Abstract
To assess the role of Fas in lesion development during genital HSV-2 infection, we used a well-established HSV-2 murine model applied to MRL-Faslpr/J (Fas−/−) and C3-Faslgld/J (FasL−/−) C57BL6 mice. In vitro infection of murine keratinocytes and epithelial cells was used to clarify molecular details of HSV-2 infection. Despite upregulation of Fas and FasL, HSV-2-infected keratinocytes and epithelial cells showed a moderate level of apoptosis due to upregulated expression of the anti-apoptotic factors Bcl-2, Akt kinase and NF-κB. Inflammatory lesions within the HSV-2-infected epithelium of C57BL6 mice consisted of infected cells upregulating Fas, FasL and Bcl-2, uninfected cells upregulating Fas and neutrophils expressing both Fas and FasL. Apoptosis was detected in HSV-2-infected cells and to even higher extent in non-infected cells surrounding HSV-2 infection sites. HSV-2 infection of Fas- and FasL-deficient mice led to increased apoptosis and stronger recruitment of neutrophils within the infection sites. We conclude that the Fas pathway participates in regulation of inflammatory response in the vaginal epithelium at the initial stage of HSV-2 infection.
Keywords: HSV-2, apoptosis, Fas/FasL
Herpes simplex viruses (HSV) type 1 and 2 are among the DNA viruses interfering at multiple levels with cellular apoptotic processes important for cellular response to virus infection.1 Moderate level of apoptosis were however observed in HSV-2-infected epithelial cells, in contrast to HSV-1-infected cells, which displayed consistent levels of apoptosis.2 HSV-2-mediated apoptosis occurs during HSV-2 infection both in vivo – in dorsal root ganglia and spinal cord pituitary gland 3– and in vitro – in peritoneal macrophages,4 monocytes,5 dendritic cells6 and human peripheral blood mononuclear cells (PBMCs).7 Other studies, however, have concluded that HSV-2 can prevent apoptosis in a manner similar to HSV-1 and that the viral US3 protein kinase8 and the ribonucleotide reductase9 participate in blocking of the apoptotic process. Furthermore, it was shown that HSV-2 ICP10PK protein, which has a role in establishment of virus latency and reactivation, inhibits caspase-3 activation through its ability to activate Ras and the downstream survival pathways Raf-1/MEK/ERK and PI3-K/Akt.9, 10
Clinical and laboratory HSV-2 strains both induced, and subsequently blocked, apoptosis in infected HEp-2 epithelial cells and these biological features were correlated with NF-kB translocation to the nuclei of HSV-2-infected cells.11 Infection of human keratinocyte cell line HaCaT with HSV-1 and HSV-2 led to a significant increase of apoptosis,12 an event depending on the p63 protein, involved in the maintenance of stratified epithelial tissues. Together, these observations suggest that whether HSV-2 infection leads to apoptotic cell death or not, depends on the cell type.
The Fas/FasL system has an important role in skin and epithelial homeostasis, carcinogenesis and inflammatory skin diseases.13, 14 Epidermal keratinocytes express Fas but not FasL.15 Abnormal expression of lytically active FasL was however found in inflammatory skin diseases as toxic epidermal necrolysis, atopic dermatitis and allergic contact dermatitis.13, 14, 16 FasL elicited a proinflammatory reaction in human keratinocyte HaCaT cell line and reconstructed human epidermis by triggering the expression of stress-responsive transcription factors and inflammatory cytokines in absence of an apoptotic response.17
HSV-2, but not HSV-1, was shown to inhibit cell surface expression of FasL in PBMCs and K562 leukemic cell line.18 However, in vivo clearance of HSV-2 infection in a murine model requires Fas and perforin-dependent cytotoxic mechanisms, as mice lacking both perforin- and Fas-mediated cytolytic mechanisms were unable to completely clear the infection from the vaginal tissue.19 The role of Fas/FasL system in the vaginal epithelium may thus not only be limited to the elimination of HSV-2 infected cells via apoptosis but also related to the development of inflammatory lesions preceding the induction of the local immune response to the infection.
We assessed the role of Fas during HSV-2 vaginal infection using keratinocytes and epithelial cells in vitro and a murine model of genital herpes. In vitro, HSV-2-infected cells upregulated Fas and FasL and anti-apoptotic proteins (Bcl-2, NF-κB, Akt kinase) and displayed moderate levels of apoptosis only during the later stage of infection. Upregulation of Fas, FasL and Bcl-2 was noticed in the HSV-2-infected epithelium of C57BL/6 mice. In the mice, the cells undergoing apoptosis were infected cells present at the site of the lesions and uninfected, Fas-expressing, epithelial cells surrounding the HSV-2-infected sites. Experiments performed in HSV-2-infected Fas (−/−) and FasL (−/−) C57BL mice showed a reduced level of apoptosis among uninfected cells and an increased infiltration of neutrophils at the vaginal site. Our results suggest a role for Fas/FasL in the regulation of inflammatory responses at the early stages of vaginal HSV-2 infection.
Results
HSV-2 infection induces moderate levels of apoptosis in epithelial cells and keratinocytes
A significantly higher number of cells, with caspase-3-specific cytokeratin 18 cleavage (M30-positive cells), was found in HSV-2-infected mouse keratinocytes (291.03C) and epithelial cells (Hepa 1–6) at 24 and 48 h postinfection (p.i.) in comparison with uninfected cultures (P≤0.05) (Figure 1a). Interestingly, in spite of the fact that both infected epithelial and keratinocyte cultures underwent apoptosis, the total cell number in these cultures was comparable to the control cell cultures (Figure 1b).
Figure 1.
(a) Kinetics of total percentage of M30-positive (apoptotic) cells in epithelial Hepa 1–6 and keratinocyte 03C cell cultures during 48 h after infection with HSV-2, (b) the total cell number in cultures of HSV-2-infected epithelial cells and keratinocytes (at 24 h p.i.) and in control, uninfected cultures, (c) the total percentage of HSV-2-infected cells in epithelial and keratinocyte cell cultures at 24 and 48 h p.i., (d) double-positive M30/HSV-2 cells in HSV-2-infected epithelial and keratinocyte cultures during 48 h of infection with HSV-2. (e) Percentage of M30-positive cells in epithelial Hepa 1–6 and keratinocyte 03C cell cultures subjected to ST (5 μM), infected or not, with HSV-2 for 18 or 22 h (including 4 h infection, before staurosporine treatment) at MOI=1. Each bar represents the mean of five independent experiments (N=5)±S.E.M.
At 24 h p.i. (HSV-2 MOI 1–10), the number of HSV-2-infected cells reached approximately 80% of cells in culture (Figure 1c). Of these, 48.23±2.34% of HSV-2-infected epithelial cells and 45.89±4.01% of HSV-2-infected keratinocytes showed apoptotic features at 24 h p.i. (Figure 1d). At 48 h p.i., the population of HSV-2-infected epithelial cells, corresponding to 90% of cells in culture (Figure 1c), included 56.55±6.78% of apoptotic cells (Figure 1d), whereas HSV-2-infected keratinocytes included 34.93±1.29% of apoptotic cells (P≤0.05) (Figure 1d). These results show that at 48 h of infection, the rate of apoptosis in keratinocyte cultures decreased and in the culture remained a population of HSV-2-infected, non-apoptotic cells. HSV-2-infected epithelial cells and keratinocytes (Figure 1e) were not susceptible to staurosporine (ST)-induced apoptosis if the treatment with ST was initiated later than 4 h p.i., thus indicating that HSV-2 induces an apoptosis-resistant status in the infected epithelial cells and keratinocytes within hours from infection through the induction of anti-apoptotic proteins.
Taking into account that HSV-2-infected cells of epithelial and neuronal origin activate anti-apoptotic mechanisms dependent on Bcl-2-family and NF-κB and PI3-K/Akt pathways,11, 20 we assessed the expression of Bcl-2, NF-κB and Akt in HSV-2-infected epithelial cells and keratinocytes. We found that Bcl-2 was significantly upregulated in both types of cells during the entire tested period in comparison with uninfected cells (P≤0.05) (Figure 2a). In our experimental conditions, both HSV-2-infected cell lines showed a significant increase in NF-κB activation, especially at 6 h of infection, in comparison with uninfected cells (P≤0.05) (Figure 2b). Between 6 and 18 h p.i., NF-κB activation decreased to levels close to baseline (P≤0.05) (Figure 2b). Similarly, a significantly increased activity of the anti-apoptotic Akt kinase (P≤0.05) occurred in HSV-2-infected epithelial and keratinocyte cultures (Figure 2c).
Figure 2.
Relative kinetics of Bcl-2 expression (a) and levels of active NF-κB (b) in epithelial Hepa 1–6 and keratinocyte 03C cell cultures infected with HSV-2. (c) Percentage of cells with active, phosphorylated form of Akt in HSV-2-infected epithelial Hepa 1–6 and keratinocyte 03C cell cultures. The presented values for HSV-2-infected cultures were subtracted from the values obtained at the same time point for the uninfected cultures. Each bar represents the mean from five experiments (N=5)±S.E.M.
HSV-2-infected epithelial cells and keratinocytes upregulate Fas and FasL but do not die through this receptor pathway
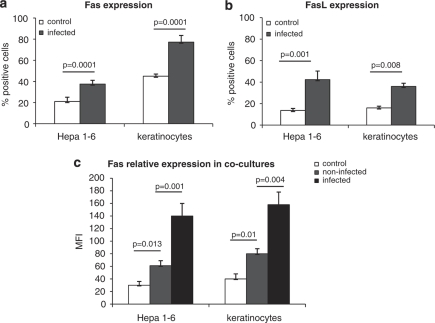
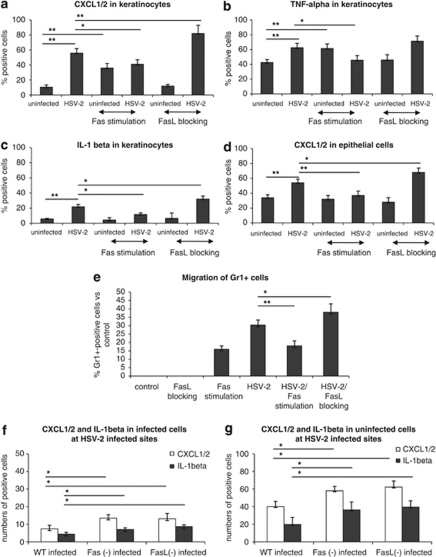
Upon infection with HSV-2, mouse keratinocyte and epithelial cell cultures significantly upregulated both Fas and FasL (P≤0.05) (Figures 3a and b). To assess whether HSV-2-infected cells may influence Fas expression on uninfected cells, we used HSV-2 at MOI 0.1, which resulted in 50% of HSV-2-infected cells and 50% of non-infected cells in culture. The intensity of Fas expression was not only higher for HSV-2-infected cells (P=0.001) but also increased for non-infected cells at 24 h p.i. (P=0.013) (Figure 3c). A similar picture was also observed for keratinocytes, with a significant increase in Fas expression on both non-infected (P=0.01) and infected cells (P=0.004) (Figure 3c).
Figure 3.
Percentage of Fas- (a) and FasL- (b) expressing cells in epithelial Hepa 1–6 and keratinocyte 03C cell cultures infected with HSV-2 24 h p.i. at MOI=1. Intensity of Fas staining (c) in HSV-2-infected cells and non-infected cells present in epithelial Hepa 1–6 and keratinocyte 03C cell cultures infected with HSV-2 at 24 h p.i. (MOI=0.1). Each bar represents mean from five experiments (N=5)±S.E.M.
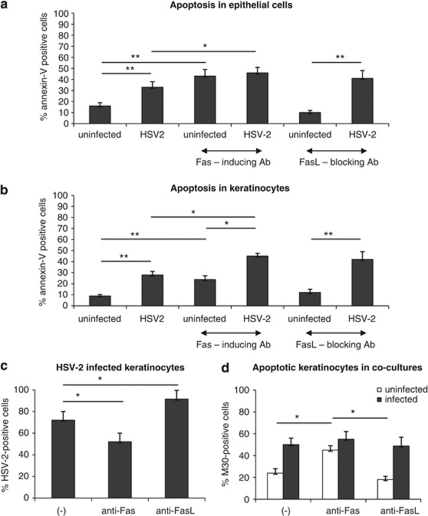
To assess the sensitivity of HSV-2-infected epithelial cells and keratinocytes to Fas-induced apoptosis we used an anti-mouse Fas cytotoxic antibody (Jo-1 clone). In both the Hepa 1–6 epithelial cell cultures and in keratinocytes, addition of the anti-Fas antibody to uninfected cultures resulted in an increased percentage of annexin V-positive cells after 24 h (P<0.001) (Figures 4a and b). Addition of anti-Fas antibody to HSV-2-infected cultures also significantly increased Fas-mediated apoptosis (P<0.05) (Figures 4a and b). FasL blocking antibody (clone MFL-4) did not decrease the numbers of apoptotic cells in HSV-2-infected epithelial cells and keratinocytes (Figures 4a and b).
Figure 4.
Percentage of annexin V-positive cells (apoptotic cells) in epithelial Hepa 1–6 (a) and keratinocyte 03C (b) cell cultures subjected to cytotoxic Fas antibody (10 μg/ml) and FasL blocking antibody (10 μg/ml), infected or not, with HSV-2 for 24 h at MOI=1. (c) Percentage of HSV-2-infected keratinocytes at 24 h p.i. with MOI=1, in presence of cytotoxic anti-Fas antibody (10 μg/ml) or FasL blocking antibody (10 μg/ml). (d) Percentage of M30-positive cells (apoptotic cells) at 24 h after infection with MOI=0.1, in presence of cytotoxic anti-Fas antibody (10 μg/ml) or FasL blocking antibody (10 μg/ml) – cells were divided into infected and uninfected keratinocytes. Each bar represents the mean from five experiments (N=3)±S.E.M. *Represents significant differences with P≤0.05, whereas **means P≤0.001
Fas/FasL system has an important role in apoptosis of keratinocytes in the skin,14 and keratinocytes are the main target cells for both HSV-1 and HSV-2.21 Therefore, we used the anti-mouse Fas cytotoxic antibody Jo-1 to study the involvement of Fas in apoptosis of HSV-2-infected and uninfected keratinocytes. Addition of the anti-Fas antibody led to a significant decrease in HSV-2-infected cells at 24 h of infection (P=0.02) (Figure 4c), whereas addition of anti-FasL blocking antibody (MFL-4) caused a significant increase of HSV-2-positive cells (P=0.032) (Figure 4c). To verify whether the decrease in HSV-2-infected cells occurring upon incubation with the cytotoxic anti-Fas antibody could result from bystander apoptosis of uninfected cells, we measured apoptosis in cultures infected with HSV-2 at MOI 0.1, when only 50% of cells were infected at 24 h p.i. (Figure 4d). Although more HSV-2-infected cells underwent apoptosis in the cultures incubated with anti-Fas and anti-FasL antibodies, this difference was not statistically significant (Figure 4d). On the contrary, in presence of cytotoxic anti-Fas antibody, more uninfected cells were apoptotic in comparison with untreated control cells and cells treated with FasL blocking antibody (P≤0.05) (Figure 4d).
Fas is involved in the development of inflammatory mucosal lesions in vivo
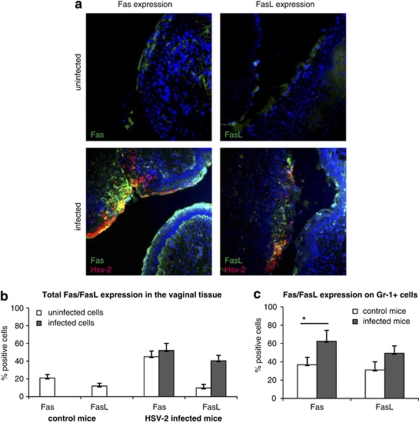
During HSV-2 infection of C57BL/6 mice, the vaginal mucosa is subjected to development of lesions, compromising its integrity and ability to protect from the virus attack.22 In our experiments, HSV-2-infected sites were present within the vaginal epithelium of C57BL/6 mice at 3 days of infection. Staining of the vaginal tissues showed that HSV-2-infected cells in the epithelium upregulated both Fas and FasL (Figures 5a and b), whereas non-infected cells surrounding the HSV-2-infected cells upregulated only Fas (Figures 5a and b). Fas and FasL expressions were also upregulated on Gr-1-positive cells (neutrophils), with only Fas showing a significant increase (P=0.01) (Figure 5c). In HSV-2-infected sites, apoptotic cells, defined as TUNEL-positive, were found not only among HSV-2-positive cells but also among non-infected cells (Figure 6a).
Figure 5.
(a) Fas and FasL expression (green) and HSV-2 antigen localization (red) in the vaginal tissue isolated from C57BL/6 mice at 3 days of HSV-2 infection. The nuclei were counterstained with Hoechst 33342 (blue). (b) Total percentage of Fas- and FasL-expressing cells from the vaginal tissue of control and HSV-2-infected mice and (c) percentage of Fas- and FasL-positive Gr-1-positive cells in cell suspensions obtained from C57BL/6 mice at 3 days of HSV-2 infection. Each bar represents the mean from three experiments (N=3)±S.E.M. *Represents significant differences with P≤0.05
Figure 6.
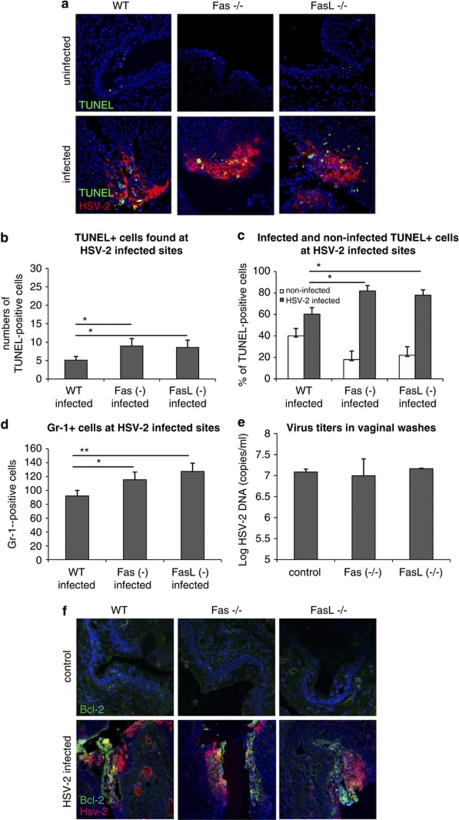
(a) Detection of apoptosis by TUNEL staining (green) and HSV-2 antigen localization (red) in the vaginal tissues from HSV-2-infected Fas (−/−), FasL (−/−) and WT (C57BL/6) mice at 3 days of infection. The nuclei were counterstained with Hoechst 33342 (blue). (b) Numbers of TUNEL-positive cells per 100 cells found at infected sites in the HSV-2-infected Fas (−/−), FasL (−/−) and WT (C57BL/6) mice. (c) Percentage of infected and uninfected TUNEL-positive cells in HSV-2-infected sites of Fas (−/−), FasL (−/−) and WT (C57BL/6) mice shown as a percentage of the total numbers of TUNEL-positive cells. (d) Mean numbers of Gr-1-positive cells (neutrophils) found in the inflammatory foci in the HSV-2- infected Fas (−/−), FasL (−/−) and WT (C57BL/6) mice. (e) HSV-2 DNA titers (copies/ml) in vaginal lavages obtained from HSV-2-infected Fas (−/−), FasL (−/−) and WT (C57BL/6) mice at day 2 of infection. (f) Bcl-2 expression (green) and HSV-2 antigen localization (red) in the vaginal tissue isolated from HSV-2-infected Fas (−/−), FasL (−/−) and WT (C57BL/6) mice at 3 days of infection. The nuclei were counterstained with Hoechst 33342 (blue). The bars represent mean from three separate experiments (N=3)±S.E.M. *Represents significant differences with P≤0.05, **P≤0.001
To estimate the role of Fas and FasL in the development of vaginal epithelium lesions induced by HSV-2, we infected mice with either a knockout in Fas gene (B6.MRL-Faslpr/J) or a knockout in FasL gene (B6Smn.C3-Faslgld/J) together with the control background strain (C57BL/6). The control, uninfected Fas (−/−) mice presented with a thin vaginal epithelium, consisting of densely packed cells, whereas uninfected FasL (−/−) mice showed no progesterone-dependent thinning of the epithelium (Figure 6a). In both Fas and FasL knockout mice, the HSV-2-infected epithelium was shedding off (Figure 6a) and apoptotic bodies could be found at all inflammatory sites (Figure 6a). A significantly higher number of TUNEL-positive cells were, however, found at the HSV-2-infected sites in Fas- and FasL-deficient mice (Figure 6b). The comparison of Fas- and FasL-deficient mice revealed that Fas (−/−) and FasL (−/−) mice showed more infected TUNEL-positive cells at the HSV-2-infected sites (Figure 6c). In Fas and FasL knockout mice, a significant increase in infiltrating Gr-1-positive cells (neutrophils) was found within the HSV-2-infected epithelium, in comparison with infected wild-type (WT) mice (P≤0.05) (Figure 6d).
A PCR method was used to measure DNA titers in vaginal lavages collected at day 2 of infection in mice; the results showed no differences in the titers of HSV-2 shed into the vaginal lumen in WT and Fas- and FasL-deficient mice (Figure 6e). To verify whether Bcl-2 expression is affected by the depletion of Fas and FasL, we stained for Bcl-2 during HSV-2 infection of the knockout mice. The stainings detected a common pattern of Bcl-2 expression in the middle part of the infection sites in all infected mice strains (Figure 6f).
Fas is involved in the regulation of keratinocyte inflammatory response in vitro
To assess whether stimulation through the Fas/FasL pathway influences the induction of inflammatory responses by HSV-2-infected keratinocytes and epithelial cells, we evaluated the expression of CXCL1/2 chemokines, TNF-α and IL-1β cytokines. HSV-2 infection of keratinocytes in vitro led to a significant upregulation of CXCL1/2, TNF-α and IL-1β expression at 18 h of infection (P≤0.001) (Figures 7a–c), whereas HSV-2-infected epithelial cells upregulated CXCL1/2 (Figure 7d), TNF-α and IL-1β (data not shown). Addition of anti-Fas cytotoxic antibody to control uninfected keratinocytes led to a significant upregulation of CXCL1/2 and TNF-α expression at 18 h of incubation (P≤0.001) (Figures 7a and b). Interestingly, addition of the cytotoxic anti-Fas antibody to HSV-2-infected keratinocyte and epithelial cultures significantly abrogated CXCL1/2 expression, whereas, upon these conditions, TNF-α and IL-1β expression was abrogated only in keratinocytes (P≤0.05, Figures 7a–d).
Figure 7.
Percentage of keratinocytes expressing CXCL1/2 (a), TNF-α (b), IL-1β (c) and epithelial cells expressing CXCL1/2 (d) in control and HSV-2-infected keratinocyte cell cultures at 18 h p.i. exposed or not to anti-Fas cytotoxic antibody (10 μg/ml) and anti-FasL blocking antibody (10 μg/ml). (e) Transwell migration assay with control and HSV-2-infected keratinocyte cell cultures at 18 h p.i. exposed or not to anti-Fas cytotoxic antibody (10 μg/ml) and anti-FasL blocking antibody (10 μg/ml). (f) Numbers of infected CXCL1/2 and IL-1β positive cells per 100 cells found at infected sites in the HSV-2-infected Fas (−/−), FasL (−/−) and WT (C57BL/6) mice at 3 days of infection (g) Numbers of uninfected CXCL1/2 and IL-1β-positive cells per 100 cells found at infected sites in the HSV-2-infected Fas (−/−), FasL (−/−) and WT (C57BL/6) mice at 3 days of infection. Each bar represents the mean from three experiments (N=3)±S.E.M. *Represents significant differences with P≤0.05, whereas **P≤0.001
To analyze how the blocking of Fas/FasL interaction influences CXCL1/2, TNF-α and IL-1β expression in HSV-2-infected keratinocyte, we used an anti-FasL blocking antibody. The anti-FasL blocking antibody showed no influence on CXCL1/2, TNF-α and IL-1β expression in uninfected keratinocytes and epithelial cells (Figures 7a–d). The biological effect of the anti-FasL blocking antibody was significantly different in HSV-2-infected cells. In fact, upon blocking of FasL, the HSV-2-infected keratinocytes significantly upregulated CXCL1/2 and IL-1β expression (P≤0.05), but not TNF-α (Figures 7a–c). HSV-2-infected epithelial cells incubated with anti-FasL blocking antibody significantly upregulated only CXCL1/2 (P≤0.05) (Figure 7d).
To verify whether the inflammatory cytokines and chemoattractants produced from keratinocytes infected and/or stimulated with the cytotoxic anti-Fas antibody exerted a chemotactic role for migration of neutrophils into HSV-2-infected vaginal tissue, we allowed neutrophils to migrate toward keratinocytes subjected to different stimuli (Figure 7e). The transwell experiment showed that Fas stimulation of uninfected and HSV-2-infected keratinocytes induced a significant migration of Gr-1-positive cells, in comparison with migration toward control, uninfected cells (P≤0.001) (Figure 7e). FasL blocking antibody significantly upregulated migration toward HSV-2-infected cells (P≤0.05), whereas Fas-stimulating antibody significantly decreased migration toward HSV-2-infected keratinocytes (P≤0.001) (Figure 7e).
Furthermore, staining for CXCL1/2 and IL-1β of the vaginal tissues from HSV-2-infected WT, Fas- and FasL-deficient mice showed a significantly increased numbers of both infected and uninfected cells expressing CXCL1/2 (Figures 7f and g) and IL-1β (Figures 7f and g) in Fas- and FasL-deficient mice (P≤0.05).
Discussion
The goal of this study was to investigate the role of Fas pathway during in vitro HSV-2 infection of mouse keratinocyte and epithelial cells and during in vivo HSV-2 vaginal infection of mice. In our study, both epithelial cells and keratinocytes underwent moderate apoptosis upon HSV-2 infection but not earlier than 12 h p.i. because of the induction of anti-apoptotic pathways early during infection. Indeed, we could observe NF-κB induction already at 6 h p.i. in both cell lines together with upregulated expression of the anti-apoptotic factor Bcl-2. Decreased NF-κB levels could be observed before apoptosis induction, remaining at low levels throughout the follow-up time of infection in both types of infected cell cultures. Activation of the NF-κB transcription factor is one of the anti-apoptotic mechanisms operating in epidermis and epithelial cells.11, 14, 23 Observations similar to the findings presented in this paper were made during infection with HSV-1, whose genome is collinear with HSV-2.24, 25
PI3K/Akt signaling is important in apoptosis suppression of epithelial cells and keratinocytes.14, 26 In HSV-2-infected cultures of epithelial cells and keratinocytes, we detected an increase in cells positive for the active form of Akt kinase, thus implying a role for this kinase in apoptosis suppression during the later stages of HSV-2 infection in vitro. The involvement of Akt in protection from apoptosis was previously shown for HSV-2-infected neurons27 and during HSV-1 infection of human epithelial HEp-2 cells.28 Bcl-2 overexpression increased the capacity of HSV-2-infected monocytoid cells to sustain a fully productive infection, while protecting against HSV-2-induced apoptosis.29 In our experiments, Bcl-2 expression was upregulated during the initial 12 h of infection, to remain at a stable, low level in the analyzed period. The induction of anti-apoptotic genes, during early events of HSV-2 infection of epithelial cells and keratinocytes, was verified by experiments conducted in presence of the proapoptotic substance ST.
It was previously shown30 that human epithelial HEp-2 cells infected with HSV-1 were resistant to Fas-induced apoptosis. In our study, HSV-2-infected murine epithelial cells and keratinocytes upregulated Fas and FasL. This event should render the HSV-2-infected cells sensitive to apoptosis delivered either by FasL present on neighboring cells or through an autocrine pathway (Figure 8). However, a closer analysis showed that induction of apoptosis in the infected epithelial and keratinocytes cultures by a cytotoxic Fas antibody involved a large portion of uninfected cells, which also upregulated Fas, therefore leading to a decrease in the number of potential target cells for HSV-2 infection; this process could be diverted by the addition of FasL blocking antibody. These results show that HSV-2-infected cells are relatively resistant to Fas-induced apoptosis and that cell death of these cells likely occurs through other pathways. Fleck et al.4 also showed that, although murine macrophages upregulated Fas and TNFR1 receptors upon HSV-2 infection, inhibition of Fas by addition of soluble Fas and blocking of TNF-α did not prevent HSV-2-induced apoptosis. In the human T-cell line Jurkat, HSV-2 induced apoptosis through extrinsic (death receptors) and intrinsic (mitochondrial) pathways.31 Therefore, it is likely that mitochondrial pathway may also be involved in apoptosis during HSV-2 infection of epithelial cells and keratinocytes.
Figure 8.
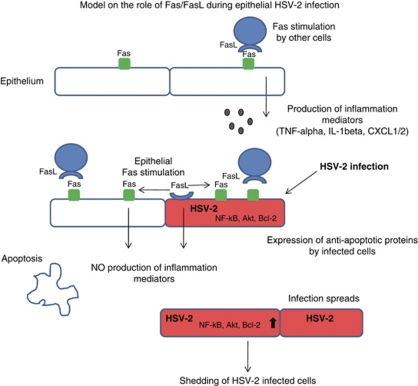
Schematic model on the role of Fas/FasL pathway in regulation of local inflammation during HSV-2 infection of epithelium. Upon stimulation by FasL-expressing cells, Fas-expressing cells respond by producing proinflammatory cytokines. Autocrine upregulation of FasL by epithelial cells, however, appears to block the effects of extrinsic Fas stimulation. HSV-2 infected cells, although upregulating Fas and FasL expression gain an apoptosis resistant status due to upregulation of anti-apoptotic proteins (Bcl-2, NF-κB, Akt kinase)
The role of Fas/FasL in induction of apoptosis is generally recognized.32 However, evidence accumulates on Fas as a mediator of apoptosis-independent processes, including proliferation, angiogenesis, fibrosis and inflammation.33 In cells resistant to Fas-induced apoptosis, activation of Fas was shown to induce activation of PI3K/AKT pathway, ultimately leading to cell migration.34 Therefore, it is possible that keratinocytes may possess the ability to activate non-apoptotic pathways through Fas receptor.
In our experimental model, consisting of murine keratinocyte 03C cells, we demonstrated an increased expression of CXCL1/2, TNF-α and IL-1β in HSV-2-infected keratinocytes. Interestingly, the expression of CXCL1/2 and TNF-α also increased significantly when uninfected keratinocytes were stimulated with the cytotoxic Fas antibody showing their ability to mount a proinflammatory response mediated through the Fas-dependent pathway (Figure 8). However, the combination of HSV-2 infection and paracrine stimulation of the Fas pathway led to downregulation of the proinflammatory response. On the contrary, blocking of FasL during HSV-2 infection of keratinocytes led to upregulation of CXCL1/2 and IL-1β responses. The production of the inflammatory cytokines and chemoattractants was reflected in the migration of Gr1-positive cells toward infected keratinocytes, in that both HSV-2 infection and anti FasL blocking antibody were important mediators for this phenomenon. Furthermore, the HSV-2-infected sites in Fas- and FasL-deficient mice showed a stronger expression of CXCL1/2 and IL-1β in comparison with HSV-2-infected WT mice, indicating the role of Fas/FasL in regulation of the inflammatory responses within the vaginal mucosa (Figure 8).
CXCL1 and CXCL2 expression is upregulated in the vagina and in the CNS of mice during acute HSV-2 infection.35 These chemokines specifically targets neutrophils through the CXCR2 receptor, promoting their chemotaxis and activation.35 IL-1β and TNF-α facilitate the recruitment of neutrophils to inflammatory sites and TNF-α stimulates differentiation of natural killer (NK) cells, a cell population critical in the control of genital HSV-2 infection.36, 37
Vaginal lesions developed during HSV-2 infection destroy the integrity of the local mucosa and lead to increased infiltration of different types of innate immune cells, including NK cells, macrophages and neutrophils. In our study we considered the possibility that Fas/FasL system may be directly involved in the development of vaginal lesions, as part of apoptosis induction following HSV-2 infection of mice. The HSV-2-infected epithelial cells upregulated Fas, FasL and also Bcl-2 in vivo. These findings, together with the increased infiltration of neutrophils in Fas- and FasL-deficient mice, suggest that HSV-2-infected cells within the epithelium may be resistant to apoptosis and that Fas/FasL interactions may serve as a mechanism to reduce inflammation (Figure 8).
Depletion of Fas and FasL did not lead to significant changes of HSV-2 titers in the vaginal fluid. It is, however, possible that Fas-dependent pathway may still influence the amounts of virus retained within the tissue and affect its latency in the ganglia. In this respect, Ishikawa et al.38 examined HSV-2 lethal infection in Fas- or FasL-deficient mice. Both the latter types of mice exhibited higher mortality than WT C57BL/6 mice after HSV-2 infection and showed significantly increased viral titers in the spinal cord compared with WT mice. However, the study38 did not assess vaginal histology.
Lack of Fas and FasL in knockout mice increased the numbers of apoptotic-infected cells found at the infection sites, but decreased the numbers of uninfected cells undergoing apoptosis. However, in the deficient mice, the sites without any visible infiltration of neutrophils corresponded to sites with scarce or absent apoptotic cells; on the contrary, the sites with infiltration showed significantly higher amounts of apoptotic cells in comparison with similar sites found in HSV-2-infected WT mice. Neutrophils are the early and predominant innate immune cells, which may contribute to virus clearance through the release of the antiviral cytokines TNF-α, IFN-α, IFN-γ, GM-CSF or oxygen and nitrogen metabolites.37 However, neutrophil-induced tissue damage by release of oxidants and proteases seen during inflammation can be controlled in part by neutrophil apoptosis,39 in which Fas/FasL pathway have an important role.39 Our study shows that lack of Fas and FasL in HSV-2-infected mice resulted in lack of control of the local inflammation induced by neutrophils, facilitating for apoptosis induction. Fas/FasL pathway in the vaginal mucosa appears to participate in the regulation of the local inflammation. Upon stimulation with extrinsic source of FasL (like NK cells, cytotoxic T cells, etc.), Fas-expressing cells produce proinflammatory cytokines, promoting the recruitment of neutrophils, NK cells and macrophages. However, the upregulation of FasL by epithelial cells appears to block the effects of extrinsic Fas stimulation, probably functioning as a self-regulatory mechanism. Fas and FasL upregulation by HSV-2 infected cells appears to have the role of protecting the virus from the effects of immune competent cells infiltrating the HSV-2 lesion, thus ensuring virus survival and further spreading (Figure 8).
Materials and Methods
Virus
The HSV-2 strain 333 was grown and titrated in African green monkey kidney cells (GMK-AH1) and prepared by one cycle of freeze–thaw and subsequent removal of cellular debris by centrifugation.40
Cell lines and in vitro HSV-2 infection
The mouse epithelial Hepa 1–6 cells and mouse keratinocyte cell line 291.03C were kindly provided by M Kulesz-Martin (Department of Dermatology, Oregon Health and Science University, Portland, OR, USA). Hepa 1–6 cells were maintained in Dulbecco's modified Eagle medium (Thermo Fisher Scientific, Lafayette, CO, USA) with 5% glucose, 10% fetal bovine serum (FBS) and 1% antibiotics (Thermo Fisher Scientific). The 291.03C line is a 7,12-dimethylbenz[a]anthracene-initiated clone, derived from non-transformed 291 cells41 and was cultured in DMEM supplemented with 5% FBS (Thermo Fisher Scientific), 10 ng/ml epidermal growth factor (Sigma, St. Louis, MO, USA) and 1% antibiotic (Sigma). The cell lines were infected with HSV-2 333 strain at MOI=10 or 0.1, incubated for up to 48 h and then collected by trypsinization or scratching. The cells were further stained with antibodies for external and internal antigens.
Apoptosis detection
Early apoptosis was detected using Annexin V-Apoptosis detection kit I (BD Biosciences, Franklin Lakes, NJ, USA), according to the producer's protocol. The annexin V-positive, propidium iodide-negative cells were scored as apoptotic cells, whereas all propidium iodide-positive cells were considered to be necrotic when analyzed in FACS Scan (BD Biosciences). Staurosporine at 5 μM was used as an inductor of apoptosis (positive control). To detect executive phase of apoptosis, M30 CytoDEATH Fluorescein kit was used (Roche, Indianapolis, IN, USA); the M30 antibody cytoDEATH recognizes a specific cleavage site within cytokeratin 18 that is not detectable in native cytokeratin 18 of normal cells. Before staining, the samples were permeabilized with Cytofix/Cytoperm fixation/permeabilization kit (BD Biosciences), according to the manufacturer's protocol and once stained, analyzed in FACS Scan (BD Biosciences) or with Leica fluorescence microscope equipped with Hamatsu C4880 cold CCD camera (Leica Microsystems GmbH, Wetzlar, Germany), after mounting in medium containing Hoechst 33342 (1 μg/ml) (Sigma).
Antibodies and immunostaining
For detection of cell surface antigens Fas and FasL, infected cells were collected by trypsinization and washed in 1% FBS/PBS. FITC-conjugated monoclonal hamster anti-mouse Fas antibody and PE-conjugated monoclonal hamster anti-mouse FasL antibody were used to detect surface expression of Fas and FasL (BD Biosciences). Intra-cellular antigens were detected using Cytofix/Cytoperm fixation/permeabilization kit (BD Biosciences), according to the manufacturer's protocol and by using the following antibodies: FITC-conjugated monoclonal hamster anti-Bcl-2 antibody (BD Biosciences), PE-conjugated monoclonal mouse anti-p65-NF-κB antibody (BD Biosciences) and polyclonal rabbit anti-p-Akt (Ser 473) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). HSV-2 antigens were detected using rabbit polyclonal HSV antibody (Dako, Glostrup, Denmark). TNF-α, CXCL1/2 and IL-1β were detected using Cytofix/Cytoperm fixation/permeabilization kit with Golgi plug (BD Biosciences) as described above, using polyclonal goat anti-GRO α/β (CXCL1/2) antibody (Santa Cruz Biotechnology), monoclonal hamster anti-IL-1β antibody (BD Biosciences) and Alexa Fluor 488-conjugated monoclonal rat anti-TNF-α antibody (BD Biosciences). Following incubation with primary antibodies, appropriate anti-rabbit PE or FITC-conjugated anti-goat or anti-hamster antibodies were used, where necessary (BD Biosciences).
Cell suspensions from the vaginal tissue were prepared as follows: vaginal tissues were cut into small pieces with scissors and then treated with collagenase/dispase (1 mg/ml) (Roche) in Iscove's medium at 37°C for 40 min. Treated tissues were pressed through a 70 μm cell strainer and washed in PBS. Gr-1-positive cells were detected using anti-CD11c-APC (BD Biosciences) and anti-Ly-6G-PE (BD Biosciences), Fas, FasL and HSV-2 were detected as described above, using FITC-conjugated antibodies. For all stainings, appropriate isotype control antibodies were used. The stained cell suspensions were analyzed in FACS Scan for the percentage of positively stained cells and/or mean intensity of fluorescence. The data were further analyzed using FlowJo software (Celeza GmbH, Olten, Switzerland).
HSV-2 infection of mice
Female mice, 6- to 8-week old, were used for all experiments. B6. MRL-Faslpr/J (Fas−/−) and B6Smn.C3-Faslgld/J (FasL−/−) mice were purchased from Charles River (Dortmund, Germany). C57BL/6 mice were used as controls. Mice were injected s.c. with 2.0 mg/kg of medroxyprogesterone (Depo-Provera; Upjohn, Puurs, Belgium) in 100 μl of PBS. At 6 days later, the mice were infected by intravaginal inoculation of 104PFU per mice (100 LD50) of HSV-2 strain 333 in 20 μl of HBSS. The animals were kept in ventilated cages under specific pathogen-free conditions at the Department of Experimental Biomedicine at the Gothenburg University. The studies were approved by the Ethics Committee for Animal Experimentation, Gothenburg, Sweden. At 3 days following intra-vaginal HSV-2 infection, the animals were killed and the vaginal tissue was used for preparation of cryostat sections.
Immunofluorescence microscopy of animal tissue
Vaginal tissue was removed, fixed in 2% paraformaldehyde in PBS for 4 h, then washed twice in 10% sucrose in PBS over 16 h before freezing and cryosectioning. Slides were washed in PBS and used for apoptosis detection via TUNEL (Millipore, Billerica, MA, USA) or M30 CytoDEATH Fluorescein kit (Roche) according to appropriate manufacturer's protocols. TUNEL and M30 CytoDEATH Fluorescein detection of apoptosis in tissues was followed by incubation with anti-HSV-2 antibody (1 : 50) (Dako) and secondary anti-rabbit-IgG-PE antibody (Vector Laboratories, Burlingame, CA, USA). For Fas, FasL, Bcl-2, IL-1β and CXCL1/2 and HSV-2 double stainings, the slides were blocked with 3% BSA in PBS/0.1% Tween for 30 min and incubated overnight at 4°C with anti-Bcl-2, anti-Fas, anti-FasL, anti-IL-1β (1 : 100) (BD Biosciences) and anti-CXCL1/2 (1 : 100) (Santa Cruz Biotechnology) and anti-HSV-2 (1 : 250) antibodies in 1% BSA in PBS/0.1% Tween. This procedure was followed by 30 min incubation with goat anti-rabbit IgG-PE antibody (Dako) and goat anti-hamster-IgG-FITC or donkey anti-goat IgG-FITC antibody (Santa Cruz Biotechnology) in 1% goat serum on PBS/0.1% Tween. After mounting in medium containing Hoechst 33342 (1 μg/ml), fluorescence was captured with Leica fluorescence microscope equipped in Hamatsu C4880 cold CCD camera. For all stainings, appropriate isotype control antibodies were used. An HSV-2-infected site was defined as the area of HSV-2 positive staining within the epithelium and the surrounding infiltration of neutrophils, without the sub-mucosal stromal layer.
Virus titration
The virus titers were determined as described by Namvar et al.42 Briefly, a 118-nucleotide segment of the gB region from HSV-2 region was amplified using a HSV-2 probe labeled with JOE (6-carboxy-4′,5′-dichloro-2′,7′-dimethoxyfluorescein) in a real-time PCR instrument ABI Prism 7000 (Applied Biosystems, Carlsbad, CA, USA) and titrated as described.42
Neutrophils isolation and transwell migration assay
Neutrophils were isolated as described by Siemsen et al.43 and stained with PKH26 Red Fluorescent Cell Linker Kit for General Cell Membrane Labeling (Sigma) as indicated by the manufacturer. Purity of isolation was determined by Gr-1-positive staining and measured by FACS. Neutrophils migration was assayed using 24 well Transwell inserts (BD Biosciences) with 3 μm pores. One million of isolated neutrophils were loaded into the top well and allowed to migrate for 6 h toward keratinocytes treated with anti-Fas antibody, anti-FasL blocking antibody, HSV-2-infected keratinocytes and HSV-2-infected keratinocytes treated with anti-Fas antibody or anti-FasL blocking antibody. Migrated cells were then collected and measured using FACS. Extent of migration was expressed as a migration index, which was defined as the number of cells that migrated in a particular assay by the number of cells that migrated toward cells kept in standard tissue culture of uninfected keratinocytes.
Statistical methods
Quantitative data were presented as means±S.E.M. In the case of normal distribution of values, confirmed by Shapiro's test, statistical comparisons were performed using the Student's t-test. With non-Gaussian distributions, non-parametric Kruskal–Wallis and Wilcoxon tests were applied. In every analysis values of P<0.05 were considered significant.
Acknowledgments
This work was supported in part by grants from the Swedish Medical Research Council, Swedish state under the ALF agreement, Karolinska Institutet (to FC), EIF Marie Curie fellowship no. 221246 (to MK), Torsten and Ragnar Söderbergs Research Foundation (to KE) and IngaBritt and Arne Lundbergs Research Foundation (to KE).
Glossary
- Bcl-2
B-cell lymphoma 2
- CXCL1/2
chemokine ligand 1/2
- GM-CSF
granulocyte macrophage colony stimulating factor
- HSV-1 or 2
herpes simplex virus type 1 or 2
- IFN
interferon
- IL
interleukin
- MOI
multiplicity of infection
- Nf-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NK cells
natural killer cells
- p.i.
post infection
- PBMC
human peripheral blood mononuclear cell
- PFU
plaque forming unit
- PI3K
phosphatidylinositol 3-kinase
- MEK
mitogen-activated protein kinase kinase
- ERK
extracellular signal-regulated kinase
- Raf-1
rapidly accelerated fibrosarcoma
- TNF
tumour necrosis factor
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick-end labelling
- JOE
6-carboxy-4′,5′-dichloro-2′,7′-dimethoxyfluorescein
- FITC
fluorescein isothiocyanate
- PE
phycoerythrin
Dr. Chiodi owns stock in the company Imed AB. The present work was not financed from Imed AB. The other authors declare no conflict of interest.
Footnotes
Edited by P Salomoni
References
- Koyama AH, Adachi A. Induction of apoptosis by herpes simplex virus type 1. J Gen Virol. 1997;78:2909–2912. doi: 10.1099/0022-1317-78-11-2909. [DOI] [PubMed] [Google Scholar]
- Koyama AH, Akari H, Adachi A, Goshima F, Nishiyama Y. Induction of apoptosis in HEp-2 cells by infection with herpes simplex virus type 2. Arch Virol. 1998;143:2435–2441. doi: 10.1007/s007050050473. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Sugiura Y, Yamamoto M, Yokoya S, Wanaka A, Nishiyama Y. Apoptosis induced in the spinal cord and dorsal root ganglion by infection of herpes simplex virus type 2 in the mouse. Neurosci Lett. 1997;228:99–102. doi: 10.1016/s0304-3940(97)00364-9. [DOI] [PubMed] [Google Scholar]
- Fleck M, Mountz JD, Hsu HC, Wu J, Edwards CK, III, Kern ER. Herpes simplex virus type 2 infection induced apoptosis in peritoneal macrophages independent of Fas and tumor necrosis factor-receptor signaling. Viral Immunol. 1999;12:263–275. doi: 10.1089/vim.1999.12.263. [DOI] [PubMed] [Google Scholar]
- Mastino A, Sciortino MT, Medici MA, Perri D, Ammendolia MG, Grelli S, et al. Herpes simplex virus 2 causes apoptotic infection in monocytoid cells. Cell Death Differ. 1997;4:629–638. doi: 10.1038/sj.cdd.4400289. [DOI] [PubMed] [Google Scholar]
- Jones CA, Fernandez M, Herc K, Bosnjak L, Miranda-Saksena M, Boadle RA, et al. Herpes simplex virus type 2 induces rapid cell death and functional impairment of murine dendritic cells in vitro. J Virol. 2003;77:11139–11149. doi: 10.1128/JVI.77.20.11139-11149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JY, Sloan DD, Aubert M, Miller SA, Dang CH, Jerome KR. Apoptosis and antigen receptor function in T and B cells following exposure to herpes simplex virus. Virology. 2007;359:253–263. doi: 10.1016/j.virol.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S, Koyama AH, Shiota H, Adachi A, Goshima F, Nishiyama Y. Antiapoptotic activity of herpes simplex virus type 2: the role of US3 protein kinase gene. Microbes Infect. 1999;1:601–607. doi: 10.1016/s1286-4579(99)80059-8. [DOI] [PubMed] [Google Scholar]
- Perkins D, Pereira EF, Aurelian L. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) functions as a dominant regulator of apoptosis in hippocampal neurons involving activation of the ERK survival pathway and upregulation of the antiapoptotic protein Bag-1. J Virol. 2003;77:1292–1305. doi: 10.1128/JVI.77.2.1292-1305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Nelson J, Aurelian L, Gober M, Goswami BB. Ras-GAP binding and phosphorylation by herpes simplex virus type 2 RR1 PK (ICP10) and activation of the Ras/MEK/MAPK mitogenic pathway are required for timely onset of virus growth. J Virol. 2000;74:10417–10429. doi: 10.1128/jvi.74.22.10417-10429.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedowitz JC, Blaho JA. Herpes simplex virus 2 modulates apoptosis and stimulates NF-kappaB nuclear translocation during infection in human epithelial HEp-2 cells. Virology. 2005;342:297–310. doi: 10.1016/j.virol.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Megyeri K, Orosz L, Kormos B, Pasztor K, Seprenyi G, Ocsovszki I, et al. The herpes simplex virus-induced demise of keratinocytes is associated with a dysregulated pattern of p63 expression. Microbes Infect. 2009;11:785–794. doi: 10.1016/j.micinf.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Trautmann A, Akdis M, Kleemann D, Altznauer F, Simon HU, Graeve T, et al. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest. 2000;106:25–35. doi: 10.1172/JCI9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippens S, Hoste E, Vandenabeele P, Agostinis P, Declercq W. Cell death in the skin. Apoptosis. 2009;14:549–569. doi: 10.1007/s10495-009-0324-z. [DOI] [PubMed] [Google Scholar]
- Viard-Leveugle I, Bullani RR, Meda P, Micheau O, Limat A, Saurat JH, et al. Intracellular localization of keratinocyte Fas ligand explains lack of cytolytic activity under physiological conditions. J Biol Chem. 2003;278:16183–16188. doi: 10.1074/jbc.M212188200. [DOI] [PubMed] [Google Scholar]
- Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998;282:490–493. doi: 10.1126/science.282.5388.490. [DOI] [PubMed] [Google Scholar]
- Farley SM, Dotson AD, Purdy DE, Sundholm AJ, Schneider P, Magun BE, et al. Fas ligand elicits a caspase-independent proinflammatory response in human keratinocytes: implications for dermatitis. J Invest Dermatol. 2006;126:2438–2451. doi: 10.1038/sj.jid.5700477. [DOI] [PubMed] [Google Scholar]
- Sieg S, Yildirim Z, Smith D, Kayagaki N, Yagita H, Huang Y, et al. Herpes simplex virus type 2 inhibition of Fas ligand expression. J Virol. 1996;70:8747–8751. doi: 10.1128/jvi.70.12.8747-8751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs ME, Strasser JE, Chu CF, Chalk C, Milligan GN. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin- or Fas-mediated cytolytic mechanisms. J Virol. 2005;79:14546–14554. doi: 10.1128/JVI.79.23.14546-14554.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gober MD, Laing JM, Thompson SM, Aurelian L. The growth compromised HSV-2 mutant DeltaRR prevents kainic acid-induced apoptosis and loss of function in organotypic hippocampal cultures. Brain Res. 2006;1119:26–39. doi: 10.1016/j.brainres.2006.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TJ, Brockman MA, McNamee EE, Knipe DM. Herpes simplex virus. Front Biosci. 2002;7:752–764. doi: 10.2741/taylor. [DOI] [PubMed] [Google Scholar]
- Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994;70:369–380. [PubMed] [Google Scholar]
- Seitz CS, Freiberg RA, Hinata K, Khavari PA. NF-kappaB determines localization and features of cell death in epidermis. J Clin Invest. 2000;105:253–260. doi: 10.1172/JCI7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin ML, Ting AT, Blaho JA. NF-kappaB is required for apoptosis prevention during herpes simplex virus type 1 infection. J Virol. 2003;77:7261–7280. doi: 10.1128/JVI.77.13.7261-7280.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici MA, Sciortino MT, Perri D, Amici C, Avitabile E, Ciotti M, et al. Protection by herpes simplex virus glycoprotein D against Fas-mediated apoptosis: role of nuclear factor kappaB. J Biol Chem. 2003;278:36059–36067. doi: 10.1074/jbc.M306198200. [DOI] [PubMed] [Google Scholar]
- Thrash BR, Menges CW, Pierce RH, McCance DJ. AKT1 provides an essential survival signal required for differentiation and stratification of primary human keratinocytes. J Biol Chem. 2006;281:12155–12162. doi: 10.1074/jbc.M512116200. [DOI] [PubMed] [Google Scholar]
- Laing JM, Smith CC, Aurelian L. Multi-targeted neuroprotection by the HSV-2 gene ICP10PK includes robust bystander activity through PI3-K/Akt and/or MEK/ERK-dependent neuronal release of vascular endothelial growth factor and fractalkine. J Neurochem. 2010;112:662–676. doi: 10.1111/j.1471-4159.2009.06475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetti L, Roizman B. Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of deltaU(S)3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1. J Virol. 2006;80:3341–3348. doi: 10.1128/JVI.80.7.3341-3348.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciortino MT, Perri D, Medici MA, Grelli S, Serafino A, Borner C, et al. Role of Bcl-2 expression for productive herpes simplex virus 2 replication. Virology. 2006;356:136–146. doi: 10.1016/j.virol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Morton ER, Blaho JA. Herpes simplex virus blocks Fas-mediated apoptosis independent of viral activation of NF-kappaB in human epithelial HEp-2 cells. J Interferon Cytokine Res. 2007;27:365–376. doi: 10.1089/jir.2006.0143. [DOI] [PubMed] [Google Scholar]
- Vanden Oever MJ, Han JY. Caspase 9 is essential for herpes simplex virus type 2-induced apoptosis in T cells. J Virol. 2010;84:3116–3120. doi: 10.1128/JVI.01726-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer PH. CD95's deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- Hohlbaum AM, Saff RR, Marshak-Rothstein A. Fas-ligand – iron fist or Achilles' heel. Clin Immunol. 2002;103:1–6. doi: 10.1006/clim.2001.5165. [DOI] [PubMed] [Google Scholar]
- Kleber S, Sancho-Martinez I, Wiestler B, Beisel A, Gieffers C, Hill O, et al. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell. 2008;13:235–248. doi: 10.1016/j.ccr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Thapa M, Carr DJ. Chemokines and chemokine receptors critical to host resistance following genital herpes simplex virus type 2 (HSV-2) infection. Open Immunol J. 2008;1:33–41. doi: 10.2174/1874226200801010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Zhu J, Klock A, Phasouk K, Huang ML, Koelle DM, et al. Evasion of the mucosal innate immune system by herpes simplex virus type 2. J Virol. 2009;83:12559–12568. doi: 10.1128/JVI.00939-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan GN, Bourne N, Dudley KL. Role of polymorphonuclear leukocytes in resolution of HSV-2 infection of the mouse vagina. J Reprod Immunol. 2001;49:49–65. doi: 10.1016/s0165-0378(00)00080-2. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Yamada H, Oyamada A, Goshima F, Nishiyama Y, Yoshikai Y. Protective role of Fas-FasL signaling in lethal infection with herpes simplex virus type 2 in mice. J Virol. 2009;83:11777–11783. doi: 10.1128/JVI.01006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgul C, Edwards SW. Regulation of neutrophil apoptosis via death receptors. Cell Mol Life Sci. 2003;60:2402–2408. doi: 10.1007/s00018-003-3110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson A, Nordstrom I, Sun JB, Eriksson K. Protective immunity to genital herpes simplex virus type 2 infection is mediated by T-bet. J Immunol. 2005;174:6266–6273. doi: 10.4049/jimmunol.174.10.6266. [DOI] [PubMed] [Google Scholar]
- Kulesz-Martin M, Yoshida MA, Prestine L, Yuspa SH, Bertram JS. Mouse cell clones for improved quantitation of carcinogen-induced altered differentiation. Carcinogenesis. 1985;6:1245–1254. doi: 10.1093/carcin/6.9.1245. [DOI] [PubMed] [Google Scholar]
- Namvar L, Olofsson S, Bergstrom T, Lindh M. Detection and typing of herpes simplex virus (HSV) in mucocutaneous samples by TaqMan PCR targeting a gB segment homologous for HSV types 1 and 2. J Clin Microbiol. 2005;43:2058–2064. doi: 10.1128/JCM.43.5.2058-2064.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemsen DW, Schepetkin IA, Kirpotina LN, Lei B, Quinn MT. Neutrophil isolation from nonhuman species. Methods Mol Biol. 2007;412:21–34. doi: 10.1007/978-1-59745-467-4_3. [DOI] [PubMed] [Google Scholar]