Abstract
We studied the evolution of nonnucleoside reverse transcriptase inhibitor (NNRTI) resistance mutations among 29 human immunodeficiency virus type 1 (HIV-1)-infected patients who experienced virologic failure when receiving an NNRTI-containing regimen (nevirapine, delavirdine, or efavirenz) and subsequently switched to antiretroviral therapy without NNRTIs. Genotypic resistance was determined from plasma samples collected at the time of NNRTI withdrawal (baseline) and during follow-up. At baseline, 83% of patients had more than two thymidine analog resistance mutations (TAMs), and all had NNRTI resistance mutations. Mutations at codons 103, 181, and 190 were found in 62, 62, and 34% of the patients, respectively. Follow-up samples were available after a median time of 6 months in all patients and at 12 months in 22 patients. The mean number of resistance mutations to NNRTIs was significantly lower at months 6 (1.34 ± 1.04) and 12 (1.18 ± 1.05) than at month 0 (2.03 ± 1.02) (P < 0.009). The percentages of patients with at least one NNRTI resistance mutation were 100, 76, and 73% at baseline, month 6, and month 12, respectively (P < 0.0044). Overall, 70% of the patients had a mutation at codon 103 or 181 at month 12. The mean number of TAMs did not vary significantly during follow-up. Our data show that, in the context of maintained antiretroviral therapy, NNRTI resistance mutations persist in two-thirds of the patients in spite of NNRTI withdrawal. These results argue for the low impact of NNRTI resistance mutations on viral fitness and suggest that resistance mutations to different classes of drugs are associated on the same genome, at least in some of the resistant strains.
Nonnucleoside reverse transcriptase inhibitors (NNRTIs) are potent inhibitors of human immunodeficiency virus type 1 (HIV-1) replication with favorable pharmacokinetics. Resistant viruses rapidly emerge in vitro, and also in vivo, when these drugs are used in the context of persistent viral replication (4). High-level phenotypic resistance is the result of single-nucleotide changes that have been identified and cluster around the NNRTI binding site (4). There is a large degree of cross-resistance between the three compounds of this class that are available today (nevirapine, efavirenz, and delavirdine), precluding the sequential use of these drugs in case of virological failure (1, 2).
In patients with multiple therapeutic failure, recycling of antiretroviral drugs has been proposed as a therapeutic option. Some studies have shown that interruption of all antiretroviral drugs was often associated with viral rebound and loss of resistance mutations to the three classes of drugs, at least when resistance was analyzed by standard genotypic methods (5, 14, 18). Reintroduction of multiple drug antiretroviral regimens following therapeutic interruption may be associated with dramatic reduction in viral load, suggesting the relevance of wild-type virus rebound (5, 6).
Data about the evolution of resistance mutations to NNRTIs when continuing antiretroviral therapy without any compound of this class are not available. Loss of NNRTI resistance mutations could allow recycling of compounds of this class when therapeutic options are limited by successive multiple failures, although different studies have shown that standard genotypic methods do not assess minority resistant viral species. Furthermore, evolution of NNRTI resistance mutations in case of persistent antiretroviral therapy without NNRTIs may be informative on the relationship between resistance mutations to different classes of antiretroviral drugs.
The objective of our study was to evaluate the evolution of NNRTI resistance mutations selected in patients who failed an NNRTI-containing regimen and subsequently switched to antiretroviral therapy without NNRTIs.
(This work was presented in part at the 1st International AIDS Society Meeting, Buenos Aires, Argentina, July 2001.)
MATERIALS AND METHODS
Patients.
The study was conducted with 29 HIV-1-infected patients who experienced virologic failure while receiving an NNRTI-containing regimen and withdrew this class of drugs. These patients were monitored in the two infectious diseases departments of Bichat-Claude Bernard Hospital. Virologic failure was defined as the presence of a plasma HIV RNA level of >200 copies/ml (Amplicor monitor, version 1.5; Roche, Meylan, France). Drug treatment history, CD4 cell count, and plasma viral load were obtained from clinical flowcharts.
Selection of plasma samples.
Genotypic resistance was determined from plasma samples stored at −80°C and collected at the time of NNRTI withdrawal (baseline) and at intervals after NNRTI withdrawal (approximately 6 and 12 months after withdrawal) according to availability.
Determination of genotypic resistance.
Plasma HIV-1 RNA was extracted, and nested PCR was used to generate the first 700 nucleotides of the reverse transcriptase (RT) gene (19). Direct dideoxy nucleoside terminator cycle sequencing of the PCR product was performed as previously described (19). Sequencing was performed in both directions. Sequencing reactions were analyzed on an ABI 3100 (Perkin-Elmer ABI, Foster City, Calif.) instrument and manually proofread and edited. RT codons 35 to 260 were analyzed and compared to the clade B consensus sequence. Genotypic resistance to NNRTIs was defined according to the ANRS Resistance Group algorithm (http://www.hivfrenchresistance.org). Only known mutations conferring resistance to NNRTIs were considered, including changes at codons 98, 100, 101, 103, 106, 108, 181, 188, 190, 225, and 236 of the RT gene. Thymidine analog resistance mutations (TAMs) were defined as the mutations M41L, D67N, K70R, L210W, T215Y/F, and K219E.
Determination of plasma NNRTI levels.
To confirm the interruption of NNRTI therapy, plasma samples stored at −80°C during follow-up were randomly selected and tested for the presence of NNRTIs. Concentrations of nevirapine, efavirenz, and delavirdine in plasma were determined separately by reverse-phase high-performance liquid chromatography assays (C18 column) coupled with UV detection, after sample pretreatment (alkaline liquid-liquid phase extractions). The nevirapine, efavirenz, and delavirdine assays were validated over concentration ranges of 50 to 10,000 ng/ml, 10 to 10,000 ng/ml, and 50 to 20,000 ng/ml, and the limits of quantification were 50, 10, and 50 ng/ml, respectively. The between-assay biases were below 10% for all assays.
Statistical analysis.
The comparison of the number of mutations per patient between day 0, month 6, and month 12 was performed with a nonparametric analysis of variance by the Kruskal-Wallis test. The comparison of the proportion of patients with at least one NNRTI mutation (or similarly for at least two) was done by a Fisher's exact test. The statistical analyses were done with SAS software.
RESULTS
Patient characteristics at baseline.
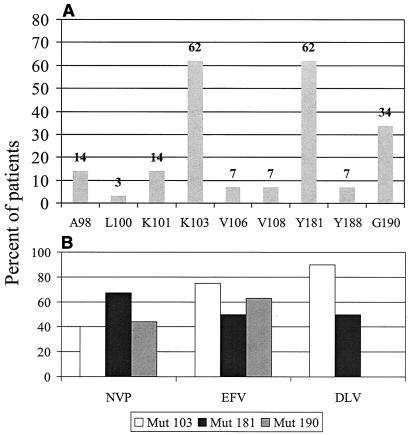
At the time of NNRTI withdrawal, the median viral load was 5.10 log copies/ml (range, 2.30 to 6.19 log copies/ml), and the median CD4 cell count was 158 cells per mm3 (range, 12 to 614). Fifteen patients had been exposed to nevirapine, 10 had been exposed to delavirdine, and 8 had been exposed to efavirenz, preceded by nevirapine in 3 subjects and followed by nevirapine in 1 subject. The median total duration of NNRTI therapy was 7 months (range, 2 to 19 months). The patients had all received at least two lines of antiretroviral therapy, and 21 patients (72%) had been exposed to protease inhibitor before NNRTI withdrawal. The incidence of associated TAMs was high: the mean number of TAMs was 3.44 ± 1.69, and 24 patients (83%) had 3 TAMs or more, in combination with the M184V mutation in 7 patients and the multidrug resistance mutation Q151M in one patient. At baseline, the mutations at codons 103, 181, and 190 were the most frequent and were found in 62, 62, and 34% of the patients, respectively. The other mutations were found in less than 20% of the subjects (Fig. 1A). Figure 1B depicts the incidence of mutations at codons 103, 181, and 190 according to the NNRTIs to which patients had been exposed. No mutation at codon 190 was found in patients exposed to delavirdine. Mutation at codon 181 was found in two of the four patients who had been exposed to efavirenz only. Ninety percent of the subjects exposed to delavirdine had the K103N mutation.
FIG. 1.
NNRTI resistance mutations at baseline. (A) Incidence of NNRTI resistance mutations at baseline, according to the different codons. (B) Incidence of mutations at codons 103, 181, and 190 at baseline, according to the NNRTI to which patients had been exposed: nevirapine (NVP), delavirdine (DLV), or efavirenz (EFV).
Patient characteristics during follow-up.
Follow-up samples were available after a median time of 6 months (range, 4 to 8 months) in all patients and 12 months in 22 patients (range, 10 to 16 months). All but two patients had a persistent viral replication during follow-up. The median viral loads were 5.05 and 4.72 log copies/ml at months 6 and 12, respectively, and were not significantly different from the baseline viral load. All patients except one received antiretroviral therapy during follow-up. Antiretroviral therapy during follow-up was a combination of nucleosides and protease inhibitor in 20 patients and 2 or 3 nucleosides in 8 patients. The mean number of TAMs did not vary significantly during follow-up: 2.93 ± 1.93 at month 6 and 3.18 ± 2.11 at month 12 versus 3.44 ± 1.69 at baseline.
Evolution of NNRTI resistance mutations during follow-up.
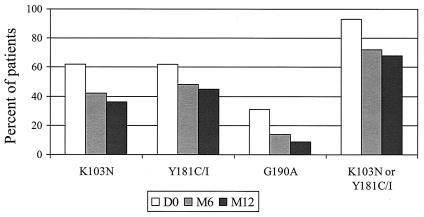
The mean number of resistance mutations to NNRTIs was significantly lower at months 6 (1.34 ± 1.04) and 12 (1.18 ± 1.05) than at month 0 (2.03 ± 1.02) (P < 0.009). The percentages of patients with at least one NNRTI-associated resistance mutation were 100, 76, and 73% at baseline, month 6, and month 12, respectively (P < 0.0044). The percentages of patients with at least two resistance mutations were 66, 45, and 32% at baseline, month 6, and month 12, respectively (P < 0.053). Persistence of mutations was not associated with a longer duration of previous NNRTI therapy, since the duration of NNRTI therapy was 8.7 ± 5.7 months in patients who lost all NNRTI mutations at month 6 compared to 7.5 ± 3.2 months in patients who had at least one NNRTI resistance mutation at month 6. Figure 2 describes the evolution of NNRTI resistance mutations over time according to the codon. Mutation at codon 190 appeared to be less stable, since it remained in less than one-third of the patients who harbored this mutation at baseline. Overall, 70% of the patients had a mutation at codon 103 or 181 at the end of follow-up.
FIG. 2.
Evolution over time of the incidence of resistance mutations at codons 103, 181, and 190. D0, day 0; M6 and M12, months 6 and 12, respectively.
We studied the evolution of NNRTI resistance mutations according to the presence or absence at baseline of the M184V mutation. The M184V mutation was detected at baseline in 7 of the 30 patients. The presence of M184V at baseline did not appear to influence the evolution of NNRTI resistance mutations. In fact, at month 6, the mean decreases in the number of NNRTI resistance mutations were −0.68 ± 0.78 and −0.71 ± 0.76 when M184V was absent or present at baseline, respectively. The percentages of patients harboring at least one NNRTI resistance mutation at month 6 were 77% (17 of 22 patients) and 71% (5 of 7 patients) when M184V was absent or present at baseline, respectively.
Plasma NNRTI levels.
Plasma samples stored during NNRTI interruption were available for 23 patients. Plasma NNRTI levels measured retrospectively were below the limit of quantification in all of these subjects.
DISCUSSION
Agents in the NNRTI class have high potency and favorable pharmacokinetic properties, but their use is complicated by the risk of high-level resistance following the development of a single mutation, as well as a high level of intraclass cross-resistance. These properties usually preclude the iterative use of NNRTIs in successive lines of antiretroviral therapy. A better knowledge of the evolution of NNRTI resistance mutations in patients interrupting NNRTI therapy could be helpful to understand the interactions between these mutations and viral replication and to assess whether NNRTI recycling is a potential therapeutic option in pretreated patients with few or no active drugs remaining available.
At baseline, genotypic testing of the HIV-1 RT gene showed NNRTI-associated mutations in 100% of patients. Mutations were found mainly at codons 103, 181, and 190. Resistance profile at baseline according to the NNRTI administered was in agreement with the results of previous studies, showing the high incidence of Y181C, G190A, and K103N mutations in patients failing nevirapine therapy (7, 11, 12), K103N in patients failing efavirenz therapy (3, 7), and Y181C and K103N in patients failing delavirdine therapy (8, 9, 15). Although it has been reported that Y181C mutation was unlikely to be selected by efavirenz (3), this mutation was found in two out of the four patients who had been exposed to efavirenz only. Preliminary studies showed that the Y181C mutation did not alter susceptibility to efavirenz in vitro (21), suggesting that this mutation would not reduce the efavirenz antiviral effect in vivo. However, further in vivo studies reported that patients with viral genotypes harboring the Y181C mutation did not respond to subsequent efavirenz-containing therapy (1; D. Baker, M. Paul, S. Jeffrey, K. Abremski, and L. T. Bacheler, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2200, 1999).
The P236L mutation, which confers resistance to delavirdine but hypersensitivity to other NNRTIs, was never noted in any of the samples tested. This is in agreement with the results of previous studies, showing that this mutation occurs infrequently in patients exposed to delavirdine (8, 9), contrary to what is observed in vitro (10).
Several studies have evaluated the evolution of resistance mutations after withdrawal of antiretroviral therapy in patients failing treatment (5, 14, 18) and reported that there was a shift from drug-resistant to drug-susceptible virus in the majority of patients. This shift was associated with increased plasma HIV-1 RNA levels and decreased CD4 T-cell counts (5, 18). Our data were apparently conflicting with the results obtained with studies evaluating structured treatment interruptions. However, the shift from resistant to wild-type virus reported in these studies appeared to be related to the release of archived wild-type virus strains that outcompete and replace the unstable, less-fit, drug-resistant strains, rather than to reversion of resistance mutations (16). Thus, the high degree of persistence of NNRTI resistance mutations in our study was not surprising. In fact, all of our patients except one continued to receive antiretroviral therapy, which was not suppressive, as judged by the median viral load at 6 and 12 months. Persistence of NNRTI resistance mutations may be explained by linkage of these mutations to other protease inhibitor and NRTI resistance mutations that were maintained under selective pressure of antiretroviral drugs.
The lack of impact of NNRTI resistance mutations on viral fitness may have also contributed to the persistence of these mutations in our patients. In vitro studies have shown that the Y181C resistant mutant was more fit than the wild type in the absence of drug, contrary to the V190A mutant, which was less fit than the wild type (13). Huang et al. reported that K103N and Y181C had little or no effect on replication capacity, contrary to V106A, P225H, and P236L (W. Huang, T. Wrin, A. Gamamik, J. Beauchaine, J. M. Whitcomb, and C. J. Retropoulos, Abstr. XI Int. HIV Drug Resist. Workshop, abstr. 72, 2002.
The M184V mutation results in diminished viral fitness, and it has been shown in vitro that M184V-containing virus was not able to mutate extensively (20). Thus, we could expect a less important decrease in the mean number of NNRTI resistance mutations in the subgroup of patients with M184V mutation at time of NNRTI withdrawal, but this was not the case. The interpretation of our data, however, remains limited by (i) the small sample size and (ii) the heterogeneity of antiretroviral treatment after NNRTI interruption, with some patients only remaining under 3TC therapy. Firm conclusions could not be drawn on the influence of M184V mutation on the evolution of genome at codons involved in resistance to NNRTIs.
Our results are in agreement with results of previous preliminary studies. In a group of 10 patients failing efavirenz-containing therapy, Baker and Bacheler reported that the K103N mutation, which was present in all patients at the time of efavirenz interruption, persisted in 7 subjects through follow-up (18 to 336 days) (D. Baker and L. Bacheler, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1545, 2000). In another study, Miller et al. reported that the K103N mutation was detected in viral strains obtained from two of three patients who had interrupted loviride therapy for at least 8 months (17).
In conclusion, our study showed the persistence of at least one resistance mutation to NNRTIs in 70% of patients discontinuing NNRTIs but still being treated with other antiretroviral drugs, at 6 and 12 months after NNRTI withdrawal. These results suggest that these mutations do not confer a selective viral fitness disadvantage. Thus, there would be little rationale for continuing a failing NNRTI-containing regimen in order to maintain a viral population with decreased fitness, in contrast to suggestions regarding the M184V mutation to lamivudine. Our results suggest that resistance mutations to different classes of drugs are associated on the same genome, at least in part of the resistant strains, and that a therapeutic strategy of selective interruption of antiretroviral therapy, suppressing one class of drugs, would not lead to reemergence of viral strains with sensitivity to the compounds of this class.
REFERENCES
- 1.Antinori, A., M. Zaccarelli, A. Cingolani, F. Forbici, M. G. Rizzo, M. P. Trotta, S. Di Giambenedetto, P. Narciso, A. Ammassari, E. Girardi, A. De Luca, and C. F. Perno. 2002. Cross-resistance among nonnucleoside reverse transcriptase inhibitors limits recycling efavirenz after nevirapine failure. AIDS Res. Hum. Retrovir. 18:835-838. [DOI] [PubMed] [Google Scholar]
- 2.Bacheler, L. T. 1999. Resistance to non-nucleoside inhibitors of HIV-1 reverse transcriptase. Drug Resist. Updates 2:56-67. [DOI] [PubMed] [Google Scholar]
- 3.Bacheler, L. T., E. D. Anton, P. Kudish, D. Baker, J. Bunville, K. Krakowski, L. Bolling, M. Aujay, X. V. Wang, D. Ellis, M. F. Becker, A. L. Lasut, H. J. George, D. R. Spalding, G. Hollis, and K. Abremski. 2000. Human immunodeficiency virus type 1 mutations selected in patients failing efavirenz combination therapy. Antimicrob. Agents Chemother. 44:2475-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deeks, S. G. 2001. Non nucleoside reverse transcriptase inhibitor resistance. J. Acquir. Immune Defic. Syndr. 26(Suppl. 1):S25-S33. [DOI] [PubMed] [Google Scholar]
- 5.Deeks S. G., T. Wrin, T. Liegler, R. Hoh, M. Hayden, J. D. Barbour, N. S. Hellmann, C. J. Petropoulos, J. M. McCune, M. K. Hellerstein, and R. M. Grant. 2001. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N. Engl. J. Med. 344:472-480. [DOI] [PubMed] [Google Scholar]
- 6.Deeks, S. G., R. M. Grant, T. Wrin, E. E. Paxinos, T. Liegler, R. Hoh, J. N. Martin, and C. J. Petropoulos. 2003. Persistence of drug-resistant HIV-1 after a structured treatment interruption and its impact on treatment response. AIDS 17:361-370. [DOI] [PubMed] [Google Scholar]
- 7.Delaguerre, C., R. Rohban, A. Simon, M. Mouroux, C. Tricot, R. Agher, J. M. Huraux, C. Katlama, and V. Calvez. 2001. Resistance profile and cross-resistance of HIV-1 among patients failing a non-nucleoside reverse transcriptase inhibitor-containing regimen. J. Med. Virol. 65:445-448. [PubMed] [Google Scholar]
- 8.Demeter, L. M., P. M. Meehan, G. Morse, P. Gerondelis, A. Dexter, L. Berrios, S. Cox, W. Freimuth, and R. C. Reichman. 1997. HIV-1 drug susceptibilities and reverse transcriptase mutations in patients receiving combination therapy with didanosine and delavirdine. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 14:136-144. [DOI] [PubMed] [Google Scholar]
- 9.Demeter, L. M., R. W. Shafer, P. M. Meehan, J. Holden-Wiltse, M. A. Fischl, W. W. Freimuth, M. F. Para, and R. C. Reichman. 2000. Delavirdine susceptibilities and associated reverse transcriptase mutations in human immunodeficiency virus type 1 isolates from patients in a phase I/II trial of delavirdine monotherapy (ACTG 260). Antimicrob. Agents Chemother. 44:794-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dueweke, T. J., S. M. Poppe, D. L. Romero, S. M. Swaney, A. G. So, K. M. Downey, I. W. Althaus, F. Reusser, M. Busso, L. Resnick et al. 1993. U-90152, a potent inhibitor of human immunodeficiency virus type 1 replication. Antimicrob. Agents Chemother. 37:1127-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eshleman, S. H., P. Krogstad, J. B. Jackson, Y. G. Wang, S. Lee, L. J. Wei, S. Cunningham, M. Wantman, A. Wiznia, G. Johnson, S. Nachman, P. Palumbo et al. 2001. Analysis of human immunodeficiency virus type 1 drug resistance in children receiving nucleoside analogue reverse-transcriptase inhibitors plus nevirapine, nelfinavir, or ritonavir. J. Infect. Dis. 183:1732-1738. [DOI] [PubMed] [Google Scholar]
- 12.Hanna, G. J., V. A. Johnson, D. R. Kuritzkes, D. D. Richman, A. J. Brown, A. V. Savara, J. D. Hazelwood, and R. T. D'Aquila. 2000. Patterns of resistance mutations selected by treatment of human immunodeficiency virus type 1 infection with zidovudine, didanosine, and nevirapine. J. Infect. Dis. 181:904-911. [DOI] [PubMed] [Google Scholar]
- 13.Iglesias-Ussel, M. D., C. Casado, E. Yuste, I. Olivares, and C. Lopez-Galindez. 2002. In vitro analysis of human immunodeficiency virus type 1 resistance to nevirapine and fitness determination of resistant variants. J. Gen. Virol. 83:93-101. [DOI] [PubMed] [Google Scholar]
- 14.Izopet, J., C. Souyris, A. Hance, K. Sandres-Saune, M. Alvarez, C. Pasquier, F. Clavel, J. Puel, and P. Massip. 2002. Evolution of human immunodeficiency virus type 1 populations after resumption of therapy following treatment interruption and shift in resistance genotype. Clin. Infect. Dis. 185:1506-1510. [DOI] [PubMed] [Google Scholar]
- 15.Joly, V., M. Moroni, E. Concia, A. Lazzarin, B. Hirschel, J. Jost, F. Chiodo, Z. Bentwich, W. C. Love, D. A. Hawkins, E. G. L. Wilkins, A. J. Gatell, N. Vetter, C. Greenwald, W. W. Freimuth, W. de Cian, and M/3331/0013B Study Group. 2000. Delavirdine in combination with zidovudine in treatment of human immunodeficiency virus type 1-infected patients: evaluation of efficacy and emergence of viral resistance in a randomized, comparative phase III trial. Antimicrob. Agents Chemother. 44:3155-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kijak, G. H., V. Simon, P. Balfe, J. Vanderhoeven, S. E. Pampuro, C. Zala, C. Ochoa, P. Cahn, M. Markowitz, and H. Salomon. 2002. Origin of human immunodeficiency virus type 1 quasispecies emerging after antiretroviral treatment interruption in patients with therapeutic failure. J. Virol. 76:7000-7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller, V., M.-P. de Bethune, A. Kober, M. Sturmer, K. Hertogs, R. Pauwels, P. Stoffels, and S. Staszewski. 1998. Patterns of resistance and cross-resistance to human immunodeficiency virus type 1 reverse transcriptase inhibitors in patients treated with the nonnucleoside reverse transcriptase inhibitor loviride. Antimicrob. Agents Chemother. 42:3123-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, V., C. Sabin, K. Hertogs, S. Bloor, J. Martinez-Picado, R. D'Aquila, B. Larder, T. Lutz, P. Gute, E. Weidmann, H. Rabenau, A. Phillips, and S. Staszewski. 2000. Virological and immunological effects of treatment interruptions in HIV-1 infected patients with treatment failure. AIDS 14:2857-2867. [DOI] [PubMed] [Google Scholar]
- 19.Pasquier, C., N. Millot, R. Njouom, K. Sandres, M. Cazabat, J. Puel, and J. Izopet. 2001. HIV-1 subtyping using phylogenetic analysis of pol gene sequences. J. Virol. Methods 94:45-54. [DOI] [PubMed] [Google Scholar]
- 20.Wei, X., C. Liang, M. Gotte, and M. A. Wainberg. 2002. The M184V mutation in HIV-1 reverse transcriptase reduces the restoration of wild-type replication by attenuated viruses. AIDS 16:2391-2398. [DOI] [PubMed] [Google Scholar]
- 21.Young, S. D., S. F. Britcher, L. O. Tran, L. S. Payne, W. C. Lumma, T. A. Lyle, J. R. Huff, P. S. Anderson, D. B. Olsen, S. S. Carroll, D. J. Pettitbone, J. A. O'Brien, R. G. Ball, S. K. Balani, J. H. Lin, I. Chen, W. A. Schleif, V. V. Sardana, W. J. Long, V. W. Byrnes, and E. A. Emini. 1995. L-743,726 (DMP-266): a novel, highly potent nonnucleoside inhibitor of the human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 39:2602-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]