Abstract
We have compared the distribution of fluconazole (FLC) with that of itraconazole (ITC) and griseofulvin (GRF) in the abdominal skin tissues after a single oral dose was administered to guinea pigs. The FLC concentrations in the stratum corneum reached a peak at 2 h after administration and were similar to those of ITC and higher than those of GRF in spite of the administration of a lower dose. GRF was eliminated from the stratum corneum faster than FLC and ITC. The FLC concentrations were also remarkably higher than those of ITC and GRF in the epidermis-cutis but lower in the subcutaneous fatty tissue. The distribution characteristics of each drug result from differences in their physicochemical properties. Following the administration of multiple doses, the FLC concentrations in the stratum corneum were highest in the abdominal skin tissues; those at 24 h after each administration increased gradually and were maintained at a level more than 10 times higher than that of the plasma concentrations. The FLC concentrations in the planta pedis stratum corneum and in the nail showed good dose proportionality and obvious accumulation and were 60 and 40 times as high as that in plasma on day 14. The extent of binding of FLC to human corneous keratin in vitro was about 10%, which is lower than those of ITC (94 to 97%) and GRF (36 to 38%). FLC, unlike ITC, therefore, is presumed to exist in the stratum corneum at high concentrations in an active nonbinding form. These excellent intracutaneous pharmacokinetic properties of FLC probably account in large part for the in vivo efficacy of FLC.
Fluconazole (FLC) is a triazole antifungal agent with broad-spectrum activity (10). FLC is not only effective for deep-seated mycosis caused by Candida, Cryptococcus spp., etc., but also for superficial infections caused by dermatophytes such as Microsporum and Trichophyton spp. (22, 24, 27). FLC was found to have extremely potent activity superior to that of ketoconazole in systemic fungal infection models in mice (9, 21) and dermal infection models with Trichophyton mentagrophytes in guinea pigs (15). Oral bioavailability of FLC in healthy adult volunteers is more than 90%, it is eliminated from plasma with a half-life of approximately 30 h, its pharmacokinetics shows good dose proportionality, and it is mostly excreted in the urine as the unchanged drug (1, 7, 16).
Since FLC was evenly distributed throughout the tissues of the body rapidly after administration and since it penetrates readily into skin (1, 7), excellent clinical efficacy in the treatment of fungal skin infections is expected following oral administration. The concentration of antifungal drug attained in the stratum corneum is an important factor in the treatment of dermatomycosis, and the presence of the therapeutically active form in the stratum corneum is closely related to the efficacy of the drug.
In the present study, following a single oral dose of FLC, itraconazole (ITC), or griseofulvin (GRF), the intracutaneous distribution of these antifungal drugs in various layers of the abdominal skin of guinea pigs, with special reference to the stratum corneum, was investigated. The FLC concentrations in the abdominal skin, the stratum corneum of the planta pedis of the hind legs, and the nails of guinea pigs following multiple doses were determined. In addition, an in vitro study was conducted to examine the extent of binding of FLC, ITC, and GRF to keratin prepared from the stratum corneum of human heels.
MATERIALS AND METHODS
Chemicals.
FLC was supplied by Pfizer Limited (Sandwich, United Kingdom). ITC was extracted from commercial preparations purchased from Janssen Kyowa Co., Ltd. (Tokyo, Japan). GRF was obtained commercially from Sigma Chemical Co. (St. Louis, Mo.).
Human-derived corneous keratin powder was obtained from the heels of healthy adult volunteers. It was cut finely, thoroughly dried, and ground in a mortar. Powders of grain 250 to 420 μm in size were used for the experiment.
Animals.
Guinea pigs of the Hartley strain (Japan SLC, Shizuoka, Japan) weighing 350 to 440 g were used in groups of four to six animals each. The animals were kept in an animal room maintained at a temperature of 23 ± 2°C and a relative humidity of 55% ± 5%, and pellets (CG-7; Oriental Yeast, Tokyo, Japan) and water were provided ad libitum. They were used in the experiments after acclimation under the same conditions. In the single-dose study, the animals fasted overnight until 4 h after the administration of the drug. The fur over the abdominal skin was sheared using electric hair clippers and a shaver on the day before drug administration, and it was sheared again using the shaver immediately before drug administration. In the multiple-dose study, the fur was lightly sheared using electric clippers immediately before sampling.
Administration.
FLC was dissolved in a normal saline solution or a 2% methyl cellulose solution containing 0.5% Tween 80. ITC was dissolved in polyethylene glycol 200 containing 2% HCl. GRF was dissolved in 2% methylcellulose containing 0.5% Tween 80. In the single-dose studies, FLC, ITC, and GRF were administered at doses of 10, 20, and 25 mg/kg of body weight, respectively, on the basis of the therapeutic efficacy data in a guinea pig model of dermatophytosis and expected clinical dosage. In the multiple-dose studies, FLC was administered at a dose of 10 mg/kg once a day for 14 days and at a dose of 16 or 64 mg/kg once a day for 28 days. Each animal was orally given the test drug at a dose of 0.4 to 0.5 ml per 100 g of body weight.
Sampling of specimens.
Four to six guinea pigs per time point were sacrificed by drawing blood directly from the heart under ether anesthesia at 1, 2, 4, 8, 24, 48, and 72 h after a single dose of FLC, ITC, or GRF (n = 4 at 2, 8, 24, and 48 h after a dose of ITC and at 72 h after a dose of GRF; n = 6 at 8 h after a dose of GRF; n = 5 at the other time points). In the multiple-dose study in which FLC was administered once a day for 14 days, four to five guinea pigs per time point were sacrificed by drawing blood directly from the heart under ether anesthesia at 1, 4, and 24 h after the initial administration of the drug, at 24 h after drug administration on days 2, 4, 7, 10, and 13, and at 1, 4, 24, 48, and 96 h after the final administration of the drug (n = 5 at 24 h after a dose on day 7 and at 1, 4, 24, and 48 h after the final dose; n = 4 at the other time points). The stratum corneum samples were obtained on 10 sheets of vinyl tape (5 by 5 cm) by applying the adhesive tape to the sheared abdominal skin and then removing the tape. This procedure was repeated until specimens were collected on all 10 sheets. The weight of abdominal stratum corneum samples obtained (determined on the basis of the mean difference between the pre- and postapplication weights of vinyl tapes in the preliminary study) was 40 mg. After extirpation of the abdominal skin tissue from the same test site, each tissue sample was divided by means of a razor into epidermis-cutis and subcutaneous fatty tissue. In the multiple-dose study in which FLC was administered once a day for 28 days, five animals per time point were sacrificed by drawing blood directly from the heart at 24 h after drug administration on days 1, 14, and 28. At the same time, the stratum corneum of the planta pedis of the hind legs was sliced thinly using a razor and the distal portions of the nails were clipped without blood contamination. Plasma was prepared by centrifugation of the obtained blood. All specimens were kept at −20°C until the analysis was performed.
Binding to skin corneous keratin.
FLC, ITC, and GRF were dissolved in dimethyl sulfoxide and diluted with 0.2 M Tris-HCl buffer (pH 7.0) to prepare solutions of 1 and 10 μg/ml.
According to the method of Uchida and Yamaguchi (23), 50 mg of corneous keratin powder was placed in a test tube; 1 ml of an FLC, ITC, or GRF solution at a concentration of 1 or 10 μg/ml was added; and the mixture was incubated for 30 min at 37°C. After incubation, the mixture was centrifuged and the drug concentration in the supernatant was determined. To determine the affinity of antifungal drugs, keratin powder obtained as the precipitate was repeatedly washed with 0.2 M Tris-HCl buffer (pH 7.0) and the drug concentration in each washing medium was measured.
The extent of binding to corneous keratin was calculated by the following formula: the extent of binding to corneous keratin = [1 − (the concentration in the supernatant/the added concentration)] × 100.
Determination of FLC concentration.
The FLC concentrations in plasma and the abdominal stratum corneum were measured by a modification of the high-performance liquid chromatography (HPLC) method of Rex et al. (14), and those in the skin tissue, the stratum corneum of planta pedis, and nail were determined by the HPLC method of Tanuma et al. (17) as follows. The 2,4-dichloro homologue of FLC was synthesized and used as the internal standard (IS). The predetermined volume of the IS methanol solution was added to test tubes. Each sample was added to this test tube after evaporating methanol to dry under a stream of nitrogen. The liquid chromatograph was equipped with a UV detector operated at 260 nm. A reverse-phase analytical column (Capcell pak C18 SG 120) (25 cm long and 4.6 mm in diameter) was used, and the flow rate was 1.0 ml/min. Recovery values for FLC and the IS were 80 and 85%, respectively. The accuracy (expressed as the deviation of the mean from the theoretical value) was not more than 7.7% for the intraday and interday means. Intra- and interprecision (expressed as the coefficient of variation) values were not more than 8.5%.
Plasma.
Plasma (1 ml) was placed in a test tube containing a predetermined amount of the IS. A total of 0.5 ml of 1 M NaOH and 6 ml of ethyl acetate was added to each tube, and the tubes were shaken vigorously for 5 min and then centrifuged at 1,500 × g for 5 min. After transferring the ethyl acetate layer to another test tube, 2 ml of 1 M HCl was added and the tube was shaken for 5 min and then centrifuged at 1,500 × g for 5 min. The organic layer was removed and discarded. To the remaining aqueous layer, 1 ml of 5 M NaOH and 6 ml of ethyl acetate were added. The tube was shaken for 5 min and centrifuged at 1,500 × g for 5 min. The organic layer was transferred to another test tube and evaporated to dryness under a stream of nitrogen. The residue was dissolved in 0.3 ml of HPLC mobile phase (0.5% ammonium acetate [pH 3.8]:acetonitrile [3:1]) to prepare the HPLC sample.
Abdominal stratum corneum.
The vinyl tapes with stratum corneum samples were each placed in a test tube containing a predetermined amount of the IS and 30 ml of methanol, and these samples were placed under ultrasonic treatment at 50°C for 1 h. After the ultrasonic treatment, the vinyl tapes were taken out and the solvent was evaporated to dryness under a stream of nitrogen. A total of 6 ml of ethyl acetate, 0.3 ml of acetonitrile, and 1 ml of 2 M HCl was added to each tube, the tubes were shaken and centrifuged, and then the ethyl acetate layer was removed and discarded. The remaining aqueous layer was washed twice with 6 ml of ethyl acetate. The HPLC samples were prepared by the same procedures as those used in the case of plasma. The mobile phase consisted of 0.5% ammonium acetate (pH 3.8):acetonitrile (3.5:1).
Skin tissue, stratum corneum of planta pedis, and nail.
Skin tissue, the stratum corneum of the planta pedis, and nail specimens were weighed, and each was placed in a test tube containing a predetermined amount of the IS. A total of 2 ml of 1 M NaOH was then added to each tube, and the tube was subjected to ultrasonic treatment at 37°C for 2 h to dissolve the specimen. After solubilization, the solvent was extracted with 6 ml of ethyl acetate and the ethyl acetate layer was transferred to another tube. To this fraction, 2 ml of 1 M HCl was added for reverse extraction, and the HCl layer was washed with 6 ml of ethyl acetate. The HPLC samples were prepared by the same procedures as those used in the case of plasma. The mobile phase consisted of 0.05 M phosphate buffer (pH 7.0):acetonitrile (3.5:1).
Determination of ITC and GRF concentrations.
ITC and GRF concentrations in plasma and tissues were measured by HPLC methods according to the methods of Woestenborghs et al. (26) and Hackett and Dusci (6), respectively. Clotrimazole and clonazepam have been extracted from commercial preparations and used as the IS for ITC and GRF, respectively. Recovery values for these drugs were more than 80%. Intra- and interday accuracy was not more than 2.3 and 2.4% for ITC and GRF. Intra- and interday precision was not more than 10.4 and 8.8% for ITC and GRF.
Standard curve.
To create a standard curve for each specimen, the predetermined volume of FLC, ITC, or GRF solution was added to a corresponding blank specimen (plasma, each skin tissue, or buffer) before processing by the method described above. From the results, the peak area ratio (each test drug/IS) was calculated and plotted against the added drug concentration to make a calibration curve by linear regression. Linear regression yielded a correlation coefficient of >0.999. Although the lower limits of quantification in the present method may differ by specimen, they were approximately 0.01 to 0.05 μg/g (or μg/ml in the case of plasma) for FLC and 0.01 μg/g (or μg/ml) for ITC and GRF, with the noise level taken into account.
Pharmacokinetic analysis.
The pharmacokinetic analysis of mean concentration profiles was performed by noncompartmental methods using an analysis program, WinNonlin Professional (version 3.1; Pharsight Corporation). Elimination half-life (t1/2) was calculated as ln 2/kel, where kel was the slope of the terminal phase of the log concentration-time points by the linear regression. The area under the concentration-time curve from time zero to the time (t) of the last quantifiable concentration (AUCt) was calculated by the linear trapezoidal method. The area under the concentration-time curve from time zero to ∞ (AUC) was calculated as AUCt + (Ct/kel), where Ct was the concentration at the last measurable time (t). The maximum concentration (Cmax) was taken directly from the recorded data. The t1/2 and AUC values for the stratum corneum could not be calculated, because the terminal phase was not identified.
RESULTS
The pharmacokinetics of FLC, ITC, and GRF in the abdominal skin of guinea pigs following a single dose of each drug was examined. We also investigated the FLC concentration profiles in the abdominal skin tissues during administration of multiple doses of this drug for 14 days and those in the stratum corneum of the planta pedis and in the nails during administration of multiple doses for 28 days. The binding of FLC, ITC, and GRF to corneous keratin was examined in an effort to find out the distribution pattern in the skin.
Single dose.
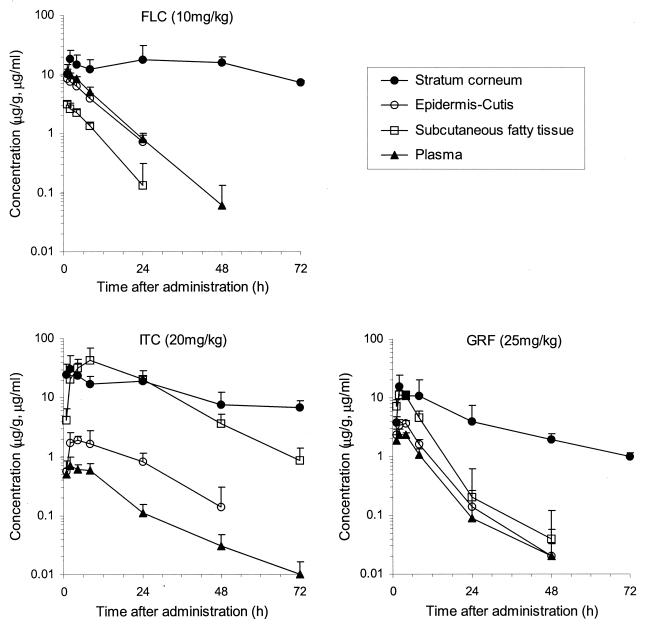
For each antifungal drug, the concentration profiles detected in the abdominal stratum corneum, epidermis-cutis, subcutaneous fatty tissue, and plasma of guinea pigs after a single oral dose of FLC at 10 mg/kg of body weight, ITC at 20 mg/kg, or GRF at 25 mg/kg are shown in Fig. 1; pharmacokinetics parameters are listed in Table 1.
FIG. 1.
Concentration profiles of FLC, ITC, and GRF in plasma and abdominal skin after administration of a single oral dose to guinea pigs (means + standard deviations [SD]; n = 4 to 6).
TABLE 1.
Mean pharmacokinetic parameters of FLC, ITC, and GRF concentrations in plasma and abdominal skin following a single oral dose to guinea pigs (n = 4 to 6)
| Drug | Dose (mg/kg) | Results
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stratum corneum
|
Epidermis-cutis
|
Subcutaneous fatty tissue
|
Plasma
|
|||||||||
| Cmax (μg/g) | AUC72 (μg · h/g) | Cmax (μg/g) | AUC (μg · h/g) | t1/2 (h) | Cmax (μg/g) | AUC (μg · h/g) | t1/2 (h) | Cmax (μg/ml) | AUC (μg · h/ml) | t1/2 (h) | ||
| FLC | 10 | 18.1 | 1,026 | 8.3 | 89.7 | 6.4 | 3.1 | 28.9 | 4.9 | 11.5 | 12.0 | 6.3 |
| ITC | 20 | 31.6 | 949 | 1.93 | 45.6 | 11.6 | 42.0 | 1,067 | 10.4 | 0.70 | 12.4 | 13.9 |
| GRF | 25 | 15.8 | 307 | 3.65 | 37.9 | 5.9 | 11.3 | 106 | 5.5 | 2.34 | 25.5 | 6.5 |
The concentration of FLC in the abdominal stratum corneum was higher than those in plasma and other skin tissues. A Cmax value of 18.1 μg/g in the abdominal stratum corneum was obtained at 2 h after administration, and this high concentration was maintained for a long duration. The value was 7.3 μg/g at 72 h after administration, when the concentration in other tissues fell below the lower limit of quantification, and the AUC72 value was 1,026 μg · h/g. FLC concentrations in plasma, epidermis-cutis, and subcutaneous fatty tissue reached a peak at 1 h after administration; the Cmax values were 11.5 μg/ml and 8.3 and 3.1 μg/g, respectively. A markedly low concentration was observed in the subcutaneous fatty tissue. The concentrations in these tissues decreased thereafter, with an elimination half-life (t1/2) of about 5 to 6 h. AUC values were 120 μg · h/ml and 89.7 and 28.9 μg · h/g in plasma and in epidermis-cutis and subcutaneous fatty tissue, respectively.
The concentration of ITC in the abdominal stratum corneum showed a Cmax of 31.6 μg/g at 2 h after administration. The value decreased gradually, and it was 6.8 μg/g at 72 h after administration. The AUC72 was 949 μg · h/g. ITC concentrations in plasma reached a peak Cmax of 0.70 μg/ml at 2 h after administration and decreased with a t1/2 of 13.9 h. The AUC was 12.4 μg · h/ml. The Cmax of ITC in the epidermis-cutis was 1.93 μg/g, as low as 1/17 of the concentration in the stratum corneum. Its concentration in subcutaneous fatty tissue, on the other hand, was higher than that in the stratum corneum, with a Cmax of 42.0 μg/g (60 times higher than that seen with plasma). AUC values were 45.6 and 1,067 μg · h/g in the epidermis-cutis and subcutaneous fatty tissue, respectively.
The concentration of GRF in the stratum corneum showed a Cmax of 15.8 μg/g at 2 h after administration, and it reached 1.0 μg/g at 72 h after administration. The AUC72 was 307 μg · h/g. The Cmax values of GRF were 2.34 μg/ml and 3.65 and 11.3 μg/g, and the AUC values were 25.5 μg · h/ml and 37.9 and 106 μg · h/g, respectively, in plasma and in epidermis-cutis and subcutaneous fatty tissue, indicating a slightly higher transfer into subcutaneous fatty tissue. The concentration in each tissue was decreased thereafter, and the concentrations reached a level below the lower limit of quantification at 72 h after administration.
Multiple doses.
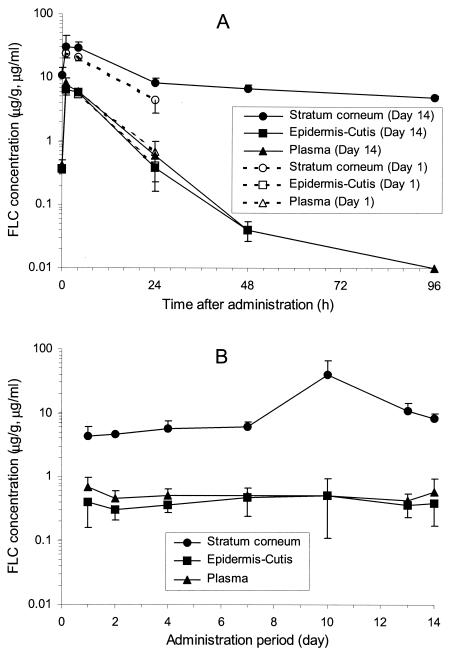
FLC at a dose of 10 mg/kg was orally administered to guinea pigs once a day for 14 days. Concentration profiles of FLC in the abdominal skin tissues and plasma are shown in Fig. 2. The concentration of FLC in the abdominal stratum corneum showed a Cmax of 24.1 μg/g at 1 h after the initial administration, and the Cmax reached 30.5 μg/g after the final administration (Fig. 2A). With multiple doses, the concentration around the peak after the final administration was about 1.3 times higher than that after the initial administration. The concentration at 24 h after each administration (Cmin) increased gradually (Fig. 2B). The Cmin after the final administration was 1.9 times higher than that after the initial administration, suggesting that the transfer of FLC to the tissues continued for a long duration during the administration period. FLC concentration in the stratum corneum maintained a high value of 5.0 μg/g even at 96 h after the final administration. FLC concentrations in plasma and epidermis-cutis after the initial and final administrations showed similar values, and the Cmin in plasma and epidermis-cutis during the administration period was in the range of 0.4 to 0.7 μg/ml and 0.3 to 0.5 μg/g, respectively, with little accumulation observed.
FIG. 2.
Concentration profiles on days 1 and 14 (A) and at 24 h after each administration (B) of FLC in plasma and abdominal skin during administration of multiple doses of FLC to guinea pigs at a dose of 10 mg/kg once a day for 14 days (means ± SD; n = 4 to 5). Specimens were obtained at 1, 4, and 24 h after the initial administration (day 1), at 1, 4, 24, 48 and 96 h after the final administration (day 14), and at 24 h after drug administration on days 2, 4, 7, 10, and 13.
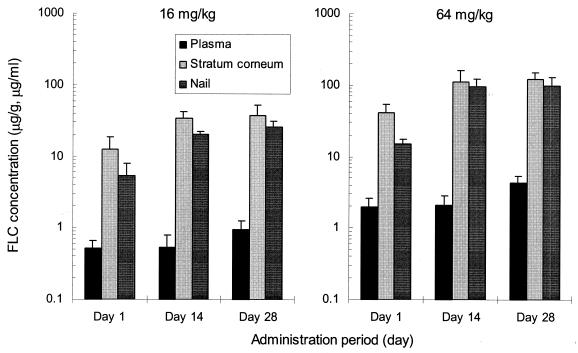
FLC at a dose of 16 or 64 mg/kg was orally administered to guinea pigs once a day for 28 days. The FLC concentrations in plasma, the stratum corneum of the planta pedis, and the nails at 24 h after administration on days 1, 14, and 28 are shown in Fig. 3. With FLC administration at 16 mg/kg, concentrations in the stratum corneum of the planta pedis and the nails at 24 h after administration on day 1 were 12.6 and 5.3 μg/g, respectively; these values increased on day 14 to 33.8 and 20.5 μg/g, respectively, an increase of about threefold for the concentration in the stratum corneum and about fourfold for the nail, showing an obvious increase in response to multiple doses. The plasma concentrations of FLC, on the other hand, showed little change, with values of 0.52 μg/ml (day 1) and 0.53 μg/ml (day 14). With administration of FLC at 64 mg/kg, concentrations in the stratum corneum and nails on day 14 showed a dose-related increase to 112.5 and 95.6 μg/g, respectively. The concentration profiles in these tissues were similar to those seen when FLC was administered at 16 mg/kg. Comparing the concentrations in the stratum corneum and nails at 24 h after administration on day 28 with those seen on day 14, a slight but not great increase in tissue concentrations in response to multiple doses was observed for each dose of FLC. It was considered that a steady state was attained by day 14 at either dosage.
FIG. 3.
FLC concentrations in plasma, the stratum corneum of the planta pedis, and the nail of guinea pigs at 24 h after administration of multiple oral doses at a dose of 16 or 64 mg/kg once a day for 28 days (means + SD; n = 5).
Binding to stratum corneum keratin.
In an effort to estimate the antidermatophytic activity in the stratum corneum, levels of binding to keratin powder prepared from the human heels of FLC were compared with the levels seen with ITC and GRF. The results are shown in Table 2.
TABLE 2.
Binding of FLC, ITC, and GRF to corneous keratin (n = 3)a
| Drug concentration (μg/ml) | % (Mean ± SD) binding to corneous keratin
|
||
|---|---|---|---|
| FLC | ITC | GRF | |
| 1 | 10.38 ± 0.51 | 93.69 ± 0.88 | 37.67 ± 0.76 |
| 10 | 9.94 ± 3.12 | 97.06 ± 0.44 | 35.96 ± 1.27 |
Corneous keratin (50 mg) was added to 1 ml of a solution of each drug, the mixture was incubated at 37°C for 30 min and centrifuged, and the drug concentration in the supernatant was determined.
Corneous keratin powder was incubated with FLC at 1 or 10 μg/ml; after centrifugation, the FLC concentration in the supernatant was measured. Since about 90% of the added drug existed in the supernatant, the extent of binding to corneous keratin was estimated to be about 10%. With ITC, only about 3 to 6% was detected in the supernatant, so the extent of binding was 94 to 97%. The extent of binding of GRF was about 36 to 38%. When the drug bound to the corneous keratin powder was washed with buffer, most of the bound FLC was recovered in supernatant after the initial washing. On the other hand, in the case of ITC the amount recovered in the supernatant was slight, indicating that its binding to the corneous keratin was strong. GRF was more easily dissociated from the corneous keratin than ITC but to a lesser degree than FLC.
DISCUSSION
We have compared the distribution of FLC in abdominal skin tissues with that of drugs with similar actions, ITC and GRF, after a single oral dose in guinea pigs. Multiple doses of FLC were also conducted to investigate the FLC concentrations in the abdominal skin, the stratum corneum of the planta pedis, and the nail of guinea pigs in an effort to elucidate the intracutaneous pharmacokinetic properties of FLC.
It was confirmed that the FLC concentrations in the abdominal stratum corneum almost reached the Cmax at 2 h after a single dose and that the drug rapidly reached the stratum corneum, the main site the fungus inhabits. The FLC concentration in the stratum corneum was lower than that of ITC at around the Cmax, reflecting the lower dose, but it maintained a level almost similar to that of ITC thereafter. The GRF concentration was the lowest at all time points. Elimination of FLC and ITC from the stratum corneum was slower than that of GRF. The AUC72 of FLC in the stratum corneum was almost similar to that of ITC and 3.3 times higher than that of GRF. The FLC concentration in the epidermis-cutis was remarkably higher than those of the other two drugs (about 2 times and 2.4 times higher than those of ITC and GRF at the AUC). On the other hand, the FLC concentration in subcutaneous fatty tissue was quite low compared with those of ITC and GRF, and the AUC values for FLC were 1/37 and 1/3.7, respectively.
Following the administration of multiple oral doses of FLC at the dose of 10 mg/kg of body weight for 14 days, FLC concentration profiles in the abdominal skin tissues were basically the same as those observed following a single dose, although somewhat wider variation was noted. That is, the FLC concentrations in the stratum corneum were the highest, and those at 24 h after each administration (Cmin) increased gradually and were maintained at a level more than 10 times higher than the plasma concentrations. This concentration remained high after the completion of the drug administration. However, these FLC concentrations in the abdominal stratum corneum during the administration of multiple doses were a little lower than the values estimated from single-dose data. On the other hand, the FLC concentrations in the stratum corneum of the planta pedis and nail on day 14 increased about threefold and fourfold, showing an obvious accumulation in response to multiple doses.
Cauwenbergh et al. (2) have reported that the back has a rather thin stratum corneum, and ITC concentrations in the stratum corneum of the back in healthy volunteers disappear within 2 weeks after the end of 100-mg-dose administration for 4 weeks. On the other hand, ITC concentrations in the stratum corneum of the palms persist at least 3 weeks after the end of therapy because of a fairly thick stratum corneum. These results suggest that the differences in accumulation ratios between abdominal stratum corneum and the planta pedis stratum corneum or nail in guinea pigs are caused by the differences of thickness of the stratum corneum layer and the turnover periods of keratinocytes at these sites. The FLC concentrations in the planta pedis stratum corneum of guinea pigs showed good dose proportionality on day 14 during the administration of multiple oral doses of FLC at 16 and 64 mg/kg. The FLC concentration in the stratum corneum on day 14 was 60 times as high as that in plasma at either dosage. Concentrations in the distal portions of the nail were approximately 40% of those in the stratum corneum at 24 h after the administration on day 1 and about 60 to 85% on days 14 and 28, suggesting good transfer. It is assumed that the concentrations in the planta pedis stratum corneum and nail achieved a steady state within 14 days, reflecting the turnover periods at these sites. It is also presumed that effective and good transfer of the drug from the epidermis-cutis to the stratum corneum and nail was effectively promoted and enhanced by the transfer accompanying the cornification of the stratum corneum and the growth of the nail plate from the nail matrix. Following the administration of multiple oral doses of FLC in humans, steady long-term transfer of the drug to keratinized adnexa associated with epidermis such as hair and nail has been demonstrated and is considered to represent continuous transference from the basal cell (25).
There are few detailed reports on the subcutaneous transfer of antifungal agents. Faergemann and Laufen (4) have investigated the distribution of FLC in the back skin following the administration of FLC to healthy adult males for 12 days and have compared it to that seen after ITC and GRF administration, as reported by Cauwenbergh et al. (2) and Epstein et al. (3). The distribution of FLC, ITC, and GRF found in human skin coincides well with the present results found with guinea pigs, which have a skin structure similar to that of humans. In humans, the highest concentration of drug in the stratum corneum is obtained with FLC in spite of the lower administered dose of FLC compared with those of ITC and GRF. Transfer rates of ITC to the sebum in humans as well as to the subcutaneous fatty tissue in the guinea pig are high. The GRF concentrations in the sweat are confirmed to be 100 to 150 times as high as that in plasma. The distribution characteristics of each drug result from differences in their physical and chemical properties. Transfer to the sebum, which is considered to be a route of transportation, is remarkably high in the case of those drugs (such as ITC) which have highly lipophilic properties, while the main route of transportation for GRF is considered to be the sweat. It is possible that these drugs reach the skin surface within hours of administration through the sweat and sebaceous glands situated in the cutis and are then associated to keratin in the stratum corneum. These drugs (transferred to the skin surface through sweat and sebum) may be excreted from the stratum corneum mainly by the normal turnover of keratinocytes and diffusion out of the stratum corneum. This is a possible reason for the slower elimination of the drugs in the stratum corneum than in plasma and other skin tissues. ITC binds to keratin strongly and is not easily dissociated from keratin in comparison to FLC and GRF. This strong binding to keratin of ITC may contribute to delayed elimination from the stratum corneum. On the other hand, FLC, which has both hydrophilic and lipophilic properties, is reported to be transferred to the stratum corneum not only through the sweat and sebum but also by direct diffusion from capillary blood vessels and through the epidermis-cutis and by accumulation into the epidermal basal cells that migrate to the surface in the normal turnover of keratinocytes (5). Passive diffusion of FLC through the layers of the epidermis may be enhanced by its low binding to keratin and would explain the rapid transport into the stratum corneum. The appearance of FLC in the distal portion of the nail on day 1 demonstrates that FLC reaches the nail not only via incorporation in the nail matrix but also by diffusion from the nail bed into the nail plate. Incorporation into the epidermal basal cells and migration during the normal turnover of keratinocytes may contribute to the slow elimination of FLC from the stratum corneum (considering the high concentrations in the epidermis-cutis).
Epstein et al. (3) have reported that in humans, GRF is found at the highest concentration in the outermost horny layers and at the lowest concentration in the innermost horny layers, with significantly reduced concentrations in epidermis. If direct diffusion were the only driving force through the stratum corneum, the gradient should be in the opposite direction. Therefore, sweating must play an important role in the development of the reversed GRF concentration gradient in the skin. The evaporation of sweat from the skin surface can result in the deposition of GRF on the stratum corneum and should cause a higher concentration in the most superficial stratum corneum layers. In contrast, Faergemann et al. (5) have reported that FLC accumulates homogeneously in each skin layer but that concentrations differ among the stratum corneum, epidermis, and cutis after a single oral dose in humans. FLC concentrations are highest in the stratum corneum, lower in the rest of the epidermis, and lowest in the cutis. This suggests the possibility that FLC is transferred to the stratum corneum not only by direct diffusion and the normal turnover of keratinocytes but also by back diffusion in the stratum corneum of FLC transferred to the skin surface through sebum and sweat on the basis of the lower affinity to keratin of FLC than GRF and ITC.
In an effort to estimate the antidermatophytic activity in the stratum corneum, we determined the extent of binding of FLC, ITC, and GRF to corneous keratin by incubating the drugs with corneous keratin powder prepared from the heels of humans. The results indicated that the in vitro binding of FLC was about 10%, which is lower than that observed with ITC (94 to 97%) and GRF (36 to 38%) at the drug concentrations of 1 and 10 μg/ml of medium. The value for the active nonbinding fraction of FLC in the stratum corneum, therefore, is presumed to be about 20 and 1.4 times higher than those for ITC and GRF. It is known that many antifungal drugs are inactivated when bound to keratin (13, 18, 19, 20). It has been reported by Niwano et al. (13) that in studies of T. mentagrophytes, the antifungal activities of analogues such as lanoconazole, bifonazole, and clotrimazole were decreased 1/32 to 1/256 in the presence of keratin. Tatsumi et al. (19) have also reported that amorolfine and terbinafine are 8- and 32-fold, respectively, less active against T. mentagrophytes in medium containing keratin due to high extents of binding to keratin of more than 90%; however, the antifungal activity of KP-103 is not affected by keratin because of its lower keratin affinity. Tatsumi et al. (20) have also reported on the basis of in vitro MICs that KP-103 is as active as neticonazole but is less active than lanoconazole and butenafine against the dermatophytes. However, KP-103 exerts therapeutic efficacy superior to that of neticonazole and comparable to those of lanoconazole and butenafine in guinea pigs with plantar tinea pedis. This is because neticonazole, lanoconazole, and butenafine are 16- to 64-fold less active in the medium with keratin, whereas KP-103 shows similar activities in both media and its potency is little affected by keratin. Uchida and Yamaguchi (23) have reported that the extent of binding of terbinafine to keratin increases with increasing keratin concentrations in the medium, showing values of about 48, 73, and 88% at the keratin concentrations of 1, 10, and 100 mg/ml of medium. This result suggests that these antifungal drugs are further inactivated in the stratum corneum, because the concentration of keratin in the stratum corneum is more than 80%. In the case of antifungal agents displaying a large extent of binding to keratin, it is suggested that a large amount of the drug is bound to keratin in the stratum corneum and that their activity with skin fungi is thereby inhibited and is much lower than that expected from the results in vitro studies.
Dermatophytes (such as Trichophyton, Epidermophyton, and Microsporum spp. and the yeast Malassezia furfur), which cause fungal skin infections, generally colonize stratum corneum or keratinized adnexa and only very seldom penetrate into the cutis. Various yeasts, such as Candida albicans and Cryptococcus neoformans, can also penetrate into nonkeratinized epidermis and cutis (17, 25). It is suggested that a high concentration of an antifungal agent is required to show sufficient efficacy against fungal skin infections not only in the stratum corneum but also in the epidermis-cutis. As described above, FLC transfers to both the stratum corneum and epidermis-cutis at high concentrations. It is assumed to directly diffuse into the epidermis-cutis from capillary blood vessels, incorporate into the epidermal basal cells, and migrate to the surface during the normal turnover of keratinocytes, and a major portion of it exists as a nonbinding form because of its small extent of binding to corneous keratin. Therefore, FLC is considered to be a drug with a favorable pharmacokinetic profile which is suitable for treating both superficial and deep-seated mycosis.
Nagino et al. have reported that the therapeutic efficacy of FLC administered orally at doses of 10 and 20 mg/kg in a guinea pig model of dermatophytosis was approximately equal to that of ITC administered at the same doses (11) and that the therapeutic efficacy of FLC administered at 16 mg/kg was approximately equal to that of GRF administered at 25 and 100 mg/kg (12). Jessup et al. (8) have reported that the means ± standard errors of the MICs of FLC, ITC, and GRF were 2.07 ± 0.29, 0.13 ± 0.01, and 0.71 ± 0.05 μg/ml, respectively, for 217 dermatophytes. The MICs of FLC were found to be about 16 and 3 times higher those of ITC and GRF. Despite the lower level of in vitro antidermatophytic activity of FLC compared to those of ITC and GRF, the in vivo efficacy of oral FLC is much higher than one would expect on the basis of FLC's in vitro activity. The AUC of FLC in the stratum corneum was almost similar to that of ITC and 3.3 times higher than that of GRF. The active nonbinding fraction of FLC in the stratum corneum is presumed to be about 20 and 1.4 times higher than those of ITC and GRF. These data suggest that the AUC of active nonbinding FLC in the stratum corneum is about 20 and 5 times higher than those of ITC and GRF. Therefore, the nonbinding drug levels with respect to stratum corneum/MIC ratios are relatively similar among these three drugs. The excellent intracutaneous pharmacokinetic properties of FLC probably account in large part for the in vivo efficacy of FLC.
REFERENCES
- 1.Brammer, K. W., P. R. Farrow, and J. K. Faulkner. 1990. Pharmacokinetics and tissue penetration of fluconazole in humans. Rev. Infect. Dis. 12:S318-S326. [DOI] [PubMed] [Google Scholar]
- 2.Cauwenbergh, G., H. Degreef, J. Heykants, R. Woestenborghs, P. Van Rooy, and K. J. Haeverans. 1988. Pharmacokinetic profile of orally administered itraconazole in human skin. J. Am. Acad. Dermatol. 18:263-268. [DOI] [PubMed] [Google Scholar]
- 3.Epstein, W. L., V. P. Shah, and S. Riegelman. 1972. Griseofulvin levels in stratum corneum. Study after oral administration in man. Arch. Dermatol. 106:344-348. [PubMed] [Google Scholar]
- 4.Faergemann, J., and H. Laufen. 1993. Levels of fluconazole in serum, stratum corneum, epidermis-dermis (without stratum corneum) and eccrine sweat. Clin. Exp. Dermatol. 18:102-106. [DOI] [PubMed] [Google Scholar]
- 5.Faergemann, J., J. Godleski, H. Laufen, and R. H. Liss. 1995. Intracutaneous transport of orally administered fluconazole to the stratum corneum. Acta Derm. Venereol. 75:361-363. [DOI] [PubMed] [Google Scholar]
- 6.Hackett, L. P., and L. J. Dusci. 1978. Determination of griseofulvin in human serum using high-performance liquid chromatography. J. Chromatogr. 155:206-208. [DOI] [PubMed] [Google Scholar]
- 7.Humphrey, M. J., S. Jevons, and M. H. Tarbit. 1985. Pharmacokinetic evaluation of UK-49,858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob. Agents Chemother. 28:648-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jessup, C. J., J. Warner, N. Isham, I. Hasan, and M. A. Ghannoum. 2000. Antifungal susceptibility testing of dermatophytes: establishing a medium for inducing conidial growth and evaluation of susceptibility of clinical isolates. J. Clin. Microbiol. 38:341-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawasaki, K., Y. Matsumura, M. Ogawa, A. Tsuji, T. Matsunaga, and S. Goto. 1991. In vivo and in vitro antifungal activity of fluconazole. Jpn. J. Antibiot. 44:552-561. [PubMed] [Google Scholar]
- 10.Marriot, M. S., and K. Richardson. 1987. The discovery and mode of action of fluconazole, p. 81-92. In R. A. Fromtling (ed.), Recent trends in the discovery, development and evaluation of antifungal agents. J. R. Prous Science Publishers, SA, Barcelona, Spain.
- 11.Nagino, K., H. Shimohira, M. Ogawa, K. Uchida, and H. Yamaguchi. 2000. Comparison of the therapeutic efficacy of oral doses of fluconazole and itraconazole in a guinea pig model of dermatophytosis. J. Infect. Chemother. 6:41-44. [DOI] [PubMed] [Google Scholar]
- 12.Nagino, K., H. Shimohira, M. Ogawa, K. Uchida, and H. Yamaguchi. 2000. Comparison of the therapeutic efficacy of oral doses of fluconazole and griseofulvin in a guinea pig model of dermatophytosis. J. Antibiot. 53:207-210. [DOI] [PubMed] [Google Scholar]
- 13.Niwano, Y., N. Kuzuhara, K. Kanai, H. Hamaguchi, and T. Ohmi. 1996. In vitro antifungal activity of lanoconazole, a topical antifungal agent. A comparative study with other topical agents developed recently, and a study on the influence of human hair as a source of horny materials. KISO TO RINSHO 30:123-130. [Google Scholar]
- 14.Rex, J. H., L. H. Hanson, M. A. Amantea, D. A. Stevens, and J. E. Bennett. 1991. Standardization of a fluconazole bioassay and correlation of results with those obtained by high-pressure liquid chromatography. Antimicrob. Agents Chemother. 35:846-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson, K., K. W. Brammer, M. S. Marriott, and P. F. Troke. 1985. Activity of UK-49,858, a bis-triazole derivative, against experimental infections with Candida albicans and Trichophyton mentagrophytes. Antimicrob. Agents Chemother. 27:832-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiba, K., A. Saito, and T. Miyahara. 1990. Safety and pharmacokinetics of single oral and intravenous doses of fluconazole in healthy subjects. Clin. Ther. 12:206-215. [PubMed] [Google Scholar]
- 17.Tanuma, H., M. Doi, A. Yaguchi, Y. Ohta, S. Nishiyama, K. Sekiguchi, and K. Katsuoka. 1998. Efficacy of oral fluconazole in tinea pedis of the hyperkeratotic type. Stratum corneum levels. Mycoses 41:153-162. [DOI] [PubMed] [Google Scholar]
- 18.Tatsumi, Y., M. Yokoo, T. Arika, and H. Yamaguchi. 2002. KP-103, a novel triazole derivative, is effective in preventing relapse and successfully treating experimental interdigital tinea pedis and tinea corporis in guinea pigs. Microbiol. Immunol. 46:425-432. [DOI] [PubMed] [Google Scholar]
- 19.Tatsumi, Y., M. Yokoo, H. Senda, and K. Kakehi. 2002. Therapeutic efficacy of topically applied KP-103 against experimental tinea unguium in guinea pigs in comparison with amorolfine and terbinafine. Antimicrob. Agents Chemother. 46:3797-3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tatsumi, Y., M. Yokoo, T. Arika, and H. Yamaguchi. 2001. In vitro antifungal activity of KP-103, a novel topical derivative, and its therapeutic efficacy against experimental plantar tinea pedis and cutaneous candidiasis in guinea pigs. Antimicrob. Agents Chemother. 45:1493-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troke, P. F., R. J. Andrews, K. W. Brammer, M. S. Marriott, and K. Richardson. 1985. Efficacy of UK-49,858 (fluconazole) against Candida albicans experimental infections in mice. Antimicrob. Agents Chemother. 28:815-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchida, K., and H. Yamaguchi. 1996. Preclinical therapeutic evaluation of agents for treating dermatophytosis. Jpn. J. Med. Mycol. 37:199-205. [Google Scholar]
- 23.Uchida, K., and H. Yamaguchi. 1993. Studies on the affinity of terbinafine with keratine. Jpn. J. Med. Mycol. 34:207-212. [Google Scholar]
- 24.Wildfeuer, A., and H. P. Seidl. 1994. The in vitro activity of fluconazole against fungi involved in dermal infections. Mycoses 37:447-449. [DOI] [PubMed] [Google Scholar]
- 25.Wildfeuer, A., J. Faergemann, H. Laufen, G. Pfaff, T. Zimmermann, H. P. Seidl, and P. Lach. 1994. Bioavailability of fluconazole in the skin after oral medication. Mycoses 37:127-130. [DOI] [PubMed] [Google Scholar]
- 26.Woestenborghs, R., W. Lorreyne, and J. Heykants. 1987. Determination of itraconazole in plasma and animal tissues by high-performance liquid chromatography. J. Chromatogr. 413:332-337. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi, H., K. Uchida, K. Kawasaki, and T. Matsunaga. 1989. In vitro activity of fluconazole, a novel bistriazole antifungal agent. Jpn. J. Antibiot. 42:1-16. [PubMed] [Google Scholar]