatin) in an environmental chamber at 26°C and 57% relative humidity on a 16:8 (light/dark) photoperiod.
Bacterial and toxin strains.
E. faecalis OG1RF was provided by Gary Dunny at the University of Minnesota. For bacterial feeding and injection assays, E. faecalis was cultured at 37°C with shaking overnight in M9-Casamino Acids-yeast extract medium with 200 mg/liter rifampin (37). Bacterial cultures were washed in phosphate-buffered saline, quantified on a hemacytometer, and diluted as necessary. Bacterial population sizes were confirmed through serial dilutions cultured on brain heart infusion (BHI) agar (supplemented with 200 mg/liter rifampin) and quantified the following day. B. thuringiensis toxin was administered via the MVPII formulation (Cry1Ac encapsulated in P. fluorescens; Dow AgroSciences, San Diego, CA). MVPII was weighed out and diluted as necessary in PBS.
Feeding and injection assays.
Bacterial cultures and/or toxin were force fed to early-5th-instar larvae in 10-µl doses using a gavage needle with a 1.25-mm tip (Fine Science Tools, Foster City, CA) mounted on a hand-held repetitive Stepper pipette (Tridak, Torrington, CT). Injections of 10-µl doses were administered to early-5th-instar larvae using a 30.5-gauge needle mounted on the Tridak Stepper. Larvae were surface sterilized, and the needle was inserted into the first proleg parallel to the epidermis to avoid injury to the alimentary canal.
For all experiments, control larvae were fed or injected with 10 µl of PBS. Larvae in mortality assays were placed in clean containers and provided with a sterile, unamended artificial diet for the duration of the assay.
E. faecalis population counts in the hemolymph and gut.
Hemolymph was collected from larvae at appropriate time points as previously described (38). Samples were serially diluted in PBS, cultured on BHI agar supplemented with 20 mg/liter rifampin, and incubated overnight at 37°C.
Hemocyte aggregate counts.
To assess hemocyte aggregation, larvae were surface sterilized and bled from the first proleg into a 1.5-ml sterile Microfuge tube with 0.02% bromophenol blue. Samples were kept on ice and counted immediately using a hemocytometer. Dead cells were excluded from the analysis, and aggregates were defined as two or more hemocytes in direct contact. Percent hemocyte aggregation was determined as follows: % hemocytes aggregated = [(number of hemocytes in aggregates)/(total number of hemocytes)] × 100.
Histological studies.
Gut tissues were fixed in 10% neutral buffered formalin supplemented with 2% dimethyl sulfoxide for 24 h. Tissues were embedded in paraffin, cut, and stained with hematoxylin and eosin for microscopic analysis.
Statistical analysis.
Statistical analysis of hemocyte aggregation was performed in GraphPad Prism 5. A one-way analysis of variance (ANOVA) with a Bonferroni correction was performed for all experimental groups.
SUPPLEMENTAL MATERIAL
Antibiotics in the larval diet do not alter the mortality rate. Larvae were reared exclusively on a diet supplemented with antibiotics or without antibiotics throughout all developmental stages. When larvae reached the early 5th instar, they were force fed PBS, E. faecalis alone (Ef), B. thuringiensis toxin alone (Toxin), or E. faecalis and B. thuringiensis toxin together (Ef + Toxin). Larval deaths were documented over time (n = 12/group, 1 representative experiment of 2). Download Figure S1, EPS file, 1.891 MB.
E. faecalis persists in the hemolymph of M. sexta. Early-5th-instar larvae were injected with 104 CFU of E. faecalis and allowed to recover. Larvae were sacrificed at each time point to collect hemolymph, and hemolymph was selectively cultured on BHI supplemented with rifampin to estimate E. faecalis populations (n = 18/group/time point, 1 representative experiment of 2). Download Figure S2, EPS file, 1.598 MB.
ACKNOWLEDGMENTS
We acknowledge Cynthia Zerillo for technical support.
K.L.M. and T.A.S. contributed equally to the manuscript.
This research was supported by NIH grant 1RC1DK086831-0 and NSF grant IOS-0924502.
Footnotes
Citation Mason KL, Stepien TA, Blum JE, Holt JF, Labbe NH, et al. 2011. From commensal to pathogen: translocation of Enterococcus faecalis from the midgut to the hemocoel of Manduca sexta. mBio 2(3):e00065-11. doi:10.1128/mBio.00065-11.
REFERENCES
- 1. Fisher K, Phillips C. 2009. The ecology, epidemiology and virulence of Enterococcus. Microbiology 155:1749–1757 [DOI] [PubMed] [Google Scholar]
- 2. Donskey CJ. 2004. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin. Infect. Dis. 39:219–226 [DOI] [PubMed] [Google Scholar]
- 3. Murray BE. 2000. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342:710–721 [DOI] [PubMed] [Google Scholar]
- 4. Sader HS, Streit JM, Fritsche TR, Jones RN. 2006. Antimicrobial susceptibility of gram-positive bacteria isolated from European medical centres: results of the Daptomycin Surveillance Programme (2002-2004). Clin. Microbiol. Infect. 12:844–852 [DOI] [PubMed] [Google Scholar]
- 5. Vasanthakumar A, Handelsman J, Schloss PD, Bauer LS, Raffa KF. 2008. Gut microbiota of an invasive subcortical beetle, Agrilus planipennis Fairmaire, across various life stages. Environ. Entomol. 37:1344–1353 [DOI] [PubMed] [Google Scholar]
- 6. Kanost MR, Jiang H, Yu XQ. 2004. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol. Rev. 198:97–105 [DOI] [PubMed] [Google Scholar]
- 7. Gill SS, Cowles EA, Pietrantonio PV. 1992. The mode of action of Bacillus thuringiensis endotoxins. Annu. Rev. Entomol. 37:615–636 [DOI] [PubMed] [Google Scholar]
- 8. Pigott CR, Ellar DJ. 2007. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol. Mol. Biol. Rev. 71:255–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Broderick NA, Raffa KF, Handelsman J. 2010. Chemical modulators of the innate immune response alter gypsy moth larval susceptibility to Bacillus thuringiensis. BMC Microbiol. 10:129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aronson AI, Beckman W, Dunn P. 1986. Bacillus thuringiensis and related insect pathogens. Microbiol. Rev. 50:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson DE, Oppert B, McGaughey WH. 1998. Spore coat protein synergizes Bacillus thuringiensis crystal toxicity for the indianmeal moth. Curr. Microbiol. 36:278–282 [DOI] [PubMed] [Google Scholar]
- 12. Tabashnik BE, et al. Inheritance of resistance to Bt toxin crylac in a field-derived strain of pink bollworm (Lepidoptera: Gelechiidae). J. Econ. Entomol. 95:1018–1026 [DOI] [PubMed] [Google Scholar]
- 13. Johnson DE, Freedman B. 1981. Toxicity of Bacillus thuringiensis Spo Cr mutants for the European corn borer Ostrinia nubilalis. Appl. Environ. Microbiol. 42:385–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heimpel A, Angus T. 1959. The site of action of crystalliferous bacteria in lepidoptera larvae. J. Insect Pathol. 1:152–170 [Google Scholar]
- 15. Park Y, et al. 2007. Clonal variation in Xenorhabdus nematophila virulence and suppression of Manduca sexta immunity. Cell. Microbiol. 9:645–656 [DOI] [PubMed] [Google Scholar]
- 16. Miller JS, Stanley DW. 2004. Lipopolysaccharide evokes microaggregation reactions in hemocytes isolated from tobacco hornworms, Manduca sexta. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 137:285–295 [DOI] [PubMed] [Google Scholar]
- 17. Toumanoff C, Vago C. 1953. Histopathological study of the silkworm with Bacillus cereus alesti. Ann. Inst. Pasteur 84:376–385 [PubMed] [Google Scholar]
- 18. Fast PG, Angus TA. 1965. Effects of parasporal inclusions of Bacillus thuringiensis var. sotto Ishiwata on the permeability of the gut wall of Bombyx mori (Linnaeus) larvae. J. Invertebr. Pathol. 20:29–32 [DOI] [PubMed] [Google Scholar]
- 19. Angus TA. 1954. A bacterial toxin paralysing silkworm larvae. Nature 173:545–546 [DOI] [PubMed] [Google Scholar]
- 20. Heimpel AM, Angus TA. 1960. Bacterial insecticides. Bacteriol. Rev. 24:266–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Broderick NA, Raffa KF, Handelsman J. 2006. Midgut bacteria required for Bacillus thuringiensis insecticidal activity. Proc. Natl. Acad. Sci. U. S. A. 103:15196–15199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raymond B, et al. 2009. A mid-gut microbiota is not required for the pathogenicity of Bacillus thuringiensis to diamondback moth larvae. Environ. Microbiol. 11:2556–2563 [DOI] [PubMed] [Google Scholar]
- 23. Johnston PR, Crickmore N. 2009. Gut bacteria are not required for the insecticidal activity of Bacillus thuringiensis toward the tobacco hornworm, Manduca sexta. Appl. Environ. Microbiol. 75:5094–5099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schnepf HE, Whiteley HR. 1981. Cloning and expression of the Bacillus thuringiensis crystal protein gene in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 78:2893–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Frankenhuyzen K. 2009. Insecticidal activity of Bacillus thuringiensis crystal proteins. J. Invertebr. Pathol. 101:1–16 [DOI] [PubMed] [Google Scholar]
- 26. van Frankenhuyzen K, et al. Specificity of activated CryIA proteins from Bacillus thuringiensis subsp. kurstaki HD-1 for defoliating forest lepidoptera. Appl. Environ. Microbiol. 57:1650–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Frankenhuyzen K, Liu Y, Tonon A. 2010. Interactions between Bacillus thuringiensis subsp. kurstaki HD-1 and midgut bacteria in larvae of gypsy moth and spruce budworm. J. Invertebr. Pathol. 103:124–131 [DOI] [PubMed] [Google Scholar]
- 28. Broderick NA, et al. Contributions of gut bacteria to Bacillus thuringiensis-induced mortality vary across a range of lepidoptera. BMC Biol. 7:11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suzuki MT, Lereclus D, Arantes OM. 2004. Fate of Bacillus thuringiensis strains in different insect larvae. Can. J. Microbiol. 50:973–975 [DOI] [PubMed] [Google Scholar]
- 30. Janmaat AF, Myers JH. 2005. The cost of resistance to Bacillus thuringiensis varies with the host plant of Trichoplusia ni. Proc. Biol. Sci. 272:1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bauce E, Kumbasli M, van Frankenhuyzen K, Carisey N. 2006. Interactions among white spruce tannins, Bacillus thuringiensis subsp. kurstaki, and spruce budworm (Lepidoptera: Tortricidae), on larval survival, growth, and development. J. Econ. Entomol. 99:2038–2047 [DOI] [PubMed] [Google Scholar]
- 32. Rahman MM, Roberts HL, Sarjan M, Asgari S, Schmidt O. 2004. Induction and transmission of Bacillus thuringiensis tolerance in the flour moth Ephestia kuehniella. Proc. Natl. Acad. Sci. U. S. A. 101:2696–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Edlund T, Sidén I, Boman HG. 1976. Evidence for two immune inhibitors from Bacillus thuringiensis interfering with the humoral defense system of saturniid pupae. Infect. Immun. 14:934–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Frankenhuyzen K, Nystrom CW, Tabashnik BE. 1995. Variation in tolerance to Bacillus thuringiensis among and within populations of the spruce budworm (Lepidoptera: Tortricidae) in Ontario. J. Econ. Entomol. 88:97–105 [DOI] [PubMed] [Google Scholar]
- 35. Mostafa AM, Fields PG, Holliday NJ. 2005. Effect of temperature and relative humidity on the cellular defense response of Ephestia kuehniella larvae fed Bacillus thuringiensis. J. Invertebr. Pathol. 90:79–84 [DOI] [PubMed] [Google Scholar]
- 36. Broderick NA, et al. 2000. Synergy between zwittermicin A and Bacillus thuringiensis subsp kurstaki against gypsy moth (Lepidoptera: Lymantriidae). Environ. Entomol. 29:101–107 [Google Scholar]
- 37. Dunny GM, Clewell DB. 1975. Transmissible toxin (hemolysin) plasmid in Streptococcus faecalis and its mobilization of a noninfectious drug resistance plasmid. J. Bacteriol. 124:784–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Orchard SS, Goodrich-Blair H. 2004. Identification and functional characterization of a Xenorhabdus nematophila oligopeptide permease. Appl. Environ. Microbiol. 70:5621–5627 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antibiotics in the larval diet do not alter the mortality rate. Larvae were reared exclusively on a diet supplemented with antibiotics or without antibiotics throughout all developmental stages. When larvae reached the early 5th instar, they were force fed PBS, E. faecalis alone (Ef), B. thuringiensis toxin alone (Toxin), or E. faecalis and B. thuringiensis toxin together (Ef + Toxin). Larval deaths were documented over time (n = 12/group, 1 representative experiment of 2). Download Figure S1, EPS file, 1.891 MB.
E. faecalis persists in the hemolymph of M. sexta. Early-5th-instar larvae were injected with 104 CFU of E. faecalis and allowed to recover. Larvae were sacrificed at each time point to collect hemolymph, and hemolymph was selectively cultured on BHI supplemented with rifampin to estimate E. faecalis populations (n = 18/group/time point, 1 representative experiment of 2). Download Figure S2, EPS file, 1.598 MB.