Abstract
The foxes at Novosibirsk, Russia, are the only population of domesticated foxes in the world. These domesticated foxes originated from farm-bred silver foxes (Vulpes vulpes), whose genetic source is unknown. In this study we examined the origin of the domesticated strain of foxes and two other farm-bred fox populations (aggressive and unselected) maintained in Novosibirsk. To identify the phylogenetic origin of these populations we sequenced two regions of mtDNA, cytochrome b and D-loop, from 24 Novosibirsk foxes (8 foxes from each population) and compared them with corresponding sequences of native red foxes from Europe, Asia, Alaska and Western Canada, Eastern Canada, and the Western Mountains of the USA. We identified seven cytochrome b - D-loop haplotypes in Novosibirsk populations, four of which were previously observed in Eastern North America. The three remaining haplotypes differed by one or two base change from the most common haplotype in Eastern Canada. ΦST analysis showed significant differentiation between Novosibirsk populations and red fox populations from all geographic regions except Eastern Canada. No haplotypes of Eurasian origin were identified in the Novosibirsk populations. These results are consistent with historical records indicating that the original breeding stock of farm-bred foxes originated from Prince Edward Island, Canada. Mitochondrial DNA data together with historical records indicate two stages in the selection of domesticated foxes: the first includes captive breeding for ~50 years with unconscious selection for behaviour; the second corresponds to over 50 further years of intensive selection for tame behaviour.
Keywords: domestication, mitochondrial DNA, phylogeography, red fox, tameness
Introduction
Uncovering the origin of domesticated species is a subject of wide interest, and has numerous practical implications in fields such as agriculture and evolutionary biology (Diamond 1999). Identification of the progenitor stock and comparison with the domesticated form allows analysis of the genetic, physiological, morphological and behavioural impacts of domestication (Trut 1999). An experimental population of domesticated silver foxes, Vulpes vulpes, a colour variant of the red fox, has been developed and maintained at the Institute of Cytology and Genetics of the Russian Academy of Sciences (ICG) in Novosibirsk since 1959 (Trut 1999). These animals were domesticated from farm bred foxes whose wild source is unknown.
The red fox has the widest geographic distribution of all Carnivora species, occurring naturally throughout the Northern Hemisphere; in North Africa, Europe, Asia and North America and, via introduction in Australia (Macdonald and Reynolds, 2004). Since the early 20th century the red fox has also become a common fur-farm animal in North America and Eurasia.
Red fox fur farming was pioneered on Prince Edward Island (PEI) in Southeastern Canada, beginning in the 1890s (Westwood 1989). Most of the original breeding stock for the fur farming industry came from PEI, and included locally caught foxes supplemented with those imported from southern Alaska (Balcom 1916; Laut 1921). Fur farmers on PEI primarily raised the silver/black colour variant of red foxes, which had the greatest economic value and were subsequently used to stock fur farms in many areas of North America and Eurasia (Petersen 1914; Westwood 1989; Nes et al., 1988). Given the high price of PEI foxes, it is possible that silver foxes from other indigenous populations were used to supplement breeding stock.
In Russia, fox farming started in the early 20th century (Generozhov, 1916; Zhaharov 1995). Small farms that maintained a few breeding pairs of local foxes or foxes captured in other regions of Russia were organised in different geographical regions, from European Russia (West of the Ural Mountains) through to Yakutia in the East (Zhaharov, 1995; Bespyatih, 2009). In the 1920s, a population of Canadian foxes and foxes of Canadian descent were imported to Russia and Baltic countries (Vahrameyev and Belyaev, 1948; Bespyatih, 2009). After World War II fox farming grew into a large industry in the former Soviet Union; among the 505,000 pelts produced worldwide in 1990, 359,000 were produced in the USSR (Bespyatih, 2009). The importation of Canadian foxes to the USSR was not well documented in the literature, but most reliable publications state that the Russian commercial silver fox population is mostly of Canadian descent (Vahrameyev and Belyaev, 1948). There are also records that indicate an introduction of local foxes into the commercial breeding stock. A unique coat colour variant “Огневк а В я т с к а я: [“Ognevka Vyatskaya”] was developed by breeding foxes with a bright red coat colour from the Kamchatka Peninsula to the standard farm-bred silver foxes (Vohmyanin, 1981; Bespyatih, 2009).
Farm-bred foxes usually show fear or a fear-aggressive response to humans. Experimental domestication of farm-bred foxes was started by Dmitry Belyaev and Lyudmila Trut at the Institute of Cytology and Genetics of the Russian Academy of Sciences, in Novosibirsk, Russia, in the late 1950s. This experiment known as “the farm-fox experiment” has been reviewed in several publications (Trut, 1999; 2001; Trut et al., 2005, 2009). Belyaev and Trut visited multiple farms across the former Soviet Union and identified a subset of commercial foxes that showed less fearful and aggressive responses to humans. These 130 foxes were brought to the experimental farm at the ICG and became the founders of the experimental population. Selection of foxes for tame behaviour was strict, with less than 10% of foxes bred to produce the next generation. At the same time, deliberate efforts were made to avoid inbreeding. A relatively low inbreeding level (0.02–0.07) has been maintained over the course of the project (Trut, 1999; Kukekova et al., 2004). The current tame fox population comprises over 300 breeding animals that show friendly responses to humans similar to that of domestic dogs: http://cbsu.tc.cornell.edu/ccgr/behaviour/Index.htm (Trut 1999, 2001; Trut et al., 2004, 2009; Kukekova et al., 2008).
In the 1970s, the ICG also started selection for an aggressive strain of foxes. Because there is deliberate selection on commercial farms against animals that show aggressive responses to humans, selection of the aggressive strain at ICG aimed to preserve this behaviour. Fifty farm-bred silver foxes with the most aggressive responses to humans were selected from several fox farms and used as founders of the aggressive population. The current aggressive population comprises ~130 breeding foxes that are aggressive and difficult to handle (Trut et al., 2009, Kukekova et al., 2004, 2008). The third fox population (unselected for behaviour) maintained at ICG originated from several commercial fox farms, some of which were the same source farms of the founders for the tame and aggressive strains.
The genetic origin of Russian farm-bred foxes has never been investigated at the molecular level. Such analysis would provide information about the history of the population and the gene pool or pools from which they stem. This information would be particularly important for studies aiming to identify regions of the fox genome implicated in fox domestication.
A recent analysis of two mitochondrial DNA regions found that red fox populations in Northern Eurasia and North America are divided into two distinct clades estimated to have diverged approximately 400 kya (Aubry et al., 2009). The Holarctic clade extends from Europe to Asia and across the Bering Strait into Alaska and Western Canada, while the Nearctic clade is restricted to North America (Figure 1). These clades of foxes were separated during the Pleistocene in disjunct glacial refugia. Within the Holarctic clade there is division into two subclades separated by the Bering Sea, one predominating in Alaska and Western Canada, the other in Eurasia. The Nearctic clade is subdivided into three subclades, with the Mountain subclade predominating in the Western Mountains of the USA, and the Eastern subclade dominating in Eastern Canada, while the Widespread subclade is older and more widely dispersed (Aubry et al., 2009). This phylogenetic framework can be used to determine the geographic source of populations of unknown origin.
Figure 1.
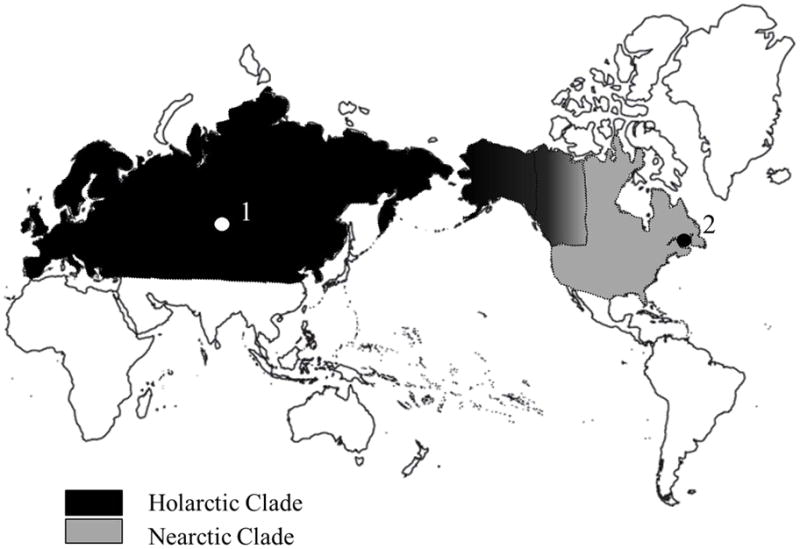
Clade breakdown of red fox lineages in Northern Eurasia and North America based on data from Aubry et al., 2009. The Nearctic clade is restricted to North America, while the Holarctic clade stretches from Europe, through Northern Asia, into Alaska and Western Canada. The zone of contact between Nearctic and Holarctic lineages in Western Canada is indicated. Point 1 is Novosibirsk, Russia; point 2 is Prince Edward Island, Canada.
The primary aim of this study was to determine the genetic source of the Novosibirsk Silver Foxes, which may be descendant from indigenous Russian foxes, and/or from animals of more distant origins. Secondarily, we examined the mitochondrial genetic diversity of this population which has been divided into three closed breeding units since the 1970s. These aims were addressed by comparing genetic sequence data of Novosibirsk silver foxes to that of native red fox populations in Northern Eurasia and North America.
Methods
Samples
Three fox populations: tame, aggressive, and unselected for behaviour are maintained at the experimental farm of the Institute of Cytology and Genetics (ICG) at the Russian Academy of Sciences, in Novosibirsk, Russia. Using pedigree information, we selected 8 foxes from each population that do not have mothers or grandmothers in common for population genetic analysis (Novosibirsk total n = 24). Animal care and use at the ICG is in compliance with Russian law regarding laboratory animals.
Laboratory procedures
We extracted DNA from blood samples collected from the Novosibirsk populations using the Qiagen Maxi Blood kit (Qiagen, CA). We amplified the 5’ region of the cytochrome b gene using primer pair RF14724 and RF15149 (Perrine et al., 2007), and the D-loop using primer pair VVDL1 and VVDL6 (Aubry et al., 2009). PCR products were purified and sequenced as described previously (Perrine et al., 2007; Aubry et al., 2009; Sacks et al., 2010).
Data analyses
To assess geographic origins we compared haplotypes obtained in this study to previously published references sequences from putative source populations. These data included a 354 bp cytochrome b (n = 220) and 342 bp D-loop (n = 174) haplotypes from Europe (Germany, Italy, Spain, and Sweden), Asia (China, Mongolia, and Eastern Siberia), Alaska and Western Canada, Eastern Canada (Manitoba, Newfoundland and Labrador, Ontario, and Quebec) and the Western Mountains of the USA (Cascade Range, Sierra Nevada and Rocky Mountains) (Perrine et al., 2007; Aubry et al., 2009). Additionally, we included all homologous portions of sequences from all published Eurasian haplotypes (Frati et al., 1998, Inouye et al., 2007) and those available in GenBank to evaluate possible Eurasian ancestry.
We estimated haplotype diversity (h) and nucleotide diversity (π) (Nei 1987) using Arlequin 3.1 (Excoffier et al., 2005) and DnaSP 4.50 (Rozas et al., 2003). Relationships among haplotypes were determined using a median-joining network (Bandelt et al., 1999) within Network 4.2.0.1 (www.fluxus-engineering.com). We estimated the extent of genetic differentiation among populations using ΦST (Nei and Li 1979) in Arlequin 3.1. This statistic is similar to FST but takes into account the divergence between haplotype sequences. Statistical significance (α = 0.05) was determined based on 1,000 permutations, and then corrected for multiple tests using the sequential Bonferroni method (Rice 1989).
Results
We identified four distinct cytochrome b haplotypes in the Novosibirsk population, three of which had previously been identified in native populations of Eastern Canada (Table 1). We identified seven distinct D-loop haplotypes, two of which (haplotypes 85 and 86) were novel (Table 1, EMBL/Genbank/DDBJ nucleotide database Accession No. HM461967-8). The cytochrome b and D-loop data, when taken together, gave 7 composite haplotypes (Table 2).
Table 1.
Occurrence of 4 cytochrome b haplotypes (354 bp) and 7 D-loop haplotypes (342 bp) among foxes from Novosibirsk and their prevalence in native red fox populations in North America and Northern Eurasia.
| Population | Cytochrome b haplotype | D-loop haplotype | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | A | E | F | G | n | 9 | 12 | 17 | 63 | 73 | 85 | 86 | |
| Novosibirsk Aggressive | 8 | - | 1 | 7 | - | 8 | 1 | - | 6 | - | - | 1 | - |
| Novosibirsk Tame | 8 | - | 2 | 5 | 1 | 8 | 1 | - | 4 | - | 1 | 1 | 1 |
| Novosibirsk Unselected | 8 | 1 | 4 | 3 | - | 8 | 1 | 1 | 2 | 1 | - | - | 3 |
| Europe | 6 | - | - | - | - | 8 | - | - | - | - | - | - | - |
| Asia | 21 | - | - | - | - | 13 | - | - | - | - | - | - | - |
| Alaska, Western Canada | 112 | - | - | 2 | 59 | 77 | 3 | 1 | - | 3 | 14 | - | - |
| Eastern Canada | 26 | 3 | - | 19 | 2 | 26 | 8 | 2 | 5 | 5 | 1 | - | - |
| Western Mountains | 55 | 31 | - | - | - | 50 | - | - | - | - | - | - | - |
Europeconsists of Germany, Italy, Spain and Sweden.
Asia consists of China, Mongolia and Russia.
Eastern Canadaconsistsof Manitoba, Newfoundland, Ontario and Quebec
Western Mountains consists of populations from the western Mountains of the USA, the Rocky Mountains, the Sierra Nevada and Cascade Range
Table 2.
Occurrence of 7 combined cytochrome b and D-loop haplotypes (696 bp) among foxes from Novosibirsk. The clade/subclade to which the haplotypes belong is indicated.
| Population | Total | Combined Cytochrome b and D-loop Haplotypes | ||||||
|---|---|---|---|---|---|---|---|---|
| A-63 | E-9 | E-86 | F-12 | F-17 | F-85 | G-73 | ||
| Cladea | Nea | Nea | Nea | Nea | Nea | Nea | Hol | |
| Subcladeb | Wide | East | East | East | East | East | Alas | |
| Novosibirsk Aggressive | 8 | - | 1 | - | - | 6 | 1 | - |
| Novosibirsk Tame | 8 | - | 1 | 1 | - | 4 | 1 | 1 |
| NovosibirskUnselected | 8 | 1 | 1 | 3 | 1 | 2 | - | - |
| Novosibirsk | 24 | 1 | 3 | 4 | 1 | 12 | 2 | 1 |
Nea = Nearctic clade; Hol = Holarctic clade
Wide = Widespread subclade of North America; East = Eastern subclade of North America; Alas = Alaska and Western Canada subclade. The Widespread subclade and the Eastern subclade are both part of the Nearctic clade. The Alaska and Western Canada subclade is part of the Holarctic clade.
Phylogenetic analyses
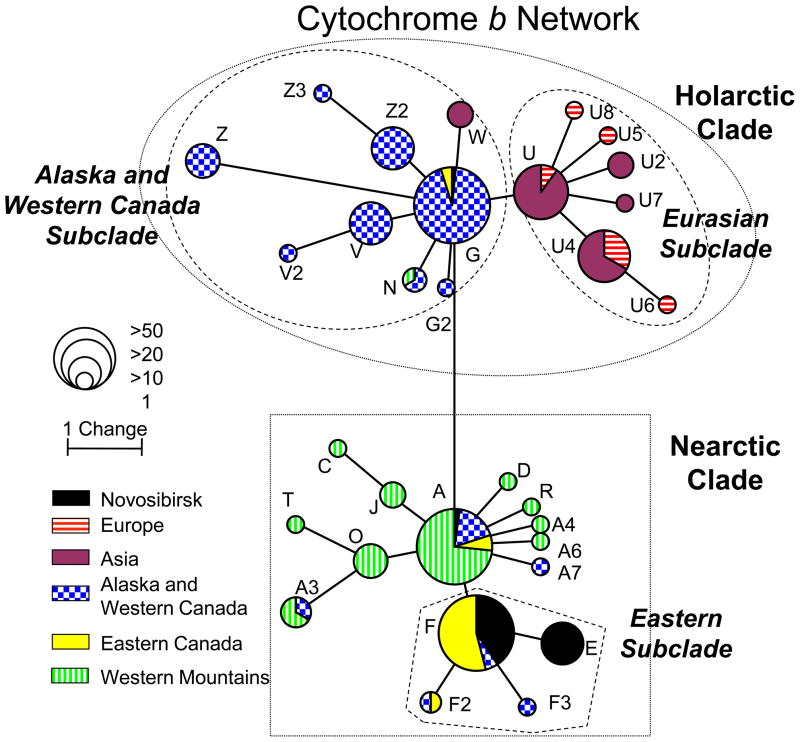
Most (92%, 22 of 24) Novosibirsk red foxes had haplotypes belonging to the Eastern subclade, within the Nearctic clade, which predominates in Eastern Canada (Table 2). One haplotype in particular, F-17 (i.e. cytochrome b haplotype F, and D-loop haplotype 17), composed half of the sample and occurred in all 3 Novosibirsk populations. This haplotype has previously been identified in Newfoundland and Labrador, in Eastern Canada (Aubry et al., 2009). Haplotype F-12 was previously found in Manitoba, Canada. Other Eastern subclade haplotypes identified in the Novosibirsk population differed by either 1 base (E-9, F-85), or 2 bases (E-86) from the most common haplotype in Eastern Canada. Haplotype G-73, part of the Holarctic clade, was previously found in several different Canadian provinces and territories from the Yukon in Western Canada through to Quebec in the East. Haplotype A-63, part of the Widespread subclade (within the Nearctic clade), has also been found at relatively high prevalence in many of the same areas. We found no Eurasian haplotypes, or haplotypes that clustered with Eurasian haplotypes, among the Novosibirsk red foxes.
Pairwise ΦST values based on both the cytochrome b and D-loop datasets indicated that the Novosibirsk population was not significantly differentiated from the Eastern Canada population, but was significantly and substantially differentiated from all other populations (Table 3).
Table 3.
Pairwise ΦST values between Novosibirsk and native red fox populations in North America and Northern Eurasia. Values in the first row are based on the cytochrome b dataset, values in the second row on the D-loop dataset. Asterisks indicate statistical significance (P < 0.05) based on sequential Bonferroni correction for multiple tests (Rice 1989).
| Population | Europe | Asia | Alaskaa | Easternb | Mountainsc |
|---|---|---|---|---|---|
| Novosibirsk Cytochrome b | 0.86* | 0.84* | 0.68* | 0.05 | 0.50* |
| Novosibirsk D-loop | 0.73* | 0.62* | 0.54* | 0.03 | 0.58* |
Alaska and Western Canada
Eastern Canada (Manitoba, Newfoundland and Labrador, Ontario, and Quebec)
the western mountains of the USA (Cascade Range, Sierra Nevada and Rocky Mountains).
The Novosibirsk population as a whole had haplotype (h) and nucleotide (π) diversities slightly lower, but on the same order of magnitude, as most of the native reference populations (Table 4). When individual lines of Novosibirsk foxes were considered, the aggressive line had the lowest level of diversity. None of the Novosibirsk lines were significantly differentiated (based on ΦST or FST) from each other.
Table 4.
Within-population statistics for Novosibirsk and native red fox populations in North America and Northern Eurasia based on mitochondrial cytochrome b and D-loop datasets.
| Population | Cyt b | Cyt b # | Cyt b | Cyt b | D-loop | D-loop # | D-loop | D-loop |
|---|---|---|---|---|---|---|---|---|
| n | Haplotypes | h | π | n | Haplotypes | h | π | |
| Novosibirsk Aggressive | 8 | 2 | 0.25 | 0.0007 | 8 | 3 | 0.46 | 0.0020 |
| Novosibirsk Tame | 8 | 3 | 0.61 | 0.0047 | 8 | 5 | 0.78 | 0.0088 |
| Novosibirsk Unselected | 8 | 3 | 0.68 | 0.0023 | 8 | 5 | 0.86 | 0.0076 |
| Novosibirsk | 24 | 4 | 0.54 | 0.0026 | 24 | 7 | 0.73 | 0.0070 |
| Europe | 6 | 4 | 0.80 | 0.0034 | 8 | 6 | 0.89 | 0.0109 |
| Asia | 21 | 5 | 0.74 | 0.0032 | 13 | 5 | 0.73 | 0.0132 |
| Alaska, Western Canada | 112 | 13 | 0.69 | 0.0055 | 78 | 23 | 0.91 | 0.0136 |
| Eastern Canada | 26 | 5 | 0.46 | 0.0032 | 26 | 7 | 0.83 | 0.0096 |
| Western Mountains | 55 | 11 | 0.61 | 0.0027 | 50 | 16 | 0.85 | 0.0110 |
Discussion
Identifying the Parent Population of Russian Silver Foxes
Our analysis clearly and unambiguously identifies Eastern Canada as the primarily, if not sole, source of ancestry for the farm-bred red fox populations maintained in Novosibirsk, Russia. Four of the seven combined cytochrome b/D-loop haplotypes found had previously been identified in Eastern Canada, including in samples collected in the 1800s prior to the advent of fur farming (Aubry et al., 2009). The remaining haplotypes were all part of the eastern subclade and were closely related to the most common haplotype in Eastern Canada. No Eurasian haplotypes were identified in the Novosibirsk population, and pairwise ΦST analysis showed significant differentiation between Novosibirsk populations and red fox populations from all geographic regions studied except Eastern Canada.
These findings are consistent with historical records indicating that the red fox fur farming industry in Russia and elsewhere traces back to the first successful fur farms in Southeastern Canada (Petersen 1914; Balcom 1916; Laut 1921; Vahrameyev and Belyaev, 1948; Westwood 1989). Further, because the coat-colour genes associated with silver morphs are indigenous to North America, it is to be expected that at least some of the ancestry of the Novosibirsk foxes was North American. It is noteworthy that we found no Eurasian haplotypes in the Novosibirsk population given that indigenous Russian red foxes were used to supplement fur-farm stock in some areas (e.g. for particular crosses; Vohmyanin, 1981; Bespyatih, 2009). Although our findings suggest Novosibirsk matrilines descend directly from North American founders, the use of biparentally inherited nuclear markers or Y chromosome markers will be necessary to determine if male Eurasian red foxes have contributed to the Novosibirsk gene pool.
Genetic Diversity
Our findings indicate that the efforts to avoid inbreeding at Novosibirsk were largely successful. The genetic diversity of the Novosibirsk population as a whole is substantially higher than that of farmed arctic foxes (Vulpes lagopus) from Norway, Sweden, and Finland where only a single D-loop haplotype was identified in a sample of 41 individuals (Norén et al., 2005). Interestingly, in a parallel situation to the Novosibirsk populations, the haplotype found in these farmed arctic foxes was not found in wild native Fennoscandian populations and likely came from a distant geographic source population. The genetic diversity of the Novosibirsk population was similar to that of the wild Eastern Canadian source population (Table 4), covering a vast geographic area spanning 4 Canadian provinces (Manitoba, Newfoundland and Labrador, Ontario, Quebec, [Aubry et. al., 2009]). Within individual lines, two haplotypes (A-63 and F-12) were restricted to the unselected population and one (G-73) was restricted to the tame. The absence of unique haplotypes in the aggressive population, along with the lower haplotype and nucleotide diversities, reflects the smaller number of founders and smaller total number of individuals maintained in this line. Considering the relatively small size (n < 1000 breeding animals), and the number of years since they became closed breeding units, a considerable degree of mitochondrial genetic diversity has been maintained in the Novosibirsk populations.
Our study indicates the common ancestry of all Novosibirsk fox populations. Two combined cytochrome b and D-loop haplotypes, F-17 and E-9, were found in all three Novosibirsk populations, making up over half of all individuals sampled. In addition, 3 of the cytochrome b haplotypes (E, F and G) found in the Novosibirsk populations were also found in a recently established red fox population likely stemming from farmed animals in southern California (Perrine et al., 2007, Sacks et al., 2010). The results of our analyses suggest that farm-bred foxes maintained in Novosibirsk descend from the foxes that were bred in captivity for over 100 years.
Further genetic analyses
With the growth of genomic technologies and reduction of sequencing cost it seems reasonable to expect that the red fox genome sequence and single nucleotide polymorphism (SNP) map will become available in the next few years. These resources will allow phylogenetic analysis of fox populations with nuclear gene markers and analysis of genetic divergence between the tame and aggressive fox strains. Genome wide association mapping across dog breeds has successfully been used for identification of loci and genes for breed specific traits (Jones et al., 2008; Chase et al., 2009; Akey et al., 2010; Boyko et al., 2010). SNP analysis of the dog and wolf-like wild canids identified regions in the dog genome demonstrating signatures of positive selection of dogs from wolves (vonHoldt et al., 2010). Similar analysis can be applied to identify selective sweeps in the fox genome produced by long-term farm-breeding. The genome wide association mapping of tame and aggressive strains maintained in Novosibirsk and pedigrees produced by cross-breeding of the two strains (Kukekova et al., 2010) will facilitate identification of genomic regions implicated in these behavioral phenotypes.
Conclusions
Identification of the origin of the Novosibirsk fox populations and estimation of the populations’ ages allows better understanding of the selection process that led to the development of the tame, dog-like Novosibirsk foxes. Mitochondrial DNA data together with historical records indicate two stages in the selection of domesticated foxes: an initial period of ~50 years of captive breeding in fur farms with conscious selection for fur quality and unconscious selection for behaviour, followed by a second 50 years of intensive selection for tame behaviour carried out at the ICG in Novosibirsk since 1959 (Trut, 1999; 2001). Understanding the phylogeographic origins of experimental populations is critical. Failure to do so can lead to spurious conclusions about selection. For example in a biometic study of aortic branches in red foxes the author compared native Polish red foxes with Polish fur farmed foxes and found significant differences between farmed and wild groups (Nowicki 2005). The author attributed this differentiation to the effects of captive breeding, without knowledge of the potential confounding effects of the considerable phylogenetic differentiation we have found in our study. Understanding the history of this population will be highly advantageous for on-going studies focused on the identification of genetic loci and genes implicated in domesticated behaviour in foxes and other species.
Figure 2.
Cytochrome b median-joining network based on 354 bp for 24 Novosibirsk and 220 native North American and Eurasian red fox specimens. Branch lengths are proportional to the number of substitutions, and circle sizes are proportional to the number of individuals represented. Clades are indicated with dotted lines and subclades with dashed lines.
Acknowledgments
We are grateful to Irina V. Pivovarova, Tatyana I. Semenova, Vasiliy V. Ivaykin, Vera I. Vladimirova, Tatyana V. Konovalova, Vera L. Haustova, and all the animal keepers at the ICG experimental farm for research assistance. Thank you to A. Statham for proof reading. Research was supported by NIH grants MH077811, EY006855, an NIH FIRCA R03TW008098, grants # 05-04-4837 and # 06-04-48142 from the Russian Fund for Basic Research, Program of the Russian Academy of Sciences: “Biodiversity and Genome Dynamics”. Partial funding was also provided by the Veterinary Genetics Laboratory at the University of California, Davis; California Department of Fish and Game (Agreements Nos. P0780029, S0810020 and subcontract No. HBSDF12); and the UC Davis Center for Population Biology. Editor-in-Chief J. Allen and three anonymous reviewers for their input on a previous version of this manuscript.
References
- Akey JM, Ruhe AL, Akey DT, Wong AK, Connelly CF, Madeoy J, Nicholas TJ, Neff MW. Tracking footprints of artificial selection in the dog genome. Proc Natl Acad Sci U S A. 2010;19;107(3):1160–5. doi: 10.1073/pnas.0909918107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry KB, Statham MJ, Sacks BN, Perrine JD, Wisely SM. Phylogeography of the North American red fox: vicariance in Pleistocene forest refugia. Mol Ecol. 2009 Jun;18(12):2668–86. doi: 10.1111/j.1365-294X.2009.04222.x. [DOI] [PubMed] [Google Scholar]
- Bandelt HJ, Forster P, Röhl A. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- Bespyatih OY. The consequences of amber acid feeding in different genotypes of farm-bred foxes. VOGIS (Russian) 2009;13(3):639–646. [Google Scholar]
- Boyko AR, Quignon P, Li L, Schoenebeck JJ, Degenhardt JD, Lohmueller KE, Zhao K, Brisbin A, Parker HG, vonHoldt BM, Cargill M, Auton A, Reynolds A, Elkahloun AG, Castelhano M, Mosher DS, Sutter NB, Johnson GS, Novembre J, Hubisz MJ, Siepel A, Wayne RK, Bustamante CD, Ostrander EA. A simple genetic architecture underlies morphological variation in dogs. PLoS Biol. 2010;10;8(8):e1000451. doi: 10.1371/journal.pbio.1000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase K, Jones P, Martin A, Ostrander EA, Lark KG. Genetic mapping of fixed phenotypes: disease frequency as a breed characteristic. J Hered. 2009 Jul–Aug;100(Suppl 1):S37–41. doi: 10.1093/jhered/esp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. Guns, Germs, and Steel. New York: Norton Press; 1999. [Google Scholar]
- Excoffier L, Laval G, Schneider S. Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- Jones P, Chase K, Martin A, Davern P, Ostrander EA, Lark KG. Single-nucleotide- polymorphism-based association mapping of dog stereotypes. Genetics. 2008;179(2):1033–44. doi: 10.1534/genetics.108.087866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frati F, Hartl GB, Lovari S, Delibes M, Markov G. Quaternary radiation and genetic structure of the red fox Vulpes vulpes in the Mediterranean Basin, as revealed by allozymes and mitochondrial DNA. Journal of Zoology. 1998;245:43–51. [Google Scholar]
- Generozhov VY. Farm breeding of silver foxes and arctic foxes in North America. Petrograd. 1916 [Google Scholar]
- Inoue T, Nonaka N, Mizuno A, Morishima A, Sato H, Katakura K, Oku Y. Mitochondrial DNA Phylogeography of the Red Fox (Vulpes vulpes) in Northern Japan. Zoological Science. 2007;24:1178–1186. doi: 10.2108/zsj.24.1178. [DOI] [PubMed] [Google Scholar]
- Kukekova AV, Trut LN, Chase K, Kharlamova AV, Johnson JL, Temnykh SV, Oskina IN, Gulevich RG, Vladimirova AV, Klebanov S, Shepeleva DV, Shikhevich SG, Acland GM, Lark KG. Mapping loci for fox domestication: deconstruction/reconstruction of a behavioral phenotype. Behavior Genetics. 2010 December; doi: 10.1007/s10519-010-9418-1. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukekova AV, Trut LN, Chase K, Shepeleva DV, Vladimirova AV, Kharlamova AV, Oskina IN, Stepika A, Klebanov S, Erb HN, Acland GM. Measurement of segregating behaviors in experimental silver fox pedigrees. Behav Genet. 2008 Mar;38(2):185–94. doi: 10.1007/s10519-007-9180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukekova AV, Trut LN, Oskina IN, Johnson JL, Temnykh SV, Kharlamova AV, Shepeleva DV, Gulievich RG, Shikhevich SG, Graphodatsky AS, Aguirre GD, Acland GM. A meiotic linkage map of the silver fox, aligned and compared to the canine genome. Genome Res. 2007 Mar;17(3):387–99. doi: 10.1101/gr.5893307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukekova AV, Trut LN, Oskina IN, Kharlamova AV, Shikhevich SG, Kirkness EF, Aguirre GD, Acland GM. A marker set for construction of a genetic map of the silver fox (Vulpes vulpes) J Hered. 2004 May–Jun;95(3):185–94. doi: 10.1093/jhered/esh033. [DOI] [PubMed] [Google Scholar]
- Macdonald DW, Reynolds JC. Red Fox. In: Sillero-Zubiri C, Hoffmann M, Macdonald DW, editors. Canids: Foxes, Wolves, Jackals and Dogs. IUCN; 2004. pp. 129–136. Total number of pages 430. [Google Scholar]
- Nei M. Molecular evolutionary genetics. Columbia University Press; New York: 1987. [Google Scholar]
- Nei M, Li WH. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proceedings of the National Academy of Sciences, USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nes N, Einarsson EJ, Lohi O. Beautiful Fur Animals and Their Colour Genetics. Scientifur. 1988:271. [Google Scholar]
- Norén K, Dalén L, Kvaløy K, Angerbjörn A. Detection of farm fox and hybrid genotypes among wild arctic foxes in Scandinavia. Conservation Genetics. 2005;6:885–894. [Google Scholar]
- Nowicki W. Comparison of biometric characters of aorta branches in farm and wild fox (Vulpes vulpes L.) Folia biol (Kraków) 2005;53 (Suppl):35–38. [Google Scholar]
- Perrine JD, Pollinger JP, Sacks BN, Barrett RH, Wayne RK. Genetic evidence for the persistence of the critically endangered Sierra Nevada red fox in California. Conservation Genetics. 2007;8:1083–1095. [Google Scholar]
- Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Rozas J, Sanchez-Delbarrio JC, Messeguer X, Rozas R. DnaSP DNA polymorphism analysis by the coalescent and other methods. Bioinformatics. 2003;19:2496–2497. doi: 10.1093/bioinformatics/btg359. [DOI] [PubMed] [Google Scholar]
- Sacks BN, Statham MJ, Perrine JD, Wisely SM, Aubry KB. North American montane red foxes: expansion, fragmentation, and the origin of the Sacramento Valley red fox. Conservation Genetics. 2010;11:1523–1539. [Google Scholar]
- Trut LN. Early Canid domestication: The Farm Fox Experiment. American Scientist. 1999;87:160–169. [Google Scholar]
- Trut LN. The Genetics of the Dog. CABI; 2001. Experimental Studies of Early Canid Domestication; pp. 15–43. [Google Scholar]
- Trut LN, Pliusnina IZ, Oskina IN. An experiment on fox domestication and debatable issues of evolution of the dog. Genetika (Russ) 2004;40:794–807. [PubMed] [Google Scholar]
- Trut L, Oskina I, Kharlamova A. Animal evolution during domestication: the domesticated fox as a model. Bioessays. 2009 Mar;31(3):349–60. doi: 10.1002/bies.200800070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Våge DI, Lu DS, Klungland H, Lien S, Adalsteinsson S, Cone RD. A non-epistatic interaction of Agouti and extension in the fox, Vulpes vulpes. Nat Genet. 1997;15:311–315. doi: 10.1038/ng0397-311. [DOI] [PubMed] [Google Scholar]
- Vahrameyev KA, Belyaev DK. Guide for fox breeding (Russian) International Book; Moscow: 1948. p. 103. [Google Scholar]
- Zhaharov VP. Fur production and trade in Yakutia (end of XIC – beginning of XX century) Novosibirsk. 1995. p. 136. [Google Scholar]