ABSTRACT
DNA lesions in the template strand block the replication fork. In Saccharomyces cerevisiae, replication through DNA lesions occurs via a Rad6/Rad18-dependent pathway where lesions can be bypassed by the action of translesion synthesis (TLS) DNA polymerases η and ζ or by Rad5-mediated template switching. An alternative Rad6/Rad18-independent but Rad52-dependent template switching pathway can also restore the continuity of the replication fork. The Mec1/Rad53-dependent replication checkpoint plays a crucial role in the maintenance of stable and functional replication forks in yeast cells with DNA damage; however, it has remained unclear which of the lesion bypass processes requires the activation of replication checkpoint-mediated fork stabilization. Here we show that postreplication repair (PRR) of newly synthesized DNA in UV-damaged yeast cells is inhibited in the absence of Mec1 and Rad53 proteins. Since TLS remains functional in cells lacking these checkpoint kinases and since template switching by the Rad5 and Rad52 pathways provides the alternative means of lesion bypass and requires Mec1/Rad53, we infer that lesion bypass by the template switching pathways occurs in conjunction with the replication fork that has been stabilized at the lesion site by the action of Mec1/Rad53-mediated replication checkpoint.
IMPORTANCE
Eukaryotic cells possess mechanisms called checkpoints that act to stop the cell cycle when DNA replication is halted by lesions in the template strand. Upon stalling of the ongoing replication at the lesion site, the recruitment of Mec1 and Rad53 kinases to the replication ensemble initiates the checkpoint wherein Mec1-mediated phosphorylation of Rad53 activates the pathway. A crucial role of replication checkpoint is to stabilize the replication fork by maintaining the association of DNA polymerases with the other replication components at the stall site. Our observations that Mec1 and Rad53 are required for lesion bypass by template switching have important implications for whether lesion bypass occurs in conjunction with the stalled replication ensemble or in gaps that could have been left behind the newly restarted forks. We discuss this important issue and suggest that lesion bypass in Saccharomyces cerevisiae cells occurs in conjunction with the stalled replication forks and not in gaps.
Introduction
DNA repair mechanisms play an important role in maintaining genomic stability. Even though eukaryotic cells possess a large variety of means via which different types of lesions can be removed and repaired, a number of lesions persist in DNA into S phase; as a consequence, the progression of the replication fork is halted when the replisome encounters a lesion in the template strand. In the yeast Saccharomyces cerevisiae, replication through DNA lesions is mediated by the Rad6-Rad18-dependent pathway, in which lesion bypass can occur by the action of translesion synthesis (TLS) DNA polymerases (Pols) (1–5) or by a Rad5-Mms2-Ubc13 pathway that promotes lesion bypass by template switching (6–8). A Rad6-Rad18-independent but Rad51-, Rad52-, and Rad54-dependent pathway can also promote fork progression through DNA lesions via template switching (9, 10).
In addition to DNA repair mechanisms that promote replication through DNA lesions, eukaryotic cells possess surveillance mechanisms called checkpoints that become activated when DNA replication is halted by DNA damage or by other perturbations that affect the progression of the replication fork. Intra-S-phase checkpoint, also known as replication checkpoint (11), plays a crucial role in the maintenance of functional replication forks and in promoting cell survival and proliferation when cells are exposed to DNA-damaging agents (12, 13). In S. cerevisiae, the key components of replication checkpoints are the Mec1 kinase and its downstream effector kinase Rad53. The recruitment of Mec1 to the stalled replication fork initiates the checkpoint pathway, where it phosphorylates and activates Rad53 (11, 14). Upon activation, this pathway affects many aspects of DNA replication that include the slowing of S-phase and cell cycle progression, downregulation of late origin firing, activation of DNA repair proteins, and stabilization of replication forks (11, 14).
The role of Mec1/Rad53 in the stabilization of replication forks is particularly important for maintaining the association of the replisome with the fork stalled at a DNA lesion. In the absence of checkpoint, the replisome dissociates and the stalled forks degenerate (12, 13, 15). Thus, in mec1Δ or rad53Δ yeast cells treated with the alkylating agent methyl methanesulfonate (MMS), replication forks collapse irreversibly, leading to incomplete replication and cell death. This lethality occurs only when cells go through S phase in the presence of MMS and is not prevented by blocking the subsequent mitotic entry (12, 13, 15). Such observations and a number of others (11, 16, 17) have provided strong evidence that replication fork stabilization by Mec1 and Rad53 is critical for maintaining cell viability in yeast cells with damaged DNA and that checkpoint-dependent induction of transcription or regulation of late origin firing plays a relatively minor role (13). However, how these checkpoint kinases prevent replisome dissociation is not known.
Since fork stabilization by the Mec1/Rad53-initiated pathway is essential for the maintenance of replication fork in yeast cells with damaged DNA, replication checkpoint must enable cells to carry out efficient lesion bypass. However, it still remains unclear as to which of the lesion bypass processes depends upon the activation of replication checkpoint. Toward this end, in a previous study we provided evidence that TLS occurs normally in UV-irradiated nucleotide excision repair (NER)-proficient wild-type yeast cells lacking Mec1 or the components of checkpoint clamp and clamp loader (18). However, when the lesion load becomes greatly accentuated, as in UV-irradiated NER-defective cells, Mec1-mediated phosphorylation of Rev1 contributes to increasing the proficiency of Polζ function in lesion bypass (18). In addition to the requirement of Mec1/Ddc2 kinase, Rev1 phosphorylation requires the components of checkpoint clamp (Mec3, Rad17, and Ddc1) and clamp loader (Rad24), but Rad53 is not required (18). Since the frequency of UV-induced mutations shows a reduction in NER-defective yeast cells in combination with the phosphorylation-defectiveness rev1 mutation, the mec1Δ mutation, or the mec3Δ, rad17Δ, ddc1Δ, or rad24Δ mutation and since an epistatic relationship is observed when the rev1 phosphorylation-defectiveness mutation is combined with deletion mutations of these checkpoint proteins, we concluded that Rev1 phosphorylation, mediated by the Mec1/Ddc2 kinase and other checkpoint proteins, contributes to increasing the efficiency of Polζ-dependent TLS, the need for which becomes more acute in NER-defective yeast cells (18). Overall, these studies have supported the inference that in UV-damaged yeast cells, Polη and Polζ can carry out TLS without the need for Mec1/Rad53-initiated fork stabilization.
Since template switching provides an alternative to TLS, in this study we determine whether replication checkpoint is required for promoting lesion bypass by template switching. We provide evidence that Mec1 and Rad53 are both required for the restoration of normal size to newly synthesized DNA in UV-irradiated yeast cells and suggest that lesion bypass by template switching occurs in conjunction with the stalled replication fork that is maintained at the lesion site by the action of Mec1/Rad53.
RESULTS
Mec1 and Rad53 promote UV survival in the absence of NER.
A major role of Mec1/Rad53-initiated checkpoint in the stabilization of replication forks in yeast cells with damaged DNA would suggest that inactivation of replication checkpoint in NER-defective cells will generate a highly deleterious effect on UV survival. That is because in NER-defective cells, all the UV-induced lesions will remain in DNA and present a block to fork progression; lesion bypass at stalled forks would then require that checkpoint-initiated fork stabilization be installed. In contrast, in mutants defective in lesion bypass processes that require fork stabilization, the absence of Mec1- and Rad53-dependent checkpoint should generate a much less adverse effect on UV survival because of the dependence of that lesion bypass process on checkpoint-mediated fork stabilization.
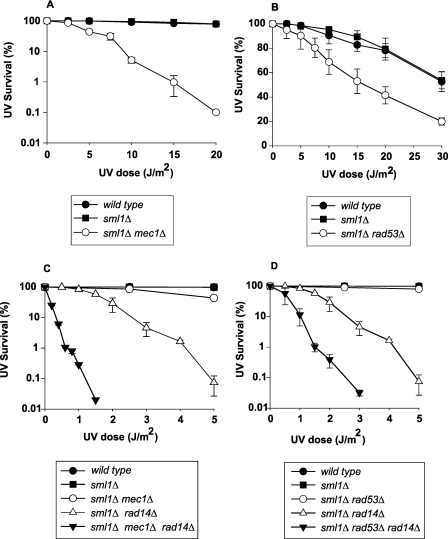
Since Mec1 and Rad53 are essential for cell viability but viability can be restored by the sml1Δ mutation, we combined the mec1Δ and rad53Δ mutations with the sml1Δ mutation. Whereas the sml1Δ mutation has no effect on UV sensitivity, the mec1Δ mutation confers a high degree of UV sensitivity, whereas the rad53Δ mutation generates a more modest increase in UV sensitivity (Fig. 1A and B). The much more pronounced effect of the mec1Δ mutation than of the rad53Δ mutation on UV survival is in keeping with the observation that in addition to its role in Rad53 activation, Mec1 can function in fork stabilization independently of Rad53 (15), and as we have shown previously, Mec1 can affect lesion bypass through its role in the activation of Polζ function via Rev1 phosphorylation, where Rad53 is not required (18).
FIG 1 .
Synergistic enhancement of UV sensitivity of rad14Δ cells in the absence of Mec1 and Rad53. Survival after UV irradiation of wild-type strain EMY74.7 and its isogenic derivative strains: sml1Δ mec1Δ strain (A), sml1Δ rad53Δ strain (B), sml1Δ mec1Δ rad14Δ strain (C), and sml1Δ rad53Δ rad14Δ strain (D). Survival curves represent averages of at least three different experiments for each strain. Error bars represent standard deviations of determinations. The apparent absence of error bars in some cases is because the error bars are very small.
We find that a large synergistic increase in UV sensitivity occurs when the NER-defective rad14Δ mutation is combined with either the mec1Δ or the rad53Δ mutation. For example, compared to the ~60% UV survival of the sml1Δ rad14Δ strain at 1.5 J/m2, UV survival declines to ~0.02% in the sml1Δ rad14Δ mec1Δ strain and to ~1% in the sml1Δ rad14Δ rad53Δ strain (Fig. 1C and D). The greatly enhanced UV sensitivity of the mec1Δ and rad53Δ mutants in the absence of NER signifies an important role for Mec1/Rad53-mediated checkpoint in affecting some aspect of lesion bypass during replication.
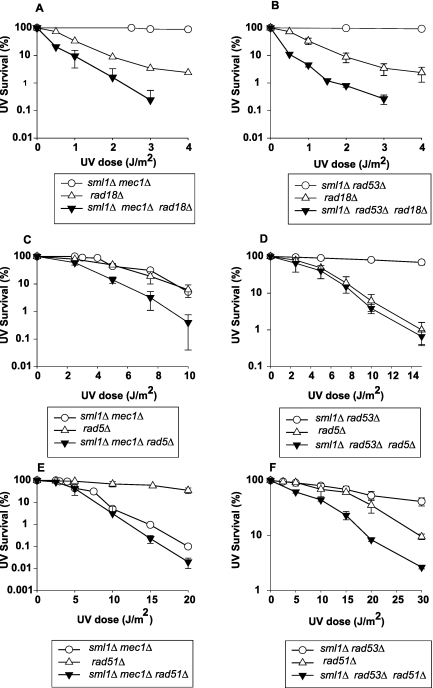
Next, we examined the UV sensitivity of mec1Δ and rad53Δ mutants in the absence of proteins that promote lesion bypass. The Rad6-Rad18 protein complex plays a major role in promoting lesion bypass in UV-damaged yeast cells, which can occur either by Polη- or Polζ-mediated TLS or by a Rad5-Mms2-Ubc13-dependent pathway in which the helicase activity of Rad5 promotes lesion bypass by template switching (6, 7, 19). The mec1Δ and rad53Δ mutations, when combined with the rad18Δ mutation, confer only a modest increase in UV sensitivity over that of the rad18Δ mutation, and the combination of rad5Δ with mec1Δ also conferred only a modest increase in UV sensitivity, whereas the UV sensitivity of the rad5Δ mutant was not affected in the absence of Rad53 (Fig. 2A to D). Since lesion bypass by template switching can additionally occur in a Rad6-Rad18-independent manner involving the action of proteins such as Rad51, -52, and -54, we examined the UV sensitivity of mec1Δ and rad53Δ mutants in combination with the rad51Δ mutation. Whereas the UV survival of the rad51Δ mutant was not affected in the absence of Mec1, a modest increase in UV sensitivity occurred in the rad53Δ mutant when it was combined with the rad51Δ mutation (Fig. 2E and F).
FIG 2 .
The absence of Mec1 or Rad53 confers only a modest increase in the UV sensitivity of mutants defective in lesion bypass by template switching. Survival after UV irradiation of isogenic derivative strains of EMY74.7: sml1Δ mec1Δ rad18Δ strain (A), sml1Δ rad53Δ rad18Δ strain (B), sml1Δ mec1Δ rad5Δ strain (C), sml1Δ rad53Δ rad5Δ strain (D), sml1Δ mec1Δ rad51Δ strain (E), and sml1Δ rad53Δ rad51Δ strain (F). Survival curves represent averages of at least three different experiments for each strain. Error bars represent standard deviations of determinations. The absence of error bars in some cases is because the error bars are very small.
These UV survival data indicating that in the absence of Mec1 or Rad53, the UV sensitivity of the rad14Δ mutant is greatly enhanced but the UV sensitivity of the rad18Δ, rad5Δ, and rad51Δ mutants shows only a rather modest increase are all consistent with a possible role of Mec1/Rad53-mediated checkpoint in affecting lesion bypass by the Rad6/Rad18/Rad5 and the Rad51/Rad52/Rad54 template switching pathways.
Requirement of Mec1 and Rad53 for postreplication repair (PRR) of UV-damaged DNA.
Since UV lesions are not removed in NER-defective cells and all the lesions go through the replication fork, when the fork stalls at lesion sites, two possible outcomes are either that a gap is left opposite from the lesion and replication reinitiates downstream or that lesion bypass occurs in coordination with the replication ensemble, in which case the role of Mec1 and Rad53 in fork stabilization would become paramount. The requirement of Mec1 and Rad53 for a lesion bypass process would then imply that the affected process takes place in conjunction with the replication fork that has been stabilized by the Mec1/Rad53-promoted checkpoint and not via gap repair in G2 after S phase has been completed. Importantly, the previously reported observations that in UV-irradiated NER-defective rad14Δ yeast cells, cell cycle is arrested in early S phase in a Mec1/Rad53-dependent manner and that Rad53 becomes heavily phosphorylated (20) have suggested that replication checkpoint-mediated fork stabilization plays an important role in promoting lesion bypass in UV-irradiated NER-defective cells.
To more directly assess the role of Mec1/Rad53-mediated checkpoint in lesion bypass, we determined whether PRR of UV-damaged DNA was affected in the absence of Mec1 and Rad53. The role of PRR in lesion bypass is more easily evaluated in NER-defective cells; since none of the UV-induced lesions, cyclobutane pyrimidine dimers (CPDs) or (6-4)pyrimidine-pyrimidone photoproducts [(6-4)PPs], are removed in the absence of NER, the high lesion load in these cells ensures that all the lesion bypass processes come into play. In wild-type cells, on the other hand, since the lower levels of DNA lesions can be proficiently replicated through by TLS, it becomes more difficult to discern the relatively small contribution of PRR processes. Using such a protocol, we have shown previously that PRR is promoted primarily via two independent pathways, a Rad6-Rad18-dependent Rad5-Mms2-Ubc13 pathway and a Rad51-Rad52-Rad54 pathway (8, 9).
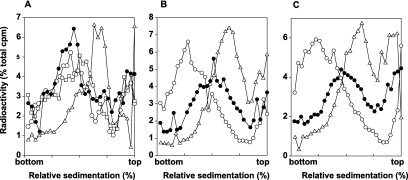
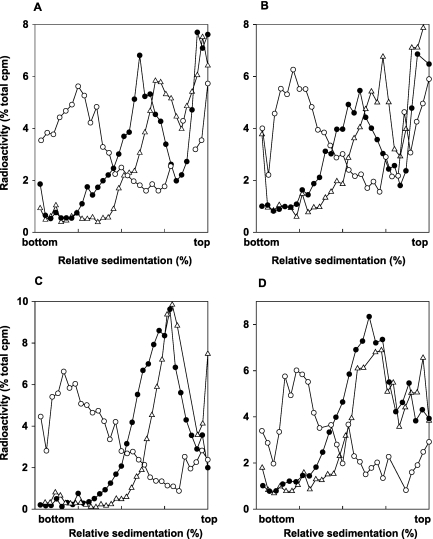
For PRR studies, the NER-defective rad1Δ mutant was combined with the sml1Δ mutation, and following UV irradiation of sml1Δ rad1Δ cells at 3.5 J/m2, the size of newly synthesized DNA from UV-damaged templates was examined by pulse-labeling of DNA with [3H]uracil for 15 min followed by a chase period of 30 min in high-uracil medium. Following this treatment, DNA sediments toward the top of the alkaline sucrose gradient (Fig. 3A); this small size of DNA results from the presence of discontinuities in the newly synthesized strand that arise from the stalling of replication forks opposite from lesion sites. In contrast, in unirradiated sml1Δ rad1Δ cells, the newly synthesized DNA attains normal size following such a 15-min pulse and 30-min chase in high-uracil medium (data not shown). In sml1Δ rad1Δ cells that were UV irradiated and where, following the 15-min pulse-labeling period, cells were allowed to repair the discontinuities for 2 h, the newly synthesized DNA attained almost the same size as that in unirradiated control cells, whereas a 4-h repair period following the 15-min pulse restored normal size to newly synthesized DNA (Fig. 3A).
FIG 3 .
Requirement of Mec1 and Rad53 for postreplication repair of UV-damaged DNA. Sedimentation in alkaline sucrose gradients of nuclear DNA from cells incubated for different periods following UV irradiation with 3.5 J/m2. sml1Δ rad1Δ (A), sml1Δ mec1Δ rad1Δ (B), and sml1Δ rad53Δ rad1Δ (C) strains, respectively, were UV irradiated at 3.5 J/m2 and then pulse-labeled with [3H]uracil for 15 min, followed by different periods to allow for repair in high-uracil medium: 30 min (Δ), 2 h (□), and 4 h (●) (A) and 30 min (Δ) and 6 h (●) (B and C). DNA from unirradiated cells was pulse-labeled with [3H]uracil for 15 min followed by incubation for 6 h (○); a similar sedimentation pattern was attained in unirradiated cells pulse-labeled for 15 min following by a chase for 30 min in high-uracil medium (data not shown).
Next we examined whether the proficiency of PRR was affected in the absence of Mec1 or Rad53 proteins. The sml1Δ rad1Δ mec1Δ and sml1Δ rad1Δ rad53Δ cells were UV irradiated at 3.5 J/m2, and the size of newly synthesized DNA was examined in experiments where the 15-min pulse-labeling period was followed by a 30-min chase period or where, following the 15-min pulse-labeling, cells were allowed to repair for up to 6 h. In both strains, the repair of discontinuities in the newly synthesized DNA is greatly diminished, since even after a 6-h repair period, small-molecular-size DNA still persists (Fig. 3B and C). However, because the size of newly synthesized DNA obtained from cells given a 6-h repair period is not coincident with the size of DNA obtained from cells given the 30-min chase period but rather shows some shift toward the larger size, a residual level of repair can occur in cells lacking Mec1 or Rad53 (Fig. 3B and C).
TLS Pols contribute to lesion bypass in the absence of Mec1 and Rad53.
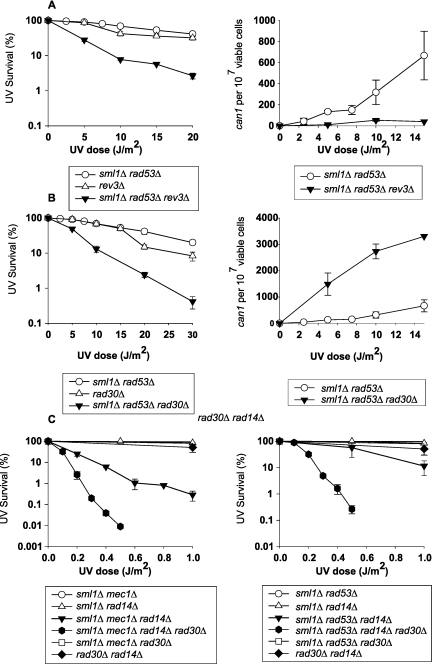
We have shown previously that Polη and Polζ can carry on their role in TLS in the absence of Mec1 in NER-proficient cells (18). This conclusion was drawn from observations that the incorporation of the rad30Δ mutation into the sml1Δ mec1Δ strains conferred a synergistic increase in UV sensitivity and UV mutagenesis, and even though the rev3Δ mutation caused only a small increase in UV sensitivity, the frequency of UV-induced mutations was greatly reduced in the sml1Δ mec1Δ rev3Δ strain compared to that in the sml1Δ mec1Δ strain (18). Since the effects of the rad53Δ mutation on TLS were not examined in the previous study, we have now determined whether TLS remains functional in the absence of Rad53 also. As shown in Fig. 4A and B, introduction of either the rev3Δ or the rad30Δ mutation into the sml1Δ rad53Δ strain conferred an increase in UV sensitivity. The frequency of UV-induced can1r mutations was greatly reduced in the sml1Δ rad53Δ rev3Δ strain compared to that in the sml1Δ rad53Δ strain (Fig. 4A), whereas the incidence of UV-induced mutations was greatly enhanced upon the introduction of the rad30Δ mutation into the sml1Δ rad53Δ strain (Fig. 4B).
FIG 4 .
Lesion bypass by translesion synthesis remains operational in the absence of Mec1 and Rad53. (A and B) UV survival (left) and frequencies of UV-induced can1r mutations (right) were determined for the sml1Δ rad53Δ strain in combination with the rev3Δ or rad30Δ mutation. (C) UV survival of sml1Δ mec1Δ rad14Δ (left) and sml1Δ rad53Δ rad14Δ (right) strains in combination with the rad30Δ mutation. Survival curves represent averages of at least three different experiments for each strain. Error bars represent standard deviations of determinations. The absence of error bars in some cases is because the error bars are very small.
Although TLS by both Polζ and Polη remains fully functional in the absence of Mec1 in NER-proficient cells, in NER-defective rad14Δ cells Mec1-mediated Rev1 phosphorylation affects the proficiency of Polζ function in TLS, since the frequency of UV-induced mutations is reduced in the rad14Δ cells carrying the rev1 S31A mutation, which confers a defect in Rev1 phosphorylation. However, since UV mutations do occur in the sml1Δ mec1Δ rad14Δ strain, albeit with a reduced frequency compared to that of the rad14Δ strain, Polζ can operate in the absence of Mec1 but with a lowered proficiency (18). Because Rev1 is the only TLS protein known to be phosphorylated by the Mec1/Ddc2 kinase, and since we could find no evidence of Mec1-mediated phosphorylation for any of the other TLS proteins, including Polη (18), we expect the TLS function of Polη to remain unperturbed in the absence of Mec1, even in NER-defective cells. Accordingly, we find that UV sensitivity of the sml1Δ mec1Δ rad14Δ or the sml1Δ rad53Δ rad14Δ strain is greatly enhanced in the absence of Polη (Fig. 4C), indicating that Polη continues to play an important role in lesion bypass in NER-defective cells in the absence of Mec1/Rad53 kinases.
Since TLS remains functional in the absence of Mec1/Rad53 kinases, we determined whether the residual repair of discontinuities in the newly synthesized DNA that occurs in UV-irradiated sml1Δ rad1Δ mec1Δ and sml1Δ rad1Δ rad53Δ cells derives from the TLS role of Polη and Polζ. To verify this possibility, we examined the size of newly synthesized DNA in sml1Δ rad1Δ mec1Δ rad30Δ and sml1Δ rad1Δ rad53Δ rad30Δ strains following UV irradiation at 3.5 J/m2. A comparison of gradient profiles in Fig. 5A and B with those in Fig. 3B and C, respectively, shows that in the absence of Polη, DNA synthesized from UV-damaged templates does not attain as large a size as it does in the presence of Polη. A much greater reduction in the capacity to repair discontinuities in the newly synthesized DNA is observed in the sml1Δ rad1Δ mec1Δ rad30Δ rev3Δ and sml1Δ rad1Δ rad53Δ rad30Δ rev3Δ strains such that the gradient profiles of DNA from UV-irradiated cells pulse-labeled for 15 min followed by a chase period of 30 min or a repair period of 6 h become more coincident (Fig. 5C and D). We conclude from these observations that Polη and Polζ are able to function in lesion bypass in the absence of Mec1 and Rad53.
FIG 5 .
Inactivation of translesion synthesis leads to further impairment of residual postreplication repair that occurs in the absence of replication checkpoint. Sedimentation in alkaline sucrose gradients of nuclear DNA from cells incubated for different periods following UV irradiation with 3.5 J/m2. sml1Δ mec1Δ rad1Δ rad30Δ (A), sml1Δ rad53Δ rad1Δ rad30Δ (B), sml1Δ mec1Δ rad1Δ rad30Δ rev3Δ (C), and sml1Δ rad53Δ rad1Δ rad30Δ rev3Δ (D) strains, respectively, were UV irradiated at 3.5 J/m2 and then pulse-labeled with [3H]uracil for 15 min, followed by a 30-min chase (Δ) or a 6-h repair period (●) in high-uracil medium. Also shown is the sedimentation pattern of DNA from unirradiated cells pulse-labeled with [3H]uracil for 15 min, followed by a 6-h incubation (○).
DISCUSSION
Requirement of Mec1 and Rad53 for lesion bypass.
Although the Mec1/Rad53-initiated replication checkpoint becomes activated in S phase and it plays a major role in fork stabilization at the site of stalled replication forks in yeast cells with damaged DNA, it has remained unclear as to which of the lesion bypass processes depends upon such fork stabilization. A role for Mec1/Rad53-dependent replication checkpoint in promoting lesion bypass has been suggested from the observation that Mec1-initiated Rad53 hyperphosphorylation and the concomitant S-phase checkpoint are activated in UV-damaged NER-defective rad14Δ cells (20), and our observation that the UV sensitivity of the rad14Δ mutant shows a synergistic increase when combined with the mec1Δ or the rad53Δ mutation is in accord with such a role for Mec1/Rad53. In this study, we provide evidence that the Mec1 and Rad53 proteins are required for promoting lesion bypass via the template switching pathways.
The roles of template switching mechanisms can be assessed more readily in NER-defective cells than in NER-proficient cells, because in wild-type yeast cells, of the two UV-induced photoproducts, CPDs and (6-4)PPs, the (6-4)PPs are removed rapidly by NER, whereas because the CPDs are removed at a lower rate, they constitute the predominant blocking lesion during replication (21, 22). However, since Polη can proficiently replicate through CPDs predominantly in an error-free way (19) and because the majority of (6-4)PPs, which are more obstructive to replication by TLS Pols, are proficiently removed, the TLS Pols, particularly Polη, and to a lesser extent Polζ, can carry out efficient lesion bypass in NER-proficient cells. Hence, in this study we examined the size of newly synthesized chromosomal DNA in UV-irradiated NER-defective rad1Δ cells which additionally harbor the mec1Δ or the rad53Δ mutation. Although the method for sizing DNA in alkaline sucrose gradients is tedious and time-consuming, we use this technique because the gradient profiles provide a good indication of the overall extent of repair in the entire yeast genome. Our observations that the newly synthesized DNA in UV-irradiated sml1Δ rad1Δ mec1Δ or sml1Δ rad1Δ rad53Δ strains shows only a low level of repair even when the cells are given a 6-h repair period, whereas in the UV-irradiated sml1Δ rad1Δ strain the newly synthesized DNA attains almost normal size in a 2-h repair period, have provided strong evidence for the requirement of Mec1 and Rad53 kinases for lesion bypass.
Mec1/Rad53-dependent and -independent pathways of lesion bypass.
Our observations that TLS by Polζ or Polη is not impaired in UV-irradiated NER-proficient yeast cells in the absence of Mec1 or Rad53 have indicated that replication checkpoint has no significant bearing on lesion bypass by TLS (18). Although Mec1-dependent Rev1 phosphorylation affects the proficiency of Polζ action in TLS in NER-defective cells (18), our observations of a large synergistic enhancement in UV sensitivity of sml1Δ mec1Δ rad14Δ or sml1Δ rad53Δ rad14Δ strains following the introduction of the rad30Δ mutation (Fig. 4C) have indicated that Polη continues to make a very prominent contribution to lesion bypass in the absence of Mec1 and Rad53, even when the load of UV photoproducts becomes very high, as in NER-defective cells. Furthermore, our findings that residual repair of discontinuities in the newly synthesized DNA strand that occurs in UV-irradiated sml1Δ mec1Δ rad1Δ or sml1Δ rad53Δ rad1Δ cells is diminished further in the absence of Polη and Polζ (Fig. 5C and D) have provided additional support to the premise that TLS remains functional in the absence of Mec1/Rad53-mediated replication checkpoint. Since lesion bypass can occur via two alternative means, TLS or template switching, and since TLS remains functional in the absence of Mec1 and Rad53, we deduce a role for Mec1 and Rad53 replication checkpoint protein kinases in promoting lesion bypass via the template switching pathways.
PRR by template switching can occur by two separate pathways, one involving Rad6-Rad18-mediated PCNA monoubiquitylation at Lys164 followed by Rad5-Mms2-Ubc13-mediated Lys63-linked polyubiquitylation of Lys164 in PCNA (23–25), and this pathway requires also the helicase function of Rad5 (6, 7), whereas the other pathway requires the Rad52 group of recombination repair proteins (9, 10). Genetic studies in rad1Δ yeast cells using a duplex plasmid system for examining the roles of various lesion bypass proteins in promoting replication through a (6-4)PP photoproduct have indicated that, whereas Rad18 contributed to the replication of ~70% of lesion-containing plasmids, Rad5 was required for replicating through ~60% and Rad52 accounted for promoting replication through ~40% of the lesion-containing plasmids (26). From these observations, one could infer that template switching by the Rad5 and Rad52 pathways accounts for the majority of lesion bypass opposite this UV-induced photoproduct.
Based upon a number of observations presented here and those published recently (18), we conclude the existence of a role of Mec1/Rad53 kinases in affecting the template switching pathways of lesion bypass. First, TLS remains functional in the absence of Mec1 and Rad53 (reference 18 and this study). Second, the large synergistic enhancement of UV sensitivity in rad14Δ cells in the absence of Mec1 or Rad53 (Fig. 1C and D) is in accord with a role of Mec1/Rad53 in lesion bypass pathways other than TLS. Third, the epistatic interactions observed for UV sensitivity of the rad5Δ mutant in combination with the rad53Δ mutation (Fig. 2D) and for the rad51Δ mutant in combination with the mec1Δ mutation (Fig. 2E) are in keeping with a role of Mec1/Rad53 kinases in Rad5- and Rad51-mediated lesion bypass pathways. Fourth, since Rad5- and Rad51/Rad52-mediated PRR pathways that act via template switching are the only pathways via which lesion bypass can occur in the absence of TLS (9), our observations that PRR becomes defective in the absence of Mec1 and Rad53 but that TLS still remains functional imply that Mec1 and Rad53 promote lesion bypass via the Rad5- and Rad51/Rad52-mediated template switching pathways.
Possible role(s) of Mec1/Rad53 in template switching pathways.
Mec1 and Rad53 could affect PRR of UV-damaged DNA in several possible ways. Foremost among these is the stabilization of replication forks stalled at DNA lesion sites. In view of the strong evidence that the Mec1/Rad53-initiated checkpoint plays a crucial role in the stabilization of replication forks in yeast cells with damaged DNA (11–13, 15–17) and the fact that replication checkpoint becomes activated in UV-irradiated NER-defective yeast cells (20), the requirement of Mec1/Rad53 kinases for PRR can best be reconciled with their role in maintaining the association of the replisome with the stalled fork. However, since Mec1 and Rad53 affect many other processes, including the activation of DNA repair and replication proteins via their phosphorylations or transcriptional induction, regulation of late origin firing, and others, these protein kinases may impact upon the Rad5- and Rad51-dependent PRR processes via their effects on these other processes as well.
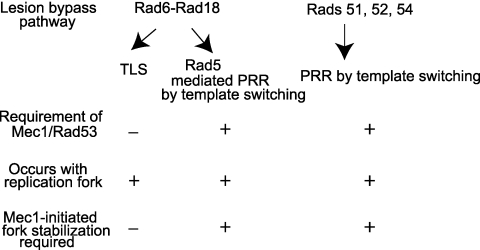
The suggested requirement of Mec1/Rad53-mediated fork stabilization for promoting lesion bypass by template switching raises the question of why a stable fork has to be maintained during this process but is not required for TLS. As we have suggested before (18), the lack of requirement of replication checkpoint for TLS could be explained if we assume that TLS occurs in a relatively rapid manner and that because of the rapidity of lesion bypass, the short duration of the fork remaining stalled does not generate enough of a signal necessary for checkpoint activation to occur. In contrast, since lesion bypass by the Rad5 and Rad51/Rad52 pathways involving template switching and copy-choice type of DNA synthesis is likely to be a much more intricate process than TLS, involving a number of discrete reactions, we expect that the fork remains stalled for a period sufficient for checkpoint activation to occur. Further, the prolonged stabilization of the replication fork needed for template switching could be maintained only if the Mec1/Rad53-initiated checkpoint were installed. A summary of these ideas is presented in Fig. 6.
FIG 6 .
Model for role of Mec1- and Rad53-mediated replication checkpoint in lesion bypass. It is proposed that lesion bypass by TLS or by template switching occurs in coordination with the replication fork and not in gaps that might have been left behind opposite from DNA lesions and then filled in later by these lesion bypass processes during the G2 phase. Since Mec1 and Rad53 are required for postreplication repair of UV-damaged DNA but TLS remains functional in the absence of these replication checkpoint proteins, we posit that both the Rad6-Rad18-Rad5-dependent and the Rad51-Rad52-Rad54-dependent template switching pathways require the Mec1/Rad53-mediated fork stabilization. From the observations that PCNA ubiquitylation is restricted primarily to S phase in UV-irradiated yeast cells (27) and that TLS remains functional in the absence of Mec1 and Rad53 (18), we infer that TLS occurs in coordination with the replication fork, but that does not necessitate the imposition of replication checkpoint. Presumably, TLS can occur in the absence of checkpoint, perhaps because of its being a less cumbersome and more efficient process than template switching.
Our suggestion that lesion bypass by TLS as well as by template switching occurs in S phase in conjunction with the replication fork is supported by the fact that in UV-damaged cells Rad6-Rad18-mediated PCNA ubiquitylation normally occurs in S phase (27). Interestingly, in experiments where the expression of lesion bypass proteins is artificially limited to G2, yeast cells can carry out lesion bypass in the G2 phase of the cell cycle (28). It remains unclear, however, whether in such cases the fork remains stalled at the lesion site until the completion of lesion bypass in the G2 phase or whether such lesion bypass occurs in gaps that might have been left behind the newly restarted forks. Also, it would be of much interest to know whether yeast cells adopt a set of regulatory mechanisms for carrying out lesion bypass in the G2 phase distinct from those utilized when lesion bypass occurs normally in S phase and whether adverse cellular consequences such as elevated rates of recombination and of chromosome rearrangements arise in cells when lesion bypass is restricted to the G2 phase of the cell cycle.
MATERIALS AND METHODS
Yeast strains.
The yeast strains were all derived from the strain EMY74.7 (MATa his3-Δ1 leu2-3,112 trp1Δ ura3-52). Genomic deletions of the checkpoint genes and of various DNA repair genes were made in the EMY74.7 strain by gene replacement. Strains used for postreplication repair studies were all made [rho0] so that the repair of only the nuclear DNA could be examined unambiguously.
UV survival and mutagenesis.
Cells were grown to logarithmic phase in synthetic complete medium (SC). Cultures were washed and sonicated to disperse cell clumps when necessary and resuspended in sterile distilled water to a density of 2 × 108 cells per ml. Cell suspensions were diluted and spread onto SC plates for viability determinations and onto SC plates lacking arginine but containing canavanine (SC-Arg+Can) for determination of CAN1S-to-can1r mutation frequencies. The plates were UV irradiated and incubated in the dark for 4 to 5 days prior to counting of colonies.
Analysis of repair of DNA synthesized from UV-irradiated templates by sedimentation in alkaline sucrose gradients.
Yeast cells grown to a density of 0.5 × 107 to 1.0 × 107 cells per ml of SC medium lacking uracil but containing 5 µg of uridine/ml were UV irradiated in 150- by 20-mm petri dishes at a dose rate of 0.1 J/m2/s. All operations were performed in yellow light to avoid photoreactivation. After UV irradiation, cells were collected by filtration and resuspended in fresh uridine medium at a density of 1 × 108 to 2 × 108 cells per ml. Pulse-labeling was achieved by the addition of 100 µCi of [5,6-3H]uracil (20 to 25 Ci/mmol, 1 mCi/ml; Moravek Biochemicals and Radiochemicals, Brea, CA) to 1 ml of cells, followed by vigorous shaking for 15 min at 30°C. Cells were then washed, resuspended in SC medium containing 1.67 mg of uracil/ml (high-uracil medium), and incubated for an additional period of 30 min or for different periods up to 6 h to allow time for repair. Cells were converted to spheroplasts, and an 0.3-ml aliquot of the spheroplast suspension was layered directly onto a 0.2-ml lysing layer (0.79 M sorbitol, 0.66 M EDTA, 2.5% Sarkosyl, 0.3 M NaCl) on top of a 15 to 30% (wt/vol) linear alkaline sucrose gradient made in 0.3 M NaOH, 0.7 M NaCl, 40 mM EDTA, 1% Sarkosyl (pH 12.5). Centrifugation and processing of samples were performed as described elsewhere (29), except that alkaline sucrose gradients were centrifuged at 21,000 rpm for 15 h 30 min at 4°C and acid precipitation of alkaline-hydrolyzed samples was carried out with 1 N HCl and 0.1 M sodium pyrophosphate.
ACKNOWLEDGMENTS
We thank Roland Klassen for helpful discussions.
This work was supported by National Institutes of Health grant CA107650.
Footnotes
Citation Gangavarapu V, et al. 2011. Requirement of replication checkpoint protein kinases Mec1/Rad53 for postreplication repair in yeast. mBio 2(3):e00079-11. doi:10.1128/mBio.00079-11.
REFERENCES
- 1. Bresson A, Fuchs RP. 2002. Lesion bypass in yeast cells: Pol η participates in a multi-DNA polymerase process. EMBO J. 21:3881–3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson RE, Prakash S, Prakash L. 1999. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Polη. Science 283:1001–1004 [DOI] [PubMed] [Google Scholar]
- 3. Johnson RE, Washington MT, Haracska L, Prakash S, Prakash L. 2000. Eukaryotic polymerases ι and ζ act sequentially to bypass DNA lesions. Nature 406:1015–1019 [DOI] [PubMed] [Google Scholar]
- 4. Pagès V, et al. 2008. Requirement of Rad5 for DNA polymerase ζ-dependent translesion synthesis in Saccharomyces cerevisiae. Genetics 180:73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pagès V, Johnson RE, Prakash L, Prakash S. 2008. Mutational specificity and genetic control of replicative bypass of an abasic site in yeast. Proc. Natl. Acad. Sci. U. S. A. 105:1170–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blastyak A, et al. 2007. Yeast Rad5 protein required for postreplication repair has a DNA helicase activity specific for replication fork regression. Mol. Cell 28:167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gangavarapu V, et al. 2006. Mms2-Ubc13-dependent and -independent roles of Rad5 ubiquitin ligase in postreplication repair and translesion DNA synthesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 26:7783–7790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torres-Ramos CA, Prakash S, Prakash L. 2002. Requirement of RAD5 and MMS2 for postreplication repair of UV-damaged DNA in Saccharomyces cerevisiae. Mol. Cell. Biol. 22:2419–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gangavarapu V, Prakash S, Prakash L. 2007. Requirement of RAD52 group genes for post-replication repair of UV-damaged DNA in Saccharomyces cerevisiae. Mol. Cell. Biol. 27:7758–7764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prakash L. 1981. Characterization of postreplication repair in Saccharomyces cerevisiae and effects of rad6, rad18, rev3 and rad52 mutations. Mol. Gen. Genet. 184:471–478 [DOI] [PubMed] [Google Scholar]
- 11. Paulsen RD, Cimprich KA. 2007. The ATR pathway: fine-tuning the fork. DNA Repair 6:953–966 [DOI] [PubMed] [Google Scholar]
- 12. Tercero JA, Diffley JF. 2001. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature 412:553–557 [DOI] [PubMed] [Google Scholar]
- 13. Tercero JA, Longhese MP, Diffley JF. 2003. A central role for DNA replication forks in checkpoint activation and response. Mol. Cell 11:1323–1336 [DOI] [PubMed] [Google Scholar]
- 14. Nyberg KA, Michelson RJ, Putnam CW, Weinert TA. 2002. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 36:617–656 [DOI] [PubMed] [Google Scholar]
- 15. Segurado M, Diffley JF. 2008. Separate roles for the DNA damage checkpoint protein kinases in stabilizing DNA replication forks. Genes Dev. 22:1816–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cobb JA, Bjergbaek L, Shimada K, Frei C, Gasser SM. 2003. DNA polymerase stabilization at stalled replication forks requires Mec1 and the RecQ helicase Sgs1. EMBO J. 22:4325–4336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cobb JA, et al. 2005. Replisome instability, fork collapse, and gross chromosomal rearrangements arise synergistically from Mec1 kinase and RecQ helicase mutations. Genes Dev. 19:3055–3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pagès V, Santa Maria SR, Prakash L, Prakash S. 2009. Role of DNA damage-induced replication checkpoint in promoting lesion bypass by translesion synthesis in yeast. Genes Dev. 23:1438–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prakash S, Johnson RE, Prakash L. 2005. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 74:317–353 [DOI] [PubMed] [Google Scholar]
- 20. Neecke H, Lucchini G, Longhese MP. 1999. Cell cycle progression in the presence of irreparable DNA damage is controlled by a Mec1- and Rad53-dependent checkpoint in budding yeast. EMBO J. 18:4485–4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfeifer GP. 1997. Formation and processing of UV photoproducts: effects of DNA sequence and chromatin environment. Photochem. Photobiol. 65:270–283 [DOI] [PubMed] [Google Scholar]
- 22. Yoon JH, Lee CS, O’Connor TR, Yasui A, Pfeifer GP. 2000. The DNA damage spectrum produced by simulated sunlight. J. Mol. Biol. 299:681–693 [DOI] [PubMed] [Google Scholar]
- 23. Haracska L, Torres-Ramos CA, Johnson RE, Prakash S, Prakash L. 2004. Opposing effects of ubiquitin conjugation and SUMO modification of PCNA on replicational bypass of DNA lesions in Saccharomyces cerevisiae. Mol. Cell. Biol. 24:4267–4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. 2002. RAD6 -dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419:135–141 [DOI] [PubMed] [Google Scholar]
- 25. Stelter P, Ulrich HD. 2003. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature 425:188–191 [DOI] [PubMed] [Google Scholar]
- 26. Zhang H, Lawrence CW. 2005. The error-free component of the RAD6/RAD18 DNA damage tolerance pathway of budding yeast employs sister-strand recombination. Proc. Natl. Acad. Sci. U. S. A. 102:15954–15959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daigaku Y, Davies AA, Ulrich HD. 2010. Ubiquitin-dependent DNA damage bypass is separable from genome replication. Nature 465:951–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karras GI, Jentsch S. 2010. The RAD6 DNA damage tolerance pathway operates uncoupled from the replication fork and is functional beyond S phase. Cell 141:255–267 [DOI] [PubMed] [Google Scholar]
- 29. Torres-Ramos CA, Yoder BL, Burgers PM, Prakash S, Prakash L. 1996. Requirement of proliferating cell nuclear antigen in RAD6-dependent postreplicational DNA repair. Proc. Natl. Acad. Sci. U. S. A. 93:9676–9681 [DOI] [PMC free article] [PubMed] [Google Scholar]