SERCA- and SPCA-family ATPases transport Ca2+ from the cytosol into intracellular stores. Proteins such as calnexin and calreticulin regulate their affinity for the cation, which controls ER and Golgi Ca2+ levels.
Abstract
The various splice variants of the three SERCA- and the two SPCA-pump genes in higher vertebrates encode P-type ATPases of the P2A group found respectively in the membranes of the endoplasmic reticulum and the secretory pathway. Of these, SERCA2b and SPCA1a represent the housekeeping isoforms. The SERCA2b form is characterized by a luminal carboxy terminus imposing a higher affinity for cytosolic Ca2+ compared to the other SERCAs. This is mediated by intramembrane and luminal interactions of this extension with the pump. Other known affinity modulators like phospholamban and sarcolipin decrease the affinity for Ca2+. The number of proteins reported to interact with SERCA is rapidly growing. Here, we limit the discussion to those for which the interaction site with the ATPase is specified: HAX-1, calumenin, histidine-rich Ca2+-binding protein, and indirectly calreticulin, calnexin, and ERp57. The role of the phylogenetically older and structurally simpler SPCAs as transporters of Ca2+, but also of Mn2+, is also addressed.
All cells invest a considerable part of their total energy budget in active transport to keep up transmembrane (TM) ion gradients (Rolfe and Brown 1997). Prokaryotes already evolved P-type ion-transport ATPases/ion pumps to that aim (Axelsen and Palmgren 1998). The name P-type refers to the transient transfer of the γ-phosphate group of ATP to a highly conserved aspartate group in the enzyme forming a phospho-intermediate. This autophosphorylation is an important step in the pump’s catalytic cycle (Kuhlbrandt 2004). Based on amino-acid sequence comparisons and on the exon/intron layout of the corresponding genes, three types of P-type Ca2+ pumps can be discerned in Eumetazoa: the SERCA-, the SPCA-, and the PMCA-type. Whereas ancestral representatives of each type are recognized in some Eubacteria and Archaea, it is also remarkable that some Eukaryotes have apparently lost either SERCA or SPCA pumps. Yeast for instance lacks SERCA pumps whereas plants thrive well without SPCAs (Mills et al. 2008). The SERCA pumps, which are found in the endoplasmic reticulum (ER) or in the sarcoplasmic reticulum (SR) of eukaryotic cells and the evolutionary older secretory pathway ATPases (SPCA) found in the Golgi apparatus, are closely related to each other and together belong to the P2A subfamily. They form the topic of this review. The plasma-membrane Ca2+-pumps (PMCA), on the other hand, appear to be phylogenetically the oldest of the three and form the P2B-subfamily branch. PMCAs are addressed in an article by Brini and Carafoli (2009). Some further information on the evolution of the three types of ATPases was recently reviewed by Palmgren and Axelsen (1998) and Vangheluwe et al. (2009). Of the three families, only SERCA pumps translocate two Ca2+ ions and hydrolyze one ATP for each catalytic turnover. They possess two Ca2+-transport sites: site I and site II; the numbers specify the sequence of filling of the respective sites. The single Ca2+-binding site of the SPCA and PMCA pumps structurally corresponds to site II of SERCA (Toyoshima 2009).
THE UBIQUITOUS SERCA2 Ca2+ PUMP
SERCA2 Splicing Variants
Vertebrates generate multiple SERCA isoforms as a result of alternative processing of the transcripts of three paralogous SERCA genes (ATP2A1-3) (Brini and Carafoli 2009). Invertebrates typically have only a single SERCA gene that is orthologous to the vertebrate housekeeping SERCA2. The two major vertebrate SERCA2 protein isoforms are the housekeeping SERCA2b and the more specialized SERCA2a isoform. The latter is found in slow skeletal muscle and cardiac muscle, but is also expressed in lower amounts in smooth muscle and in neuronal cells (Vandecaetsbeek et al. 2009a). Recently novel SERCA2c (Dally et al. 2006) and SERCA2d (Kimura et al. 2005) isoforms were discovered in the heart, but are expressed at low levels and their physiological meaning remains to be further explored.
Physiological Role of SERCA2
The housekeeping SERCA2b Ca2+ pump serves a dual role. By translocating Ca2+ from the cytosol into the lumen of the ER, it restores the cytosolic Ca2+ concentration to its low resting level (circa 100 nM). At the same time, SERCA2b maintains a sufficiently high (circa 500 µM) luminal ER Ca2+ concentration. The ER not only serves as a useful Ca2+ store for the release of Ca2+ that activates an impressive number of cellular activities (e.g., contraction, fertilization, insulin release, etc.) but it also creates the luminal environment necessary for almost all local enzyme activities (such as protein folding and synthesis of lipids and steroids) and that controls cell fate (proliferation, apoptosis, growth, or differentiation) (Wuytack et al. 2002).
The muscle variant SERCA2a removes the Ca2+ stimulus for contraction by pumping myoplasmic Ca2+ into the SR and thereby determines the Ca2+ load of the SR, which in turn determines the amount of Ca2+ that can be released for the next contraction. Together, SERCA2a is a major determinant of the speed and force of cardiac contraction and relaxation (Periasamy and Huke 2001). SERCA2 expression is reduced in end-stage heart failure, contributing to an impaired contractility of the heart (Hasenfuss et al. 1994).
Ablation in mice of the two Atp2a2 alleles is incompatible with life (Periasamy et al. 1999). But in light of the central role SERCA2a exerts in the heart, it is quite surprising that in an inducible cardiac-specific knock-out mouse model at 4 weeks following Atp2a2 gene deletion, cardiac function remained near normal in spite of the drop of the myocardial SERCA2 levels below 5% of controls (Andersson et al. 2009). However, end-stage heart failure developed at 7 weeks. These results show the remarkable power of a compensatory (albeit ultimately failing) response to such a major acute reduction in SERCA2 function (Andersson et al. 2009). The effect of heterozygous knock-out of Atp2a2 in mice is also paralleled by compensatory responses, with only slight impact on cardiac contractility and relaxation without eliciting cardiac disease (Periasamy et al. 1999; Ji et al. 2000). With age, these heterozygotes are prone to develop squamous cell tumors, which supports the notion that altered Ca2+ homeostasis plays a significant role in cancer (Liu et al. 2001; Prasad et al. 2005). Likewise, humans lacking one functional ATP2A2 allele do not develop cardiomyopathy (Tavadia et al. 2001), but the effect of reduced Ca2+ uptake activity is manifested in keratinocytes, where it triggers the onset of the skin disorder of Darier (Sakuntabhai et al. 1999).
Whereas previous studies suggest that changes in SERCA2 expression levels are reasonably well tolerated in the heart (Ji et al. 2000; Tavadia et al. 2001), other studies point to a more critical regulation of the apparent affinity of the Ca2+ pump for cytosolic Ca2+ ions (MacLennan and Kranias 2003; Vandecaetsbeek et al. 2009a; Sipido and Vangheluwe 2010). For normal cardiac function, the affinity of SERCA2a in the cardiac SR needs to be controlled within a tight window (Vangheluwe et al. 2005a; Vandecaetsbeek et al. 2009a). Genetic manipulations in mouse that lead to the expression of the high Ca2+-affinity variant SERCA2b in the cardiomyocyte instead of the normal SERCA2a, triggers cardiac hypertrophy and heart failure (Ver Heyen et al. 2001; Vangheluwe et al. 2006b). Likewise, in humans (Haghighi et al. 2003), but not in mice (Luo et al. 1994), the increased Ca2+ affinity resulting from the absence of phospholamban (PLN, i.e., an affinity modulator of the pump, discussed below) triggers heart failure (Haghighi et al. 2003). On the contrary, a chronic reduction in the Ca2+ affinity triggered by a higher activity of PLN is also associated with dilated cardiomyopathy in humans (Haghighi et al. 2001; Schmitt et al. 2003; Haghighi et al. 2006).
The Ca2+-Pumping Mechanism
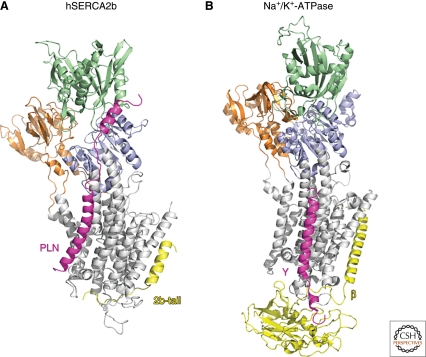
Ten years ago, the first high-resolution crystal structure of the fast-twitch skeletal-muscle isoform SERCA1a was published (Toyoshima et al. 2000). Since then, we have been spoilt by high-resolution crystal structures of SERCA1a in nine different conformations, yielding detailed molecular insights of the Ca2+-pumping process (reviewed in Moller et al. 2005; Toyoshima 2008; Toyoshima 2009). In addition, structures of other archetypical P-type ATPases (Na+/K+-ATPase [Morth et al. 2007; Shinoda et al. 2009] and H+-ATPase [Pedersen et al. 2007]) were reported. The basic structure of these P-type ATPases is very well conserved, even if the overall sequence similarity is low (Fig. 1). Three cytosolic domains can be recognized in the P-type ATPases: a nucleotide-binding (N), phosphorylation (P), and actuator (A) domain (Fig. 1). ATP binds on the N-domain, whereas the P-domain drives ATP hydrolysis leading to phosphorylation of a highly conserved aspartate in the P-domain. The A-domain then contains a conserved glutamate that catalyzes the dephosphorylation of the P-domain (Kuhlbrandt 2004; Vangheluwe et al. 2009). The large headpiece is intimately connected with and partially embedded in the TM region that contains the ion-binding sites. This connection assures tight coupling between ATP hydrolysis in the cytosolic domains and ion transport across the membrane. Surprisingly, the overall structure of the TM region is also highly conserved with only subtle differences accounting for ion specificity (Gadsby 2007). The accessibility of the TM Ca2+-binding sites in SERCA1a is controlled by both a cytosolic and a luminal gate, which are under control of the phosphorylation and dephosphorylation events, respectively, in the headpiece (Moller et al. 2005; Toyoshima 2008; Toyoshima 2009). Moreover, a feedback mechanism associated with ion binding guarantees that ATP hydrolysis can only occur when ions are bound. This tight coupling assures that first the cytosolic gate closes and Ca2+ ions are occluded before ATP hydrolysis and opening of the luminal gate can occur (Moller et al. 2005; Toyoshima 2008; Toyoshima 2009). This allows Ca2+ ions to be pumped against an almost 10000-fold gradient across the ER/SR membrane (Toyoshima 2009).
Figure 1.
Interesting structural similarities between SERCA2b and Na+/K+-ATPase. (A) The PLN NMR structure (Seidel et al. 2008) and the carboxyl terminus of SERCA2b (Vandecaetsbeek et al. 2009b) modeled on the E2 crystal structure of rabbit SERCA1a (2AGV) (Obara et al. 2005). (B) Crystal structure of the pig renal Na+/K+-ATPase α-subunit (2ZXE) (Shinoda et al. 2009) in the E2 conformation, together with its regulatory β- and γ-subunits. Interesting similarities exist between the binding sites of the regulatory β- and γ-subunits on the Na+/K+-ATPase and, respectively, the 2b-tail and PLN on the SERCA1a pump. Orange: A-domain; Blue: P-domain; Green: N-domain; Gray: TM-domain. PLN, phospholamban; SLN, sarcolipin; 2b-tail, SERCA2b carboxyl terminus.
Structure of the Ubiquitous SERCA2b Pump
Although the ubiquitous SERCA2b pump shares an overall 85% sequence identity with SERCA1a, which points to a common Ca2+-pumping mechanism (Toyoshima 2009), three related properties discriminate the SERCA2b isoform from SERCA1a or SERCA2a: the characteristic two-fold higher affinity for cytosolic Ca2+ ions, the lower maximal turnover rate and the presence of a unique carboxy-terminal extension (2b-tail) comprising an additional TM segment (TM11) and a luminal extension (LE) (Lytton et al. 1992; Verboomen et al. 1994; Dode et al. 2003; Vandecaetsbeek et al. 2009b). Functional measurements on SERCA2b mutants and SERCA1a-2b chimeras revealed that both of these regions contribute to the functional effect of the 2b-tail (Verboomen et al. 1994; Vandecaetsbeek et al. 2009b). Based on the known SERCA1a crystal structures and the solved NMR structure of TM11, a structural model for SERCA2b was proposed that is backed up by extensive mutagenesis results (see Fig. 1A in Vandecaetsbeek et al. 2009b). According to that model, TM11 is interacting with TM7 and TM10 of the Ca2+ ATPase, a relatively immobile part of the pump. A groove between luminal loops L5-6 and L7-8 is opened at the luminal side of TM11, for the descent of LE. This displacement allows that the peptide consisting of the last four, crucial amino-acids at the pump’s carboxyl terminus (1039-MFWS) reaches a luminal binding pocket that is formed by the five luminal loops of the pump (Vandecaetsbeek et al. 2009b). This intramolecular interaction stabilizes the pump in the Ca2+-bound E1 conformation with high-affinity binding sites facing the cytosol. Mathematical modeling confirmed that this could explain the increased apparent affinity for Ca2+ (Vandecaetsbeek et al. 2009b). Moreover, the experimentally observed slower E1-P to E2-P and E2-P to E2 transitions (Dode et al. 2003) are tightly coupled to extensive rearrangements of the proposed luminal docking site of the 2b-tail (Vandecaetsbeek et al. 2009b). How the short TM11 α-helix alters the enzymatic properties at the distant and relatively immobile TM helices TM7 and TM10, remains to be clarified.
Regulators of the ER Ca2+ Pump
Given its central position in cellular Ca2+ homeostasis, the activity of SERCA2 is prone to tight regulation. At least a dozen of different proteins were suggested to regulate SERCA2 activity (previously reviewed in Vangheluwe et al. 2005a; Vandecaetsbeek et al. 2009a). This suggests that as for the intracellular Ca2+ channels inositol-1,4,5-trisphosphate receptor (IP3R) or the ryanodine receptor (RyR) (Foskett et al. 2007), SERCA2 might form a multi-protein complex varying in composition in different cell types. However, because of its smaller size and the requirement to undergo major conformational changes during its enzymatic cycle, formation of a macromolecular SERCA complex is probably more restricted.
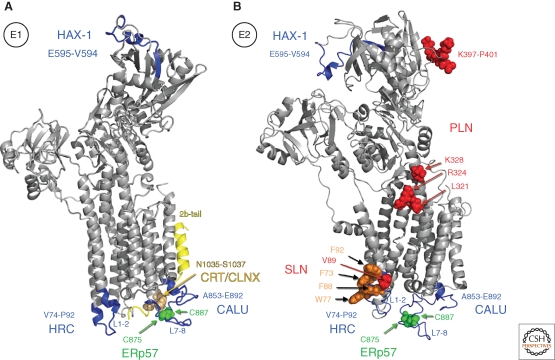
It is of some concern that studies on the effect of putative SERCA modulators often rely on overexpression, which on itself can lead to ER stress via the unfolded protein response (UPR) that includes up-regulation of SERCA2b expression and activity (Caspersen et al. 2000). In addition, the effect of these modulators is almost never confined to SERCA because they are nearly always part of the Ca2+ signalome also affecting Ca2+ release channels. Finally, direct interaction of these regulators with the pump is often documented by immunoprecipitation, which for TM proteins is technically very challenging. The thriving literature of putative SERCA regulators should therefore be viewed with caution as long as the interaction site is not properly identified. Here, we will only focus on those regulators for which the binding site on the Ca2+ pump is defined and well-documented (Fig. 2).
Figure 2.
Interaction sites of different SERCA regulators. Different interaction sites are depicted on the crystal structure of rSERCA1a in the E1 conformation (1SU4) (Toyoshima et al. 2000) (A) and in E2 (2AGV) (Obara et al. 2005) (B). Note that PLN and SLN only interact in E2, and the 2b-tail predominantly in E1, and therefore are only depicted in the respective conformations. CALU, Calumenin; PLN, phospholamban; SLN, sarcolipin; HAX-1, HS1-associated protein; CRT, calreticulin; CLNX, calnexin; ERp57, endoplasmic reticulum thiol-disulfide oxidoreductase; HRC, histidine-rich Ca2+-binding protein; 2b-tail, SERCA2b carboxyl terminus.
Phospholamban and Sarcolipin
The related small TM proteins PLN and sarcolipin (SLN) are the best-studied regulators of the SERCA pump (reviewed in MacLennan and Kranias 2003; Vangheluwe et al. 2006a; Bhupathy et al. 2007; Periasamy et al. 2008). In contrast to the 2b-tail, these proteins interact with the pump to reduce the apparent affinity for cytosolic Ca2+ ions, which inhibits overall Ca2+ transport (Lee 2003; MacLennan and Kranias 2003). In vivo, PLN is mainly coexpressed with SERCA2a in the heart, smooth muscle, and slow-twitch skeletal-muscle fibers. During the β-adrenergic response in cardiac muscle, phosphorylation of PLN by protein kinase A and/or Ca2+-calmodulin kinase II (CaMKII) promotes dissociation of the complex, which reverses the inhibition of SERCA2a (reviewed in MacLennan and Kranias 2003). Dissociated PLN also exists in a stable but inactive, pentameric state, which is promoted by phosphorylation (Kimura et al. 1997). PLN-SERCA2a dissociation causes a dramatic increase in SR Ca2+ transport leading to improved cardiac contraction and relaxation (Luo et al. 1994). Studies in numerous PLN animal models further showed its central role in cardiac contractility (reviewed in MacLennan and Kranias 2003). Moreover, human PLN mutations leading to either a chronic increase like L39stop (Haghighi et al. 2003) or decrease like R14del (Haghighi et al. 2006) or R9C (Schmitt et al. 2003) of the apparent Ca2+ affinity of the pump trigger the onset of dilated cardiomyopathy and heart failure at a young age. In line with the effect of the SERCA2a→b isoform switch (Vangheluwe et al. 2006b), these studies further indicate that regulating the Ca2+ affinity of the pump is of vital importance to maintain normal cardiac function and development (Vangheluwe et al. 2006a). This appears to be more important in humans than in mice (Haghighi et al. 2003; MacLennan et al. 2003; Zhao et al. 2006). More recent studies suggest that the regulation of the pump by PLN phosphorylation is crucial for maintaining some cardiac reserve to prevent heart failure (Schmitt et al. 2009). This is in line with an increased morbidity and mortality in heart failure patients with a lower response to β-agonists (Wu et al. 2004; Kobayashi et al. 2008).
PLN inhibits the SERCA2a and SERCA2b isoforms to the same extent (Verboomen et al. 1992), thus occupying a different affinity-regulating site on the Ca2+ pump than the 2b-tail (see Fig. 1A in Vandecaetsbeek et al. 2009b). In fact, extensive crosslinking, site-directed mutagenesis and structural modeling studies have shown that residues in both the cytoplasmic and the TM portions of PLN are involved in direct interaction with SERCA2a (Fig. 2B) (James et al. 1989; Kimura et al. 1996; Asahi et al. 1999; Asahi et al. 2001; Toyoshima et al. 2003). First proof of the direct interaction between SERCA and PLN came from a homobifunctional crosslink between a lysine in the N-domain of SERCA2a (in the region 397-401) and a lysine in the cytosolic region of PLN (K3) (James et al. 1989). To date, evidence for at least three sites of close association between SERCA1a and PLN was provided by robust homobifunctional crosslinking: between V89C positioned on TM2 of SERCA1a with V49C (Toyoshima et al. 2003), between L321C at the cytosol-membrane boundary of SERCA1a TM4 with N27C (Toyoshima et al. 2003), and between K328C in the cytosolic domain with Q23C (Morita et al. 2008). Additional heterobifunctional crosslinks were observed between the SERCA2a isoform and PLN, but unexpectedly and in apparent contrast with earlier studies, no such crosslinks were observed involving K3 of PLN (Chen et al. 2003). Phosphorylation of PLN or high Ca2+ concentrations lead to the (partial) dissociation of the PLN-SERCA2a complex preventing crosslinking (Chen et al. 2007; Morita et al. 2008). Together, these studies showed that the interaction of the PLN TM region occurs in a hydrophobic cleft only present in the Ca2+-free E2 conformation that is formed by TM2,4,6,9 (Toyoshima et al. 2003). This interaction occurs at the border between the highly mobile (TM1-6) and the immobile (TM7-10) parts of the pump and inhibits the closing of the cleft during the transition from E2 to E1. The profound effect of PLN phosphorylation on the functional and physical interaction with the Ca2+ pump already indicates that the cytosolic interaction with the N-domain could be equally important. This is further corroborated by the functional effect of mutating the cytosolic domain of PLN (reviewed in MacLennan et al. 2003). Phosphorylation would partially unwind the cytosolic region, indicating an order-to-disorder transition (Metcalfe et al. 2005; Karim et al. 2006), which would prevent more distant interactions such as a crucial H-bridge between R324 and Q26 (Traaseth et al. 2006; Traaseth et al. 2008). Together this would loosen the interaction or even cause a complete dissociation of the PLN-SERCA2a complex.
Although several lines of evidence indicate that monomeric PLN is the active species (Kimura et al. 1997), recent structural observations indicate that PLN pentamers might also interact with the pump, although at a different site (close to TM3) than the monomer. It remains unknown whether this serves a physiological function (Stokes et al. 2006).
SLN appeared to act as the functional counterpart of PLN in fast-twitch skeletal-muscle. But SLN is also found together with PLN in the atria of the heart (Minamisawa et al. 2003; Vangheluwe et al. 2005b; Babu et al. 2007a), where it modulates the activity of the SERCA2a pump and is under control of β-adrenergic stimulation (Babu et al. 2007b), presumably via CaMKII-dependent phosphorylation of T5 (Bhupathy et al. 2009). By analogy, the conservation in sequence, structure and dynamics between SLN and PLN suggest that SLN would fit into the same hydrophobic groove as PLN having similar regulatory properties (Traaseth et al. 2008). The aromatic residues of the highly conserved luminal extension RSYQY of SLN are functionally relevant (Odermatt et al. 1998) and would interact with aromatic residues on the face of luminal loop L1-2 of SERCA (possibly with the side chains of F73, W77, F88 or F92), opposite to that which constitutes the luminal interaction site of the 2b-tail (Fig. 2) (Hughes et al. 2007). TM1 undergoes a strong upward movement during the enzymatic cycle, which might be affected by this interaction. Notably, this SLN luminal tail is also crucial for proper integration of SLN in the membrane (Gramolini et al. 2004).
PLN and SLN would fit together side-by-side into the same TM cleft TM2,4,6,9, leading to a tighter functional interaction (Fig. 2B) (Asahi et al. 2003). This would explain the super-inhibitory properties of the PLN-SLN heterodimers observed in vitro (Asahi et al. 2002). Given the functional importance of the cytosolic domain of PLN and luminal extension of SLN, an additional stabilization of the complex might arise from their combined interaction with the pump (Hughes et al. 2007). So far, there is no clear evidence for this super-inhibition under physiological circumstances in the atria of the heart where both SERCA regulators are found (Bhupathy et al. 2007; Periasamy et al. 2008; Vandecaetsbeek et al. 2009a).
Surprisingly, the proposed positions of the 2b-tail and PLN/SLN on the Ca2+ pump strikingly mirrors the observed interaction site of the Na+/K+-ATPase β- and γ-subunits (Fig. 1) (Toyoshima et al. 2003; Morth et al. 2007; Vandecaetsbeek et al. 2009b). Although these modulators evolved independently from each other, they seem to occupy similar binding sites on the corresponding pump sharing similar molecular mechanisms. In all cases, the functional effect is related to a combined interaction of a TM region and luminal or cytosolic extensions with the pump, which might stabilize one of the conformational intermediates of the enzyme (Vandecaetsbeek et al. 2009b). Notably, the site of interaction of the γ-subunit was determined from the E2 Na+/K+-ATPase crystal structure (Morth et al. 2007; Shinoda et al. 2009), but in contrast to earlier modeling of PLN on SERCA1a (Toyoshima et al. 2003) and the γ-subunit on Na+/K+-ATPase (Li et al. 2004), the binding occurs on TM9, at the outside of the proposed cleft.
Antiapoptotic Proteins HAX-1 and Bcl-2
The HS1-associated protein HAX-1 (35 kDa) is an integral membrane protein normally residing in the outer mitochondrial membrane (Suzuki et al. 1997; Vafiadaki et al. 2009b). It interacts with a multitude of proteins. It was proposed that depending on the available interaction partners, the subcellular localization and functional properties of HAX-1 might vary among different tissues (Vafiadaki et al. 2009b). Recently, PLN was identified via yeast two-hybrid screen and GST-pull-down experiments as a novel interaction partner of HAX-1 (Vafiadaki et al. 2007). The site of the HAX-1 and PLN interaction is well documented and is confined to the regions 203-245 of HAX-1 and 16-22 of PLN, overlapping with the PLN phosphorylation sites (Vafiadaki et al. 2007). The direct association between HAX-1 and PLN was further established in vivo (Zhao et al. 2009). HAX-1 serves an inhibitory role on basal contractility of the heart by stabilizing the PLN monomers and lowering the apparent Ca2+ affinity of SERCA2a. Notably, this effect is reversed during β-adrenergic stimulation (Zhao et al. 2009).
The HAX-1 GST-pull-down experiments also detected SERCA2a, which implies that PLN can interact simultaneously with HAX-1 and SERCA2a, notably with similar binding affinities (KD of 0.70 µM and 1 µM, respectively (Kimura and Inui 2002; Vafiadaki et al. 2007). The HAX-1 interaction is confined to residues 575-594 in the SERCA2 N-domain, enclosing an accessible and highly conserved loop, on the opposite site of the proposed cytosolic PLN interaction region 397-401 (Fig. 2) (Vafiadaki et al. 2009a). Whether this interaction also occurs in the physiological setting of the heart remains to be investigated.
The preferential mitochondrial localization of HAX-1 in HEK-293 cells can be changed to an ER distribution on cotransfection with PLN (Vafiadaki et al. 2007), but not on cotransfection with SERCA1a or SERCA2 (Vafiadaki et al. 2009a). Interaction of HAX-1 in the outer membrane of the mitochondria and with the ER-based SERCA could be possible at the ER-mitochondrial nexus sites, which are considered crucial for eliciting apoptosis. HAX-1 overexpression in HEK-293 cells results in a posttranscriptional downregulation of SERCA2 protein levels. The resulting lower ER Ca2+ content could explain the antiapoptotic role of HAX-1 (Vafiadaki et al. 2009a). In addition, because of its association with PLN and SERCA2 on one hand, and interaction with caspase-9 on the other hand, HAX-1 might link two Ca2+-regulated processes in the heart: contractility and cell survival (Han et al. 2006).
These observations on HAX-1 are remarkably parallel to the effects of Bcl-2, another antiapoptotic protein (reviewed in Vafiadaki et al. 2009b; Vandecaetsbeek et al. 2009a). Bcl-2 is also located in the mitochondria and can be found in the ER, where it is able to interact with SERCA2. However, the putative interaction site of Bcl-2 on the pump remains to be defined, and how Bcl-2 affects ER Ca2+ reuptake remains somewhat controversial. Experimental evidence supports different alternatives: a) the interaction between SERCA and Bcl-2 inactivates the pump, presumably by destabilizing the protein (Dremina et al. 2004), b) Bcl-2 would regulate the SERCA expression levels (Kuo et al. 1998; Vanden Abeele et al. 2002), and c) Bcl-2 could inactivate SERCA by extraction of the ATPase from caveolae-related domains in the SR (Dremina et al. 2006).
SERCA Complexes Involving Luminal Proteins Calreticulin, Calnexin, and ERp57
Two of the earliest proposed SERCA2b interactors are the lectin molecular chaperones: the 46-kDa ER luminal Ca2+-binding calreticulin (CRT) and its 90-kDa homolog the type-I ER integral protein calnexin (CLNX). Both proteins contain a globular N-domain involved in glucose or oligo-saccharide binding, an extended P-domain mediating ERp57 binding and an acidic Ca2+-binding C-domain (Michalak et al. 2009). The C-domain of CRT can bind 25 mol of Ca2+ with low (2 mM) affinity (Baksh and Michalak 1991) and thus CRT complexes over half of all ER luminal Ca2+. Luminal Ca2+ buffering by CLNX is much less pronounced because it contains much less Ca2+-binding sites and its acidic carboxyl terminus protrudes into the cytosol. The direct interaction between these luminal ER Ca2+ buffers and the Ca2+ pump and release channels might represent an elegant feed-back system that controls ER Ca2+ filling (John et al. 1998; Roderick et al. 2000).
According to some early reports Ca2+-loaded CRT or CLNX would interact with the N-linked carbohydrates inserted on residues 1035-NFS in the isoform-specific luminal extension of SERCA2b (Fig. 2A) (John et al. 1998). Although this is a consensus N-glycosylation site (N1035), glycosylation was never experimentally observed (John et al. 1998; Roderick et al. 2000; Vandecaetsbeek et al. 2009b). The lack of glycosylation does however not a priori exclude CLNX or CRT binding to SERCA because these ER chaperones can occasionally also bind nonglycosylated targets (Roderick et al. 2000; Ireland et al. 2008). The interaction with CRT or CLNX would exert an inhibitory effect on the Ca2+-wave propagation in Xenopus oocytes (John et al. 1998; Roderick et al. 2000). However, mutants in this site retain normal Ca2+-dependent ATPase-activity when overexpressed in COS cells (Vandecaetsbeek et al. 2009b). According to the SERCA2b molecular model, the 2b-tail is buried in luminal loops of the pump making its interaction with other proteins less likely (Vandecaetsbeek et al. 2009b).
ERp57, a member of the PDI family with thio-oxidoreductase activity catalyzing disulfide-bond formation of glycoproteins (Ni and Lee 2007) is recruited into the SERCA2b-chaperone complex and establishes a disulfide bridge between C875 and C887 in L7-8 of SERCA2 (Fig. 2) (Li and Camacho 2004). According to the proposed model, SERCA2 with an oxidized loop (S-S bridge is present) would be inhibited and remain so as long as ERp57 is bound (Li and Camacho 2004). The conclusion that reduced C875 and C887 in L7-8 are required for full SERCA2 activity is difficult to reconcile with the observation that mutations of either or both of the cysteine residues resulted in a loss of transport without loss of Ca2+-dependent ATPase activity in SERCA1 (Daiho et al. 2001). Note that these cysteine residues are conserved in SERCA1-3, and that the C875G mutation is a known Darier mutant (Ruiz-Perez et al. 1999). Finally, we want to remark that ERp57 does not require interactions with CLNX and CRT to recognize its substrate (Zhang et al. 2009) and that CRT binds to SERCA2a oxidatively damaged by H2O2 treatment, which leads to SERCA degradation via a proteasome-dependent pathway (Ihara et al. 2005).
SERCA-Calumenin Interaction
Calumenin (CALU; 50 kDa) is a ubiquitously expressed protein, conserved from invertebrates to vertebrates, which is found in the lumen of the ER and SR (Sahoo et al. 2009). Because of its nonconsensus ER-retention signal, the protein can escape from the ER and even be secreted (Vorum et al. 1999). CALU belongs to the CREC family, which members share multiple EF-hand Ca2+-binding motifs (Honore 2009). CALU binds in its Ca2+-loaded form to the luminal domain of SERCA2 and presumably also the other SERCAs. GST-pull-down experiments with the different luminal loops of the pump showed that CALU interacts with L7-8 of the ATPase (presumably region 853-892, Fig. 2), i.e., close to or overlapping with the ERp57 interaction area, but apparently on the other side of the 2b-tail interaction site. CALU prefers the Ca2+-bound E1 conformation of SERCA, and when bound decreases the apparent Ca2+ affinity of the ATPase (Sahoo et al. 2009). Overexpression of CALU in rat neonatal cardiomyocytes reduced SR Ca2+ uptake and decreased fractional release. Thus, interaction with the ryanodine receptor RyR2 is also suggested from these experiments. CALU would be essential during the early stages of development, similar to other Ca2+-binding ER chaperone proteins like CRT, and ERp57. Much lower levels of CALU than calsequestrin are present in the adult heart.
Of note, the longest luminal loop L7-8 of SERCA2 apparently is the interaction site of several regulators (Fig. 2): the 2b-tail (Vandecaetsbeek et al. 2009b), ERp57 (Li and Camacho 2004) and CALU (Sahoo et al. 2009). Also, the extracellular loop L7-8 of the Na+/K+-ATPase α-subunit is functionally interacting with the extracellular region of the β-subunit (Morth et al. 2007). The long L7-8 would predominantly serve a regulatory function, because Ca2+ transport is supported with a much shorter L7-8, as in the closely related SPCA Ca2+ pump (Vangheluwe et al. 2009). This loop may regulate the Ca2+-binding affinity of SERCA2 through modulation of the Ca2+-binding pocket in TM8 (a true Ca2+-affinity effect) or via stabilization of an intermediate of the pump exerting a kinetic effect on the apparent Ca2+ affinity (Vandecaetsbeek et al. 2009b).
Histidine-Rich Ca2+-Binding Protein
Another luminal Ca2+-binding protein that interacts with SERCA2 is the histidine-rich Ca2+-binding protein (HRC; 170 kDa), which shows an inhibitory interaction with the luminal domain of SERCA2 where it binds to L1-2 (region 74-90, Fig. 2) (Arvanitis et al. 2007). Note that this site potentially overlaps with the binding site of SLN or the luminal extension of the 2b-tail (Hughes et al. 2007). HRC binds Ca2+ with high capacity, but low affinity (Hofmann et al. 1989; Picello et al. 1992). HRC shares similarities with calsequestrin, the major SR Ca2+ buffer protein, but is much less abundant (1% of skeletal muscle SR) (Damiani et al. 1997; Pritchard and Kranias 2009). Using different regions HRC binds in a Ca2+-dependent manner with the SERCA pump and with triadin, which is part of the RyR Ca2+-release complex (Pritchard and Kranias 2009). If the Ca2+ load in the SR is low, HRC would interact with SERCA. If HRC becomes saturated with Ca2+, it dissociates from SERCA and interacts with triadin to modulate Ca2+ release (Arvanitis et al. 2007). This dual interaction would ensure a cross-talk between SR Ca2+ uptake and release in the heart (Pritchard and Kranias 2009). However, the functional effect of HRC on SERCA2a is less clear. Overexpression of HRC in mouse results in depressed cardiomyocyte Ca2+ uptake (Gregory et al. 2006), indicating that HRC would inhibit SERCA2 activity. The fact that such inhibition would occur at low SR Ca2+, when high activity should be more appropriate to refill the SR, is somewhat counter-intuitive. Direct measurements of SERCA activity and cardiomyocyte SR Ca2+ handling in the presence and absence of HRC are needed to clarify this further.
Other SERCA Isoforms
SERCA1
SERCA1 represents a highly specialized pump isoform which, with the notable exception of brown adipose tissue (de Meis 2003), that is, a cell type embryologically closely related to muscle (Enerback 2009), appears to be almost exclusively expressed in fast skeletal muscle fibers of all vertebrates from fish to mammals. Expression of SERCA1 is spatially controlled by the type of innervation the muscle fiber receives (Hamalainen and Pette 1997). Humans and some large animals tolerate the absence of SERCA1 reasonably well as is seen in some forms of human Brody myopathy (Odermatt et al. 1996) and in congenital pseudomyotonia in Chianina cattle (Drogemuller et al. 2008), but the lack of SERCA1 is lethal in mice (Pan et al. 2003) and zebra fish (Hirata et al. 2004).
The transcript of the ATP2A1 gene can be processed into two different SERCA1 mRNAs coding for an adult SERCA1a and for SERCA1b, a form found only in neonatal or regenerating muscle (Zador et al. 2007). In SERCA1b, a highly-conserved octapeptide (-DPEDERRK) replaces the carboxy-terminal Gly residue of SERCA1a. The physiological and functional relevance of this extension remains unknown (Maruyama and MacLennan 1988; Zador et al. 2007). Insertion of the aberrant isoform into the ER reduces the ER Ca2+ concentration and induces apoptosis (Chami et al. 2000; Chami et al. 2001).
SERCA3
SERCA3 represents the last described and most enigmatic member of the SERCA family. It shows a limited cell-specific and differentiation-stage dependent expression pattern and a bewildering number of splice variants. At least six different variants in human (SERCA3a-f) are known, three in mice (SERCA3a-c) and two in rats (SERCA3a,b/c) (Dally et al. 2009). High expression of SERCA3 is found in various types of blood cells including lymphocytes, platelets, and mast cells, in endothelial cells, in epithelia of the intestinal or respiratory tract and in cerebellar Purkinje neurons (Wuytack et al. 1994; Baba-Aissa et al. 1996a). It should be mentioned, however, that in these cells SERCA3 is always coexpressed with the housekeeping SERCA2b isoform (Papp et al. 1991; Wootton and Michelangeli 2006).
All six SERCA3 splice variants present a 5- to 10-fold lower apparent affinity for cytosolic Ca2+ than SERCA2b (Chandrasekera et al. 2009). The obvious question that then arises is what the meaning is of the coexpression in a cell of the high-affinity SERCA2b with a low-affinity SERCA3. Especially, SERCA3 knock-out mice do not display any overt phenotype, further questioning the physiological importance of SERCA3.
Cells belonging to the hematopoietic lineage and epithelial or endocrine secretory cells are endowed with a complex Ca2+-signaling network (Guse et al. 1993). SERCA3 would here help to shape spatiotemporal cytosolic Ca2+ oscillation patterns (Arredouani et al. 2002). A differential subcellular localization of SERCA3 versus SERCA2, whereby SERCA3 would then most likely face an environment with locally higher Ca2+ concentration would also help in this respect. In epithelial cells, SERCA3 resides in a distinct subcellular localization positioned more at the basal region of the cell (Lee et al. 1997; Petersen 2003). A complex subcellular distribution of various SERCA3 splice variants was also described in human cardiomyocytes, although the expression levels of the various splice variants must be rather low (Dally et al. 2009). Of these, SERCA3f was found close to the plasma membrane and to be up-regulated in human failing heart (Dally et al. 2009).
In human platelets, SERCA3 is thought to reside in membranes of an acidic lysosome-related Ca2+ store, from which it can possibly be released via NAADP-gated two-pore channels (Calcraft et al. 2009; Brailoiu et al. 2010) whereas SERCA2b is confined to the so-called dense tubular system. The latter store is derived from the ER and its Ca2+ can be discharged by IP3R-mediated Ca2+-release (Juska et al. 2008). On Ca2+ depletion, each of both types of stores activates its own store-operated Ca2+-entry mechanism (SOCE) (Redondo et al. 2008b), although in the case of the acidic store SOCE appears to be more pronounced (Rosado et al. 2004). SOCE thereby relies on the formation of macromolecular complexes involving the respective SERCA isoforms. Complexes of SERCA3 and IP3R-2 in the acidic store and of a transient receptor potential channel TRPC1–TRPC6 heterodimer in the adjacent plasma membrane have been shown (Redondo et al. 2008a). On depletion of the acidic Ca2+ stores in platelets with thrombin or with a combination of thapsigargin and ionomycin, SERCA3 also forms complexes with STIM1 and Orai1 (Lopez et al. 2008).
Yet another indication for a specific role of SERCA3 in cellular Ca2+ signaling is found in its specific up-regulation during cell differentiation. Differentiation of vascular endothelium (Mountian et al. 1999), myeloid cells (Launay et al. 1999) or colon epithelial cells (Gelebart et al. 2002) is accompanied by an up-regulation of SERCA3 rather than of SERCA2b. Conversely, on malignant transformation colon cells loose their SERCA3 expression (Brouland et al. 2005) and both Epstein-Barr virus-mediated immortalization of B-lymphocytes with its accompanying lymphomagenesis and normal B-lymphocyte activation in lymph nodes are also paralleled by SERCA3 down-regulation (Dellis et al. 2009).
A number of reported germ-line mutations in the ATP2A3 gene may predispose to cancer development (Korosec et al. 2008; Korosec et al. 2009). Presumably, haploinsufficiency of this gene underlies this predisposition. Remarkably, one of these mutants is also more frequently found in type II diabetic patients (Varadi et al. 1999).
Normal SERCA3 activity in vascular endothelium (Liu et al. 1997) and in respiratory epithelium (Kao et al. 1999) is important for relaxation of the adjacent smooth muscle as shown by defects in the relaxation in SERCA3 KO mice. The reported higher resistance of SERCA3 versus SERCA2b to oxidative damage might be considered as a meaningful adaptation in these local environments (Grover et al. 2003).
SPCAs
The SPCAs, together with the SERCAs, are responsible for loading the Golgi complex and the secretory compartment with Ca2+. In contrast to SERCAs, SPCAs are also equipped to transport Mn2+ and thus supply this essential trace metal to the Golgi lumen. A number of comprehensive reviews have been published recently by our group (Vanoevelen et al. 2007; Vangheluwe et al. 2009) and by others (Dhitavat et al. 2004; Foggia and Hovnanian 2004; Brini and Carafoli 2009).
Short History
The archetypal member of the SPCA family was independently discovered in yeast (Saccharomyces cerevisiae) by two laboratories and named Plasma membrane ATPase-related, or Pmr1. Smith et al. (Smith et al. 1985) cloned PMR1 by complementation of “super-secreting” yeast mutants (ssc) while Serrano et al. (Serrano et al. 1986) identified the same gene by hybridization with a PMA1 (plasma-membrane H+-ATPase) probe. Later on, homologs were studied in many animal species and in other fungi because of its value for biotechnology (efficient secretion of heterologously expressed proteins).
In humans, the ATP2C1 gene-encoding SPCA1 was mapped to chromosome 3 and gained interest when it proved to be the gene that causes Hailey-Hailey disease (OMIM 169600), an acantholytic skin disease (Hu et al. 2000; Sudbrak et al. 2000).
A novel paralogue, ATP2C2, was found in the genome of higher vertebrates. Its protein product SPCA2 was characterized independently by two groups (Vanoevelen et al. 2005; Xiang et al. 2005). Its expression pattern suggests a more specific cellular role.
Structural Aspects of SPCAs
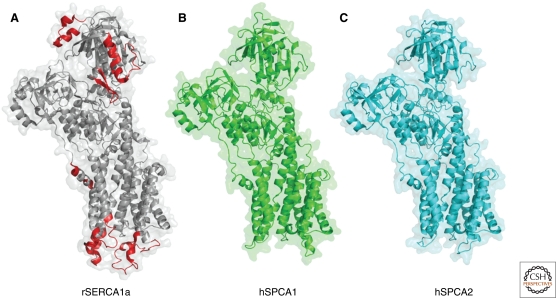
SPCAs differ from SERCAs mainly by the presence of only one ion-binding site (corresponding to site II in SERCA1). The structure of this site and its access pathway is probably affected by more distant residues and the packing of the TM helices allowing also for the transport of Mn2+ with high affinity (Wei et al. 1999; Wei et al. 2000; Van Baelen et al. 2001; Vangheluwe et al. 2009). In SPCAs, the E1 conformation is stabilized with respect to E2, explaining the observation that SPCAs have much higher apparent affinity for the transported ions than SERCAs (Dode et al. 2006). Compared to SERCA1a, structures of the two SPCA isoforms are more compact as shown by the shorter luminal and cytosolic loops (Fig. 3) (Vangheluwe et al. 2009). As indicated above, at least some of these longer loops of the SERCA pump represent specific binding sites for regulatory proteins. The homology models of SPCA1 and SPCA2 look almost identical (Fig. 3). Only minor differences are apparent, especially in the amino terminus and carboxyl terminus. In Pmr1, the amino terminus contains an EF-hand-like motif that binds Ca2+ and is crucial for Ca2+ transport (Wei et al. 1999). Although the EF-hand like motif in hSPCA1 is even more degenerate compared to PMR1, 45Ca2+-overlay experiments on the GST-purified amino terminus of hSPCA1 also indicated the binding of Ca2+ (Vanoevelen, unpublished).
Figure 3.
Comparison between the rSERCA1a, hSPCA1, and hSPCA2 structures. Homology models of hSPCA1 (B) and hSPCA2 (C) based on the E2 rSERCA1a structure (A) (1WPG) (Toyoshima et al. 2004). Homology models were obtained from the SWISS-MODEL repository (Kiefer et al. 2009). SPCA1 and SPCA2 are very similar, but in general more compact than SERCA1a. The longer loops in SERCA are indicated in red and are predominantly found in the N-domain and in the luminal loops.
Expression Pattern
SPCA1
SPCA1 is the housekeeping Ca2+ and Mn2+ pump of the secretory pathway because it is expressed in all cell types studied. However, different laboratories described different relative expression levels in various tissues. Wootton et al. (Wootton et al. 2004) observed much higher mRNA and protein expression in rat brain and testis than in other tissues whereas this difference was not observed in the corresponding tissues of humans (Hu et al. 2000; Vanoevelen et al. 2005).
The human ATP2C1 gene transcript is alternatively spliced, giving rise to different protein products. Although there has been some confusion about the various splice variants, Fairclough et al. presented a unifying study describing four isoforms (Fairclough et al. 2003). The corresponding proteins are termed SPCA1a-d and only differ in their carboxyl termini. Three splice variants SPCA1a, b, and d are functional whereas SPCA1c, which is truncated within the last TM segment, is nonfunctional and rapidly degraded (Dode et al. 2006). Exploring the ATP2C1 gene structure in the database points to the interesting peculiarity that the terminal exon of SPCA1b overlaps with the coding region of the neighboring gene Asteroid 1 whose open reading frame is oriented in the opposite direction with respect to that of ATP2C1.
The yeast Pmr1 is localized in the Golgi apparatus possibly restricted to some of its subcompartments (Antebi and Fink 1992). SPCA from Caenorhabditis elegans heterologously expressed in COS-1 cells (Van Baelen et al. 2001) and the human SPCA1 expressed in CHO cells (Ton et al. 2002) showed a localization largely coinciding with Golgi markers. It is now well established that both overexpressed SPCA and the endogenous SPCAs in a whole range of cell types are present in the Golgi compartment (reviewed in Missiaen et al. 2007).
In human spermatozoa, SPCA1 displays an unusual subcellular distribution: it is found in the area behind the nucleus extending into the midpiece. SPCA1 is believed to be the only intracellular Ca2+ pump in these cells because both functional and immunocytochemical tests failed to show the presence of SERCAs (Harper et al. 2005). A similar picture arises from sea-urchin sperm cells, which lack SERCAs. Their SPCAs are located in the zone occupied by the single giant mitochondrion where also the main ATPases involved in Ca2+-store filling are situated (Gunaratne and Vacquier 2006, 2007).
In the fly (Drosophila melanogaster), three SPCA splice-variants (SPoCk-A; SPoCk-B; SPoCk-C) are expressed. Of these isoforms, only SPoCk-A is targeted to the Golgi apparatus. The subcellular localization of SPoCk-B and SPoCk-C is less clear and unexpected targeting to, respectively, the ER and the peroxisomes was reported (Southall et al. 2006). Furthermore, expression of the SPoCk-C variant was shown to be sexually dimorphic (Southall et al. 2006).
Expression analysis in developing mouse brain showed that SPCA1 expression is prominent and at constant levels during the entire development of brain cortex, hippocampus, and cerebellum. In spite of the apparently unchanged expression levels, SPCA-associated Ca2+-ATPase activity increased with the stage of development (Sepulveda et al. 2008). SPCA1 was localized in Golgi stacks of the soma and the initial part of the primary dendritic trunk in main cortical, hippocampal and cerebellar neurons, and is present from the earliest postnatal stages onward. Although SPCA1 expression has been reported in different glial cultures (Murin et al. 2006), other efforts to show SPCA- or SERCA-pump expression in glial cells in nervous tissue were unsuccessful (Baba-Aissa et al. 1996b; Sepulveda et al. 2007; Sepulveda et al. 2008). Because glial cells express high levels of the Mn2+-dependent glutamine synthetase (Wedler and Denman 1984), the low levels of SPCAs argues against a role of SPCAs in Mn2+ uptake. However, in rat brain SPCA1 is upregulated following Mn2+ exposure (Zhang et al. 2005), which would be compatible with a role in Mn2+ detoxification, as also observed in yeast (Lapinskas et al. 1995).
SPCA2
Screening of the genome databases shows that besides the ancestral housekeeping ATP2C1 gene, a second paralogue, ATP2C2, emerged in the genomes of vertebrates higher than fish. The corresponding gene is also lacking in invertebrates.
In human tissues, SPCA2 expression is more restricted than that of SPCA1, suggesting a more specialized physiological function of the former. Its mRNA is most abundant throughout the gastrointestinal tract, in trachea, thyroid, salivary gland, mammary gland and in prostate (Vanoevelen et al. 2005). It is striking that SPCA2 is most abundantly expressed in cells possessing a highly active secretion system like the mammary gland cells during lactation (Faddy et al. 2008) and the mucin-secreting goblet cells in human colon (Dmitriev et al. 2005; Vanoevelen et al. 2005). This indicates an important role for SPCA2 in protein secretion. However, reported SPCA2 expression in keratinocytes and hippocampal neurons does not fit this picture. These data on mRNA expression should however be confirmed at the protein level. So far, the presently available antibodies could only show SPCA2 expression in cultured hippocampal neurons (Mattiazzi et al. 2005), in the colon (Vanoevelen et al. 2005), in the secretory acini of the mouse mammary gland (Faddy et al. 2008) and in neutrophil granulocytes (Baron et al. 2009).
The precise subcellular localization of SPCA2 is not completely unambiguous. In human goblet cells, both SPCA2 and SPCA1 colocalized with Golgi markers in a compact structure near the apical pole of the nucleus (Vanoevelen et al. 2005). In addition, on heterologous expression in COS-1 cells, SPCA2 appeared predominantly in the Golgi area (Missiaen et al. 2007). In cultured mouse hippocampal neurons, however, SPCA2 staining showed a punctate distribution in the cell body and in the dendrites (Xiang et al. 2005). Although in neurons the Golgi apparatus does in general appear as a more fragmented structure, SPCA2 only partially colocalized with the trans-Golgi marker TGN38. It was therefore argued that in hippocampal neurons SPCA2 is, at least partially localized in downstream, post-Golgi segments of the secretory pathway (Xiang et al. 2005). Taken together, the available data indicate that SPCA2 can be found in the Golgi complex and in more downstream compartments of the secretory pathway.
Role of SPCAs in Cellular Physiology
Insights from PMR1 Mutants in Yeast
Although homozygous null mutations in the ATP2C1 gene encoding SPCA1 seem to be lethal in mammals (Okunade et al. 2007), they are tolerated in lower eukaryotes, including fungi and C. elegans (Rudolph et al. 1989; Cho et al. 2005), where compensatory mechanisms presumably suffice to allow viability. An attractive model for understanding such mechanisms is the yeast orthologue Pmr1. PMR1 mutants in yeast display pleiotropic changes in Ca2+-dependent growth (Antebi and Fink 1992), secretion of unprocessed proteins (Antebi and Fink 1992), outer-chain glycosylation (Rudolph et al. 1989), Mn2+ tolerance (Lapinskas et al. 1995), salt tolerance (Park et al. 2001), cell shape (Cortes et al. 2004), virulence (Bates et al. 2005) and viability (Agaphonov et al. 2007). The characterization of the diverse PMR1-mutant phenotypes in yeast has been invaluable in providing the basis for studies on the role of metazoan SPCA orthologues. Some of these studies will be discussed in the following parts.
Studies in Cell Systems
Van Baelen et al. used RNA interference to understand the role of SPCA1 in HeLa cells (Van Baelen et al. 2003). Luminal [Ca2+] measurements using Golgi-targeted aequorin showed that endogenous SPCA1 was responsible for Ca2+ uptake in a subcompartment of the Golgi. On knock-down, the frequency of histamine- induced baseline Ca2+-oscillations was reduced, indicating that in these cells a SPCA1-related Ca2+-store may affect cytosolic Ca2+ signals.
SPCA1 also seems to be an important component of Ca2+ signaling in insulin-secreting cells (Mitchell et al. 2004). Knock-down of SPCA1 diminished Ca2+ uptake into the ER and in dense-core secretory vesicles, increased Ca2+ influx through L-type Ca2+ channels and increased the response to glucose. The time course of glucose-induced Ca2+ oscillations was also modified (Mitchell et al. 2004).
The same approach in cell lines expressing misfolded proteins revealed defects in protein processing and degradation (Ramos-Castaneda et al. 2005). Furthermore, SPCA1 deficiency rendered cells hypersensitive to ER stress.
Down-regulating SPCA1 in neurons compromises differentiation. The affected neurons displayed increased numbers of neurites of reduced length as compared to control cells. Additionally, Golgi Ca2+-signalling was disturbed and trafficking of proteins through the Golgi was also hampered (Sepulveda et al. 2009). It is also known that both expression and activity of SPCA1 changes on ischemic events in the brain (Pavlikova et al. 2009).
Studies in Other Model Organisms
Knockdown of SPCA1 in C. elegans rendered the worms highly sensitive to Ca2+-deficient and Mn2+-enriched conditions and made them more resistant to oxidative stress (Cho et al. 2005). These defects are reminiscent of the mutant phenotype observed in yeast, as discussed earlier.
Using a genetically transmissible RNA-interference strategy in Drosophila, Southall et al. also showed aberrant Ca2+ signaling combined with defective neuropeptide-stimulated diuresis in the Malpighian tubes of transgenic flies (Southall et al. 2006).
Expression levels and activity of SPCAs change in response to altered physiological needs. In response to changes in glucose concentration, SPCA1 expression levels significantly increased in smooth muscle cells cultured in high-glucose medium versus normal medium. Functional consequences consisted of increased ATPase activity and altered thapsigargin-insensitive AVP (arginine-vasopressin)-induced cytosolic Ca2+ transients. These results indicate that SPCA can play a role in Ca2+ uptake within smooth muscle cells (Lai and Michelangeli 2009).
Expression of SPCA1 and especially SPCA2 rapidly adapts to lactation. SPCA2 is up-regulated 35-fold whereas SPCA1 expression only rises two-fold. These results clearly suggest an important role for SPCA2 specifically (in addition to PMCA2) in the transport of high amounts of proteins and Ca2+ into milk (Faddy et al. 2008). Conversely, on mammary gland involution the expression level of both pumps is reduced 80–95% in an early phase and subsequently up-regulated again to meet normal physiological needs (Reinhardt and Lippolis 2008).
The description of the phenotype of SPCA1−/− mice has shown the important housekeeping function of SPCA (Okunade et al. 2007). Homozygous mutant mice died in utero before gestation day 10.5. The animals showed growth retardation and had an open rostral neural tube. At the subcellular level, the Golgi membranes were dilated, expanded in amount and with fewer stacked leaflets. In addition, the number of Golgi-associated vesicles was increased although processing and trafficking of proteins in the secretory pathway was apparently normal. Apoptosis was increased and a large increase of cytoplasmic lipids was observed, consistent with impaired handling of lipids by the Golgi complex. The authors introduced the concept of Golgi stress to summarize these defects (Okunade et al. 2007). Adult SPCA1 heterozygous mice were found to have an increased incidence of squamous cell tumors of epithelial cells in the skin and esophagus (Okunade et al. 2007). In addition, SERCA2 heterozygous mice developed such tumors (Graef et al. 2001). The development of squamous cell tumors in aged ATP2A2+/− and ATP2C1+/− mice indicates that SERCA2 and SPCA1 haploinsufficiency predisposes murine keratinocytes to neoplasia. The possible links between Ca2+-transporting proteins and cancer have been reviewed in detail by Monteith et al. (Monteith et al. 2007).
SPCAs and Human Disease
Hailey-Hailey disease (OMIM 169600) is an autosomal-dominant skin disease caused by the loss of one functional copy of the ATP2C1 gene encoding SPCA1 (Hu et al. 2000; Sudbrak et al. 2000). It is characterized by an increased propensity for the formation of erosive and oozing skin lesions in the flexural areas (Hailey and Hailey 1939) from the second decade of life on. In recent years, a large number of causative mutations have been described (Cialfi et al. 2009). One cannot miss the remarkable parallels between the inactivation of one allele of the ATP2A2 or ATP2C1 genes causing very similar dermatological problems, respectively, Darier and Hailey-Hailey disease (Dhitavat et al. 2004). However, in contrast to keratinocytes of Darier patients, keratinocytes of Hailey-Hailey patients show an abnormal response to extracellular Ca2+. Apparently, Darier keratinocytes behave normally in this respect because SPCA1 is up-regulated and can compensate for the partial loss of SERCA2 function (Foggia et al. 2006).
Very recently, ATP2C2 in addition to the CMIP (c-maf inducing protein) gene has genetically been linked to both a human developmental disorder termed specific language impairment (SLI) and to phonological short-term memory. Detailed analysis indicates that both genes are independenly involved. This study provides molecular evidence for a role of phonological short-term memory in language acquisition (Newbury et al. 2009).
CONCLUSIONS
SERCA and SPCA pumps help to establish and maintain low cytosolic and high luminal free Ca2+ concentration in respectively the ER and the organelles of the secretory pathway. Failure to keep this vital Ca2+ gradient results in ER stress, Golgi stress and cell death. It is thus physiologically important to maintain the activity of the pump, which is mainly accomplished by meticulously controlling the affinity of the pump for Ca2+. To that extent, the cell has at its disposal several SERCA isoforms displaying differences in Ca2+ affinity and of affinity modulators of the pump, such as phospholamban and sarcolipin. Furthermore, multitudes of additional SERCA2 modulators were recently identified, although more work is needed to clarify their functional and physiological roles.
The role of the SPCA pumps in the secretory pathway is less well understood, but a remarkable property of SPCA is its ability to transport Mn2+. Transport of Mn2+ from the cytosol to the lumen of the secretory pathway organelles provides these with a necessary cofactor for several of the resident enzymes and may be important for Mn2+ detoxification of the cells.
ACKNOWLEDGMENTS
P.V. and J.V. are Postdoctoral Fellows of the Fonds voor Wetenschappelijk Onderzoek (F.W.O.)—Vlaanderen (Research Foundation—Flanders). This work was also supported by the Interuniversity Attraction Poles Program—Belgian Science Policy IUAP P6/28, and by the F.W.O.—Vlaanderen G.0646.08 (to F.W.).
REFERENCES
- Agaphonov MO, Plotnikova TA, Fokina AV, Romanova NV, Packeiser AN, Kang HA, Ter-Avanesyan MD 2007. Inactivation of the Hansenula polymorpha PMR1 gene affects cell viability and functioning of the secretory pathway. FEMS Yeast Res 7: 1145–1152 [DOI] [PubMed] [Google Scholar]
- Andersson KB, Birkeland JA, Finsen AV, Louch WE, Sjaastad I, Wang Y, Chen J, Molkentin JD, Chien KR, Sejersted OM, et al. 2009. Moderate heart dysfunction in mice with inducible cardiomyocyte-specific excision of the Serca2 gene. J Mol Cell Cardiol 47: 180–187 [DOI] [PubMed] [Google Scholar]
- Antebi A, Fink GR 1992. The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol Biol Cell 3: 633–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredouani A, Guiot Y, Jonas JC, Liu LH, Nenquin M, Pertusa JA, Rahier J, Rolland JF, Shull GE, Stevens M, et al. 2002. SERCA3 ablation does not impair insulin secretion but suggests distinct roles of different sarcoendoplasmic reticulum Ca2+ pumps for Ca2+ homeostasis in pancreatic β-cells. Diabetes 51: 3245–3253 [DOI] [PubMed] [Google Scholar]
- Arvanitis DA, Vafiadaki E, Fan GC, Mitton BA, Gregory KN, Del Monte F, Kontrogianni-Konstantopoulos A, Sanoudou D, Kranias EG 2007. Histidine-rich Ca2+-binding protein interacts with sarcoplasmic reticulum Ca2+-ATPase. Am J Physiol Heart Circ Physiol 293: H1581–H1589 [DOI] [PubMed] [Google Scholar]
- Asahi M, Green NM, Kurzydlowski K, Tada M, MacLennan DH 2001. Phospholamban domain IB forms an interaction site with the loop between transmembrane helices M6 and M7 of sarco(endo)plasmic reticulum Ca2+ ATPases. Proc Natl Acad Sci 98: 10061–10066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi M, Kimura Y, Kurzydlowski K, Tada M, MacLennan DH 1999. Transmembrane helix M6 in sarco(endo)plasmic reticulum Ca2+-ATPase forms a functional interaction site with phospholamban. Evidence for physical interactions at other sites. J Biol Chem 274: 32855–32862 [DOI] [PubMed] [Google Scholar]
- Asahi M, Kurzydlowski K, Tada M, MacLennan DH 2002. Sarcolipin inhibits polymerization of phospholamban to induce superinhibition of sarco(endo)plasmic reticulum Ca2+-ATPases (SERCAs). J Biol Chem 277: 26725–26728 [DOI] [PubMed] [Google Scholar]
- Asahi M, Sugita Y, Kurzydlowski K, De Leon S, Tada M, Toyoshima C, MacLennan DH 2003. Sarcolipin regulates sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) by binding to transmembrane helices alone or in association with phospholamban. Proc Natl Acad Sci 100: 5040–5045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsen KB, Palmgren MG 1998. Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol 46: 84–101 [DOI] [PubMed] [Google Scholar]
- Baba-Aissa F, Raeymaekers L, Wuytack F, Callewaert G, Dode L, Missiaen L, Casteels R 1996a. Purkinje neurons express the SERCA3 isoform of the organellar type Ca2+-transport ATPase. Brain Res Mol Brain Res 41: 169–174 [DOI] [PubMed] [Google Scholar]
- Baba-Aissa F, Raeymaekers L, Wuytack F, De Greef C, Missiaen L, Casteels R 1996b. Distribution of the organellar Ca2+ transport ATPase SERCA2 isoforms in the cat brain. Brain Res 743: 141–153 [DOI] [PubMed] [Google Scholar]
- Babu GJ, Bhupathy P, Carnes CA, Billman GE, Periasamy M 2007a. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J Mol Cell Cardiol 43: 215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu GJ, Bhupathy P, Timofeyev V, Petrashevskaya NN, Reiser PJ, Chiamvimonvat N, Periasamy M 2007b. Ablation of sarcolipin enhances sarcoplasmic reticulum calcium transport and atrial contractility. Proc Natl Acad Sci 104: 17867–17872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baksh S, Michalak M 1991. Expression of calreticulin in Escherichia coli and identification of its Ca2+ binding domains. J Biol Chem 266: 21458–21465 [PubMed] [Google Scholar]
- Baron S, Struyf S, Wuytack F, Van Damme J, Missiaen L, Raeymaekers L, Vanoevelen J 2009. Contribution of intracellular Ca2+ stores to Ca2+ signaling during chemokinesis of human neutrophil granulocytes. Biochim Biophys Acta 1793: 1041–1049 [DOI] [PubMed] [Google Scholar]
- Bates S, MacCallum DM, Bertram G, Munro CA, Hughes HB, Buurman ET, Brown AJ, Odds FC, Gow NA 2005. Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence. J Biol Chem 280: 23408–23415 [DOI] [PubMed] [Google Scholar]
- Bhupathy P, Babu GJ, Periasamy M 2007. Sarcolipin and phospholamban as regulators of cardiac sarcoplasmic reticulum Ca2+ ATPase. J Mol Cell Cardiol 42: 903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhupathy P, Babu GJ, Ito M, Periasamy M 2009. Threonine-5 at the N-terminus can modulate sarcolipin function in cardiac myocytes. J Mol Cell Cardiol 47: 723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Hooper R, Cai X, Brailoiu GC, Keebler MV, Dun NJ, Marchant JS, Patel S 2010. An ancestral deuterostome family of two-pore channels mediates nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J Biol Chem 285: 2897–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M, Carafoli E 2009. Calcium pumps in health and disease. Physiol Rev 89: 1341–1378 [DOI] [PubMed] [Google Scholar]
- Brouland JP, Gelebart P, Kovacs T, Enouf J, Grossmann J, Papp B 2005. The loss of sarco/endoplasmic reticulum calcium transport ATPase 3 expression is an early event during the multistep process of colon carcinogenesis. Am J Pathol 167: 233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, et al. 2009. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459: 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen C, Pedersen PS, Treiman M 2000. The sarco/endoplasmic reticulum calcium-ATPase 2b is an endoplasmic reticulum stress-inducible protein. J Biol Chem 275: 22363–22372 [DOI] [PubMed] [Google Scholar]
- Chami M, Gozuacik D, Lagorce D, Brini M, Falson P, Peaucellier G, Pinton P, Lecoeur H, Gougeon ML, le Maire M, et al. 2001. SERCA1 truncated proteins unable to pump calcium reduce the endoplasmic reticulum calcium concentration and induce apoptosis. J Cell Biol 153: 1301–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chami M, Gozuacik D, Saigo K, Capiod T, Falson P, Lecoeur H, Urashima T, Beckmann J, Gougeon ML, Claret M, et al. 2000. Hepatitis B virus-related insertional mutagenesis implicates SERCA1 gene in the control of apoptosis. Oncogene 19: 2877–2886 [DOI] [PubMed] [Google Scholar]
- Chandrasekera PC, Kargacin ME, Deans JP, Lytton J 2009. Determination of apparent calcium affinity for endogenously expressed human sarco(endo)plasmic reticulum calcium-ATPase isoform SERCA3. Am J Physiol Cell Physiol 296: C1105–C1114 [DOI] [PubMed] [Google Scholar]
- Chen Z, Akin BL, Jones LR 2007. Mechanism of reversal of phospholamban inhibition of the cardiac Ca2+-ATPase by protein kinase A and by anti-phospholamban monoclonal antibody 2D12. J Biol Chem 282: 20968–20976 [DOI] [PubMed] [Google Scholar]
- Chen Z, Stokes DL, Rice WJ, Jones LR 2003. Spatial and dynamic interactions between phospholamban and the canine cardiac Ca2+ pump revealed with use of heterobifunctional cross-linking agents. J Biol Chem 278: 48348–48356 [DOI] [PubMed] [Google Scholar]
- Cho JH, Ko KM, Singaravelu G, Ahnn J 2005. Caenorhabditis elegans PMR1, a P-type calcium ATPase, is important for calcium/manganese homeostasis and oxidative stress response. FEBS Lett 579: 778–782 [DOI] [PubMed] [Google Scholar]
- Cialfi S, Oliviero C, Ceccarelli S, Marchese C, Barbieri L, Biolcati G, Uccelletti D, Palleschi C, Barboni L, De Bernardo C, et al. 2009. Complex multipathways alterations and oxidative stress are associated with Hailey-Hailey disease. Br J Dermatol 162: 518–526 [DOI] [PubMed] [Google Scholar]
- Cortes JC, Katoh-Fukui R, Moto K, Ribas JC, Ishiguro J 2004. Schizosaccharomyces pombe Pmr1p is essential for cell wall integrity and is required for polarized cell growth and cytokinesis. Eukaryot Cell 3: 1124–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiho T, Yamasaki K, Saino T, Kamidochi M, Satoh K, Iizuka H, Suzuki H 2001. Mutations of either or both Cys876 and Cys888 residues of sarcoplasmic reticulum Ca2+-ATPase result in a complete loss of Ca2+ transport activity without a loss of Ca2+-dependent ATPase activity. J Biol Chem 276: 32771–32778 [DOI] [PubMed] [Google Scholar]
- Dally S, Bredoux R, Corvazier E, Andersen JP, Clausen JD, Dode L, Fanchaouy M, Gelebart P, Monceau V, Del Monte F, et al. 2006. Ca2+-ATPases in non-failing and failing heart: evidence for a novel cardiac sarco/endoplasmic reticulum Ca2+-ATPase 2 isoform (SERCA2c). Biochem J 395: 249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dally S, Monceau V, Corvazier E, Bredoux R, Raies A, Bobe R, del Monte F, Enouf J 2009. Compartmentalized expression of three novel sarco/endoplasmic reticulum Ca2+ATPase 3 isoforms including the switch to ER stress, SERCA3f, in non-failing and failing human heart. Cell Calcium 45: 144–154 [DOI] [PubMed] [Google Scholar]
- Damiani E, Tobaldin G, Bortoloso E, Margreth A 1997. Functional behaviour of the ryanodine receptor/Ca2+-release channel in vesiculated derivatives of the junctional membrane of terminal cisternae of rabbit fast muscle sarcoplasmic reticulum. Cell Calcium 22: 129–150 [DOI] [PubMed] [Google Scholar]
- de Meis L 2003. Brown adipose tissue Ca2+-ATPase: uncoupled ATP hydrolysis and thermogenic activity. J Biol Chem 278: 41856–41861 [DOI] [PubMed] [Google Scholar]
- Dellis O, Arbabian A, Brouland JP, Kovacs T, Rowe M, Chomienne C, Joab I, Papp B 2009. Modulation of B-cell endoplasmic reticulum calcium homeostasis by Epstein-Barr virus latent membrane protein-1. Mol Cancer 8: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhitavat J, Fairclough RJ, Hovnanian A, Burge SM 2004. Calcium pumps and keratinocytes: lessons from Darier’s disease and Hailey-Hailey disease. Br J Dermatol 150: 821–828 [DOI] [PubMed] [Google Scholar]
- Dmitriev RI, Pestov NB, Korneenko TV, Kostina MB, Shakhparonov MI 2005. Characterization of Second Isoform of Secretory Pathway Ca2+/Mn2+-ATPase. J Gen Physiol 126: 71a–72a15955877 [Google Scholar]
- Dode L, Andersen JP, Leslie N, Dhitavat J, Vilsen B, Hovnanian A 2003. Dissection of the functional differences between sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) 1 and 2 isoforms and characterization of Darier disease (SERCA2) mutants by steady-state and transient kinetic analyses. J Biol Chem 278: 47877–47889 [DOI] [PubMed] [Google Scholar]
- Dode L, Andersen JP, Vanoevelen J, Raeymaekers L, Missiaen L, Vilsen B, Wuytack F 2006. Dissection of the functional differences between human secretory pathway Ca2+/Mn2+-ATPase (SPCA) 1 and 2 isoenzymes by steady-state and transient kinetic analyses. J Biol Chem 281: 3182–3189 [DOI] [PubMed] [Google Scholar]
- Dremina ES, Sharov VS, Schoneich C 2006. Displacement of SERCA from SR lipid caveolae-related domains by Bcl-2: a possible mechanism for SERCA inactivation. Biochemistry 45: 175–184 [DOI] [PubMed] [Google Scholar]
- Dremina ES, Sharov VS, Kumar K, Zaidi A, Michaelis EK, Schoneich C 2004. Anti-apoptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA). Biochem J 383: 361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drogemuller C, Drogemuller M, Leeb T, Mascarello F, Testoni S, Rossi M, Gentile A, Damiani E, Sacchetto R 2008. Identification of a missense mutation in the bovine ATP2A1 gene in congenital pseudomyotonia of Chianina cattle: An animal model of human Brody disease. Genomics 92: 474–477 [DOI] [PubMed] [Google Scholar]
- Enerback S 2009. The origins of brown adipose tissue. N Engl J Med 360: 2021–2023 [DOI] [PubMed] [Google Scholar]
- Faddy HM, Smart CE, Xu R, Lee GY, Kenny PA, Feng M, Rao R, Brown MA, Bissell MJ, Roberts-Thomson SJ, et al. 2008. Localization of plasma membrane and secretory calcium pumps in the mammary gland. Biochem Biophys Res Commun 369: 977–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough RJ, Dode L, Vanoevelen J, Andersen JP, Missiaen L, Raeymaekers L, Wuytack F, Hovnanian A 2003. Effect of Hailey-Hailey Disease mutations on the function of a new variant of human secretory pathway Ca2+/Mn2+-ATPase (hSPCA1). J Biol Chem 278: 24721–24730 [DOI] [PubMed] [Google Scholar]
- Foggia L, Hovnanian A 2004. Calcium pump disorders of the skin. Am J Med Genet C Semin Med Genet 131C: 20–31 [DOI] [PubMed] [Google Scholar]
- Foggia L, Aronchik I, Aberg K, Brown B, Hovnanian A, Mauro TM 2006. Activity of the hSPCA1 Golgi Ca2+ pump is essential for Ca2+-mediated Ca2+ response and cell viability in Darier disease. J Cell Sci 119: 671–679 [DOI] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH, Mak DO 2007. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87: 593–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby DC 2007. Structural biology: ion pumps made crystal clear. Nature 450: 957–959 [DOI] [PubMed] [Google Scholar]
- Gelebart P, Kovacs T, Brouland JP, van Gorp R, Grossmann J, Rivard N, Panis Y, Martin V, Bredoux R, Enouf J, et al. 2002. Expression of endomembrane calcium pumps in colon and gastric cancer cells. Induction of SERCA3 expression during differentiation. J Biol Chem 277: 26310–26320 [DOI] [PubMed] [Google Scholar]
- Graef IA, Chen F, Chen L, Kuo A, Crabtree GR 2001. Signals transduced by Ca2+/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell 105: 863–875 [DOI] [PubMed] [Google Scholar]
- Gramolini AO, Kislinger T, Asahi M, Li W, Emili A, MacLennan DH 2004. Sarcolipin retention in the endoplasmic reticulum depends on its C-terminal RSYQY sequence and its interaction with sarco(endo)plasmic Ca2+-ATPases. Proc Natl Acad Sci 101: 16807–16812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KN, Ginsburg KS, Bodi I, Hahn H, Marreez YM, Song Q, Padmanabhan PA, Mitton BA, Waggoner JR, Del Monte F, et al. 2006. Histidine-rich Ca2+ binding protein: a regulator of sarcoplasmic reticulum calcium sequestration and cardiac function. J Mol Cell Cardiol 40: 653–665 [DOI] [PubMed] [Google Scholar]
- Grover AK, Kwan CY, Samson SE 2003. Effects of peroxynitrite on sarco/endoplasmic reticulum Ca2+ pump isoforms SERCA2b and SERCA3a. Am J Physiol Cell Physiol 285: C1537–C1543 [DOI] [PubMed] [Google Scholar]
- Gunaratne JH, Vacquier VD 2006. Evidence for a secretory pathway Ca2+-ATPase in sea urchin spermatozoa. FEBS Lett 580: 3900–3904 [DOI] [PubMed] [Google Scholar]
- Gunaratne JH, Vacquier VD 2007. Sequence, annotation and developmental expression of the sea urchin Ca2+ ATPase family. Gene 397: 67–75 [DOI] [PubMed] [Google Scholar]
- Guse AH, Roth E, Emmrich F 1993. Intracellular Ca2+ pools in Jurkat T-lymphocytes. Biochem J 291: 447–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi K, Kolokathis F, Gramolini AO, Waggoner JR, Pater L, Lynch RA, Fan GC, Tsiapras D, Parekh RR, Dorn GW, et al. 2006. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci 103: 1388–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, Fan GC, Tsiapras D, Hahn HS, Adamopoulos S, et al. 2003. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest 111: 869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi K, Schmidt AG, Hoit BD, Brittsan AG, Yatani A, Lester JW, Zhai J, Kimura Y, Dorn GW, MacLennan DH, et al. 2001. Superinhibition of sarcoplasmic reticulum function by phospholamban induces cardiac contractile failure. J Biol Chem 276: 24145–24152 [DOI] [PubMed] [Google Scholar]
- Hailey HW, Hailey HE 1939. Familial benign chronic pemphigus. Arch Dermatol Syphilol 39: 679–685 [Google Scholar]
- Hamalainen N, Pette D 1997. Coordinated fast-to-slow transitions of myosin and SERCA isoforms in chronically stimulated muscles of euthyroid and hyperthyroid rabbits. J Muscle Res Cell Motil 18: 545–554 [DOI] [PubMed] [Google Scholar]
- Han Y, Chen YS, Liu Z, Bodyak N, Rigor D, Bisping E, Pu WT, Kang PM 2006. Overexpression of HAX-1 protects cardiac myocytes from apoptosis through caspase-9 inhibition. Circ Res 99: 415–423 [DOI] [PubMed] [Google Scholar]
- Harper C, Wootton L, Michelangeli F, Lefievre L, Barratt C, Publicover S 2005. Secretory pathway Ca2+-ATPase (SPCA1) Ca2+ pumps, not SERCAs, regulate complex [Ca(2+)]i signals in human spermatozoa. J Cell Sci 118: 1673–1685 [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, Holubarsch C, Posival H, Just H, Drexler H 1994. Relation between myocardial function and expression of sarcoplasmic reticulum Ca2+-ATPase in failing and nonfailing human myocardium. Circ Res 75: 434–442 [DOI] [PubMed] [Google Scholar]
- Hirata H, Saint-Amant L, Waterbury J, Cui W, Zhou W, Li Q, Goldman D, Granato M, Kuwada JY 2004. accordion, a zebrafish behavioral mutant, has a muscle relaxation defect due to a mutation in the ATPase Ca2+ pump SERCA1. Development 131: 5457–5468 [DOI] [PubMed] [Google Scholar]
- Hofmann SL, Goldstein JL, Orth K, Moomaw CR, Slaughter CA, Brown MS 1989. Molecular cloning of a histidine-rich Ca2+-binding protein of sarcoplasmic reticulum that contains highly conserved repeated elements. J Biol Chem 264: 18083–18090 [PubMed] [Google Scholar]
- Honore B 2009. The rapidly expanding CREC protein family: members, localization, function, and role in disease. Bioessays 31: 262–277 [DOI] [PubMed] [Google Scholar]
- Hu Z, Bonifas JM, Beech J, Bench G, Shigihara T, Ogawa H, Ikeda S, Mauro T, Epstein EH Jr 2000. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet 24: 61–65 [DOI] [PubMed] [Google Scholar]
- Hughes E, Clayton JC, Kitmitto A, Esmann M, Middleton DA 2007. Solid-state NMR and functional measurements indicate that the conserved tyrosine residues of sarcolipin are involved directly in the inhibition of SERCA1. J Biol Chem 282: 26603–26613 [DOI] [PubMed] [Google Scholar]
- Ihara Y, Kageyama K, Kondo T 2005. Overexpression of calreticulin sensitizes SERCA2a to oxidative stress. Biochem Biophys Res Commun 329: 1343–1349 [DOI] [PubMed] [Google Scholar]
- Ireland BS, Brockmeier U, Howe CM, Elliott T, Williams DB 2008. Lectin-deficient calreticulin retains full functionality as a chaperone for class I histocompatibility molecules. Mol Biol Cell 19: 2413–2423 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- James P, Inui M, Tada M, Chiesi M, Carafoli E 1989. Nature and site of phospholamban regulation of the Ca2+ pump of sarcoplasmic reticulum. Nature 342: 90–92 [DOI] [PubMed] [Google Scholar]
- Ji Y, Lalli MJ, Babu GJ, Xu Y, Kirkpatrick DL, Liu LH, Chiamvimonvat N, Walsh RA, Shull GE, Periasamy M 2000. Disruption of a single copy of the SERCA2 gene results in altered Ca2+ homeostasis and cardiomyocyte function. J Biol Chem 275: 38073–38080 [DOI] [PubMed] [Google Scholar]
- John LM, Lechleiter JD, Camacho P 1998. Differential modulation of SERCA2 isoforms by calreticulin. J Cell Biol 142: 963–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juska A, Jardin I, Rosado JA 2008. Physical properties of two types of calcium stores and SERCAs in human platelets. Mol Cell Biochem 311: 9–18 [DOI] [PubMed] [Google Scholar]
- Kao J, Fortner CN, Liu LH, Shull GE, Paul RJ 1999. Ablation of the SERCA3 gene alters epithelium-dependent relaxation in mouse tracheal smooth muscle. Am J Physiol 277: L264–L270 [DOI] [PubMed] [Google Scholar]
- Karim CB, Zhang Z, Howard EC, Torgersen KD, Thomas DD 2006. Phosphorylation-dependent conformational switch in spin-labeled phospholamban bound to SERCA. J Mol Biol 358: 1032–1040 [DOI] [PubMed] [Google Scholar]
- Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T 2009. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res 37: D387–D392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Nakamori M, Lueck JD, Pouliquin P, Aoike F, Fujimura H, Dirksen RT, Takahashi MP, Dulhunty AF, Sakoda S 2005. Altered mRNA splicing of the skeletal muscle ryanodine receptor and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase in myotonic dystrophy type 1. Hum Mol Genet 14: 2189–2200 [DOI] [PubMed] [Google Scholar]
- Kimura Y, Inui M 2002. Reconstitution of the cytoplasmic interaction between phospholamban and Ca2+-ATPase of cardiac sarcoplasmic reticulum. Mol Pharmacol 61: 667–673 [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kurzydlowski K, Tada M, MacLennan DH 1996. Phospholamban regulates the Ca2+-ATPase through intramembrane interactions. J Biol Chem 271: 21726–21731 [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kurzydlowski K, Tada M, MacLennan DH 1997. Phospholamban inhibitory function is activated by depolymerization. J Biol Chem 272: 15061–15064 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Izawa H, Cheng XW, Asano H, Hirashiki A, Unno K, Ohshima S, Yamada T, Murase Y, Kato TS, et al. 2008. Dobutamine stress testing as a diagnostic tool for evaluation of myocardial contractile reserve in asymptomatic or mildly symptomatic patients with dilated cardiomyopathy. J Am Coll Cardiol Img 1: 718–726 [DOI] [PubMed] [Google Scholar]
- Korosec B, Glavac D, Volavsek M, Ravnik-Glavac M 2008. Alterations in genes encoding sarcoplasmic-endoplasmic reticulum Ca2+ pumps in association with head and neck squamous cell carcinoma. Cancer Genet Cytogenet 181: 112–118 [DOI] [PubMed] [Google Scholar]
- Korosec B, Glavac D, Volavsek M, Ravnik-Glavac M 2009. ATP2A3 gene is involved in cancer susceptibility. Cancer Genet Cytogenet 188: 88–94 [DOI] [PubMed] [Google Scholar]
- Kuhlbrandt W 2004. Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol 5: 282–295 [DOI] [PubMed] [Google Scholar]
- Kuo TH, Kim HR, Zhu L, Yu Y, Lin HM, Tsang W 1998. Modulation of endoplasmic reticulum calcium pump by Bcl-2. Oncogene 17: 1903–1910 [DOI] [PubMed] [Google Scholar]
- Lai P, Michelangeli F 2009. Changes in expression and activity of the secretory pathway Ca2+ ATPase 1 (SPCA1) in A7r5 vascular smooth muscle cells cultured at different glucose concentrations. Biosci Rep 29: 397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapinskas PJ, Cunningham KW, Liu XF, Fink GR, Culotta VC 1995. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol Cell Biol 15: 1382–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay S, Gianni M, Kovacs T, Bredoux R, Bruel A, Gelebart P, Zassadowski F, Chomienne C, Enouf J, Papp B 1999. Lineage-specific modulation of calcium pump expression during myeloid differentiation. Blood 93: 4395–4405 [PubMed] [Google Scholar]
- Lee AG 2003. How phospholamban could affect the apparent affinity of Ca2+-ATPase for Ca2+ in kinetic experiments. FEBS Lett 551: 37–41 [DOI] [PubMed] [Google Scholar]
- Lee MG, Xu X, Zeng W, Diaz J, Kuo TH, Wuytack F, Racymaekers L, Muallem S 1997. Polarized expression of Ca2+ pumps in pancreatic and salivary gland cells. Role in initiation and propagation of [Ca2+]i waves. J Biol Chem 272: 15771–15776 [DOI] [PubMed] [Google Scholar]
- Li Y, Camacho P 2004. Ca2+-dependent redox modulation of SERCA 2b by ERp57. J Cell Biol 164: 35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Grosdidier A, Crambert G, Horisberger JD, Michielin O, Geering K 2004. Structural and functional interaction sites between Na+,K+-ATPase and FXYD proteins. J Biol Chem 279: 38895–38902 [DOI] [PubMed] [Google Scholar]
- Liu LH, Boivin GP, Prasad V, Periasamy M, Shull GE 2001. Squamous cell tumors in mice heterozygous for a null allele of Atp2a2, encoding the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 Ca2+ pump. J Biol Chem 276: 26737–26740 [DOI] [PubMed] [Google Scholar]
- Liu LH, Paul RJ, Sutliff RL, Miller ML, Lorenz JN, Pun RY, Duffy JJ, Doetschman T, Kimura Y, MacLennan DH, et al. 1997. Defective endothelium-dependent relaxation of vascular smooth muscle and endothelial cell Ca2+ signaling in mice lacking sarco(endo)plasmic reticulum Ca2+-ATPase isoform 3. J Biol Chem 272: 30538–30545 [DOI] [PubMed] [Google Scholar]
- Lopez JJ, Jardin I, Bobe R, Pariente JA, Enouf J, Salido GM, Rosado JA 2008. STIM1 regulates acidic Ca2+ store refilling by interaction with SERCA3 in human platelets. Biochem Pharmacol 75: 2157–2164 [DOI] [PubMed] [Google Scholar]
- Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG 1994. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of β-agonist stimulation. Circ Res 75: 401–409 [DOI] [PubMed] [Google Scholar]
- Lytton J, Westlin M, Burk SE, Shull GE, MacLennan DH 1992. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem 267: 14483–14489 [PubMed] [Google Scholar]
- MacLennan DH, Kranias EG 2003. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 4: 566–577 [DOI] [PubMed] [Google Scholar]
- MacLennan DH, Asahi M, Tupling AR 2003. The regulation of SERCA-type pumps by phospholamban and sarcolipin. Ann N Y Acad Sci 986: 472–480 [DOI] [PubMed] [Google Scholar]
- Maruyama K, MacLennan DH 1988. Mutation of aspartic acid-351, lysine-352, and lysine-515 alters the Ca2+ transport activity of the Ca2+-ATPase expressed in COS-1 cells. Proc Natl Acad Sci 85: 3314–3318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiazzi A, Mundina-Weilenmann C, Guoxiang C, Vittone L, Kranias E 2005. Role of phospholamban phosphorylation on Thr17 in cardiac physiological and pathological conditions. Cardiovasc Res 68: 366–375 [DOI] [PubMed] [Google Scholar]
- Metcalfe EE, Traaseth NJ, Veglia G 2005. Serine 16 phosphorylation induces an order-to-disorder transition in monomeric phospholamban. Biochemistry 44: 4386–4396 [DOI] [PubMed] [Google Scholar]
- Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M 2009. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J 417: 651–666 [DOI] [PubMed] [Google Scholar]
- Mills RF, Doherty ML, Lopez-Marques RL, Weimar T, Dupree P, Palmgren MG, Pittman JK, Williams LE 2008. ECA3, a Golgi-localized P2A-type ATPase, plays a crucial role in manganese nutrition in Arabidopsis. Plant Physiol 146: 116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamisawa S, Wang Y, Chen J, Ishikawa Y, Chien KR, Matsuoka R 2003. Atrial chamber-specific expression of sarcolipin is regulated during development and hypertrophic remodeling. J Biol Chem 278: 9570–9575 [DOI] [PubMed] [Google Scholar]
- Missiaen L, Dode L, Vanoevelen J, Raeymaekers L, Wuytack F 2007. Calcium in the Golgi apparatus. Cell Calcium 41: 405–416 [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Tsuboi T, Rutter GA 2004. Role for Plasma Membrane-Related Ca2+-ATPase-1 (ATP2C1) in Pancreatic β-Cell Ca2+ Homeostasis Revealed by RNA Silencing. Diabetes 53: 393–400 [DOI] [PubMed] [Google Scholar]
- Moller JV, Nissen P, Sorensen TL, le Maire M 2005. Transport mechanism of the sarcoplasmic reticulum Ca2+ -ATPase pump. Curr Opin Struct Biol 15: 387–393 [DOI] [PubMed] [Google Scholar]
- Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ 2007. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer 7: 519–530 [DOI] [PubMed] [Google Scholar]
- Morita T, Hussain D, Asahi M, Tsuda T, Kurzydlowski K, Toyoshima C, MacLennan DH 2008. Interaction sites among phospholamban, sarcolipin, and the sarco(endo)plasmic reticulum Ca2+-ATPase. Biochem Biophys Res Commun 369: 188–194 [DOI] [PubMed] [Google Scholar]
- Morth JP, Pedersen BP, Toustrup-Jensen MS, Sorensen TL, Petersen J, Andersen JP, Vilsen B, Nissen P 2007. Crystal structure of the sodium-potassium pump. Nature 450: 1043–1049 [DOI] [PubMed] [Google Scholar]
- Mountian I, Manolopoulos VG, De Smedt H, Parys JB, Missiaen L, Wuytack F 1999. Expression patterns of sarco/endoplasmic reticulum Ca2+-ATPase and inositol 1,4,5-trisphosphate receptor isoforms in vascular endothelial cells. Cell Calcium 25: 371–380 [DOI] [PubMed] [Google Scholar]
- Murin R, Verleysdonk S, Raeymaekers L, Kaplan P, Lehotsky J 2006. Distribution of secretory pathway Ca2+ ATPase (SPCA1) in neuronal and glial cell cultures. Cell Mol Neurobiol 26: 1355–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury DF, Winchester L, Addis L, Paracchini S, Buckingham LL, Clark A, Cohen W, Cowie H, Dworzynski K, Everitt A, et al. 2009. CMIP and ATP2C2 modulate phonological short-term memory in language impairment. Am J Hum Genet 85: 264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Lee AS 2007. ER chaperones in mammalian development and human diseases. FEBS Lett 581: 3641–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara K, Miyashita N, Xu C, Toyoshima I, Sugita Y, Inesi G, Toyoshima C 2005. Structural role of countertransport revealed in Ca2+ pump crystal structure in the absence of Ca2+. Proc Natl Acad Sci 102: 14489–14496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt A, Becker S, Khanna VK, Kurzydlowski K, Leisner E, Pette D, MacLennan DH 1998. Sarcolipin regulates the activity of SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem 273: 12360–12369 [DOI] [PubMed] [Google Scholar]
- Odermatt A, Taschner PE, Khanna VK, Busch HF, Karpati G, Jablecki CK, Breuning MH, MacLennan DH 1996. Mutations in the gene-encoding SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+ ATPase, are associated with Brody disease. Nat Genet 14: 191–194 [DOI] [PubMed] [Google Scholar]
- Okunade GW, Miller ML, Azhar M, Andringa A, Sanford LP, Doetschman T, Prasad V, Shull GE 2007. Loss of the Atp2c1 secretory pathway Ca2+-ATPase (SPCA1) in mice causes Golgi stress, apoptosis, and midgestational death in homozygous embryos and squamous cell tumors in adult heterozygotes. J Biol Chem 282: 26517–26527 [DOI] [PubMed] [Google Scholar]
- Palmgren MG, Axelsen KB 1998. Evolution of P-type ATPases. Biochim Biophys Acta 1365: 37–45 [DOI] [PubMed] [Google Scholar]
- Pan Y, Zvaritch E, Tupling AR, Rice WJ, de Leon S, Rudnicki M, McKerlie C, Banwell BL, MacLennan DH 2003. Targeted disruption of the ATP2A1 gene encoding the sarco(endo)plasmic reticulum Ca2+ ATPase isoform 1 (SERCA1) impairs diaphragm function and is lethal in neonatal mice. J Biol Chem 278: 13367–13375 [DOI] [PubMed] [Google Scholar]
- Papp B, Enyedi A, Kovacs T, Sarkadi B, Wuytack F, Thastrup O, Gardos G, Bredoux R, Levy-Toledano S, Enouf J 1991. Demonstration of two forms of calcium pumps by thapsigargin inhibition and radioimmunoblotting in platelet membrane vesicles. J Biol Chem 266: 14593–14596 [PubMed] [Google Scholar]
- Park SY, Seo SB, Lee SJ, Na JG, Kim YJ 2001. Mutation in PMR1, a Ca2+-ATPase in Golgi, confers salt tolerance in Saccharomyces cerevisiae by inducing expression of PMR2, a Na+-ATPase in plasma membrane. J Biol Chem 276: 28694–28699 [DOI] [PubMed] [Google Scholar]
- Pavlikova M, Tatarkova Z, Sivonova M, Kaplan P, Krizanova O, Lehotsky J 2009. Alterations induced by ischemic preconditioning on secretory pathways Ca2+-ATPase (SPCA) gene expression and oxidative damage after global cerebral ischemia/reperfusion in rats. Cell Mol Neurobiol 29: 909–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BP, Buch-Pedersen MJ, Morth JP, Palmgren MG, Nissen P 2007. Crystal structure of the plasma membrane proton pump. Nature 450: 1111–1114 [DOI] [PubMed] [Google Scholar]
- Periasamy M, Bhupathy P, Babu GJ 2008. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res 77: 265–273 [DOI] [PubMed] [Google Scholar]
- Periasamy M, Huke S 2001. SERCA pump level is a critical determinant of Ca2+ homeostasis and cardiac contractility. J Mol Cell Cardiol 33: 1053–1063 [DOI] [PubMed] [Google Scholar]
- Periasamy M, Reed TD, Liu LH, Ji Y, Loukianov E, Paul RJ, Nieman ML, Riddle T, Duffy JJ, Doetschman T, et al. 1999. Impaired cardiac performance in heterozygous mice with a null mutation in the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 (SERCA2) gene. J Biol Chem 274: 2556–2562 [DOI] [PubMed] [Google Scholar]
- Petersen OH 2003. Localization and regulation of Ca2+ entry and exit pathways in exocrine gland cells. Cell Calcium 33: 337–344 [DOI] [PubMed] [Google Scholar]
- Picello E, Damiani E, Margreth A 1992. Low-affinity Ca2+-binding sites versus Zn2+-binding sites in histidine-rich Ca2+-binding protein of skeletal muscle sarcoplasmic reticulum. Biochem Biophys Res Commun 186: 659–667 [DOI] [PubMed] [Google Scholar]
- Prasad V, Boivin GP, Miller ML, Liu LH, Erwin CR, Warner BW, Shull GE 2005. Haploinsufficiency of Atp2a2, encoding the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 Ca2+ pump, predisposes mice to squamous cell tumors via a novel mode of cancer susceptibility. Cancer Res 65: 8655–8661 [DOI] [PubMed] [Google Scholar]
- Pritchard TJ, Kranias EG 2009. Junctin and the histidine-rich Ca2+ binding protein: potential roles in heart failure and arrhythmogenesis. J Physiol 587: 3125–3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Castaneda J, Park YN, Liu M, Hauser K, Rudolph H, Shull GE, Jonkman MF, Mori K, Ikeda S, Ogawa H, et al. 2005. Deficiency of ATP2C1, a Golgi ion pump, induces secretory pathway defects in endoplasmic reticulum (ER)-associated degradation and sensitivity to ER stress. J Biol Chem 280: 9467–9473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo PC, Jardin I, Lopez JJ, Salido GM, Rosado JA 2008a. Intracellular Ca2+ store depletion induces the formation of macromolecular complexes involving hTRPC1, hTRPC6, the type II IP3 receptor and SERCA3 in human platelets. Biochim Biophys Acta 1783: 1163–1176 [DOI] [PubMed] [Google Scholar]
- Redondo PC, Salido GM, Pariente JA, Sage SO, Rosado JA 2008b. SERCA2b and 3 play a regulatory role in store-operated calcium entry in human platelets. Cell Signal 20: 337–346 [DOI] [PubMed] [Google Scholar]
- Reinhardt TA, Lippolis JD 2008. Mammary gland involution is associated with rapid down regulation of major mammary Ca2+-ATPases. Biochem Biophys Res Commun 378: 99–102 [DOI] [PubMed] [Google Scholar]
- Roderick HL, Lechleiter JD, Camacho P 2000. Cytosolic phosphorylation of calnexin controls intracellular Ca2+ oscillations via an interaction with SERCA2b. J Cell Biol 149: 1235–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe DF, Brown GC 1997. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77: 731–758 [DOI] [PubMed] [Google Scholar]
- Rosado JA, Lopez JJ, Harper AG, Harper MT, Redondo PC, Pariente JA, Sage SO, Salido GM 2004. Two pathways for store-mediated calcium entry differentially dependent on the actin cytoskeleton in human platelets. J Biol Chem 279: 29231–29235 [DOI] [PubMed] [Google Scholar]
- Rudolph HK, Antebi A, Fink GR, Buckley CM, Dorman TE, LeVitre J, Davidow LS, Mao JI, Moir DT 1989. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell 58: 133–145 [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Carter SA, Healy E, Todd C, Rees JL, Steijlen PM, Carmichael AJ, Lewis HM, Hohl D, Itin P, et al. 1999. ATP2A2 mutations in Darier’s disease: variant cutaneous phenotypes are associated with missense mutations, but neuropsychiatric features are independent of mutation class. Hum Mol Genet 8: 1621–1630 [DOI] [PubMed] [Google Scholar]
- Sahoo SK, Kim T, Kang GB, Lee JG, Eom SH, Kim do H 2009. Characterization of calumenin-SERCA2 interaction in mouse cardiac sarcoplasmic reticulum. J Biol Chem 284: 31109–31121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuntabhai A, Ruiz-Perez V, Carter S, Jacobsen N, Burge S, Monk S, Smith M, Munro CS, O’Donovan M, Craddock N, et al. 1999. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet 21: 271–277 [DOI] [PubMed] [Google Scholar]
- Schmitt JP, Ahmad F, Lorenz K, Hein L, Schulz S, Asahi M, Maclennan DH, Seidman CE, Seidman JG, Lohse MJ 2009. Alterations of phospholamban function can exhibit cardiotoxic effects independent of excessive sarcoplasmic reticulum Ca2+-ATPase inhibition. Circulation 119: 436–444 [DOI] [PubMed] [Google Scholar]
- Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, Kranias EG, MacLennan DH, Seidman JG, Seidman CE 2003. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 299: 1410–1413 [DOI] [PubMed] [Google Scholar]
- Seidel K, Andronesi OC, Krebs J, Griesinger C, Young HS, Becker S, Baldus M 2008. Structural characterization of Ca2+-ATPase-bound phospholamban in lipid bilayers by solid-state nuclear magnetic resonance (NMR) spectroscopy. Biochemistry 47: 4369–4376 [DOI] [PubMed] [Google Scholar]
- Sepulveda MR, Berrocal M, Marcos D, Wuytack F, Mata AM 2007. Functional and immunocytochemical evidence for the expression and localization of the secretory pathway Ca2+-ATPase isoform 1 (SPCA1) in cerebellum relative to other Ca2+ pumps. J Neurochem 103: 1009–1018 [DOI] [PubMed] [Google Scholar]
- Sepulveda MR, Marcos D, Berrocal M, Raeymaekers L, Mata AM, Wuytack F 2008. Activity and localization of the Secretory Pathway Ca2+-ATPase isoform 1 (SPCA1) in different areas of the mouse brain during postnatal development. Mol Cell Neurosci 38: 461–473 [DOI] [PubMed] [Google Scholar]
- Sepulveda MR, Vanoevelen J, Raeymaekers L, Mata AM, Wuytack F 2009. Silencing the SPCA1 (secretory pathway Ca2+-ATPase isoform 1) impairs Ca2+ homeostasis in the Golgi and disturbs neural polarity. J Neurosci 29: 12174–12182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R, Kielland-Brandt MC, Fink GR 1986. Yeast plasma membrane ATPase is essential for growth and has homology with (Na++K+), K+- and Ca2+-ATPases. Nature 319: 689–693 [DOI] [PubMed] [Google Scholar]
- Shinoda T, Ogawa H, Cornelius F, Toyoshima C 2009. Crystal structure of the sodium-potassium pump at 2.4 A resolution. Nature 459: 446–450 [DOI] [PubMed] [Google Scholar]
- Sipido KR, Vangheluwe P 2010. Targeting sarcoplasmic reticulum Ca2+ uptake to improve heart failure: hit or miss. Circ Res 106: 230–233 [DOI] [PubMed] [Google Scholar]
- Smith RA, Duncan MJ, Moir DT 1985. Heterologous protein secretion from yeast. Science 229: 1219–1224 [DOI] [PubMed] [Google Scholar]
- Southall TD, Terhzaz S, Cabrero P, Chintapalli VR, Evans JM, Dow JAT, Davies S-A 2006. Novel subcellular locations and functions for secretory pathway Ca2+/Mn2+-ATPases. Physiol Genomics 26: 35–45 [DOI] [PubMed] [Google Scholar]
- Stokes DL, Pomfret AJ, Rice WJ, Glaves JP, Young HS 2006. Interactions between Ca2+-ATPase and the pentameric form of phospholamban in two-dimensional co-crystals. Biophys J 90: 4213–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbrak R, Brown J, Dobson-Stone C, Carter S, Ramser J, White J, Healy E, Dissanayake M, Larregue M, Perrussel M, et al. 2000. Hailey-Hailey disease is caused by mutations in ATP2C1 encoding a novel Ca2+ pump. Hum Mol Genet 9: 1131–1140 [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Demoliere C, Kitamura D, Takeshita H, Deuschle U, Watanabe T 1997. HAX-1, a novel intracellular protein, localized on mitochondria, directly associates with HS1, a substrate of Src family tyrosine kinases. J Immunol 158: 2736–2744 [PubMed] [Google Scholar]
- Tavadia S, Tait RC, McDonagh TA, Munro CS 2001. Platelet and cardiac function in Darier’s disease. Clin Exp Dermatol 26: 696–699 [DOI] [PubMed] [Google Scholar]
- Ton VK, Mandal D, Vahadji C, Rao R 2002. Functional expression in yeast of the human secretory pathway Ca2+, Mn2+-ATPase defective in Hailey-Hailey disease. J Biol Chem 277: 6422–6427 [DOI] [PubMed] [Google Scholar]
- Toyoshima C 2008. Structural aspects of ion pumping by Ca2+-ATPase of sarcoplasmic reticulum. Arch Biochem Biophys 476: 3–11 [DOI] [PubMed] [Google Scholar]
- Toyoshima C 2009. How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim Biophys Acta 1793: 941–946 [DOI] [PubMed] [Google Scholar]
- Toyoshima C, Nomura H, Tsuda T 2004. Lumenal gating mechanism revealed in calcium pump crystal structures with phosphate analogues. Nature 432: 361–368 [DOI] [PubMed] [Google Scholar]
- Toyoshima C, Asahi M, Sugita Y, Khanna R, Tsuda T, MacLennan DH 2003. Modeling of the inhibitory interaction of phospholamban with the Ca2+ ATPase. Proc Natl Acad Sci 100: 467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima C, Nakasako M, Nomura H, Ogawa H 2000. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature 405: 647–655 [DOI] [PubMed] [Google Scholar]
- Traaseth NJ, Thomas DD, Veglia G 2006. Effects of Ser16 phosphorylation on the allosteric transitions of phospholamban/Ca2+-ATPase complex. J Mol Biol 358: 1041–1050 [DOI] [PubMed] [Google Scholar]
- Traaseth NJ, Ha KN, Verardi R, Shi L, Buffy JJ, Masterson LR, Veglia G 2008. Structural and dynamic basis of phospholamban and sarcolipin inhibition of Ca2+-ATPase. Biochemistry 47: 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafiadaki E, Arvanitis DA, Pagakis SN, Papalouka V, Sanoudou D, Kontrogianni-Konstantopoulos A, Kranias EG 2009a. The Anti-apoptotic Protein HAX-1 Interacts with SERCA2 and Regulates Its Protein Levels to Promote Cell Survival. Mol Biol Cell 20: 306–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafiadaki E, Papalouka V, Arvanitis DA, Kranias EG, Sanoudou D 2009b. The role of SERCA2a/PLN complex, Ca2+ homeostasis, and anti-apoptotic proteins in determining cell fate. Pflugers Arch 457: 687–700 [DOI] [PubMed] [Google Scholar]
- Vafiadaki E, Sanoudou D, Arvanitis DA, Catino DH, Kranias EG, Kontrogianni-Konstantopoulos A 2007. Phospholamban interacts with HAX-1, a mitochondrial protein with anti-apoptotic function. J Mol Biol 367: 65–79 [DOI] [PubMed] [Google Scholar]
- Van Baelen K, Vanoevelen J, Callewaert G, Parys JB, De Smedt H, Raeymaekers L, Rizzuto R, Missiaen L, Wuytack F 2003. The contribution of the SPCA1 Ca2+ pump to the Ca2+ accumulation in the Golgi apparatus of HeLa cells assessed via RNA-mediated interference. Biochem Biophys Res Commun 306: 430–436 [DOI] [PubMed] [Google Scholar]
- Van Baelen K, Vanoevelen J, Missiaen L, Raeymaekers L, Wuytack F 2001. The Golgi PMR1 P-type ATPase of Caenorhabditis elegans. Identification of the gene and demonstration of calcium and manganese transport. J Biol Chem 276: 10683–10691 [DOI] [PubMed] [Google Scholar]
- Vandecaetsbeek I, Raeymaekers L, Wuytack F, Vangheluwe P 2009a. Factors controlling the activity of the SERCA2a pump in the normal and failing heart. Biofactors 35: 484–499 [DOI] [PubMed] [Google Scholar]
- Vandecaetsbeek I, Trekels M, De Maeyer M, Ceulemans H, Lescrinier E, Raeymaekers L, Wuytack F, Vangheluwe P 2009b. Structural basis for the high Ca2+ affinity of the ubiquitous SERCA2b Ca2+ pump. Proc Natl Acad Sci 106: 18533–18538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Abeele F, Skryma R, Shuba Y, Van Coppenolle F, Slomianny C, Roudbaraki M, Mauroy B, Wuytack F, Prevarskaya N 2002. Bcl-2-dependent modulation of Ca2+ homeostasis and store-operated channels in prostate cancer cells. Cancer Cell 1: 169–179 [DOI] [PubMed] [Google Scholar]
- Vangheluwe P, Raeymaekers L, Dode L, Wuytack F 2005a. Modulating sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: cell biological implications. Cell Calcium 38: 291–302 [DOI] [PubMed] [Google Scholar]
- Vangheluwe P, Schuermans M, Zador E, Waelkens E, Raeymaekers L, Wuytack F 2005b. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem J 389: 151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangheluwe P, Sepulveda MR, Missiaen L, Raeymaekers L, Wuytack F, Vanoevelen J 2009. Intracellular Ca2+- and Mn2+-transport ATPases. Chem Rev 109: 4733–4759 [DOI] [PubMed] [Google Scholar]
- Vangheluwe P, Sipido KR, Raeymaekers L, Wuytack F 2006a. New perspectives on the role of SERCA2’s Ca2+ affinity in cardiac function. Biochim Biophys Acta 1763: 1216–1228 [DOI] [PubMed] [Google Scholar]
- Vangheluwe P, Tjwa M, Van Den Bergh A, Louch WE, Beullens M, Dode L, Carmeliet P, Kranias E, Herijgers P, Sipido KR, et al. 2006b. A SERCA2 pump with an increased Ca2+ affinity can lead to severe cardiac hypertrophy, stress intolerance and reduced life span. J Mol Cell Cardiol 41: 308–317 [DOI] [PubMed] [Google Scholar]
- Vanoevelen J, Dode L, Raeymaekers L, Wuytack F, Missiaen L 2007. Diseases involving the Golgi calcium pump. Subcell Biochem 45: 385–404 [DOI] [PubMed] [Google Scholar]
- Vanoevelen J, Dode L, Van Baelen K, Fairclough RJ, Missiaen L, Raeymaekers L, Wuytack F 2005. The secretory pathway Ca2+/Mn2+-ATPase 2 is a Golgi-localized pump with high affinity for Ca2+ ions. J Biol Chem 280: 22800–22808 [DOI] [PubMed] [Google Scholar]
- Varadi A, Lebel L, Hashim Y, Mehta Z, Ashcroft SJ, Turner R 1999. Sequence variants of the sarco(endo)plasmic reticulum Ca2+-transport ATPase 3 gene (SERCA3) in Caucasian type II diabetic patients (UK Prospective Diabetes Study 48). Diabetologia 42: 1240–1243 [DOI] [PubMed] [Google Scholar]
- Ver Heyen M, Heymans S, Antoons G, Reed T, Periasamy M, Awede B, Lebacq J, Vangheluwe P, Dewerchin M, Collen D, et al. 2001. Replacement of the muscle-specific sarcoplasmic reticulum Ca2+-ATPase isoform SERCA2a by the nonmuscle SERCA2b homologue causes mild concentric hypertrophy and impairs contraction-relaxation of the heart. Circ Res 89: 838–846 [DOI] [PubMed] [Google Scholar]
- Verboomen H, Wuytack F, De Smedt H, Himpens B, Casteels R 1992. Functional difference between SERCA2a and SERCA2b Ca2+ pumps and their modulation by phospholamban. Biochem J 286: 591–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verboomen H, Wuytack F, Van den Bosch L, Mertens L, Casteels R 1994. The functional importance of the extreme C-terminal tail in the gene 2 organellar Ca2+-transport ATPase (SERCA2a/b). Biochem J 303: 979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorum H, Hager H, Christensen BM, Nielsen S, Honore B 1999. Human calumenin localizes to the secretory pathway and is secreted to the medium. Exp Cell Res 248: 473–481 [DOI] [PubMed] [Google Scholar]
- Wedler FC, Denman RB 1984. Glutamine synthetase: the major Mn(II) enzyme in mammalian brain. Curr Top Cell Regul 24: 153–169 [DOI] [PubMed] [Google Scholar]
- Wei Y, Chen J, Rosas G, Tompkins DA, Holt PA, Rao R 2000. Phenotypic screening of mutations in Pmr1, the yeast secretory pathway Ca2+/Mn2+-ATPase, reveals residues critical for ion selectivity and transport. J Biol Chem 275: 23927–23932 [DOI] [PubMed] [Google Scholar]
- Wei Y, Marchi V, Wang R, Rao R 1999. An N-terminal EF hand-like motif modulates ion transport by Pmr1, the yeast Golgi Ca2+/Mn2+-ATPase. Biochemistry 38: 14534–14541 [DOI] [PubMed] [Google Scholar]
- Wootton LL, Argent CC, Wheatley M, Michelangeli F 2004. The expression, activity and localisation of the secretory pathway Ca2+ -ATPase (SPCA1) in different mammalian tissues. Biochim Biophys Acta 1664: 189–197 [DOI] [PubMed] [Google Scholar]
- Wootton LL, Michelangeli F 2006. The effects of the phenylalanine 256 to valine mutation on the sensitivity of sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) Ca2+ pump isoforms 1, 2, and 3 to thapsigargin and other inhibitors. J Biol Chem 281: 6970–6976 [DOI] [PubMed] [Google Scholar]
- Wu WC, Bhavsar JH, Aziz GF, Sadaniantz A 2004. An overview of stress echocardiography in the study of patients with dilated or hypertrophic cardiomyopathy. Echocardiography 21: 467–475 [DOI] [PubMed] [Google Scholar]
- Wuytack F, Papp B, Verboomen H, Raeymaekers L, Dode L, Bobe R, Enouf J, Bokkala S, Authi KS, Casteels R 1994. A sarco/endoplasmic reticulum Ca2+-ATPase 3-type Ca2+ pump is expressed in platelets, in lymphoid cells, and in mast cells. J Biol Chem 269: 1410–1416 [PubMed] [Google Scholar]
- Wuytack F, Raeymaekers L, Missiaen L 2002. Molecular physiology of the SERCA and SPCA pumps. Cell Calcium 32: 279–305 [DOI] [PubMed] [Google Scholar]
- Xiang M, Mohamalawari D, Rao R 2005. A novel isoform of the secretory pathway Ca2+,Mn2+-ATPase, hSPCA2, has unusual properties and is expressed in the brain. J Biol Chem 280: 11608–11614 [DOI] [PubMed] [Google Scholar]
- Zador E, Vangheluwe P, Wuytack F 2007. The expression of the neonatal sarcoplasmic reticulum Ca2+ pump (SERCA1b) hints to a role in muscle growth and development. Cell Calcium 41: 379–388 [DOI] [PubMed] [Google Scholar]
- Zhang S, Fu J, Zhou Z 2005. Changes in the brain mitochondrial proteome of male Sprague-Dawley rats treated with manganese chloride. Toxicol Appl Pharmacol 202: 13–17 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kozlov G, Pocanschi CL, Brockmeier U, Ireland BS, Maattanen P, Howe C, Elliott T, Gehring K, Williams DB 2009. ERp57 does not require interactions with calnexin and calreticulin to promote assembly of class I histocompatibility molecules, and it enhances peptide loading independently of its redox activity. J Biol Chem 284: 10160–10173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Waggoner JR, Zhang ZG, Lam CK, Han P, Qian J, Schroder PM, Mitton B, Kontrogianni-Konstantopoulos A, Robia SL, et al. 2009. The anti-apoptotic protein HAX-1 is a regulator of cardiac function. Proc Natl Acad Sci 106: 20776–20781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Yuan Q, Qian J, Waggoner JR, Pathak A, Chu G, Mitton B, Sun X, Jin J, Braz JC, et al. 2006. The presence of Lys27 instead of Asn27 in human phospholamban promotes sarcoplasmic reticulum Ca2+-ATPase superinhibition and cardiac remodeling. Circulation 113: 995–1004 [DOI] [PubMed] [Google Scholar]