Abstract
We have recently developed a convenient method of screening a broad range of microorganisms that produce ɛ-poly-l-lysine (M. Nishikawa and K. Ogawa, Appl. Environ. Microbiol. 68:3575-3581, 2002). Using this method, we found an ergot fungus that secretes a charged polypeptide other than ɛ-poly-l-lysine. It was identified as a new species on the basis of its 28S rRNA sequence and was named Verticillium kibiense (formerly Epichloe kibiensis). Peptide sequencing and mass spectrometry revealed that the polypeptide is a linear peptide composed of repeated units of arginyl-histidine. The numbers of repeated units were in most cases five and in some cases four or six. This peptide showed activity against a broad range of bacteria and fungi but lost its activity under conditions of high ionic strength. Zinc and copper ions specifically changed the circular dichroism spectra of the peptide and restored the antimicrobial activity from abrogation under high ionic conditions, although these ions had no reinforcing effect on antimicrobial activity when they were added to solutions at a low ionic strength. The peptide labeled with fluorescein was able to permeate the cell membranes of target microbes, but its ability to permeate cell membranes decreased under conditions of high ionic strength. This decreased ability was partially recovered specifically by the addition of zinc and copper ions. These results indicate that poly(arginyl-histidine) is a cationic polypeptide characterized by specific metal binding and resistance to salts.
Many organisms produce arginine- and lysine-rich polycationic peptides to protect themselves from the menaces of competitive, invasive, and pathogenic microbes. Because the specificities of target microbes are not stringent, this broad-spectrum strategy is considered the primary defense system prior to the target-specific strategy, such as the antigen-antibody reaction. For example, it has been well established that the following polypeptides exhibit antimicrobial activities: polymyxin (18), nisin (7), and ɛ-poly-l-lysine (ɛ-PL) from bacteria (11, 13, 14); ɛ-PL-like peptides from fungi (17); cecropin from insects (5); magainin from amphibians (19); and defensins from higher animals and plants (2, 9). In most cases, such polypeptides are composed of at least four kinds of amino acids, and they may have more complex structures and functions than poly(amino acids) composed of only one or two kinds of amino acids. ɛ-PL is considered the only example of a biosynthesized antimicrobial poly(amino acid), although the mechanism underlying its antimicrobial activity is unknown. In general, however, simple polycationic polymers such as α-poly-l-lysine, α-poly-l-arginine, and chitosan have antimicrobial activities similar to that of ɛ-PL.
Electrostatic interaction, which possibly occurs between polycationic peptides and anionic lipids in the cell membrane of the target microbes, is important, particularly in the initial stage of antimicrobial activity, and is affected by pH and ionic strength. The electrolytic groups of polycations liberate protons at high pHs and compete with coexisting cations for interaction with the anionic target under conditions of high ionic strength. As a result, polycationic peptides are inactivated under such severe conditions. However, it remains obscure how microorganisms develop an effective means of overcoming the problems caused by these phenomena.
Recently, we have developed a simple and sensitive screening method of detecting polycationic and, probably, antimicrobial polymers in microbial secretions (11). This method detects cationic or anionic secretions that interact with electrically charged dyes embedded in agar plates and is unique in that its detection ability does not depend on antimicrobial activity. We report here on the characterization of a novel antimicrobial poly(amino acid) composed of arginyl-histidine, which was first isolated from the secretions of an ergot fungus by our method. Arginine-rich and histidine-rich peptides have been found among antimicrobial peptides, but peptides with an alternate arrangement of arginine and histidine have not. We investigated whether the putative metal binding caused by the chelatable property of histidine affects antimicrobial activity, especially under conditions of high ionic strength.
MATERIALS AND METHODS
Strain identification.
The fungus, strain E18, producing the novel polypeptide was isolated from forest soil. On the basis of the DNA sequence complementary to its 28S rRNA sequence, it was taxonomically identified as a new species and was named Verticillium kibiense. When the sequence data were registered in DDBJ (accession no. AB087373), it was tentatively named Epichloe kibiensis.
Polypeptide production.
Strain E18 was grown in a synthetic galactose medium by either solid or liquid culture. The synthetic galactose medium was composed of 10 g of d-galactose, 0.66 g of ammonium sulfate, 0.68 g of potassium dihydrogen phosphate, 0.25 g of magnesium sulfate heptahydrate, 0.1 g of yeast extract, and 1 ml of Kirk's mineral solution (6) in 1 liter. Its pH was adjusted to 7.0 with an aqueous solution of sodium hydroxide. For solidification, 1.5% agar was added. After incubation at 28°C for 1 to 2 weeks, the basic polypeptide secreted in liquid culture was purified by cation-exchange chromatography with Amberlite IRC-50 resin (Organo, Tokyo, Japan) (14). Alternatively, the polypeptide secreted in the agar matrix in solid culture was extracted by maceration of the agar by acid hydrolysis (1 M HCl, 100°C, 1 h) prior to chromatographic purification. Depending on the requisites of analyses, the polypeptide was passed through Sep-Pak C18 (octadecyl group) and AC-2 (activated charcoal) cartridges (Waters, Milford, Mass.) to eliminate impurities.
TLC.
To determine the amino acid compositions and the amino acid linking patterns, an extracted polypeptide was directly hydrolyzed by heating at 100°C for 22 h in the presence of 6 M HCl or was hydrolyzed under the same conditions after derivatization with the 2,4-dinitrophenol (DNP) group. The resulting samples were analyzed by thin-layer chromatography (TLC; silica gel 60 or cellulose plates [Merck, Darmstadt, Germany]; mobile phase, n-butanol, acetic acid, pyridine, and water [4:1:1:2]). For the general detection of amino acids and the specific detection of arginine and histidine, the ninhydrin reaction, Sakaguchi's reaction, and Pauly's reaction, respectively, were used.
Amino acid sequencing and mass spectrometry.
The amino acid sequence of the polypeptide was determined with an automated peptide sequencer (model 492; Applied Biosystems, Foster City, Calif.). The molecular weight was determined by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF-MS; model Voyager DE spectrometer; PerSeptive Biosystems, Framingham, Mass.) with α-cyano-4-hydroxycinnamic acid as the matrix.
Antimicrobial assay.
The microbial strains were from either the Institute for Fermentation, Osaka (IFO; Osaka, Japan) or our laboratory stocks. The culture media used in this assay were as follows (per liter): for nutrient broth, 3 g of beef extract and 5 g of peptone (pH 6.8); IFO702, 10 g of polypeptone, 2 g of yeast extract, and 1 g of magnesium sulfate heptahydrate (pH 7.0); and for potato dextrose broth, 4 g of potato infusion (approximately 200 g of raw potatoes) and 20 g of d-glucose (pH 5.1). Chemically defined media (DMBm1 and DMBm2) were also prepared by modifying Davis's minimal broth (8). DMBm1 (ionic strength, 168.3 mM) was composed of 1 g of d-glucose, 1 g of ammonium sulfate, 7 g of dipotassium hydrogen phosphate, 2 g of potassium dihydrogen phosphate, 0.5 g of trisodium citrate dihydrate, 10 mg of magnesium sulfate heptahydrate, 0.1 g of Casamino Acids, and 2 mg of thiamine hydrochloride (pH 7.1) in 1 liter. The composition of DMBm2 (ionic strength, 37.4 mM) was essentially the same as that of DMBm1, except that the K2HPO4, KH2PO4, and sodium citrate contents were 1/10 those in DMBm1. Test microbes were inoculated into 1 ml of the liquid culture medium containing serially diluted polypeptides that were purified from the culture supernatant of strain E18 or chemically synthesized. In most cases, the concentration of microbes added was 104 to 105 cells/ml at the start of culture. After appropriate periods of incubation at the optimum temperature, growth was evaluated by measurement of the optical density at 600 or 660 nm. In the case of nondispersed cells, visible growth was evaluated.
Hemolytic assay.
Sheep whole blood was suspended in 1 ml of a solution of 10% d-maltose and 5 mM sodium phosphate (pH 6.9). A synthetic peptide, RHRHRHRHRH [(RH)5], was added to the suspension at 10 μM. Similarly, melittin (Sigma, St. Louis, Mo.), a hemolytic peptide from bees, was added to the suspension as a control. After incubation at 28°C for 10 min, the absorbance at 540 nm of the supernatant obtained after centrifugation was measured. The intensity (in percent) of hemolysis was evaluated by the following equation: (A − A0)/(Ap − A0) × 100, where A, A0, and Ap denote the absorbances of samples spiked with peptides, blank, and completely hemolyzed samples by the addition of Triton X-100, respectively.
Metal binding assay.
Metal binding activity was determined by MALDI-TOF-MS. The solution of (RH)5 and chloride or sulfate salts of metals at a molar ratio of 1:50 was dehydrated in the presence of 2,5-dihydroxybenzoic acid as the matrix and was then subjected to MS.
CD spectrometry.
The synthetic polypeptide, (RH)5 (25.08 nmol), was added to a series of metal solutions with various metal contents (0 to 250.8 nmol) in 20 mM potassium phosphate buffer (pH 7.0). The final volume of each solution was 2.5 ml, which was placed in a glass cuvette (optical pass length, 1 cm). The circular dichroism (CD) spectra were measured at 25°C with a spectropolarimeter (model J-720WI; JASCO, Tokyo, Japan).
Membrane disruption assay.
To test the abilities of the peptides to form pores on lipid layers or to break on lipid layers, liposomes that encapsulate fluorescent dyes were used. A mixture of phosphatidylcholine dipalmitoyl (C16:0) and phosphatidyl-d,l-glycerol dipalmitoyl (C16:0) at a weight ratio of 7:3 was dissolved and dried under vacuum. The lipids were then suspended in 25 mM sodium phosphate buffer (pH 7.0) containing 50 mM carboxyfluorescein (FAM) with an ultrasonic generator. The liposomes were separated from the free dyes with a gel filtration column (PD-10; Amersham Biosciences, Piscataway, N.J.) and kept in the phosphate buffer. Synthetic (RH)5 was added to a suspension of liposomes at 10 μM. As a control experiment, mastoparan (Sigma), a membrane-active peptide from bees, was added to the liposomes. The leakage of the dyes from liposomes was monitored by measuring the fluorescence with a fluorescence spectrophotometer (model F-4500; Hitachi, Tokyo, Japan).
Fluorescence microscopy.
In the chemical peptide synthesis, an amino group at the N terminus of (RH)5 was labeled with FAM. Candida boidinii yeast cells were suspended in either 5 mM potassium phosphate buffer (pH 7.0) for experiments under conditions of low ionic strength or DMBm1 for experiments under conditions of high ionic strength. Synthetic Nα-FAM-(RH)5 and 4′,6-diamino-2-phenylindole (DAPI; Sigma) were each added to the cell suspension at a concentration of 10 μM. As control experiments, free FAM dye was added alone or together with the nonlabeled synthetic (RH)5 peptide. After incubation at 25°C for 5 min, the cells were washed with the same buffer or medium to remove excess dyes and peptides. Moreover, another experiment with DMBm1 containing 1 mM copper chloride was also carried out. The cells were mounted on a glass slide and observed under a fluorescence microscope (model Axiovert 135 M; Carl Zeiss, Oberkochen, Germany).
RESULTS
Isolation of a novel peptide and its structure.
Strain E18 secreted into the culture medium a basic substance at a concentration of 40 μg/ml, as estimated by electrophoresis. To examine the structure of the basic substance, it was hydrolyzed with acid, separated by TLC, and subjected to qualitative tests. The substance was a polypeptide and showed positive signals in Sakaguchi's and Pauly's reactions, which are specific for the guanidino group (arginine) and the imidazole ring (histidine) or phenol group (tyrosine), respectively. Because the positive signals were observed not only with a hydrolyzed sample (monomer) but also with an intact form (polymer), these reactive groups are definitely free in the polymer. The signal of the hydrolysates of the DNP-labeled polymer corresponded to authentic Nim-DNP-l-histidine. On the basis of these results, the secreted basic polypeptide is considered a composite of arginine and histidine, which are likely linked by α-amide bonds.
In order to determine the primary structure of the polypeptide, it was subjected to degradation by Edman's method. The N-terminal residue was identified to be arginine. The second residue was histidine. The other residues alternated between arginine (at odd positions) and histidine (at even positions). By cycling of Edman's reaction, we were able to determine the amino acid sequence up to residue 10.
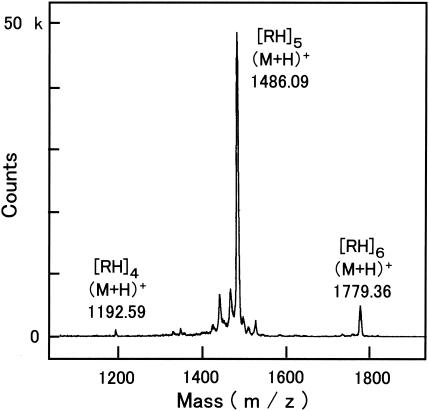
The MALDI-TOF-MS spectrum of the polypeptide is shown in Fig. 1. The major signal was found at m/z 1,486.09. This value corresponds to the pseudomolecular ion ([M + H]+) of a decapeptide (NH2-RHRHRHRHRH-COOH). Additional peaks were detected at m/z 1,192.59 and 1,779.36, which correspond to an octapeptide (NH2-RHRHRHRH-COOH) and a dodecapeptide (NH2-RHRHRHRHRHRH-COOH), respectively. It is notable that no signals corresponding to either a nonapeptide (NH2-RHRHRHRHR-COOH) or an undecapeptide (NH2-RHRHRHRHRHR-COOH) were detected. This result strongly indicates that the polypeptide is synthesized from a dipeptide (RH) unit. Because of the alternating order of arginine and histidine, the polypeptides are hereafter referred to as poly(arginyl-histidine) or (RH)n [e.g., (RH)5 indicates a decapeptide].
FIG. 1.
MALDI-TOF mass spectrum of poly(arginyl-histidine) secreted by V. kibiense strain E18. Ionization was carried out in the presence of α-cyano-4-hydroxycinnamic acid. Polypeptides are referred to as [RH]n (where n indicates the number of arginyl-histidine units).
Antimicrobial activity.
Poly(arginyl-histidine) peptides were polycationic and secretory. Such characteristics are common in many antimicrobial peptides. Whether the poly(arginyl-histidine) has antimicrobial activity was determined first under conditions of low ionic strength. Poly(arginyl-histidine) peptides partially purified from the culture filtrate of strain E18 had an inhibitory effect on microbe growth (Table 1). Almost all species of gram-negative and gram-positive bacteria and fungi tested were inhibited by the peptide at a concentration of 60 μg/ml or lower. To exclude the possibility that antibiotics other than poly(arginyl-histidine) suppressed the growth of the test microbes, a chemically synthesized peptide was used. Except for the growth of Salmonella enterica serovar Typhimurium and Bacillus cereus, synthetic (RH)5 inhibited the growth of the test microbes as much as the purified fungal peptides did. An amphipathic derivative of the poly(arginyl-histidine) peptide, which was generated by conjugating hydrophobic myristic acid to hydrophilic (RH)5, inhibited the growth of S. enterica serovar Typhimurium and B. cereus. The free guanidino groups of arginine residues have a pKa (pKa = 12.5) higher than that of the α-amino groups (pKa = 8.9) of lysine residues in ɛ-PL. Positive charges of ɛ-PL are eliminated at pH >9. Alkaliphilic bacteria grown at pH 11.2 were resistant to ɛ-PL but sensitive to (RH)5. The MICs of (RH)5 were 10 μg/ml for Bacillus alcalophilus IFO15653 and 50 μg/ml for both Bacillus cohnii IFO15565 and Exiguobacterium aurantiacum IFO14763. Thus, it was confirmed that poly(arginyl-histidine) has activity against a broad range of microbes even under alkaline conditions.
TABLE 1.
Antimicrobial spectrum of fungal poly(arginyl-histidine) and synthetic polypeptides
| Microbe group and microbe species and strain | Mediuma | MIC (μg/ml)b
|
||
|---|---|---|---|---|
| Fungal (RH)n | Synthetic (RH)5 | Synthetic myristoyl-(RH)5 | ||
| Gram-negative bacteria | ||||
| Agrobacterium tumefaciens LBA4404 | N | 8 | 10 | 10 |
| Escherichia coli IFO3301 | N | 4 | 10 | 10 |
| Pseudomonas aeruginosa IFO3445 | N | 8 | 10 | 10 |
| Salmonella enterica serovar Typhimurium IFO12529 | N | 20 | 150 | 10 |
| Gram-positive bacteria | ||||
| Bacillus cereus IFO3514 | N | 60 | >400 | 10 |
| Bacillus subtilis IFO3336 | N | 4 | 10 | 10 |
| Enterococcus faecalis JCM8726 | N | 4 | (10) | 10 |
| Geobacillus stearothermophilus IFO12550 | 702 | 4 | 10 | NT |
| Kitasatospora kifunense MN1 | N | (200) | >400 | 25 |
| Lactobacillus brevis IFO3345 | 702 | 4 | 10 | NT |
| Staphylococcus aureus IFO13276 | N | 20 | 50 | 10 |
| Staphylococcus aureus (methicillin resistant) ATCC 33591 | N | 20 | 50 | 10 |
| Yeasts and filamentous fungi | ||||
| Saccharomyces cerevisiae KK4 | PD | 20 | 100 | NT |
| Candida albicans IFO1385 | PD | 40 | 100 | NT |
| Candida boidinii MIP104 | PD | 20 | 10 | NT |
| Aspergillus niger IFO4416 | PD | (200) | (50) | NT |
| Verticillium kibiense E18 | PD | NT | >400 | NT |
N, nutrient broth; PD, potato dextrose broth; 702, IFO702 broth.
Parentheses indicate that growth was retarded but not stopped at a concentration greater than that indicated. NT, not tested.
On the other hand, (RH)5 did not cause the hemolysis of sheep erythrocytes at 10 μM (about 15 μg/ml), at which the growth of bacteria such as Escherichia coli was inhibited. Melittin caused 49.7% hemolysis, adopting the value of complete hemolysis induced with a detergent as 100%, and (RH)5 had no significant hemolytic activity (below 0.5%).
To determine which amino acid residues exert antimicrobial action, several synthetic substitute peptides were examined in assays with the nutrient broth (Table 2). Neither arginine nor histidine by itself was toxic, but an alternative repeat composed of six residues, (RH)3, inhibited the growth of Bacillus subtilis and E. coli at 100 μg/ml. The MICs of the longer repeats, (RH)4, (RH)5, and (RH)6, reached a plateau at 10 μg/ml. Together with the observation that the MIC of (HR)5 was equivalent to that of (RH)5, the results presented above indicate that three units of arginyl-histidine is sufficient for antimicrobial activity. The replacement of the original arginine residues with the neutral amino acid glycine resulted in the loss of the antimicrobial activity, but replacement with lysine did not. Replacement of the original histidine residues with glycine reduced the antimicrobial activity to 1/10 of that of the original peptide, whereas replacement with cysteine, which has chelating activity similar to that of histidine, did not. These results indicate that the basic property of the arginine residues is indispensable to the antimicrobial activity of poly(arginyl-histidine) and that the chelatable property of the histidine residues reinforces such activity under conditions of low ionic strength.
TABLE 2.
Antimicrobial activities of synthetic polypeptides mimicking fungal poly(arginyl-histidine)a
| Polypeptide | Structure | MIC (μg/ml)
|
|
|---|---|---|---|
| B. subtilis | E. coli | ||
| (RH)1 | NH2-RH-COOH | >500 | >500 |
| (RH)2 | NH2-RHRH-COOH | >500 | >500 |
| (RH)3 | NH2-RHRHRH-COOH | 100 | 100 |
| (RH)4 | NH2-RHRHRHRH-COOH | 10 | 10 |
| (RH)5 | NH2-RHRHRHRHRH-COOH | 10 | 10 |
| (RH)6 | NH2-RHRHRHRHRHRH-COOH | 10 | 10 |
| (HR)5 | NH2-HRHRHRHRHR-COOH | 10 | 10 |
| (KH)5 | NH2-KHKHKHKHKH-COOH | 10 | 10 |
| (RG)5 | NH2-RGRGRGRGRG-COOH | 100 | 100 |
| (GH)5 | NH2-GHGHGHGHGH-COOH | >500 | >500 |
| (RC)5 | NH2-RCRCRCRCRC-COOH | 10 | 20 |
The test bacteria were B. subtilis IFO3336 and E. coli IFO3301; the medium was nutrient broth.
Since the antimicrobial activities of polycationic peptides are generally influenced by coexisting cations and anions, we investigated how much coexisting cations and anions influence poly(arginyl-histidine) (Table 3). The growth inhibition caused by 13.2 μM synthetic (RH)5 was abrogated by the addition of salts to the nutrient broth. Chlorides and sulfates of both univalent and divalent cations abrogated the antimicrobial activity at ionic strengths of 30 to 50 and 40 to 60 mM, respectively. The antimicrobial activity of (RH)5 was abrogated by coexisting electrolytes, regardless of the type of salt.
TABLE 3.
Abrogation of antimicrobial activity caused by saltsa
| Salt | Minimum concn (mM)b
|
|
|---|---|---|
| Molarity | Ionic strengthc | |
| LiCl | 40 | 40 |
| NaCl | 40 | 40 |
| KCl | 50 | 50 |
| NH4Cl | 50 | 50 |
| MgCl2 | 10 | 30 |
| CaCl2 | 10 | 30 |
| Na2SO4 | 20 | 60 |
| K2SO4 | 20 | 60 |
| (NH4)2SO4 | 20 | 60 |
| MgSO4 | 10 | 40 |
The test bacterium was E. coli IFO3301; the medium was nutrient broth containing synthetic (RH)5 at 20 μg/ml (13.2 μM).
Salt concentration when the abrogation of antimicrobial activity was observed.
The sum total of the molarity (mi) of each type of ion present multiplied by the square of its charge (zi) is divided by 2 (I = 1/2 Σmi zi2, where I is ionic strength).
Relationship of metal ions to antimicrobial activity.
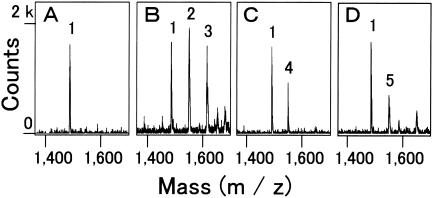
Since it seemed likely that poly(arginyl-histidine) acts as a chelator, the association between synthetic (RH)5 molecules and metal ions was investigated by MALDI-TOF-MS. In the presence of metal ions (Fig. 2B-D), the m/z values of the signals increased from the m/z value of about 1,486 for the metal-free (RH)5 molecules, as shown in Fig. 2A. The increment corresponded to the mass of the metal ions. For example, the mixture of (RH)5 and CuCl2 yielded signals at 1,549.2 and 1,612.0, in addition to the signal at 1,486.6 (Fig. 2B). The signals at the middle and highest m/z values probably represent the peptide adducts with one and two copper ions, respectively. All the other metal ions examined, namely, those liberated from CaCl2, CoCl2, NiCl2, MgSO4, MnSO4, and ZnSO4, were found to be associated with the (RH)5 peptide at a molar ratio of 1:1, whereas the molar ratio was 1:1 or 1:2 for copper ions, although there was a difference in the strengths between the signals.
FIG. 2.
MALDI-TOF mass spectra of metal-polypeptide complex. Synthetic (RH)5 peptides (60 pmol) alone (A) or premixed with CuCl2 (B), CoCl2 (C), and ZnSO4 (D) at a molar ratio of 1:50 were ionized in the presence of 2,5-dihydroxybenzoic acid. Peaks: 1, (M + H)+, m/z 1486.0 to 1486.6; 2, (M + Cu)+, m/z 1549.2; 3, (M − H + 2Cu)+, m/z 1612.0; 4, (M − H + Co)+, m/z 1542.9; 5, (M − H + Zn)+, m/z 1550.2.
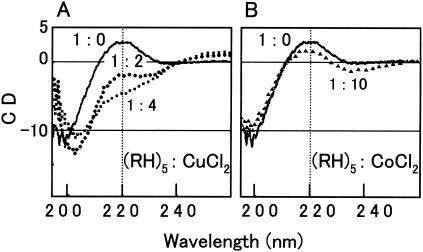
In addition to MALDI-TOF-MS, CD spectrometry showed the metal binding that led to the structural changes of poly(arginyl-histidine), although the observation was specific to copper and zinc ions. Whereas synthetic (RH)5 peptides had no ordered structure by themselves, a certain conformation gradually appeared in the series of mixtures of the peptides and copper ions with molar ratios of 1:2 to 1:4 (Fig. 3A). The signal intensities in the CD spectra decreased at 220 nm and increased at 195 and 255 nm. This supports the idea that (RH)5 peptides have an ordered but unknown conformation in a solution with copper ions. Zinc ions also induced the ordered structure of (RH)5 peptides at molar ratios greater than or equal to 1:1, but the maximum signal intensity-decreasing effect of zinc ions at 220 nm was smaller than that of copper ions (data not shown). Unlike copper and zinc ions from CuCl2 and ZnSO4, other metal ions from MgCl2, MnSO4, CoCl2, NiCl2, and CdCl2 did not affect the CD spectra of the (RH)5 peptides. Representative CD spectra obtained with the mixture of (RH)5 and CoCl2 are shown in Fig. 3B.
FIG. 3.
CD spectra of synthetic (RH)5 alone and with copper and cobalt ions at different ratios. Molar ratios of peptide to metal salts were 1:0 (solid lines), 1:2 (circles), 1:4 (squares), and 1:10 (triangles). The peptide concentration was 10 μM.
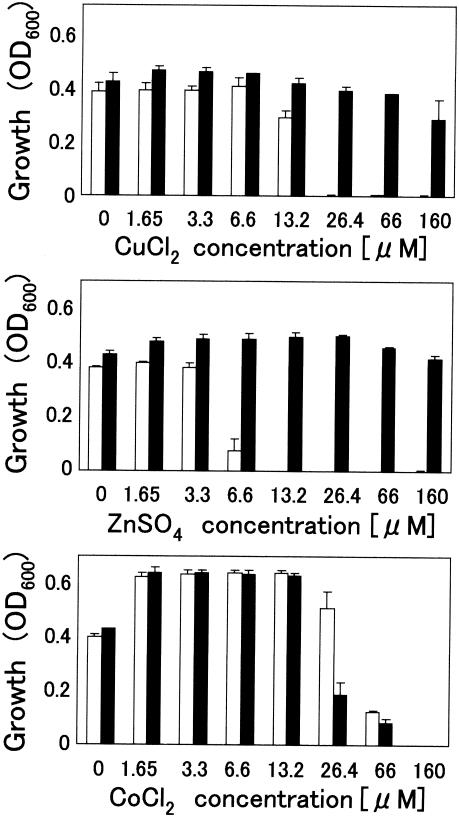
To investigate chelating activity with reference to antimicrobial activity, poly(arginyl-histidine) was subjected to an antimicrobial assay in a chemically defined medium, DMBm1, with or without trace amounts of copper, zinc, and cobalt ions. This metal-depleted medium had sufficient ionic strength (168.3 mM, equivalent to that of a 0.98% aqueous solution of NaCl) to abrogate the antimicrobial activity of (RH)5, which was confirmed by the result that the addition of (RH)5 peptides at 6.6 μM to DMBm1 did not inhibit the growth of the test bacterium, E. coli strain IFO3301 (Fig. 4). Zinc ions at concentrations more than 6.6 μM restored the antimicrobial activity. In the case of copper ions, antimicrobial activity was restored when the copper ions were present at at least a twofold concentration of (RH)5 molecules. Unlike zinc and copper ions, cobalt ions did not restore the antimicrobial activity. Over the range of concentrations examined, neither zinc nor copper ions alone affected the growth of the microorganisms tested, whereas cobalt ions at concentrations greater than 26.4 μM were toxic to the bacteria tested. The toxicity was counteracted by 6.6 μM (RH)5 peptides. This was probably due to the formation of the (RH)5-Co2+ complex, as determined by MALDI-TOF-MS (Fig. 2C). Similar results were obtained with B. subtilis IFO3336 (data not shown). In DMBm2, which has a lower ionic strength (37.4 mM), (RH)5 exhibited antimicrobial activity in the absence of zinc or copper ions (data not shown). Therefore, it was concluded that copper and zinc metals are not required for the antimicrobial activity of poly(arginyl-histidine) under conditions of low ionic strength but serve as a reinforcement factor for the activity under conditions of high ionic strength.
FIG. 4.
Effects of metal ions on antimicrobial activity under conditions of high ionic strength. The growth of E. coli strain IFO3301 in chemically defined medium (DMBm1) was monitored by measuring the optical density at 600 nm (OD600). Open columns, addition of synthetic (RH)5 at a final concentration of 6.6 μM; closed columns, no addition of peptide. Values are means ± standard deviations (n = 3).
Effects of poly(arginyl-histidine) on liposomes and living cells.
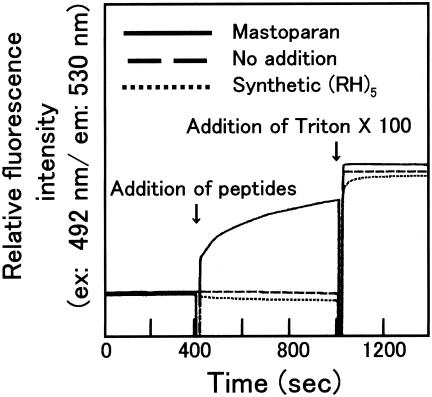
To address whether the antimicrobial activity of poly(arginyl-histidine) is attributed to the damaged cell membrane of the target microbes, the synthetic phospholipid vesicle liposomes containing a fluorescent dye were challenged with (RH)5 peptides at 10 μM, which is sufficient to inhibit the growth of bacteria such as E. coli (Table 1), but no leakage of fluorescent dye from the liposomes was observed (Fig. 5). Therefore, it is considered that the poly(arginyl-histidine) peptide neither acts as a detergent nor forms micropores on the cell membrane to exert its antimicrobial activity.
FIG. 5.
Membrane disruption assay by using liposomes that encapsulate fluorescent dyes. The leakage of dyes caused by peptide addition was measured with a fluorescence spectrophotometer. At 400 and 1,000 s after the start of measurement, the liposome suspension was spiked with either mastoparan or synthetic poly(arginyl-histidine) at a final concentration of 10 μM and detergents, respectively.
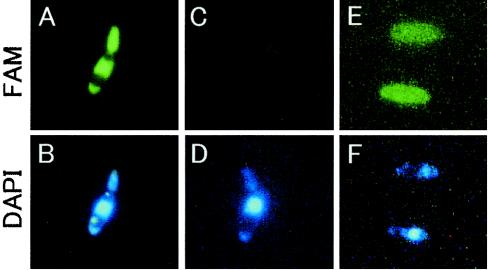
To investigate where poly(arginyl-histidine) localizes in target microbial cells, fluorescence-labeled peptides, Nα-FAM-(RH)5, were applied to a suspension of C. boidinii MIP104 cells. The fluorescent labels localized evenly throughout the cell except in the vacuoles and accumulated a little in the nuclei (Fig. 6A). The peptides did not alter the intracellular structure, as visualized with DAPI (Fig. 6B). Free (nonconjugated) FAM dye was not taken up by the cells in the absence or presence of (RH)5. These results indicate that intact Nα-FAM-(RH)5 entered living cells. The uptake of Nα-FAM-(RH)5 was perturbed in DMBm1, which had a high ionic strength (Fig. 6C). However, adsorption was observed following the addition of copper ions, although uptake was not observed (Fig. 6E). The same results were obtained in experiments with Saccharomyces cerevisiae KK4 and E. coli IFO3301 (data not shown).
FIG. 6.
Fluorescence microscopic observation of uptake of fluorescence-labeled (RH)5 [Nα-FAM-(RH)5] by C. boidinii cells. The cells were suspended in 5 mM potassium phosphate (A and B) or DMBm1 (C to F) in the presence of both 10 μM Nα-FAM-(RH)5 and 10 μM DAPI. In panels E and F, 1 mM CuCl2 was also added to the cell suspension.
DISCUSSION
Although it has been established that many organisms produce antimicrobial polypeptides, no organisms with an alternating series of arginine and histidine residues has yet been reported. Poly(arginyl-histidine), produced by the fungus Verticillium, is the fifth biosynthesized poly(amino acid) to be discovered, following ɛ-poly-l-lysine (ɛ-PL), γ-poly-d,l-glutamic acid, multi-l-arginyl-poly-l-aspartic acid (cyanophycin [15, 16]) and poly (γ-glutamyl-cysteinyl)glycine (phytochelatin [12]). Poly(arginyl-histidine) has the unique feature of dipeptide repetition, while other poly(amino acids) have special structural linkages involving ɛ-amino and γ- and β-carboxylic groups. Phytochelatin has both isopeptide bonds and repetitions of dipeptides. Generally, it is considered that short poly(amino acids) cannot form complex secondary structures. Likewise, CD spectra showed that the (RH)5 peptide is structurally amorphous in a metal-free state in aqueous phase but that coexisting metal induced an ordered structure.
Poly(arginyl-histidine) has moderate activity against a broad range of bacteria and fungi. This is likely attributed to the polycationic property generated by its arginine residues as well as by a primary structure of simple biopolymers, such as ɛ-PL and chitosan. Such polycations are considered to act electrostatically on the acidic surfaces of target microbial cells. For more complex peptides, such as nisin, magainin, and defensin, many lines of evidence indicate that electrostatic interaction is important at the initial stage of antimicrobial activity, prior to micropore formation in the cell membrane (1, 3, 10). Because the electrostatic interaction is destroyed by coexisting electrolytes, the antimicrobial activities of polycations, including poly(arginyl-histidine), are hampered under electrolyte-rich conditions. However, in the case of poly(arginyl-histidine), specific binding to copper and zinc ions maintains the antimicrobial activity even under conditions of high ionic strength. A Cu2+ or Zn2+ to poly(arginyl-histidine) peptide at molar ratio of 3:1 or 2:1 is sufficient to maintain the antimicrobial activity of the peptide under conditions of high ionic strength. Even though metal binding is not necessary for antimicrobial activity, it is important when poly(arginyl-histidine) functions under conditions of high ionic strength. The CD spectra indicated that poly(arginyl-histidine) forms a certain structure induced by the association of Cu2+ or Zn2+ ions when they are present at a molar ratio to the peptide greater than 2:1 (copper) or 1:1 (zinc). The amount of metal ions necessary to maintain antimicrobial activity is almost equal to that necessary to induce a certain structure. However, further studies are required to define the interaction between the structure and function of the peptide.
Three models that explain the antimicrobial action of polycationic peptides are proposed: (i) simple adhesion to the negatively charged surface of a cell to suppress the functions of the cell membrane; (ii) an amphipathic effect, similar to the amphipathic effects of detergents, that disrupts the cell membrane and micropore formation on the cell membrane, which leads to the loss of the selective abilities of substances to permeate the cell membrane; and (iii) distortion of intracellular functions. At the initial stage of its action on target cells, highly positively charged poly(arginyl-histidine) probably attaches to the cell membrane. Poly(arginyl-histidine) was considered to suppress the putative membrane functions of target cells. However, on the basis of the result that poly(arginyl-histidine) did not induce dye leakage from liposomes (Fig. 5), it is unlikely that poly(arginyl-histidine) disrupts the cell membrane or forms micropores in the cell membrane. The fluorescence microscopic observation indicated that fluorescent dye-labeled poly(arginyl-histidine) can cross the cell membranes of living cells. Although arginine-rich peptides were reported to enter animal cells (4), to our knowledge poly(arginyl-histidine) is the first example of a structurally simple antimicrobial peptide that enters living cells and that may act inside the cell. Indeed, on the basis of the localization of FAM-labeled (RH)5 in the yeast nucleus (Fig. 6), it is likely that peptide binding to nucleic acids affects replication and transcription in target cells in a manner similar to that in which basic dyes interrupt cell functions. At present, it is difficult to specify the antimicrobial mechanism of poly(arginyl-histidine).
Acknowledgments
We thank T. Negishi (RIBS) for assistance with measuring the CD spectra and M. Iwabuchi (RIBS) for helpful discussions.
REFERENCES
- 1.Breukink, E., C. van Kraaij, R. A. Demel, R. J. Siezen, O. P. Kuipers, and B. de Kruijff. 1997. The C-terminal region of nisin is responsible for the initial interaction of nisin with the target membrane. Biochemistry 36:6968-6976. [DOI] [PubMed] [Google Scholar]
- 2.Broekaert, W. F., F. R. G. Terras, B. P. A. Cammue, and R. W. Osborn. 1995. Plant defensins: novel antimicrobial peptides as components of the host defense system. Plant Physiol. 108:1353-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujii, G., M. E. Selsted, and D. Eisenberg. 1993. Defensins promote fusion and lysis of negatively charged membranes. Protein Sci. 2:1301-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Futaki, S., T. Suzuki, W. Ohashi, T. Yagami, S. Tanaka, K. Ueda, and Y. Sugiura. 2001. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 276:5836-5840. [DOI] [PubMed] [Google Scholar]
- 5.Kimbrell, D. A. 1991. Insect antibacterial proteins: not just for insects and against bacteria. Bioessays 13:657-663. [DOI] [PubMed] [Google Scholar]
- 6.Kirk, T., K. E. Schultz, W. J. Connors, L. F. Lorenz, and J. G. Zeikus. 1978. Influence of culture parameters on lignin metabolism by Phanerochaete chrysosporium. Arch. Microbiol. 117:277-285. [Google Scholar]
- 7.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-85. [DOI] [PubMed] [Google Scholar]
- 8.Lederberg, J. 1950. Isolation and characterization of biochemical mutants of bacteria. Methods Med. Res. 3:5-21. [Google Scholar]
- 9.Lehrer, R. I., A. K. Lichtenstein, and T. Ganz. 1993. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu. Rev. Immunol. 11:105-128. [DOI] [PubMed] [Google Scholar]
- 10.Matsuzaki, K., M. Harada, S. Funakoshi, N. Fujii, and K. Miyajima. 1991. Physicochemical determinants for the interactions of magainins 1 and 2 with acidic lipid bilayers. Biochim. Biophys. Acta. 1063:162-170. [DOI] [PubMed] [Google Scholar]
- 11.Nishikawa, M., and K. Ogawa. 2002. Distribution of microbes producing antimicrobial epsilon-poly-l-lysine polymers in soil microflora determined by a novel method. Appl. Environ. Microbiol. 68:3575-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rauser, W. E. 1995. Phytochelatins and related peptides. Structure, biosynthesis, and function. Plant Physiol. 109:1141-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shima, S., and H. Sakai. 1981. Poly-l-lysine produced by Streptomyces. Part II. Taxonomy and fermentation studies. Agric. Biol. Chem. 45:2497-2502. [Google Scholar]
- 14.Shima, S., and H. Sakai. 1981. Poly-l-lysine produced by Streptomyces. Part III. Chemical studies. Agric. Biol. Chem. 45:2503-2508. [Google Scholar]
- 15.Simon, R. D. 1971. Cyanophycin granules from the blue-green alga Anabaena cylindrica: a reverse material consisting of copolymers of aspartic acid and arginine. Proc. Natl. Acad. Sci. USA 68:265-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon, R. D., and P. Weathers. 1976. Determination of the structure of the novel polypeptide containing aspartic acid and arginine which is found in cyanobacteria. Biochim. Biophys. Acta 420:165-176. [DOI] [PubMed] [Google Scholar]
- 17.Szokan, G., M. Almas, K. Krizsan, A. R. Khlafulla, E. Tyihak, and B. Szende. 1997. Structure determination and synthesis of lysine isopeptides influencing on cell proliferation. Biopolymers 42:305-318. [DOI] [PubMed] [Google Scholar]
- 18.Vogler, K., and R. O. Studer. 1966. The chemistry of the polymyxin antibiotics. Experientia 22:345-354. [DOI] [PubMed] [Google Scholar]
- 19.Zasloff, M. 1987. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 84:5449-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]