ABSTRACT
A key challenge in managing transmissible spongiform encephalopathies (TSEs) or prion diseases in medicine, agriculture, and wildlife biology is the development of practical tests for prions that are at or below infectious levels. Of particular interest are tests capable of detecting prions in blood components such as plasma, but blood typically has extremely low prion concentrations and contains inhibitors of the most sensitive prion tests. One of the latter tests is quaking-induced conversion (QuIC), which can be as sensitive as in vivo bioassays, but much more rapid, higher throughput, and less expensive. Now we have integrated antibody 15B3-based immunoprecipitation with QuIC reactions to increase sensitivity and isolate prions from inhibitors such as those in plasma samples. Coupling of immunoprecipitation and an improved real-time QuIC reaction dramatically enhanced detection of variant Creutzfeldt-Jakob disease (vCJD) brain tissue diluted into human plasma. Dilutions of 1014-fold, containing ~2 attogram (ag) per ml of proteinase K-resistant prion protein, were readily detected, indicating ~10,000-fold greater sensitivity for vCJD brain than has previously been reported. We also discriminated between plasma and serum samples from scrapie-infected and uninfected hamsters, even in early preclinical stages. This combined assay, which we call “enhanced QuIC” (eQuIC), markedly improves prospects for routine detection of low levels of prions in tissues, fluids, or environmental samples.
IMPORTANCE
Transmissible spongiform encephalopathies (TSEs) are largely untreatable and are difficult to diagnose definitively prior to irreversible clinical decline or death. The transmissibility of TSEs within and between species highlights the need for practical tests for even the smallest amounts of infectivity. A few sufficiently sensitive in vitro methods have been reported, but most have major limitations that would preclude their use in routine diagnostic or screening applications. Our new assay improves the outlook for such critical applications. We focused initially on blood plasma because a practical blood test for prions would be especially valuable for TSE diagnostics and risk reduction. Variant Creutzfeldt-Jakob disease (vCJD) in particular has been transmitted between humans via blood transfusions. Enhanced real-time quaking-induced conversion (eQuIC) provides by far the most sensitive detection of vCJD to date. The 15B3 antibody binds prions of multiple species, suggesting that our assay may be useful for clinical and fundamental studies of a variety of TSEs of humans and animals.
Introduction
The transmissible spongiform encephalopathies (TSEs) or prion diseases are fatal neurodegenerative disorders that include human Creutzfeldt-Jakob disease (CJD), bovine spongiform encephalopathy (BSE), sheep scrapie, and cervid chronic wasting disease (CWD). The TSE infectious agent, or prion, is composed primarily of an abnormal, misfolded, multimeric, and usually partially protease-resistant form of the host’s prion protein (e.g., PrPres, PrPvCJD, and PrPSc), which can induce, or seed, its own propagation by recruiting and converting the normal protease-sensitive prion protein, PrPC (1). For brevity we will use the term “prion” to refer to either TSE infectivity or TSE-associated PrP seeding activity, bearing in mind that the two activities may not always be strictly proportional to one another.
The apparent lack of prion-specific nucleic acid genomes, polypeptides, or immune responses has prevented the use of most conventional means for detecting pathogens and diagnosing infections. Moreover, the unusual stability of prions makes decontamination difficult. Presymptomatic detection of prion infections in individuals will be critical in expediting potential treatments and preventing further transmissions. In various mammalian hosts, prions can be found in a wide variety of tissues and accessible bodily fluids, including blood (2–7), saliva (4, 8), and nasal fluids (9, 10). These materials not only have potential diagnostic value, but also can be sources of TSE contagion. Of particular concern is the safety of the blood supply (11, 12) due to transfusion-based transmissions of BSE and scrapie in sheep (13) and of variant CJD (vCJD) in humans (14–16). The prevalence of subclinical vCJD infections is far from clear but may be as high as 1 per 4,000 in certain United Kingdom age cohorts (http://www.seac.gov.uk/papers/104-2.pdf). Unfortunately, the typically low concentrations of prions in blood and other bodily fluids have usually prevented rapid detection. In scrapie-affected hamsters, for example, blood contains only ~13 infectious doses per milliliter (17).
A number of in vitro methods for detecting minimally infectious or subinfectious amounts of prions have been reported (5, 6, 10, 18–32), but most have limitations that would preclude their widespread use in routine diagnostic or screening applications. Among the most rapid of the ultrasensitive prion tests are the quaking-induced conversion (QuIC) reactions (20, 24), in which prions induce the polymerization of recombinant PrPC (rPrPC) into amyloid fibrils. The original format (herein called “standard QuIC” [S-QuIC]) involved tube-based reactions and immunoblotting of protease-resistant rPrP conversion products (9, 10, 20, 24). A higher-throughput format, called “real-time QuIC” (10, 32), or RT-QuIC, employs multiwell plates and a thioflavin T (ThT)-based fluorescence detection of the prion-seeded amyloid, as has been described for many other amyloid seeding assays (e.g., references 22 and 33). RT-QuIC sensitivity rivals that of animal bioassays and allows detection of prions endogenous to cerebrospinal fluid (CSF) and nasal fluids (10, 32). Moreover, in an endpoint dilution mode, the RT-QuIC can be quantitative (10). The original RT-QuIC has been shown recently to have >80% sensitivity and 100% specificity in discriminating between sporadic CJD (sCJD) and non-CJD patients based on CSF samples (32).
Here we show that the combination of prion/PrPSc immunoprecipitation (IP) with the S-QuIC and RT-QuIC prion amplification assays (IP-S-QuIC and IP-RT-QuIC, respectively) markedly improves their sensitivities and applicability to the detection of prions in dilute, inhibitor-laden fluids such as blood plasma. The IP step employs the PrP aggregate-specific monoclonal IgM antibody 15B3 (34–36). When coupled with a substrate replacement step, the IP-RT-QuIC assay is orders of magnitude more sensitive than previously described tests for vCJD (30, 37).
RESULTS
Immunoaffinity capture of prions from blood plasma.
To develop a blood test for prions, we initially attempted to detect prions spiked into human and sheep plasma samples by directly adding spiked plasma to S-QuIC and RT-QuIC reaction mixtures. However, plasma components strongly inhibited both assays (data not shown), consistent with previously reported inhibition of protein misfolding cyclic amplification (PMCA) (38) and flow cytometric assays (23). Accordingly, we sought methods to capture and concentrate prions in a detectable form from plasma. Prion immunoaffinity beads were prepared by coupling monoclonal antibody 15B3 (34) to magnetic beads. This antibody selectively binds PrPres and other PrP oligomers but not monomeric PrPC (34, 36). The ability of 15B3-coupled beads to immunoprecipitate prion activity from plasma was first tested with the S-QuIC assay. We spiked 0.5 ml of human plasma with hamster scrapie and human vCJD brain homogenate dilutions or comparable TSE-negative brain homogenates and incubated them with the beads. The beads were then washed, added directly to the S-QuIC reaction, and subjected to cycles of shaking and rest. As described previously (20, 21, 24), positive prion-seeded S-QuIC reactions were indicated by the characteristic pattern of 17-, 13-, 12, and 11-kDa proteinase K-resistant products [called rPrP-res(Sc)] in immunoblots. We performed two-round reactions by seeding aliquots of first-round reaction products into fresh rPrPC substrate. Control (mock) beads coated only with anti-IgM antibodies (without 15B3) had some affinity for prions, as indicated, for example, by the positive rPrP-res(Sc) products generated in one of the two replicate single-round reaction mixtures seeded with beads incubated with plasma spiked with a 1 × 10−9 dilution of scrapie brain homogenate containing ~100 fg PrPres (see the lane marked by the asterisk in Fig. S1a in the supplemental material). However, 15B3-coated beads were ~100-fold more efficient at capturing lower levels of prions from plasma, enabling detection of dilutions containing ≥1 fg PrPres (Fig. S1a and b). This IP-S-QuIC protocol gave positive reactions from as little as 4 × 10−10 dilutions of vCJD brain homogenate containing ~10 fg of human PrPres (Fig. 1) and 2 × 10−11 dilutions of scrapie hamster brain containing ~1 fg of PrPres (Fig. S1). In contrast, no positive rPrP-res(Sc) reaction products were obtained in reactions seeded with non-TSE human or hamster brain homogenates. Moreover, 15B3 IP-S-QuIC detected prion activity naturally present in 0.5 ml of plasma from nine near-terminal scrapie-infected hamsters, while no positive S-QuIC reactions were seeded by plasma from a negative control hamster in two-round reactions (Fig. 2).
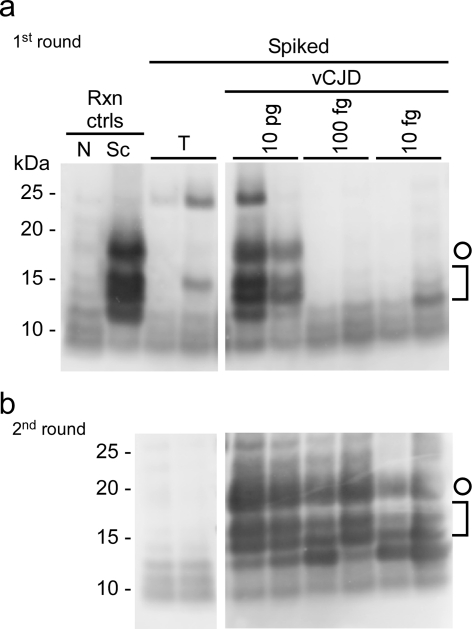
FIG 1 .
IP-S-QuIC detection of ≥10 fg human vCJD PrPres spiked into human plasma. Dilutions of human nonprion (tumor [T]) control or vCJD brain homogenates were spiked into 500 µl of human plasma to give final tissue dilutions of 4 × 10−7 (T) and 4 × 10−7, 4 × 10−9, and 4 × 10−10 (vCJD; containing ~10 pg, 100 fg, and 10 fg PrPres, respectively). PrPvCJD was immunoprecipitated and subjected to S-QuIC as described in Materials and Methods. The first S-QuIC round was at 50°C for 8 h (a), and one-tenth of the first-round reaction volume was used to seed the second round (45°C for 10 h) (b). Plasma-free positive and negative control reaction (Rxn ctrls) mixtures were seeded directly with 2 µl of 5 × 10−7 dilutions of hamster uninfected (N) or scrapie (Sc) brain, the latter containing ~100 fg PrPres seed. Hamster rPrPC23–231 was used as a substrate in all reactions and comigrated with the 25-kDa marker. PK-digested products were analyzed by immunoblotting using the polyclonal R20 antibody as previously reported (24). Open circles mark 17-kDa fragments, and brackets indicate the lower-molecular-mass bands (10 to 13 kDa).
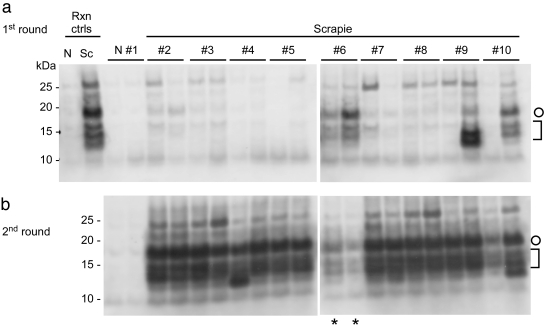
FIG 2 .
Detection of endogenous PrPSc in plasma of scrapie-infected hamsters by IP-S-QuIC. (a) Plasma samples from scrapie 263K-infected and uninfected (N) hamsters (500 µl) were subjected to IP-SQ as described in Materials and Methods with the first round of S-QuIC at 50°C for 10 h and the second round (b) at 50°C for 8 h, except for lanes marked with asterisks, which show the first-round products seeded with sample no. 6 for comparison. Plasma-free positive and negative control reactions (Rxn ctrls), rPrPC23–231 substrate, and analysis of PK-digested products were as described for Fig. 1. Open circles mark 17-kDa fragments, and brackets indicate the lower-molecular-mass bands (10 to 13 kDa).
15B3 IP of prions in plasma for detection by RT-QuIC (IP-RT-QuIC).
Because of the greater potential for high-throughput testing, we then focused mainly on adapting the 15B3 IP to detection by RT-QuIC (designated IP-RT-QuIC). The RT-QuIC assay uses intermittent shaking of reactions in 96-well plates, rPrPc as the substrate, virtually detergent-free (≤0.002% SDS) and chaotrope-free conditions, and ThT-based detection of prion-seeded amyloid fibrils (10, 32). Positive reactions are indicated by an enhancement of ThT fluorescence in the presence of rPrP amyloid fibrils, which we plot as the average fluorescence from replicate wells. In screening for conditions that allow the detection of prions captured on 15B3 beads from blood plasma with the RT-QuIC assay, we found that preincubation of the prion-bound beads with 0.05% SDS for ~20 min at room temperature, in addition to a Sarkosyl wash of the beads, accelerated prion amplification in the otherwise detergent-free RT-QuIC (see Fig. S2 in the supplemental material).
The IP-RT-QuIC protocol detected ~10−10 dilutions of scrapie brain in human plasma (data not shown), but was less sensitive for vCJD brain (see Fig. S3C in the supplemental material). For detection of scrapie, we used hamster rPrPC comprising residues 90 to 231 (rPrPC90–231) as a substrate. For vCJD, we found that a chimeric rPrPC molecule, comprised of Syrian hamster residues 23 to 137 followed by sheep residues 141 to 234 (R154 Q171 polymorph), provided for greater sensitivity and less spontaneous (prion-independent) conversion to ThT-positive products than was observed with the homologous human PrPC23–231 construct (Fig. S3).
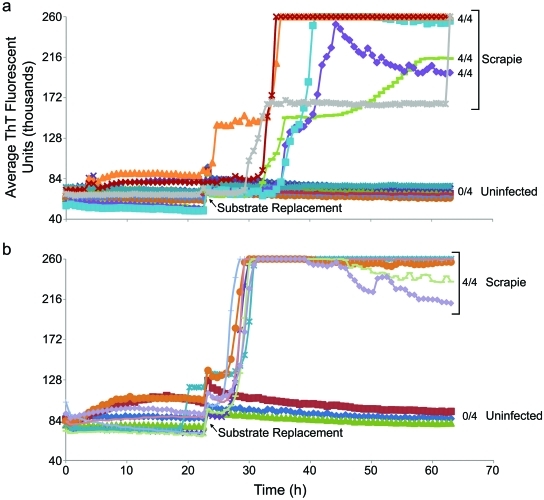
Using the hamster rPrPC90–231 substrate,15B3 IP of PrPSc endogenous to 0.5 ml plasma or serum from scrapie-affected hamsters yielded some, but usually not all, positive replicate reactions, indicating that the PrPSc levels in these samples were at or near the detection limit (Fig. 3). Collectively, these initial results showed that 15B3 beads captured highly diluted prions from plasma or serum in a manner compatible with both S-QuIC or RT-QuIC detection, but the sensitivity of IP-RT-QuIC was borderline for detection of prions endogenous to scrapie hamster plasma.
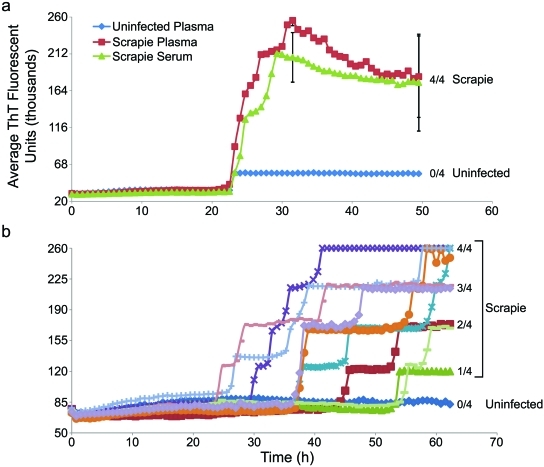
FIG 3 .
IP-RT-QuIC detection of endogenous PrPSc in plasma and serum of scrapie-infected hamsters. (a) IP-RT-QuIC analyses of plasma samples from a scrapie 263K-infected hamster and a normal hamster, and a serum sample from a scrapie 263K-infected hamster. (b) Analyses of plasma samples from nine scrapie 263K-infected hamsters and one uninfected hamster. In all cases, 500-µl samples were immunoprecipitated using 15B3-coated beads for ~20 h at 37°C. One-fifth of the beads were preincubated with 0.05% SDS in PBS at room temperature for ~20 min and used to seed RT-QuIC containing 300 mM NaCl. RT-QuIC reaction mixtures were incubated at 42°C, and hamster rPrPC90–231 was used as a substrate in all reactions. The vertical axes indicate the average fluorescence from four replicate reaction wells. Error bars show standard deviations for selected sets of replicates in panel a. In panel b, all individual reactions that registered positive fluorescence achieved nearly identical maximal fluorescence values (~260,000 U), but in many of the scrapie-seeded cases, only a subset of replicate reactions rose above background fluorescence within 63 h. With such all-or-nothing responses among replicates, it is of little value to calculate standard deviations from all of the replicates; instead, on the right, we indicate the fraction of positive wells per total number of replicates at the end of the reactions. Error bars representing standard deviations calculated for the positive replicates (only) barely, if at all, exceeded the sizes of the symbols and therefore are not shown.
eRTQ detection of 15B3-captured prions with substrate replacement.
To improve the sensitivity of IP-RT-QuIC we introduced a substrate replacement step after ~24 h of the RT-QuIC reaction. In IP-RT-QuIC reactions, the beads and associated prions or prion-induced RT-QuIC conversion products tended to adhere to the bottom of reaction wells. Thus, reaction fluid could be removed and fresh rPrPC added while retaining most of the beads and bead-bound reaction products in the well. This combination of IP and RT-QuIC with substrate replacement, which we call “enhanced RT-QuIC” (eQuIC), allowed detection of 4 × 10−14 dilutions of vCJD brain tissue (~1 attogram [ag] vCJD PrPres) within ~28 h in all replicate reactions (n = 4) in three independent experiments (e.g., Fig. 4A; see Fig. S4A in the supplemental material) performed using four different lots of human plasma. With a further 4 × 10−15 dilution, three of four replicate reactions were positive in a single experiment (data not shown). By comparison, Alzheimer’s and tumor brain negative control dilutions gave uniformly negative reactions in each of these eQuIC experiments. Mock beads lacking 15B3 gave much reduced sensitivity and consistency (Fig. 4b). Moreover, the 15B3-coupled beads provided for ≥106-fold more sensitive eQuIC detection than superparamagnetic nanoparticles that were reported recently to have prion-binding capacity (39) (Fig. S4). These results showed the ability of the 15B3-based eQuIC to detect extremely low concentrations of prions spiked into human plasma.
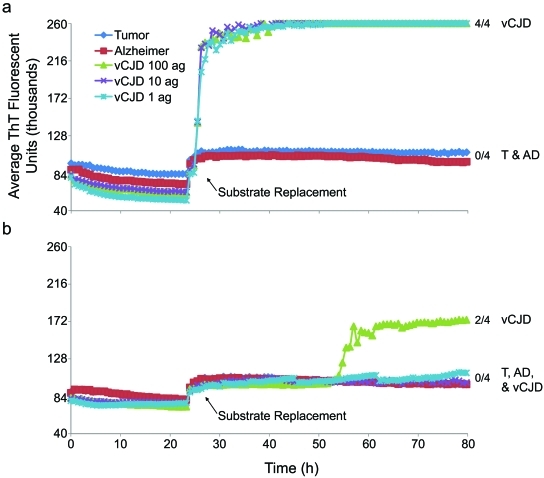
FIG 4 .
eQuIC detection of human PrPvCJD spiked into human plasma. Dilutions of human nonprion (tumor and Alzheimer’s disease) control or vCJD brain tissues were spiked into 500 µl of human plasma to give final dilutions of 4 × 10−7 (tumor and Alzheimer’s disease) and 4 × 10−12, 4 × 10−13, and 4 × 10−14 (vCJD; containing ~100, 10, and 1 ag PrPres, respectively). PrPvCJD was immunoprecipitated with 15B3-coated beads (a) or mock anti-IgM-coated beads (b), and a portion of the beads were used to seed replicate eQuIC reaction mixtures containing 400 mM NaCl. After 24 h, the substrate was replaced. The chimeric Ha-S rPrPC was used as a substrate in all reactions. The vertical axes indicate the average fluorescence from four replicate wells, and the fractions on the right indicate the positive/total replicate reactions associated with the adjacent traces.
eQuIC detection of endogenous prions in hamster plasma samples.
We also tested if eQuIC improved the detection of prions endogenous to plasma from scrapie-infected hamsters. In contrast to the earlier results with the unenhanced RT-QuIC (Fig. 3), all of the replicate eQuIC reactions from a total of 13 scrapie hamsters were positive, while none of those from 11 uninfected hamsters were positive within 65 h (Fig. 5). Of the scrapie-infected hamsters, nine were clinically affected (80 days postinfection [dpi]), and four were subclinically affected (3 at 30 dpi and 1 at 10 dpi). Thus, eQuIC detected prions in plasma long before clinical signs of scrapie, which in this model begin at ~60 dpi. With some scrapie samples the replicate wells, although all individually positive, gave submaximal average fluorescence values (Fig. 5a). This variability, as well as lag-phase variability, appeared to be due to aggregated plasma components because these variations were not seen with samples that were precleared by brief centrifugation immediately prior to immunoprecipitation (Fig. 5b).
FIG 5 .
eQuIC detection of endogenous PrPSc in plasma of scrapie-infected hamsters. (a) eQuIC analysis of plasma samples (without preclearing) from eight uninfected hamsters and six scrapie-infected hamsters, with one collected at 30 dpi (preclinical) and five at 80 dpi (near-terminal). The vertical axis indicates the average fluorescence from four replicate wells, and the fractions on the right indicate the positive/total replicate reactions associated with the adjacent traces. Although all replicate reactions seeded with the scrapie samples were positive, submaximal average fluorescence was observed for three of the samples at 60 h. In the latter cases, the bead distribution in the well partially interfered with fluorescence readings; when such wells (n = 3) were reread at 64 h after manual stirring with a pipette, the fluorescence achieved maximal levels (gray trace). In contrast, stirring of uninfected control wells (n = 4) did not increase their fluorescence. Hamster rPrPC90–231 was used as a substrate. (b) eQuIC analysis of precleared plasma samples from three uninfected and seven scrapie-infected hamsters (three collected at 80 dpi, two at 30 dpi, and one at 10 dpi).
DISCUSSION
Collectively, these results showed that prions can be captured from a complex inhibitor-laden biological fluid in a manner that is compatible with ultrasensitive detection by in vitro prion amplification assays. The eQuIC assay in particular provides a practical, high-throughput, and rapid means of testing for amounts of PrPres (e.g., 1 ag) that are several orders of magnitude below those typically required to cause prion disease by intracerebral inoculation into animals. The ability of eQuIC to detect prions in plasma samples raises the possibility that this assay could be used to improve prion disease diagnosis in humans and animals and to screen the blood supply for prion contamination. We have demonstrated discrimination of scrapie-infected and uninfected hamsters based on eQuIC analysis of their blood plasma samples; thus, the assay has at least some capability to detect natural blood-borne prion seeding activity. However, although we have also shown extreme sensitivity of the eQuIC in detecting brain-derived spikes of vCJD into human plasma, the sensitivity of eQuIC detection of vCJD seeding activity that is endogenous to plasma remains to be determined. We focused initially on plasma because it is a main target fluid for the development of an in vivo vCJD test. However, as 5- to 10-fold more CJD infectivity has been found in leukocyte fractions of blood (40), it is possible that somewhat greater sensitivity could be obtained by eQuIC analysis of leukocytes. 15B3-based prion capture might also enhance the utility of other prion amplification assays. Ultimately, it will be important for TSE surveillance labs to have multiple assays at their disposal so that positive tests can be reevaluated by other types of tests.
The two-stage substrate addition that we describe here for the eQuIC differs from serial (multiple-round) amplification steps that have been described previously for protein misfolding cyclic amplification (PMCA) (5, 18), rPrP-PMCA (21), and QuIC (20, 24) reactions because most of the bead-bound prions and prion-seeded products are retained in the reaction vessel so that the substrate can be replaced without removing most of the seed particles. In contrast, in serial PMCA and S-QuIC reactions, only a small proportion (typically ≤10%) of the total reaction mixture is transferred to a new vessel containing fresh substrate, so that much of the seeding activity from the first round is lost.
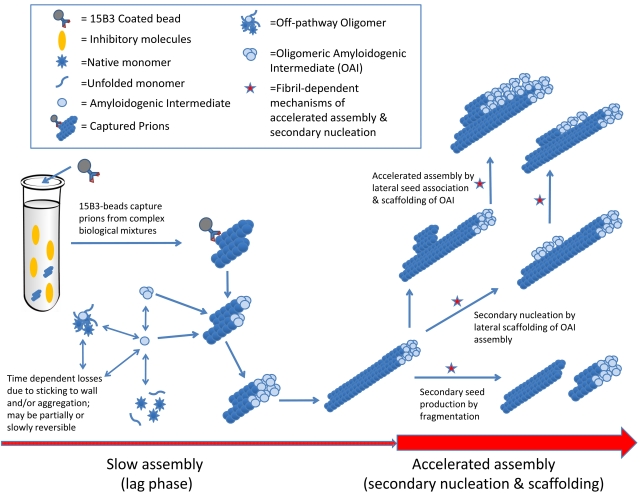
The mechanistic basis for the dramatic improvements in assay time and sensitivity due to the substrate replacement is not yet clear, but our results suggest that at least two processes are occurring during the initial lag phase of the eQuIC reaction, i.e., between the addition of seed and substrate replacement (Fig. 6). First, the rPrPC must be moving into a pool that is less rapidly accessible to prion-seeded fibril assembly, such as an off-pathway oligomer (OO); otherwise, the addition of fresh rPrPC after 20 h, but before the initial substrate is converted to detectable ThT-positive fibrillar products, would not accelerate the reaction. We suggest a “less rapidly accessible” pool rather than an inaccessible pool because even without substrate replenishment, the vast majority of the substrate can still be converted if given enough time. Second, the initial seed must be being altered and primed in some way to seed more rapid fibril assembly upon the addition of fresh substrate; otherwise, it would have been capable of seeding rapid ThT-positive rPrP-res(Sc) assembly at the beginning of the reaction, when there was the same concentration of fresh substrate. This priming effect might be explained by secondary nucleation mechanisms (41, 42), such as those marked with red stars in Fig. 6. For example, during the lag phase, prion seeds may elongate by incorporating rPrPC at a relatively slow and largely undetected rate determined in part by the concentration of seed particles. With continued elongation, the seeded rPrP fibrils would become long enough to be sheared by agitation, increasing the seed particle concentration and accelerating overall fibril assembly. Moreover, other types of fibril-dependent secondary nucleation might contribute to the acceleration of fibril assembly. For instance, fibril assembly might be hastened by the prealignment or scaffolding of rPrPC substrate or amyloidogenic intermediate (AI) along the sides of an existing fibril, either with or without the need for a similarly aligned seed. In any case, further studies will be required to define the mechanistic underpinnings of the effects of two-phase substrate addition.
FIG 6 .
Potential mechanisms of substrate replacement effect.
As we have seen before with the S-QuIC assay (24), the best rPrPC substrate for the RT-QuIC is not always the one that is the most homologous with the type of prion/PrPres being assayed. For example, we were surprised to find that the substrate that worked best for the detection of human vCJD was the chimeric hamster-sheep construct (Ha-S rPrPC) rather than a human rPrPC molecule. The reasons for this are not clear, but may relate to factors such as (i) the relative tendencies of the rPrPC to be consumed by off-pathway changes that remove substrate from the assembly-competent pool or promote spontaneous, prion-independent fibril formation (i.e., false positives) or (ii) differences in propensities to form an amyloidogenic precursor or intermediate which interacts most favorably with prion seeds (Fig. 6). Such a precursor may be distinct from native rPrPC, as has been described for other types of seeded polymerizations of PrPC (43–45). These factors are likely to be condition dependent and, for the practical purposes of detecting vCJD PrPres, appear to override problems associated with sequence mismatches between the vCJD seed and the Ha-S rPrPC substrate.
The eQuIC assay offers advantages compared to other currently established ultrasensitive prion/PrPSc assays. Relative to the first-generation RT-QuIC assay, the eQuIC not only allows for prion detection in inhibitor-laden samples such as plasma, but also enhances the sensitivity for vCJD brain homogenate dilutions into human plasma by at least 10,000-fold. Compared to PMCA reactions that have been described, the eQuIC is more rapid for a given sensitivity level, more practical by using bacteria rather than brain as the source of PrPC substrate, more easily replicated by using shaking rather than sonication, and more amenable to high-throughput analyses due to multiwell-plate-based reactions and fluorescence detection. Fluorescence plate readers should be more commonly available than the specialized instrumentation required for some other tests (25–29). Recently, Edgeworth and colleagues described a new vCJD PrPres detection assay which includes prion capture on stainless steel beads and an enzyme-linked immunosorbent assay (ELISA) detection method (30). Whereas this capture ELISA detected 1010-fold dilutions of vCJD brain in whole blood, our eQuIC assay detected 1014-fold dilutions in plasma. The Edgeworth assay detected PrPvCJD in blood from 15 symptomatic patients with ~70% sensitivity and 100% specificity, which is nearly as effective as the RT-QuIC in diagnosing sporadic CJD by using CSF samples (32). However, the ~10,000-fold greater sensitivity of the eQuIC in detecting brain-derived vCJD seeding activity suggests that it has considerable potential to improve the sensitivity of vCJD and sCJD diagnosis using blood, plasma, CSF, or other samples. However, we have not yet gained access to vCJD patient plasma or CSF samples, and further studies will be required to assess the diagnostic utility of eQuIC based on the detection of vCJD seeding activity that is endogenous to these and other specimens. The remarkable resistance to inactivation of prions relative to other pathogens also makes it important to develop practical assays for prion contamination in a wide variety of materials, such as foods, feeds, transplanted tissues, medical devices, agricultural wastes and by-products, soils, water sources, and other environmental samples. The ability to immunoprecipitate prions from complex matrices and detect them with high sensitivity should foster the further development of such assays.
MATERIALS AND METHODS
Recombinant prion protein purification.
Syrian golden hamster (residues 23 to 231; accession no. K02234), human (residues 23 to 231; accession no. M13899.1) rPrPC and hamster-sheep chimera rPrPC (Syrian hamster residues 23 to 137 followed by sheep residues 141 to 234 of the R154 Q171 polymorph [accession no. AY907689]) were amplified and ligated into the pET41 vector (EMD Biosciences), and sequences were verified. Protein expression and purification were performed as previously described (10, 20, 21, 24). Purity of rPrPC proteins was ≥99%, as estimated by SDS-PAGE, immunoblotting, and mass spectrometry (data not shown).
Plasma sample collection and tissue homogenate preparation.
Syrian golden hamsters were inoculated intracerebrally with 50 µl 0.5% brain homogenate (BH) (Fig. 5) or 108-fold-diluted BH (Fig. 2 and 3) from hamsters clinically affected with the 263K scrapie strain and held for the designated time periods prior to brain tissue or blood collection. The hamsters inoculated with the lower dose of scrapie took longer to become ill, so tissues were collected from “near-terminal” hamsters at 103 to 116 dpi for Fig. 2 and 3 compared to 80 dpi for Fig. 5. For plasma collections, hamsters were euthanized by deep isoflurane anesthesia and exsanguinated via heart stick. Blood was immediately transferred to BD Vacutainer (sodium citrate; Becton-Dickinson) tube and mixed gently. Samples were centrifuged at 3,000 rpm in a Beckman J6-HC centrifuge for 15 min. Plasma was transferred to a new tube and stored at −80°C. Hamster serum samples were collected in a similar fashion, but with no sodium citrate. When designated (Fig. 5B), plasma samples were centrifuged at 16,000 relative centrifugal force (RCF) for 30 s after thawing and immediately prior to the immunoprecipitation step (using the plasma supernatant). Pooled human plasma (Innovative Research) was stored at −20°C. For brain BH dilution spiking experiments, human plasma aliquots were thawed over night at 4°C and subjected to a 10-min 2,000 × g spin to eliminate precipitated fraction.
Hamster and human 10% (wt/vol) BH were made as previously reported (18), aliquoted, and stored at −80°C. For spiking experiments, BH was serially diluted in either 1% or 0.1% SDS in phosphate-buffered saline (PBS) with 130 mM NaCl and N2 medium supplement (Gibco) (20, 21, 24) for the S-QuIC and RT-QuIC assays, respectively. Two microliters of the designated BH dilutions was used to spike 0.5 ml of human plasma.
15B3 coating of magnetic beads.
Rat anti-mouse IgM Dynabeads (Invitrogen) were vortexed for 30 s, and 250 µl of beads (1 × 108 total beads) was transferred to a new tube for the coating procedure. Following incubation on the magnet for 2 min, bead storage buffer was discarded and two washes were performed with 5 original suspended bead volumes, using coating buffer (0.1% bovine serum albumin [BSA] in PBS made fresh, filtered, and kept at 4°C). A ratio of 1 × 106 beads per µg of 15B3 antibody (Prionics) was used. Tubes were incubated with “end-over-end” rotation at room temperature for 2 h. Next, three more washes with coating buffer were carried out, and beads were resuspended in coating buffer (initial bead volume) and stored at 4°C. Mock control beads were prepared as described for 15B3 beads but with no addition of 15B3 antibody.
Preparation of MagnaBind beads.
MagnaBind beads (Pierce, Rockford, IL) were vortexed for 30 s, and 1.2 × 108 total beads were transferred to a new tube. The beads were rinsed twice with 500 µl of 0.5% Triton X-100 in PBS and resuspended in their initial volume with assay buffer (Tris-buffered saline [TBS], 1% Triton X-100, 1% Tween 20).
Immunoprecipitation of 263K and vCJD PrPres in plasma.
15B3-coated beads, mock beads, or MagnaBind beads were briefly vortexed, and 1.6 × 107 total beads were transferred to a new tube. Following 2 min of incubation on the magnet, the storage (coating) buffer was discarded and 500 µl of immunoprecipitation buffer (Prionics) was added. Next, 500 µl of BH-spiked human plasma or 500 µl of hamster plasma from uninfected or scrapie-positive animals was added to the beads. Samples were incubated with “end-over-end” rotation at room temperature or 37°C overnight (ON). Subsequently, samples were incubated on the magnet for 2 min, plasma-buffer mix was discarded, and beads were washed twice with 500 µl of wash buffer (Prionics). All beads were resuspended into 10 µl of PBS and used fresh.
S-QuIC and RT-QuIC.
The S-QuIC assay was performed as previously described (20, 24). 15B3-coated- or mock bead S-QuIC reaction mixtures were each seeded with 2 µl of beads in PBS. The RT-QuIC was performed as previously described (10), except for a few modifications. Briefly, 15B3-coated, mock, or MagnaBind beads from the immunoprecipitation step (resuspended in 10 µl PBS) were combined with 0.05% SDS–PBS (1:1 ratio) and incubated at room temperature for 20 min, and reaction mixtures were seeded with 4 µl of 0.05% SDS–PBS–bead mix. RT-QuIC reaction mixtures were incubated at 46°C unless indicated otherwise in figure legends. Substrate replacement was performed by interrupting the RT-QuIC reaction after 24 h and spinning the plate at 3,000 × g for 10 min at 4°C. Next, 90 µl of supernatant was removed from each well, taking care not to perturb the beads, and 100 µl of new reaction buffer containing fresh rPrPC was gently added to each well. RT-QuIC was continued for an additional 36 to 60 h.
SUPPLEMENTAL MATERIAL
Better IP-S-QuIC sensitivity and consistency of PrPSc Detection in spiked human plasma using 15B3 versus mock beads. (a) Comparison of 15B3 versus mock beads with 2-h IP from 100 µl plasma and two-round S-QuIC. Dilutions of hamster uninfected “normal” (N) or scrapie 263K (Sc) brain homogenates were spiked into 100 µl of human plasma to give final brain dilutions of 10−8 (N) and 10−8, 10−9, and 10−10 (Sc) (~100, 10, and 1 fg PrPres, respectively). PrPSc was immunoprecipitated using 40 µl (1.6 × 107 total beads) of 15B3-coated beads (15B3) or mock anti-IgM-coated beads (C) for 2 h at 37°C. Beads were resuspended in 10 µl of PBS. One-fifth of the beads were used to seed a first-round S-QuIC at 50°C for 10 h, and one-tenth of the first-round reaction volume was used to seed the second round (50°C for 10 h). (b) 15B3 beads with 20-h IP from 500 µl plasma and single-round S-QuIC. Dilutions of N or Sc brain homogenates were spiked into 500 µl of human plasma to give final brain tissue dilutions of 2 × 10−8 (N) and 2 × 10−8 to 2 × 10−11 (Sc) (containing ~1 pg to 1 fg PrPres, respectively). PrPSc was immunoprecipitated with 15B3 beads for ~20 h at 37°C. The remainder of the protocol was performed as in panel a, except that only a single-round S-QuIC at 50°C for 10 h was performed. Plasma-free positive and negative control reaction mixtures were seeded directly with 2 µl of 5 × 10−7 dilutions of hamster N or Sc brain, the latter containing ~100 fg PrPres seed. Hamster rPrPC23–231 was used as a substrate in all S-QuIC reactions. PK-digested products were analyzed by immunoblotting with the polyclonal R20 antibody as previously reported (24). Open circles mark 17-kDa fragments, and brackets indicate the lower-molecular-mass bands (10 to 13 kDa). Download Figure S1, TIF file, 0.445 MB.
SDS pretreatment of 15B3-bound PrPSc accelerates RT-QuIC detection. Dilutions of hamster N or Sc 263K brain homogenates were spiked into 500 µl of human plasma to give final brain dilutions of 2 × 10−7 (containing ~10 pg PrPres in the case of Sc). IP incubations with beads were for ~20 h at 37°C. One-fifth of the beads were used to seed RT-QuIC (a), and an equivalent number of beads were preincubated with 0.05% SDS in PBS at room temperature for ~20 min and used to seed RT-QuIC reaction mixtures containing 300 mM NaCl (b). Reaction mixtures were incubated at 42°C, and hamster rPrPC90–231 was used as a substrate in all reactions. The vertical axis indicates the average fluorescence from four replicate wells, and the fractions on the right indicate the positive/total replicate reactions associated with the adjacent traces. Download Figure S2, TIF file, 1.830 MB.
Better RT-QuIC detection of 15B3-bound human PrPvCJD with hamster-sheep chimeric rPrPC (Ha-S rPrPC) versus human rPrPC23–231 with NaCl variation. Dilutions of human nonprion (tumor) control or vCJD brain tissues were spiked into 500 µl of human plasma to give final dilutions of 4 × 10−7 (a and b) (containing ~10 pg PrPres, in the case of vCJD) or 4 × 10−7 and 4 × 10−9 and 4 × 10−10 (c) (containing ~10 pg, 100 fg, and 10 fg PrPrres, respectively, in the case of vCJD). The samples were subjected to IP-RT-QuIC as described in Materials and Methods, except for indicated variations in NaCl concentration and rPrPC substrate. The vertical axis indicates the average fluorescence from four replicate wells, and the fractions on the right indicate the positive/total replicate reactions associated with the adjacent traces. Download Figure S3, TIF file, 4.302 MB.
Comparison of 15B3 beads to MagnaBind beads in eQuIC. Dilutions of human non-TSE Alzheimer’s disease (AD) control or vCJD brain tissues were spiked into 0.5 ml of human plasma to give final dilutions of 4 × 10−7 (AD) and 4 × 10−7, 4 × 10−10, 4 × 10−13, and 4 × 10−14 (vCJD) (containing ~10 pg, 10 fg, 10 ag, and 1 ag PrPres, respectively). PrPvCJD was immunoprecipitated using 1.6 × 107 total 15B3-coated beads (a) or an equivalent number of MagnaBind beads (b) for ~20 h at 37°C using immunoprecipitation buffer (Prionics). Beads were washed twice with wash buffer (Prionics) and resuspended in 10 µl of PBS. The remainder of the protocol was as described in Material and Methods, starting with the preincubation in 0.05% SDS in PBS. The vertical axis indicates the average fluorescence from four replicate wells, and the fractions on the right indicate the positive/total replicate reactions associated with the adjacent traces. Similar results were obtained using the MagnaBind beads PrPSc capture conditions described by Miller and Supattapone (39; data not shown). Download Figure S4, TIF file, 2.686 MB.
ACKNOWLEDGMENTS
This research was funded by the Intramural Research Program of the NIAID, NIH; Prionics AG; and the EU grant PRIORITY.
We thank Pierluigi Gambetti (Case Western Reserve University, National Prion Disease Surveillance Center) for providing human tissue samples; Andrew Hughson, Kimberly Meade-White, and Brent Race for providing us with plasma samples from uninfected and scrapie-affected hamsters; and Gregory Raymond for overseeing our sample acquisition and experimental rodent facilities. We also appreciate the animal caretaking by Jeff Severson. We are grateful to Karin Peterson, Brent Race, and Gregory Raymond for critical review of the manuscript and Anita Mora for assistance with graphics. We thank Kristin McNally and Marshall Bloom for providing expression plasmids for human rPrPC23–231 and hamster rPrPC23–231, respectively. We thank Michael Coulthart (Canadian CJD Surveillance System and Prion Diseases Program) for suggesting the acronym “eQuIC.”
C.D.O., B.C., B.S., and A.R. designed the project. C.D.O. performed all experiments shown. L.D.R. constructed expression vectors for the rPrPC substrates. J.W. provided SQ and RTQ expertise, as well as experimental, analytical, editorial, and graphics support. F.K., B.S. and A.R. provided the 15B3 antibody and advice on its use. C.D.O. and B.C. wrote the manuscript.
Footnotes
Citation Orrú CD, et al. 2011. Prion disease blood test using immunoprecipitation and improved quaking-induced conversion. mBio 2(3):e00078-11. doi:10.1128/mBio.00078-11.
REFERENCES
- 1. Caughey B, Baron GS, Chesebro B, Jeffrey M. 2009. Getting a grip on prions: oligomers, amyloids, and pathological membrane interactions. Annu. Rev. Biochem. 78:177–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown P, et al. 1998. The distribution of infectivity in blood components and plasma derivatives in experimental models of transmissible spongiform encephalopathy. Transfusion 38:810–816 [DOI] [PubMed] [Google Scholar]
- 3. Manuelidis EE, Gorgacz EJ, Manuelidis L. 1978. Viremia in experimental Creutzfeldt-Jakob disease. Science 200:1069–1071 [DOI] [PubMed] [Google Scholar]
- 4. Mathiason CK, et al. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314:133–136 [DOI] [PubMed] [Google Scholar]
- 5. Saá P, Castilla J, Soto C. 2006. Presymptomatic detection of prions in blood. Science 313:92–94 [DOI] [PubMed] [Google Scholar]
- 6. Terry LA, et al. 2009. Detection of PrPsc in blood from sheep infected with the scrapie and bovine spongiform encephalopathy agents. J. Virol. 83:12552–12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thorne L, Terry LA. 2008. In vitro amplification of PrPSc derived from the brain and blood of sheep infected with scrapie. J. Gen. Virol. 89:3177–3184 [DOI] [PubMed] [Google Scholar]
- 8. Vascellari M, et al. 2007. PrPSc in salivary glands of scrapie-affected sheep. J. Virol. 81:4872–4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bessen RA, et al. 2010. Prion shedding from olfactory neurons into nasal secretions. PLoS Pathog. 6:e1000837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilham JM, et al. 2010. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog. 6:e1001217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ironside JW. 2010. Variant Creutzfeldt-Jakob disease. Haemophilia 16(Suppl. 5):175–180 [DOI] [PubMed] [Google Scholar]
- 12. Brown P. 2008. Transmissible spongiform encephalopathy in the 21st century: neuroscience for the clinical neurologist. Neurology 70:713–722 [DOI] [PubMed] [Google Scholar]
- 13. Houston F, et al. 2008. Prion diseases are efficiently transmitted by blood transfusion in sheep. Blood 112:4739–4745 [DOI] [PubMed] [Google Scholar]
- 14. Peden AH, Head MW, Ritchie DL, Bell JE, Ironside JW. 2004. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet 364:527–529 [DOI] [PubMed] [Google Scholar]
- 15. Llewelyn CA, et al. 2004. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet 363:417–421 [DOI] [PubMed] [Google Scholar]
- 16. Wroe SJ, et al. 2006. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: a case report. Lancet 368:2061–2067 [DOI] [PubMed] [Google Scholar]
- 17. Gregori L, et al. 2004. Effectiveness of leucoreduction for removal of infectivity of transmissible spongiform encephalopathies from blood. Lancet 364:529–531 [DOI] [PubMed] [Google Scholar]
- 18. Saá P, Castilla J, Soto C. 2006. Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. J. Biol. Chem. 281:35245–35252 [DOI] [PubMed] [Google Scholar]
- 19. Fujihara A, et al. 2009. Hyperefficient PrP Sc amplification of mouse-adapted BSE and scrapie strain by protein misfolding cyclic amplification technique. FEBS J. 276:2841–2848 [DOI] [PubMed] [Google Scholar]
- 20. Atarashi R, et al. 2008. Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nat. Methods 5:211–212 [DOI] [PubMed] [Google Scholar]
- 21. Atarashi R, et al. 2007. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat. Methods 4:645–650 [DOI] [PubMed] [Google Scholar]
- 22. Colby DW, et al. 2007. Prion detection by an amyloid seeding assay. Proc. Natl. Acad. Sci. U. S. A. 104:20914–20919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trieschmann L, et al. 2005. Ultra-sensitive detection of prion protein fibrils by flow cytometry in blood from cattle affected with bovine spongiform encephalopathy. BMC Biotechnol. 5:26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Orrú CD, et al. 2009. Human variant Creutzfeldt-Jakob disease and sheep scrapie PrP(res) detection using seeded conversion of recombinant prion protein. Protein Eng. Des. Sel. 22:515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bieschke J, et al. 2000. Ultrasensitive detection of pathological prion protein aggregates by dual-color scanning for intensely fluorescent targets. Proc. Natl. Acad. Sci. U. S. A. 97:5468–5473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang B, et al. 2009. Surround optical fiber immunoassay (SOFIA): an ultra-sensitive assay for prion protein detection. J. Virol. Methods 159:15–22 [DOI] [PubMed] [Google Scholar]
- 27. Rubenstein R, et al. 2010. A novel method for preclinical detection of PrPSc in blood. J. Gen. Virol. 91:1883–1892 [DOI] [PubMed] [Google Scholar]
- 28. Birkmann E, et al. 2008. A highly sensitive diagnostic assay for aggregate-related diseases, including prion diseases and Alzheimer’s disease. Rejuvenation Res. 11:359–363 [DOI] [PubMed] [Google Scholar]
- 29. Birkmann E, et al. 2006. Detection of prion particles in samples of BSE and scrapie by fluorescence correlation spectroscopy without proteinase K digestion. Biol. Chem. 387:95–102 [DOI] [PubMed] [Google Scholar]
- 30. Edgeworth JA, et al. 2011. Detection of prion infection in variant Creutzfeldt-Jakob disease: a blood-based assay. Lancet 377:487–493 [DOI] [PubMed] [Google Scholar]
- 31. Edgeworth JA, Jackson GS, Clarke AR, Weissmann C, Collinge J. 2009. Highly sensitive, quantitative cell-based assay for prions adsorbed to solid surfaces. Proc. Natl. Acad. Sci. U. S. A. 106:3479–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Atarashi R, et al. 2011. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat. Med. 17:175–178 [DOI] [PubMed] [Google Scholar]
- 33. LeVine H., III 1999. Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 309:274–284 [DOI] [PubMed] [Google Scholar]
- 34. Korth C, et al. 1997. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature 390:74–77 [DOI] [PubMed] [Google Scholar]
- 35. Biasini E, et al. 2009. Immunopurification of pathological prion protein aggregates. PLoS One 4:e7816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Biasini E, et al. 2008. Non-infectious aggregates of the prion protein react with several PrPSc-directed antibodies. J. Neurochem. 105:2190–2204 [DOI] [PubMed] [Google Scholar]
- 37. Jones M, et al. 2009. Human platelets as a substrate source for the in vitro amplification of the abnormal prion protein (PrP) associated with variant Creutzfeldt-Jakob disease. Transfusion 49:376–384 [DOI] [PubMed] [Google Scholar]
- 38. Castilla J, et al. 2006. Protein misfolding cyclic amplification for diagnosis and prion propagation studies. Methods Enzymol. 412:3–21 [DOI] [PubMed] [Google Scholar]
- 39. Miller MB, Supattapone S. 2011. Superparamagnetic nanoparticle capture of prions for amplification. J. Virol. 85:2813–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brown P. 2007. Creutzfeldt-Jakob disease: reflections on the risk from blood product therapy. Haemophilia 13(Suppl. 5):33–40 [DOI] [PubMed] [Google Scholar]
- 41. Ferrone FA, Hofrichter J, Eaton WA. 1985. Kinetics of sickle hemoglobin polymerization. II. A double nucleation mechanism. J. Mol. Biol. 183:611–631 [DOI] [PubMed] [Google Scholar]
- 42. Padrick SB, Miranker AD. 2002. Islet amyloid: phase partitioning and secondary nucleation are central to the mechanism of fibrillogenesis. Biochemistry 41:4694–4703 [DOI] [PubMed] [Google Scholar]
- 43. Panza G, et al. 2008. Spontaneous and BSE-prion-seeded amyloid formation of full length recombinant bovine prion protein. Biochem. Biophys. Res. Commun. 373:493–497 [DOI] [PubMed] [Google Scholar]
- 44. Stöhr J, et al. 2008. Mechanisms of prion protein assembly into amyloid. Proc. Natl. Acad. Sci. U. S. A. 105:2409–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khan MQ, et al. 2010. Prion disease susceptibility is affected by beta-structure folding propensity and local side-chain interactions in PrP. Proc. Natl. Acad. Sci. U. S. A. 107:19808–19813 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Better IP-S-QuIC sensitivity and consistency of PrPSc Detection in spiked human plasma using 15B3 versus mock beads. (a) Comparison of 15B3 versus mock beads with 2-h IP from 100 µl plasma and two-round S-QuIC. Dilutions of hamster uninfected “normal” (N) or scrapie 263K (Sc) brain homogenates were spiked into 100 µl of human plasma to give final brain dilutions of 10−8 (N) and 10−8, 10−9, and 10−10 (Sc) (~100, 10, and 1 fg PrPres, respectively). PrPSc was immunoprecipitated using 40 µl (1.6 × 107 total beads) of 15B3-coated beads (15B3) or mock anti-IgM-coated beads (C) for 2 h at 37°C. Beads were resuspended in 10 µl of PBS. One-fifth of the beads were used to seed a first-round S-QuIC at 50°C for 10 h, and one-tenth of the first-round reaction volume was used to seed the second round (50°C for 10 h). (b) 15B3 beads with 20-h IP from 500 µl plasma and single-round S-QuIC. Dilutions of N or Sc brain homogenates were spiked into 500 µl of human plasma to give final brain tissue dilutions of 2 × 10−8 (N) and 2 × 10−8 to 2 × 10−11 (Sc) (containing ~1 pg to 1 fg PrPres, respectively). PrPSc was immunoprecipitated with 15B3 beads for ~20 h at 37°C. The remainder of the protocol was performed as in panel a, except that only a single-round S-QuIC at 50°C for 10 h was performed. Plasma-free positive and negative control reaction mixtures were seeded directly with 2 µl of 5 × 10−7 dilutions of hamster N or Sc brain, the latter containing ~100 fg PrPres seed. Hamster rPrPC23–231 was used as a substrate in all S-QuIC reactions. PK-digested products were analyzed by immunoblotting with the polyclonal R20 antibody as previously reported (24). Open circles mark 17-kDa fragments, and brackets indicate the lower-molecular-mass bands (10 to 13 kDa). Download Figure S1, TIF file, 0.445 MB.
SDS pretreatment of 15B3-bound PrPSc accelerates RT-QuIC detection. Dilutions of hamster N or Sc 263K brain homogenates were spiked into 500 µl of human plasma to give final brain dilutions of 2 × 10−7 (containing ~10 pg PrPres in the case of Sc). IP incubations with beads were for ~20 h at 37°C. One-fifth of the beads were used to seed RT-QuIC (a), and an equivalent number of beads were preincubated with 0.05% SDS in PBS at room temperature for ~20 min and used to seed RT-QuIC reaction mixtures containing 300 mM NaCl (b). Reaction mixtures were incubated at 42°C, and hamster rPrPC90–231 was used as a substrate in all reactions. The vertical axis indicates the average fluorescence from four replicate wells, and the fractions on the right indicate the positive/total replicate reactions associated with the adjacent traces. Download Figure S2, TIF file, 1.830 MB.
Better RT-QuIC detection of 15B3-bound human PrPvCJD with hamster-sheep chimeric rPrPC (Ha-S rPrPC) versus human rPrPC23–231 with NaCl variation. Dilutions of human nonprion (tumor) control or vCJD brain tissues were spiked into 500 µl of human plasma to give final dilutions of 4 × 10−7 (a and b) (containing ~10 pg PrPres, in the case of vCJD) or 4 × 10−7 and 4 × 10−9 and 4 × 10−10 (c) (containing ~10 pg, 100 fg, and 10 fg PrPrres, respectively, in the case of vCJD). The samples were subjected to IP-RT-QuIC as described in Materials and Methods, except for indicated variations in NaCl concentration and rPrPC substrate. The vertical axis indicates the average fluorescence from four replicate wells, and the fractions on the right indicate the positive/total replicate reactions associated with the adjacent traces. Download Figure S3, TIF file, 4.302 MB.
Comparison of 15B3 beads to MagnaBind beads in eQuIC. Dilutions of human non-TSE Alzheimer’s disease (AD) control or vCJD brain tissues were spiked into 0.5 ml of human plasma to give final dilutions of 4 × 10−7 (AD) and 4 × 10−7, 4 × 10−10, 4 × 10−13, and 4 × 10−14 (vCJD) (containing ~10 pg, 10 fg, 10 ag, and 1 ag PrPres, respectively). PrPvCJD was immunoprecipitated using 1.6 × 107 total 15B3-coated beads (a) or an equivalent number of MagnaBind beads (b) for ~20 h at 37°C using immunoprecipitation buffer (Prionics). Beads were washed twice with wash buffer (Prionics) and resuspended in 10 µl of PBS. The remainder of the protocol was as described in Material and Methods, starting with the preincubation in 0.05% SDS in PBS. The vertical axis indicates the average fluorescence from four replicate wells, and the fractions on the right indicate the positive/total replicate reactions associated with the adjacent traces. Similar results were obtained using the MagnaBind beads PrPSc capture conditions described by Miller and Supattapone (39; data not shown). Download Figure S4, TIF file, 2.686 MB.