Abstract
Introduction
Tuberculosis (TB) transmission is determined by contact between infectious and susceptible individuals. A recent study reported a 4% annual risk of child TB infection (ARTI) in a Southern African township. A model was used to explore the interactions between prevalence of adult TB infection, adult-to-child contacts and household ventilation which could result in such a high ARTI.
Methods
Number of residents per household and TB incidence were derived from a household census and community TB registers. Using the “Wells-Riley” equation and probability analyses of contact between TB infectious adults and pre-school children, we estimated the ARTI within and outside of the home.
Results
There was a mean of 2.2 adults per child-household with a 1.35% annual adult smear-positive TB notification rate. The maximal household ARTI was 3% which was primarily determined by the number of resident adults. Transmission risk outside the home increased with numbers of households visited. Transmission probabilities were sensitive to exposure time, ventilation and period of adult infectivity. The benefits of increased ventilation were greatest when the period of infectivity was reduced. Similar reductions in household transmission could be achieved by increasing ventilation from 2 to 6 air changes/hour or separating child and adult sleeping areas.
Conclusions
The ARTI of pre-school children predominantly results from infectious residents in the home. However, even with limited social interactions, a substantial proportion of transmission may occur from non-resident adults. The benefits of increased ventilation are maximized when the period of infectivity is reduced by prompt treatment of source cases.
Keywords: Tuberculosis, Transmission, Infectiousness, Children, Ventilation
Introduction
South Africa is now the 5th highest tuberculosis (TB) burdened country in the world, with high rates of both adult and childhood (0-15 yrs) TB notifications 1. Total TB notifications in the South African city of Cape Town with 3.2 million people, reached 27,000 in 2006.2 However the distribution of TB cases within the city is very unequal, with unprecedentedly high burdens in the crowded and socially deprived African townships. In these townships housing consists largely of informal shack dwellings where the annual TB notification rates exceed 1500/100,000 3-5. While adult TB disease is due to a combination of reactivation of remote infection together with rapid progression of recent adult to adult transmission 6, childhood disease reflects rapid progression from recent adult to child transmission 7. Childhood (0-15 yrs) TB notification rates have been reported to be 3.5 times the adult rate in specific high burden Cape Town communities, where childhood TB contributed 39% of the total TB case load 8. Recent studies of childhood TB infection rates in southern Africa townships have reported annual risks of TB infection (ARTI) as high as 4 percent per annum 9, 10, 11. This ARTI is similar to reported values from sixty years ago, before implementation of national TB control programs 12.
Childhood TB infection is quantitatively related to exposure of susceptible children to sputum smear-positive adults 13, 14. The prevalence of infectious adults is determined by the annual incidence rate of adult smear-positive TB and the mean time period of infectivity between becoming infective and either initiating effective therapy or death. The prevalence of untreated TB is therefore primarily determined by the effectiveness of the TB control program to identify, diagnose and effectively treat infective TB cases. The risk of a possible transmission event is related to the number of contacts a child has with infectious adults. The efficiency of transmission in turn is determined by the infectiousness of the source, the length of contact and the environmental characteristics at the site of a contact. TB transmission therefore results from the interplay between social interactions, environmental factors and prevalence of infective adults. The period of infectiousness (Δ) of adults is the only modeled parameter impacted by the activities of the TB control program.
We therefore modeled pre-school child (0-5 yrs) TB transmission, both in and outside of their primary residence using the distribution of resident adults per household and prevalence of adult infectious TB. The modeled transmission probabilities were adjusted for length of exposure time and variable household ventilation characteristics. We also explored the reduction of the period of adult infectivity and increased household ventilation which would be required to achieve significant reductions in transmission.
Methods
Study design
The study aim was to explore transmission probabilities from adults to pre-school children within and outside of households in a South African township. The Wells-Riley equation is a well known transmission model that has been used to describe airborne transmission probabilities of a single enclosed room or space with defined ventilation 14. The Wells-Riley equation which has been applied to a wide range of transmission scenarios 15-19 was used in combination with the distribution of adults per household and their probability of being infectious in order to explore adult to child TB transmission probabilities.
Study community
The study site used to provide data inputs to these modeling analyses is a peri-urban township (Site-M) near Cape Town, South Africa, which was established in 1992 and has grown to a 2008 population of 14,788 people. The township is home to an almost exclusively African population, the adult HIV-prevalence in 2005 was 23% and the majority of persons have low socioeconomic status 3. Unemployment exceeds 50%, and housing predominately consists of closely aggregated, formal and informal structures. The township has clearly demarcated boundaries and constitutes a well-defined population for research studies and community health interventions.
Tuberculosis control program
The study community is served by a single government primary health care clinic with a dedicated TB service. All patients with TB in the community are treated at this facility. The program adhered to the South African National TB Control Program guidelines and included the WHO DOTS strategy 20. DOTS coverage in this community was complete, and treatment completion rates for smear-positive disease exceeded 80%.13 Adult pulmonary sputum-positive TB was diagnosed on the basis at least 1 positive sputum culture of Mycobacterium tuberculosis or 2 sputum smears containing acid-fast bacilli in the context of a compatible clinical illness. Childhood TB diagnosis utilized a scoring system using a combination of clinical and radiological features 20. A score of 7 or greater indicated a high likelihood of TB using length of illness(1-3), nutritional status (1-3), family history of smear positive disease (3), tuberculin skin test reactivity (3), enlarged lymph nodes (3), abdominal mass (3), central nervous signs (3), chest radiography (3) and spinal angling (4). All sputum testing was performed at the National Health Laboratory Services facilities in Cape Town.
Data sources
TB definitions used for notification data were as defined by the South African TB Control Program 20. TB is a notifiable condition in South Africa, and each TB clinic is required to maintain and report TB statistics. Numbers of TB notifications, demographic characteristics, history of previous TB, sputum microbiologic test findings, and TB classification data were obtained from the community TB clinic register. Tuberculosis program data were collected for the years 2004 to 2008 in order to cover the period of potential TB exposure for children aged 5 years or less in 2008. Demographic data for the community were derived from household censuses performed in 2004, 2006 and 2008 as part of an ongoing health research program. This research was approved by the Research Ethics Committee of the University of Cape Town.
Mathematical transmission model
The number of childhood tuberculosis infections (C) within a household with susceptible children (S) was assumed to be a function of the number of infectious adults (I), their infectivity (q), the time of exposure (t), susceptible respiration rate (p), and germ-free ventilation (Q) as given by Wells Riley equation C=S(1-e-Iptq/Q). The number of infectious adults at any time is given by the smear positive incidence rate (M) and the period of infectivity (Δ). The risk of contact with an infectious adult is given by the Poisson distribution (λI/I!)e-λ, where λ=M*A is the expected number of infectious adults in a household with “A” adults. Prevalence was defined as M/[365/Δ].
Modeled inputs
Germ-free ventilation (Q) was calculated as air changes per hour (ACH) for a standard shack dwelling of 30 cubic meters. Three values of ACH were modeled; 2 ACH (poor ventilation), 6 ACH (moderate ventilation) and 12 ACH the WHO recommendation for an airborne precaution room 21. Shacks with closed windows and doors would have ACH of 2 or less. Shacks with an open window (0.25 M2) facing prevailing wind and open door on leeward side would achieve 6 ACH with low prevailing wind speeds between 4 and 5 km/hour and 12 ACH in winds of 8 to10 km/hour 22.
The rate of production of infectious TB quanta (q) was modeled at a value of 1 infectious quantum per hour which is the mean measured value of smear positive inpatients of a TB ward 15. Sensitivity analyses were performed for values of q between 0.1 and 10 infectious quanta per hour.
The mean respiratory rate of pre-school children aged 0-5 years was estimated to be 225 liters per hour, which approximates to a respiratory volume of 150-200 mls/kg/min 23. The period of diagnostic delay during which adult may be infective (Δ) has been estimated in a systematic review to be very variable but is frequently reported to be between 60 days to 90 days 24. Since Δ may exceed the period of diagnostic delay and is the primary modeled parameter influenced by the functioning of the TB control program, it was allowed to take values of 30, 60, 90 and 120 days were included in the model.
For modeling purposes, child time allocation within a 24 hour period was 12 hours within the home to allow interaction with resident adults during the evening and night, including 8 hours for sleeping and 4 hours for other family activities. Similar exposure to non-resident adults can result from adults visiting the primary home or the child visiting other households. For modeling purposes, 12 hours of day time was allocated to 3 hours outdoors during which TB transmission was assumed negligible and 9 hours allocated equally between 1 to 3 additional households with similar numbers of residents as the primary residence.
Results
Household survey 2008
The total population of the study community in December 2008 was 14,788 of whom 12,097 were adolescents and adults over the age of 15 years, and 2,691 children aged less than 15 years of age including 1,051 children aged less than 5 years of age. The total number of households was 6,654 of which 1,708 contained children less than 15 years of age and 918 with children less than 5 years of age. The age distribution of residents of households with adults only and adults and children is shown in table 1. Crowding was twice as high in child-containing households as in adult-only households. Of the 918 households with children less than 5 years of age, 800 contained a single child, 109 two children and 9 three children less than 5 years of age. The median number of adults in these households was 2.27 per household and only 28% of these households had 3 or more resident adults (Figure 1).
Table 1.
Age distribution of residents in all Site-M households, households with adults residents only and household with children under 15 years or under 5 years of age.
| No of households | No under 5 years | No 5-15 years | No of Adults | Adults per HH | Total Residents | Residents per HH | |
|---|---|---|---|---|---|---|---|
| All households | 6654 | 1051 | 1640 | 12097 | 1.82 | 14788 | 2.22 |
| Households with only adults | 4946 | 0 | 0 | 8148 | 1.65 | 8148 | 1.65 |
| Households with children under 15 years | 1708 | 1051 | 1640 | 3949 | 2.31 | 6640 | 3.89 |
| Households with children under 5 years | 918 | 1051 | 516 | 2083 | 2.27 | 3650 | 3.98 |
Figure 1.
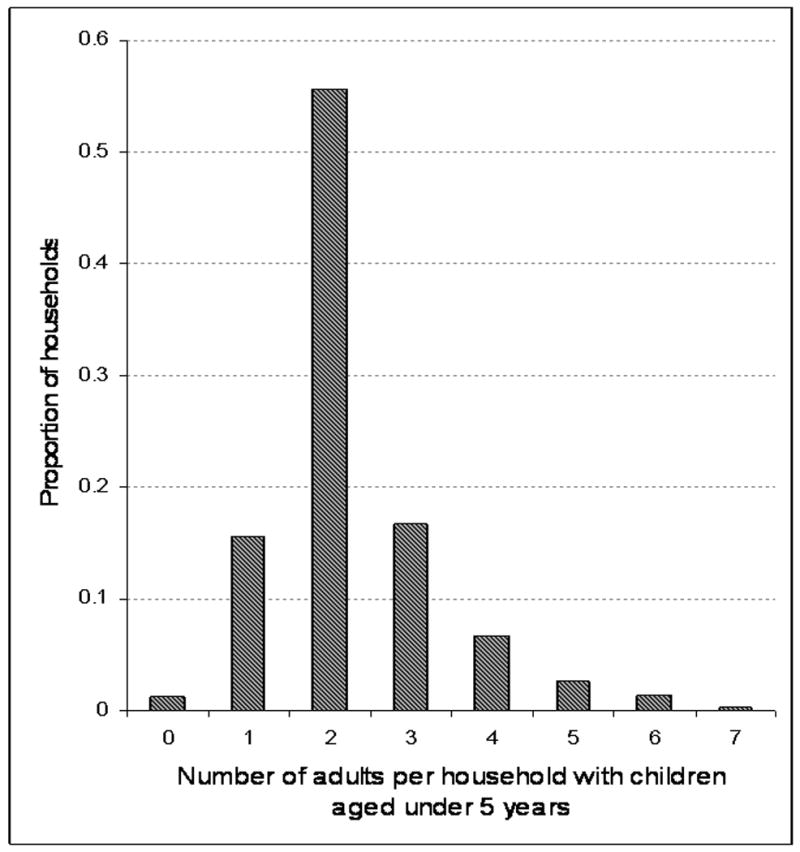
The proportions of households with pre-school children in which different numbers of adults are resident. 2083 adults and 1051 pre-school children were resident in 918 households. Data derived from a 2008 household survey performed in Site M.
Tuberculosis notifications
Between 2004 and 2008, 1,289 cases of TB were notified to the national TB control program of which 90% were in adults and 10% in children 15 years or less. Of the childhood TB, 66% occurred in children less than 5 years of age (table 2). The population increased from 12,803 in 2004 to 14,788 in 2008 resulting in a total of 67,747 person years of residence. The population growth was restricted to adults as the child population less than 5 years remained relatively constant at 1,057 in 2004 and 1,051 in 2008. A mean of 1.35% of the adult population were identified with sputum smear-positive tuberculosis each year (table 2).
Table 2.
The number of tuberculosis notifications in Site-M reported between 2004 and 2008 stratified by age with corresponding population denominators. Tuberculosis rates per 100,000 populations shown together with 95% confidence limits in brackets.
| Total population | Adults >15 years | Children 5-15 years | Children under 5 years | Adult smear positive TB | |
|---|---|---|---|---|---|
| Tuberculosis notifications | 1289 | 1158 | 45 | 86 | 670 |
| Population years of exposure | 67747 | 53056 | 9181 | 5510 | 53056 |
| Tuberculosis rate per 100,000 population | 1909 | 2137 | 546 | 1522 | 1347 |
| [95% confidence limits] | [1799-2018] | [1984-2339] | [346-546] | [1419-1533] | [1108-1437] |
Transmission from resident adults
Ventilation
The modeled impact of increasing the shack ventilation on the probability of a child becoming TB infected is shown for 4 periods of adult infectivity (Δ=30, 60, 90 & 120 days) in figure 2A. Maximal risk of TB transmission even under poor environmental ventilation and prolonged period of adult infectiousness only reached 3%. This maximal condition was primarily determined by the average number of adults resident in the household and their TB incidence rate. Transmission could be reduced by a combination of high ventilation and reduction of infectivity period. For example, a reduction of risk of transmission to 1.5% would require either 4, 8 or 12 ACH for Δ (period of infectiousness) values of 30, 60 and 90 days respectively. Sensitivity analyses with low values of infectious quanta (q=0.1) were unable to reach significant transmission probabilities and values of high infectious quanta (q=10) reached values above 2.75% at all achievable values of Δ and ventilation.
Figure 2A&B.
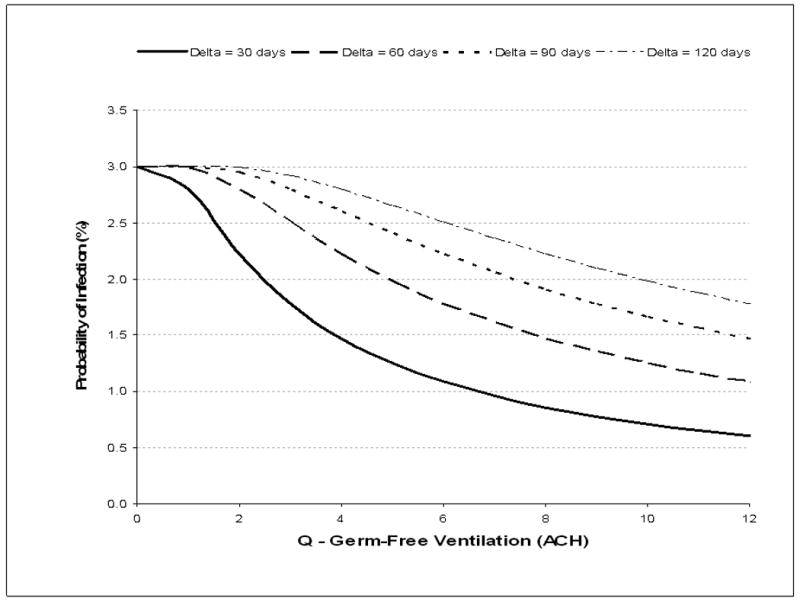
A: The effect of ventilation (air changes per hour, ACH) and mean period of infectivity (delta, Δ) on the mean annual risk of TB infection resulting from a child sleeping in a shack shared with adults. Values are plotted for mean periods of adult infectivity of 30, 60, 90 and 120 days.
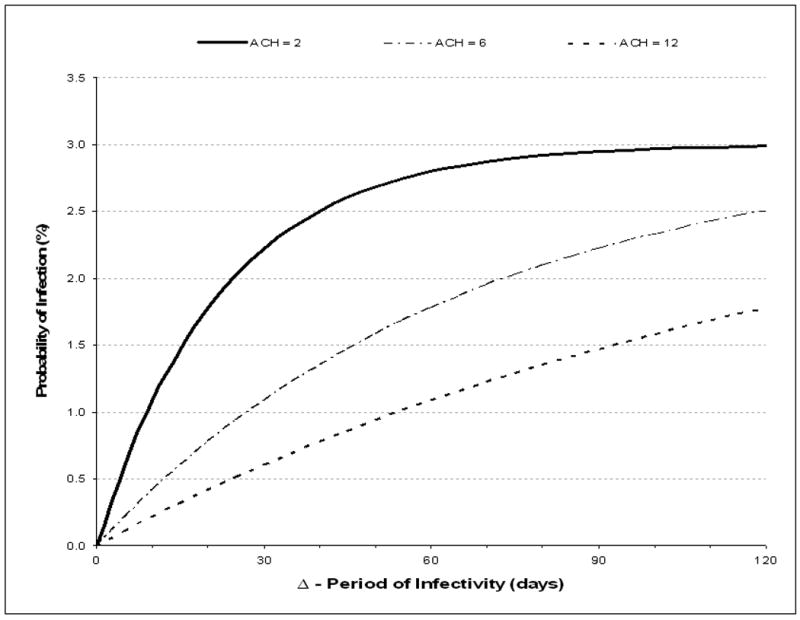
B: The effect of period of infectivity (delta, Δ) and ventilation (air changes per hour, ACH) on the mean annual risk of TB infection resulting from a child sleeping in a shack shared with adults. Values are plotted for 2, 6 and 12 air exchanges per hour (ACH).
N.B. The period of infectivity (delta, Δ) is the mean time from onset of infective tuberculosis until initiation of effective antituberculosis chemotherapy. Modeled estimations are based on a potential night-time exposure of 12 hours, a median of 2.2 adult residents per shack, a 1.35% annual risk for smear-positive tuberculosis and a mean production of 1 infectious air-borne quantum of TB per hour during untreated smear-positive disease.
Infective period
The modeled impact of increasing periods of adult infectiousness (Δ) on the probability of a child less than 5 years of age becoming TB infected is shown for 3 levels of ventilation (2, 6 and 12 ACH) in figure 2B. The benefits of increased ventilation are greatest when Δ is low. Increasing ventilation from 2 to 6 ACH reduces transmission to 2.5% (-16%), to 2.2% (-25%), 1.8% (-36%) and 1.1% (-51%) for Δ of 120, 90, 60 and 30, days respectively. Identical reductions in transmission could be alternatively obtained by reducing the child exposure time by 8 hours per day. A reduction in exposure time could be achieved by separation of child sleeping areas from those of adults for an 8 hour sleeping period. When the modeled infective number of infectious quanta were low (q= 0.1/hour) the transmission probabilities of transmission did not reach 1% and when the number of quanta were high (q=10/hour) the TB transmission probabilities rapidly became maximal at 3% with minimal sensitivity to either increased ventilation or shortened Δ. The modeled proportions of ARTI due to transmission from resident adults were 70% and 74% for periods of infectivity of 60 and 90 days, respectively. Therefore we went on to explore additional transmission (26%-30%) that might occur due to contact with other potentially infective adults in addition to those residents in the home.
Transmission from non-resident adults
The probability of TB infection as a result of spending 75% of daytime indoors and visiting between 1 to 3 households other than child's home is shown for visited household with poor ventilation (2 ACH) in figure 3A. Increasing the number of visited households increased exposure to an additional 2.2 potentially infective adults per household which resulted in greatly increased probabilities of infection when Δ exceeded 30 days. When multiple poorly ventilated households were visited, ARTIs exceeding 4%, 5.5% and 6% could be achieved when Δ was 60, 90 and 120 days respectively. Sensitivity analyses of low infectious quanta (q=0.1) resulted in transmission risks of <1% and high infectious quanta (q=10) resulted in rates of transmission that were directly related to number of households visited for all modeled values of Δ.
Figure 3A&B.
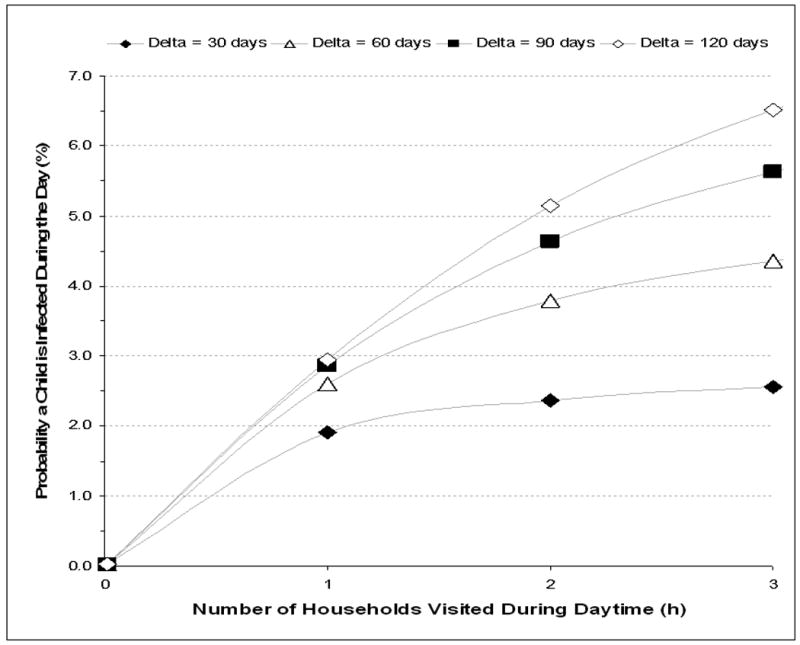
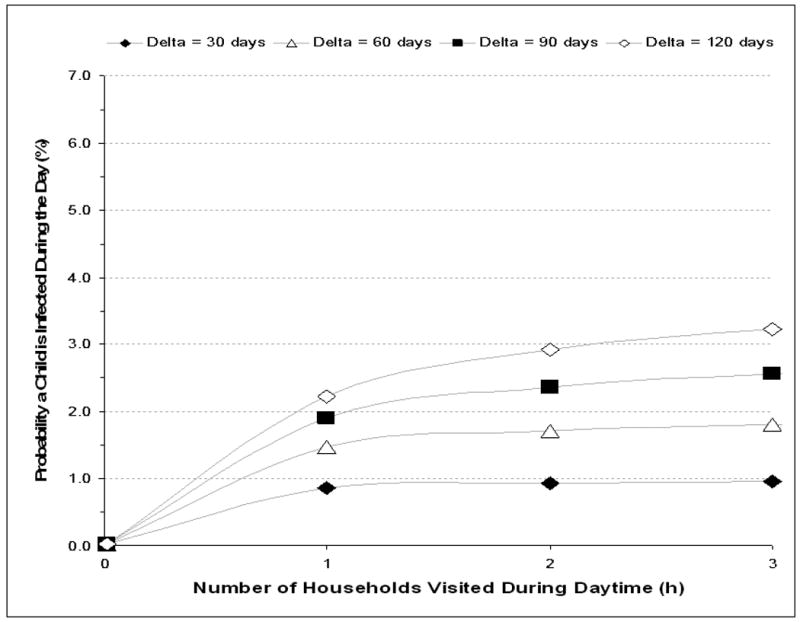
The mean annual risk of TB infection for a child visiting 1 to 3 households other than own residential household during the day each with (Figure A) ventilation of 2 air changes per hour and (Figure B) ventilation of 6 air changes per hour. Values are plotted for mean periods of adult TB infectivity (delta, Δ) of 30, 60, 90 and 120 days.
N.B. The period of infectivity (delta, Δ) is the time from onset of infective tuberculosis until initiation of effective antituberculosis chemotherapy. Modeled estimations are for a pre-school child spending 75% of day-time indoors, a median of 2.2 resident adults per visited shack, a 1.35% annual risk for smear-positive tuberculosis and a mean production of 1 infectious air-borne quantum of TB per hour during untreated smear-positive disease.
In contrast, the corresponding risks for infection when visiting household with 6 ACH showed minimal increase of transmission risk with increasing number of households visited. Under these moderate ventilation conditions Δ became the major determinant of TB transmission risk. Sensitivity analyses of low infectious quanta (q=0.1) resulted in transmission risks of <0.4% and high infectious quanta (q=10) resulted in rates of transmission of 6-9% that were directly related to number of households visited for all values of Δ.
Discussion
These modeling analyses demonstrate that the high reported rates of community TB transmission to children in Southern Africa 9-13 can be explained by the interplay between the prevalence of adult infectious TB, social mixing between adults and children and the prevailing domestic ventilation characteristics.
The model in this study was based on the Wells-Riley equation which has been used to examine airborne TB disease transmission since the 1970s 14 in a wide variety of medical and non-medical settings and thus has been useful in examining the relative importance of transmission factors in real-life situations 15-19. This study used a novel approach of incorporating population data from a specific South African township to populate the Well-Riley equation in order to explore household and non-household TB transmission to pre-school children.
Of particular interest our model indicated that the potential of the existing clinic-based TB control program to reduce transmission to children is somewhat limited. Even reductions in Δ (period of infectiousness) to 30 days by active case finding and rapid TB diagnosis would only have significant impact on transmission to children when ventilation rates in households exceed 6 ACH. However, such high ventilation rates of informal dwellings during cold Cape Town winters might be difficult to achieve throughout the year. As similar reductions in TB transmission could be achieved by separating child and adult sleeping areas, this might be a more practicable stratagem.
Another major finding of our study was that a maximum of 75% of the total annual risk of infection could possibly be explained by the interaction between a child and the limited number of adults resident in a primary household. Pre-school children are susceptible to TB infection predominantly due to exposure to infectious adults 6, 7 and therefore the main determinant of maximal transmission risk in either setting was the number of adults to whom a child was exposed.
Our model also indicated that at least 25% of the risk of infection resulted from exposure to non-resident adults. In well ventilated settings transmission was related to Δ rather than number of households visited. In contrast to transmission risks from adult household residents, transmission from non-resident adults can be markedly influenced by the TB control program's ability to decrease Δ by active case finding. In poorly ventilated non-residence settings transmission risks increased markedly with the numbers of households visited. These analyses indicate that as children become more socially mobile, the potential for transmission in poorly ventilated non-household settings might become the largest contributor to total transmission risk. Indeed, we have reported increasing TB infection rates throughout childhood in this community, which peak at approximately 8% at 15 years of age 11.
The strength of this study was the availability of accurate information specific for this community including the ARTI, number of adults and children per household and smear-positive TB notification rates. A caveat is that some important parameters such as Δ and numbers of infective quanta produced by TB diseased adults are difficult to measure directly and estimates were derived from published data. Indeed, Δ may not be identical to the period of diagnostic delay in published studies and smear-positive TB incidence may only approximate to the smear-positive notification rate. The model used the epidemiologic assumption that the TB epidemic was generalized with equal mixing of infectivity and contact risks. However, stochastic transmission events such as close non-household contact with highly infectious individuals are not captured in this model. Despite these limitations, the outputs from the model were robust and were compatible with the previously observed ARTI in this and similar communities 9-11.
Our findings may give insight to why TB rates of transmission in South Africa have remained very high, despite apparent improvement in case management by the TB control program 1. The conditions within crowded African townships with high unemployment rates may have much in common with those conditions present during the industrial revolution of 18th and 19th centuries when TB burdens were also extremely high. Children lived and worked side by side with adults 25 but successive factory acts in UK and USA reduced the childhood exposure to adults in the workplace 26, 27. Improvement in housing and schooling further reduced close exposures between children and adults. The crèche movement further limited the frequency of contacts between young children and potentially infectious adults 28 and may have potential to decrease non-household transmission in crowded townships. Reduction in household TB transmission in poor informal housing will be difficult to achieve. However, improvement of housing stock should particularly focus on improved ventilation and separation of child from adult sleeping quarters.
These modeled analyses have identified social and environmental factors which contribute to high rates of TB transmission in this community. Social mixing patterns of pre-school children result in TB transmission within the extended rather than nuclear family. Where TB is highly endemic, interruption of community TB transmission requires prompt treatment of source cases. TB control will therefore necessitate an increased focus on active case finding and reduction of diagnostic delays.
Acknowledgments
RW and LGB are funded in part by the National Institutes of Health through a CIPRA grant 1U19AI53217-01 and RO1 grant A1058736-01A1. SDL is funded by the Wellcome Trust, London, UK.
Footnotes
Conflict of Interest Statement: No conflicts of interest to declare
References
- 1.World Health Organization. Epidemiology, strategy, financing. WHO/HTM/TB/2009.411. World Health Organization; Geneva: Global tuberculosis control 2009. [Google Scholar]
- 2.Health Systems Trust. District Health Barometer 2006/2007. [Accessed 16 February 2010]; Available at URL: http://www.hst.org.za/publications/717.
- 3.Wood R, Middelkoop K, Myer L, et al. The burden of undiagnosed tuberculosis in an African community with high HIV-prevalence: implications for TB control. Am J Resp Crit Care Med. 2007;175(1):87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R. Impact of HIV on age-specific tuberculosis notification rates in a peri-urban community in South Africa. Clin Inf Dis. 2006;42:1040–1047. doi: 10.1086/501018. [DOI] [PubMed] [Google Scholar]
- 5.Middelkoop K, Bekker LG, Mathema B, et al. Molecular epidemiology of Mycobacterium tuberculosis in a South African community with high HIV prevalence. JID. 2009;200(8):1207–11. doi: 10.1086/605930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reider HL. Epidemiological basis of Tuberculosis Control. Paris: International Union against Tuberculosis and Lung Disease; 1999. [Google Scholar]
- 7.Opie E, McPhedran FM, Putnam P. The fate of children in contact with tuberculosis: the exogenous infection of children and adults. Am J Hyg. 1935;22:644–682. [Google Scholar]
- 8.van Rie A, Beyers N, Gie RP, Kunneke M, Zietsman L, Donald PR. Childhood tuberculosis in an urban population in South Africa: burden and risk factor. Arch Dis Child. 1999;80:433–437. doi: 10.1136/adc.80.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanaube K, Sismanidis C, Ayles H, et al. Annual Risk of Tuberculous Infection Using Different Methods in Communities with a High Prevalence of TB and HIV in Zambia and South Africa. PLoS One. 2009;4(11):e7749. doi: 10.1371/journal.pone.0007749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Middelkoop K, Bekker LG, Myer L, Dawson R, Wood R. Rates of tuberculosis transmission to children and adolescents in a community with a high prevalence of HIV infection among adults. Clin Infect Dis. 2008;47:349–55. doi: 10.1086/589750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood R, Liang H, Wu H, et al. Changing prevalence of TB infection with increasing age in high TB burden townships in South Africa. Int J Tuberc Lung Dis. 2010 in press. [PMC free article] [PubMed] [Google Scholar]
- 12.Roelsgaard E, Iversen E, Blocher C. Tuberculosis in tropical Africa: an epidemiological study. Bull World Health Organ. 1964;30:459–518. [PMC free article] [PubMed] [Google Scholar]
- 13.Middelkoop K, Bekker LG, Morrow C, Zwane E, Wood R. Childhood tuberculosis infection and disease in a South African township: a spatial and temporal transmission analysis. SAMJ. 2009;99(10):738–43. [PMC free article] [PubMed] [Google Scholar]
- 14.Wells WF. Airborne Contagion and Air Hygiene. Cambridge, MA: Harvard University Press; 1955. [Google Scholar]
- 15.Riley Rl, Wells WF, Mills CC, Nyka W, McLean RL. Air hygiene in tuberculosis: quantative studies of infectivity and control in a pilot ward. Am Rev Tuberc. 1957 Mar;75(3):420–31. doi: 10.1164/artpd.1957.75.3.420. [DOI] [PubMed] [Google Scholar]
- 16.Cantazaro A. Nosochomial tuberculosis. Am Rev Respir Dis. 1982;125:559–562. doi: 10.1164/arrd.1982.125.5.559. [DOI] [PubMed] [Google Scholar]
- 17.Noakes CJ, Sleigh PA. Mathematical models for assessing the role of airflow on the risk of airborne infection in hospital wards. J R Soc Interface. 2009;6:S791–S800. doi: 10.1098/rsif.2009.0305.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nardell EA, Keegan J, Cheney SA, Etkind SC. Airborne infection: theoretical limits of protection achievable by building ventilation. Am Rev Respir Dis. 1991;144:302–306. doi: 10.1164/ajrccm/144.2.302. [DOI] [PubMed] [Google Scholar]
- 19.Furuya H, Nagamine M, Watanabe T. Use of a mathematical model to estimate tuberculosis transmission risk in an Internet café. Environ Health Prev Med. 2009;14:96–102. doi: 10.1007/s12199-008-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The South African Tuberculosis Control Program; Department of Health, South Africa. Practical guidelines 2000. [Accessed 16 February 2010]; Available at URL. http://www.capegateway.gov.za/Text/2003/tb_guidelines2000.pdf.
- 21.WHO; Geneva, Switzerland: 2009. [Accessed 16 February 2010]. WHO Policy on TB Infection Control in Health-Care Facilities, Congregate Settings and Households. Available at URL http://whqlibdoc.who.int/publications/2009/9789241598323_eng.pdf. [PubMed] [Google Scholar]
- 22.Lindament Martin W. Air Infiltration and Ventilation Centre, ECBCS Annex 5, International Energy Agency; [Accessed 13th April 2010]. A guide to energy efficient ventilation. Available at URL http://www.aivc.org. [Google Scholar]
- 23.Marcus CL, Carroll JL, Donnelly DF, Loughlin GM, editors. Sleep and breathing in children: developmental changes in breathing during sleep. Informa Healthcare, USA Inc.; New York, NY 10017, USA: 2008. Published by. [Google Scholar]
- 24.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8(15):1–9. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Making of the English Working Class. Penguin Books; London: 1968. Published 1991. [Google Scholar]
- 26.Cornish WR, de N Clark G. Sweet & Maxwell; London, UK: 1989. [Accessed 16 February 2010]. Law and Society in England 1750-1950. Available at http://www.law.cam.ac.uk/faculty-resources/download/law-and-society-in-england-1750-1950/2624. [Google Scholar]
- 27.United States Department of Labor; [Accessed 16 February 2010]. Child Labor Laws and Enforcement. Youth and Labor. Available at: http://www.dol.gov/dol/topic/youthlabor/childlaborstatistics.htm. [Google Scholar]
- 28.Parker-Rees Rod, Willan Jenny. Early Years Education: Major Themes in Education. Routledge 2, Park Square. Milton Park, Abingdon, Oxon 14 4RN. UK: 2006. The Development of Infant Schools and of Separate Nursery Schools from 1905 to the Present Time. Published. [Google Scholar]


