Abstract
Clarithromycin was administered intravenously to 55 rabbits to evaluate its effect on experimental sepsis caused by multidrug-resistant Pseudomonas aeruginosa. Acute pyelonephritis was induced after ligation of the right ureter and injection of 108 CFU of the test isolate per kg of body weight into the renal pelvis. The animals were divided into six groups: group A, controls; group B, rabbits that received one intravenous dose of 80 mg of clarithromycin per kg concomitantly with bacterial challenge; group C, rabbits that received two doses of clarithromycin, the second one of which was given 2 h after the first one; group D, rabbits that received 15 mg of amikacin per kg; group E, rabbits that received one dose of clarithromycin and amikacin; and group F, rabbits that received two doses of clarithromycin and amikacin. Serum endotoxin levels were estimated by the QCL-1000 Limulus amoebocyte lysate assay, tumor necrosis factor alpha (TNF-α) levels were measured by a bioassay, and malondialdehyde (MDA) levels were measured by the thiobarbiturate assay. Viable bacterial counts in various tissue samples were also assessed. The mean survival times of the animals in groups A, B, C, D, E, and F were 4.50, 7.69, 4.07, 4.55, 11.55, and 11.60 days, respectively (P = 0.033 for group D versus group F, P = 0.006 for group D versus group E, P = not significant for group B versus group E, P = 0.042 for group C versus group F). Serum endotoxin levels were similar between groups at all sampling times; TNF-α and MDA levels in groups B, C, E, and F decreased significantly over follow-up. The numbers of viable bacterial cells in the infected kidney were similar among the groups; those in the liver, spleen, lungs, and mesenteral lymph nodes were significantly decreased in groups B, E, and F compared to those in groups A and D. It is concluded that a prolongation of survival in animals with experimental sepsis caused by multidrug-resistant P. aeruginosa was achieved after coadministration of clarithromycin and amikacin and that the increased survival was probably attributable to the immunomodulatory properties of clarithromycin.
Clarithromycin is a macrolide classically known to possess an antimicrobial spectrum that includes gram-positive cocci and atypical pathogens (23). However, an increasing body of evidence suggests that clarithromycin possesses considerable anti-inflammatory and immunomodulatory properties, recommending its administration for the treatment of chronic inflammatory conditions like diffuse panbronchiolitis and cystic fibrosis (11, 21, 26). Its immunomodulatory properties are observed in vitro at concentrations close to 10 μg/ml (14). On the basis of that finding, intravenous administration of clarithromycin leading to the same levels in blood might be beneficial for the treatment of an acute inflammatory state like sepsis.
Pseudomonas aeruginosa is a common pathogen that is involved in nosocomial sepsis and that is often characterized nowadays by multidrug resistance (18). In the present study, intravenous clarithromycin was coadministered with amikacin for the treatment of experimental sepsis in animals with acute pyelonephritis caused by multidrug-resistant P. aeruginosa. The study focused on assessment of the effect of clarithromycin on (i) the survival of experimental animals, (ii) the host's immune response, and (iii) the recovery of bacteria from various organs.
MATERIALS AND METHODS
Animals.
A total of 60 male New Zealand White rabbits (mean ± standard deviation weight, 3.29 ± 0.41 kg) were enrolled in the study. The study received a permit from the Veterinary Directorate of the Perfecture of Athens, according to Greek legislation, in conformance with the Council Directive of the European Union. Animals were housed individually in metal cages and had access to tap water and standard balanced rabbit chow ad libitum. The temperature ranged from 18 to 22°C, the relative humidity ranged from 55 to 65%, and the light-dark cycle was 12 h (lights on at 6 a.m. and off at 6 p.m.). Five rabbits were used for determination of the dosage of clarithromycin that could be administered safely and yield a concentration in serum close to 10 μg/ml (14), and the remaining 55 animals were used in the final study design.
Preliminary pharmacokinetic study.
Five animals were sedated by intramuscular injection of 25 mg of ketamine per kg of body weight and 5 mg of xylazine per kg. Clarithromycin for intravenous use (Abbott Laboratories, Abbott Park, Ill.) was provided as a white amorphous powder in vials; it was reconstituted in pyrogen-free dextrose water (5%) to a solution of 50 mg/ml. Appropriate amounts of that solution were further diluted with sodium chloride (0.9%) to a final volume of 30 ml; the latter was administered in the right ear vein with a 25-gauge catheter under sterile conditions. Doses equal to 20, 40, and 80 mg/kg were infused over 15 and 30 min; they were administered to all animals, with a 7-day interval between each dose. Blood was then sampled 1 h after the end of the clarithromycin infusion for estimation of the levels of drug in serum by a microbiological assay, as described below. At the end of the administration the animals were monitored each hour for 12 h. Pharmacokinetic analysis revealed that the regimen that yielded a concentration close to 10 μg/ml was 80 mg/kg infused over 30 min. This regimen was selected for the entire study design.
Bacterial isolate.
One multidrug-resistant isolate of P. aeruginosa derived from the blood of a patient with nosocomial sepsis was used. The MICs of ticarcillin-clavulanate, piperacillin, ceftazidime, imipenem, meropenem, ciprofloxacin, clarithromycin, and amikacin were determined by a microdilution technique with final volumes of 0.1 ml. The MIC was considered the lowest concentration of the antimicrobial tested that limited visible bacterial growth after 18 h of incubation at 35°C.
Time-kill assay.
A log-phase inoculum of the P. aeruginosa isolate equal to 5 × 105 CFU/ml was incubated in separate tubes with Mueller-Hinton broth (Becton Dickinson, Cockeysville, Md.) in the presence of clarithromycin, amikacin, or the combination. Clarithromycin (Abbott Laboratories) and amikacin (Bristol, Syracuse, N.Y.) were provided as amorphous powders. Clarithromycin was diluted in methanol (99%; Merck, Darmstadt, Germany), and amikacin was diluted in pyrogen-free water. They were then added to the test tubes so that the final concentrations were 10 and 20 μg/ml, respectively; 0.002% (vol/vol) methanol (99%) was present in each test tube. The concentrations of antimicrobials were selected to represent the mean levels achieved in serum in the present study. The final volume in each tube was 10 ml. One growth control tube was applied.
The tubes were left to incubate at 37°C in a shaking water bath; and at 2, 4, 6, and 24 h of growth, aliquots of 0.1 ml were taken for estimation of viable bacterial counts. Aliquots were consecutively diluted 1:10 five times in sterile water; one 0.1-ml aliquot of each dilution was plated onto MacConkey agar (BBL, Becton Dickinson, Cockeysville, Md.) to avoid any possible antimicrobial carryover effect. The lowest detection limit was 30 CFU/ml. All tubes were assayed in duplicate.
Study design.
Acute pyelonephritis was induced in 55 rabbits by a modification of a protocol described by others (2, 7, 13). The animals were initially sedated by the intramuscular injection of 25 mg of ketamine per kg and 5 mg of xylazine per kg. Anesthesia was maintained by the intramuscular administration of 15 mg of xylazine per kg at 30-min intervals. The peritoneal cavity was entered through an abdominal midline incision, and the intestines were displaced to the left. The right ureter was located and ligated with a 3.0 suture just below the pelvis. A total of 108 CFU of the P. aeruginosa isolate per kg in a volume of 0.1 ml was injected into the renal pelvis, proximal to the suture, with a 26-gauge needle. The bacterial inoculum was selected on the basis of those used in previous studies (9). The peritoneal cavity and the abdominal wall were then closed in layers. Anesthesia was maintained until the end of the administration of antimicrobials for each study group.
The animals were then divided into six study groups, as follows: group A (n = 10), control animals that received 30 ml of normal saline intravenously; group B (n = 8), animals that received the clarithromycin regimen concomitantly with the inoculation of P. aeruginosa; group C (n = 7), animals that received clarithromycin, as for animals in group B, and a second dose of clarithromycin exactly 2 h after bacterial challenge; group D (n = 10), animals that received amikacin (Bristol) 30 min after bacterial challenge at a single intravenous bolus dose of 15 mg/kg, as proposed by others (19); group E (n = 10); animals that received the clarithromycin regimen concomitantly with the inoculation of P. aeruginosa and amikacin 30 min after bacterial challenge; and group F (n = 10), animals that received clarithromycin and amikacin, as for the animals in group E, and a second dose of clarithromycin exactly 2 h after bacterial challenge.
The time interval selected for the administration of clarithromycin was based on previous experience. Pretreatment with potent immunomodulatory agents in animal studies may have led to the failure of previous clinical trials (1). Administration of clarithromycin in parallel with bacterial challenge allows (i) avoidance of pretreatment; (ii) the ability to achieve the desired drug levels within 30 to 60 min after intravenous infusion, i.e., later than bacterial inoculation; and (iii) documentation of whether the secretion of proinflammatory mediators is prevented, which constitutes the necessary step before proceeding to drug administration in the event of the presence of clinical symptoms of sepsis (9).
A 2-ml volume of blood was sampled from the vein of the left ear of each animal before the start of the operation and at 1, 2, 24, and 48 h after bacterial inoculation; it was applied for culture and for estimation of endotoxin (lipopolysaccharide [LPS]), tumor necrosis factor alpha (TNF-α), malondialdehyde (MDA), and drug levels. One more sample was drawn 3 h after bacterial challenge for estimation of drug levels in animals in groups B, C, and F. Blood was collected in pyrogen-free tubes (Vacutainer; Becton Dickinson) and centrifuged; serum was kept frozen at −70°C until it was assayed.
The survival of the animals was recorded every 12 h for a total period of follow-up of 21 days. A necropsy was performed after death; the animals that remained alive after 21 days of follow-up were killed by the intravenous administration of sodium thiopental. Segments of the right kidney, liver, spleen, the lower lobe of the right lung, and one mesenteral lymph node were taken under sterile conditions and placed in sterile plastic containers for quantitative cultures.
Assays for LPS, TNF-α, and MDA levels.
For the estimation of LPS levels, serum samples were diluted 1:10 in sterile pyrogen-free water (BioWhitaker, Walkersville, Md.) and were incubated for 5 min at 70°C. The concentration of LPS (in E units [EU] per milliliter) was then measured by the QCL-1000 Limulus amoebocyte lysate assay (lower limit of detection, 1 IU/ml; BioWhitaker) by using a standard curve created with known concentrations of Escherichia coli serotype O111:B4 LPS. All determinations were performed in duplicate, and the mean of two observations was used.
TNF-α levels were measured by a bioassay with the L929 fibrosarcoma cell line, as described previously (6). Briefly, confluent cells were thoroughly washed with Hanks solution (Biochrom AG, Berlin, Germany) and harvested with 0.25% thrypsin-0.02% EDTA (Biochrom AG). Cells were centrifuged, resuspended in RMPI 1640 (Biochrom AG) supplemented with 10% fetal bovine serum (Biochrom AG) and 2 mM glutamine (Biochrom AG), and distributed into a 96-well cell culture plate at a density of 105 cells/well. The final volume of fluid in each well was 0.05 ml. After incubation for 2 to 3 h at 37°C in an atmosphere with 5% CO2, 0.06 ml of serum was added to each well, followed by the addition of 0.05 ml of a 0.3-mg/ml dilution of cycloheximide (Sigma Chemical Co., St. Louis, Mo.). Known concentrations of human TNF-α (concentration range, 5.75 to 375.00 pg/ml; Sigma) were added at the same time. Incubation continued overnight, and then the supernatant of each well was removed by aspiration and 0.1 ml of a 0.5-mg/ml methylene blue solution in methanol (99%) was added. After 10 min, the dye was removed and wells were thoroughly washed three times with 0.9% sodium chloride. The wells were left to dry, and the remnants of the dye in each well were solubilized by the addition of 0.1 ml of 50% glacial acetic acid (Merck). The optical density of each well was read at 495 nm (Hitachi Spectophotometer, Tokyo, Japan) and compared with those of blank wells and control wells to which serum was not added. The concentrations of TNF-α were estimated by determination of the reduction of the optical density of unknown samples compared with those of the control wells by applying a standard curve generated with standard concentrations. All determinations were performed in quadruplicate. The interday variation of the assay was 13.5%.
Lipid peroxidation was estimated by determination of the MDA concentration by the thiobarbiturate assay, as described by others (8, 16). Briefly, a 0.1-ml aliquot of each sample was mixed with 0.9 ml of trichloroacetic acid (20%; Merck), and the mixture was centrifuged at 12,000 × g and 4°C for 10 min. The supernatant was removed and incubated with 1 ml of phosphate-buffered saline (Merck) and 1 ml of thiobarbituric acid (0.6%; Merck) for 20 min at 90°C. The optical density was then read at 535 nm with a spectrophotometer (Hitachi). The MDA concentration (in millimolar) was determined from a standard curve created with 1,1,3,3-tetramethoxypropane (Merck). A water sample treated in the same way was applied as a blank. All determinations were performed in duplicate.
Blood and tissue cultures.
Blood (volume, 0.2 ml) was added to glass tubes with 4 ml of thioglycolate medium (Becton Dickinson) and incubated at 35°C for a total period of 7 days. One milliliter was then plated onto MacConkey agar and incubated under the same conditions. The colonies were identified as described below.
Tissue segments were weighed and homogenized; a 0.1-ml aliquot was then diluted 1:10 in sterile sodium chloride four consecutive times. Another 0.1-ml aliquot of each dilution was plated onto MacConkey agar. The plates were incubated at 35°C for 48 h, and the numbers of viable colonies in each dilution were counted and multiplied by the appropriate dilution factor. Identification of colonies was performed with the API 20E and the API 20NE systems (bioMérieux, Paris, France). The number of viable cells was expressed as the number of CFU per gram.
Pharmacokinetic analysis.
The concentrations of each antimicrobial agent were estimated by a microbiological assay. In order to discriminate the activities of clarithromycin and amikacin, two different reference strains were required, as proposed elsewhere (4); one strain was resistant to amikacin and susceptible to clarithromycin, and the other strain was resistant to clarithromycin and susceptible to amikacin. Mutants of Bacillus subtilis ICB6633 resistant to amikacin (MIC > 1,028 μg/ml) were selected after serial passage of the parent strain onto MacConkey agar (BBL, Becton Dickinson) impregnated with increasing concentrations of amikacin. After thorough testing of the acquisition of resistance by MIC determination, these colonies were applied for the estimation of the clarithromycin levels. E. coli ICB4004, which is resistant to clarithromycin and susceptible to amikacin, was applied for the determination of amikacin levels. All determinations were performed in triplicate, and the means were applied. Concentrations were determined by comparison with those on a standard semilogarithmic curve prepared with known concentrations of antimicrobials. The lower limits of detection were 0.25 μg/ml for clarithromycin and 0.7 μg/ml for amikacin. The standardized interday coefficients of variation of the assay were 5.8% for clarithromycin and 1% for amikacin.
Statistical analysis.
The results for LPS, TNF-α, and MDA are expressed as medians, and those of the drug concentrations are expressed as means ± standard errors (SEs). The log10 number of viable cells in tissue segments was applied and is presented as the mean ± SE.
The number of observations over follow-up for each group did not remain constant due to the death of the animals, so comparisons of group-time curves could not be applied for any proinflammatory mediator. As a consequence, comparisons between groups for a specific time interval and between consecutive time intervals within the same group were performed by analysis of variance; values were adjusted according to the Bonferroni correction to avoid any random correlation.
Survival was estimated by Kaplan-Meier analysis; groups were compared by the log-rank test. Survival time and the number of viable cells of tissue segments were correlated by linear regression analysis.
Any value of P equal to or less than 0.05 was considered significant.
RESULTS
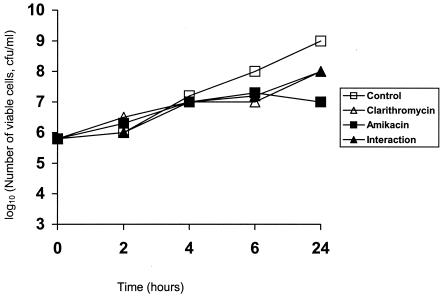
The MICs of ticarcillin-clavulanate, piperacillin, ceftazidime, imipenem, meropenem, ciprofloxacin, clarithromycin, and amikacin for the isolate used in this study were 256/2, 512, 16, 64, 32, 128, 512, and 512 μg/ml, respectively. Time-kill curves for the test isolate exposed to clarithromycin, amikacin, and the combination are shown in Fig. 1.
FIG. 1.
Time-kill curves for a multidrug-resistant P. aeruginosa isolate exposed to 10 μg of clarithromycin per ml, 20 μg of amikacin per ml, and to a combination of both drugs (interaction).
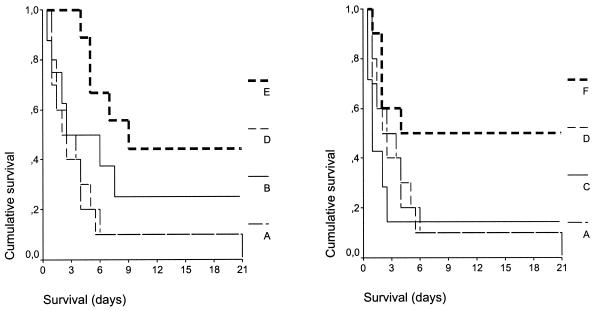
Over the 21-day follow-up, death occurred in all animals in group A (mortality rate, 100%), six animals in group B (mortality rate, 75%), six animals in group C (mortality rate, 85.7%), all animals in group D (mortality rate, 100%), six animals in group E (mortality rate, 60%), and five animals in group F (mortality rate, 50%). The mean ± SE survival times of the animals in the different groups were as follows: group A, 4.50 ± 1.81 days; group B, 7.69 ± 2.83 days; group C, 4.07 ± 2.63 days; group D, 4.55 ± 1.90 days; group E, 11.55 ± 2.51 days; and group F, 11.60 ± 2.98 days. Statistically significant differences were found between groups D and F (P = 0.033), groups D and E (P = 0.006), groups A and E (P = 0.021), and groups C and F (P = 0.042). No statistically significant differences were found between groups E and F or between groups B and E. Survival curves for all treatment groups are shown in Fig. 2.
FIG. 2.
Comparative survival of rabbits in groups A, B, D, and E and groups A, C, D, and F with acute pyelonephritis caused by multidrug-resistant P. aeruginosa. P values were as follows: group A versus group D, not significant; group B versus group E, not significant; group E versus group F, not significant; group D versus group F, 0.033; group D versus group E, 0.006; group A versus E, 0.021; group C versus group E, 0.042.
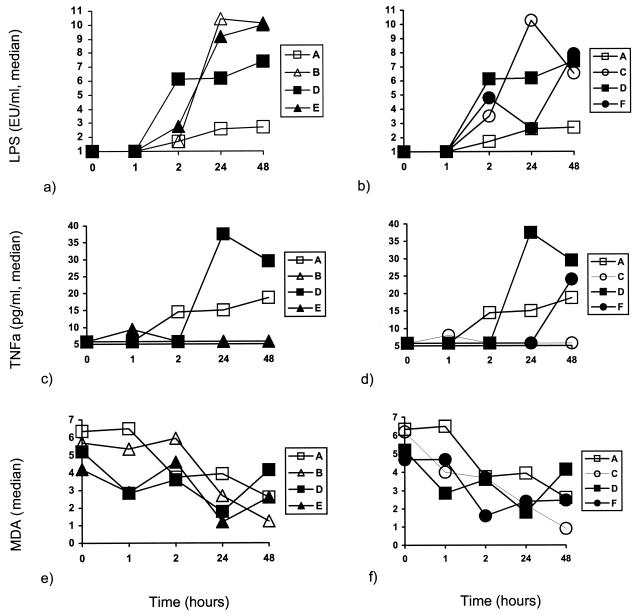
The concentrations of LPS, TNF-α, and MDA detected in serum over the time of follow-up for animals in groups A, B, D, and E and animals in groups A, C, D, and F are compared in Fig. 3. The concentrations of LPS were similar in all groups at each time interval. No statistically significant differences in TNF-α levels were recorded between treatment groups with the exception of those at 24 and 48 h. At 24 h the serum TNF-α concentrations for groups E and F were significantly lower than those for group D (P = 0.032 and 0.012, respectively); at 48 h the serum TNF-α concentrations for group C were significantly lower than those for group D (P = 0.030). The concentrations of MDA remained unaltered in groups A and B over the follow-up period. However, a significant decrease was noted at 48 h in group B (P = 0.038), at 48 h in group C (P = 0.020), at 2 h in group E (P = 0.040), and at 24 and 48 h in group F (P = 0.022 and 0.022, respectively).
FIG. 3.
Concentrations of endotoxins (LPS) (a and b), TNF-α (c and d), and MDA (millimolar) (e and f) in rabbits in groups A, B, D, and E (a, c, and e) and rabbits in groups A, C, D, and F (b, d, and f) with acute pyelonephritis caused by multidrug-resistant P. aeruginosa.
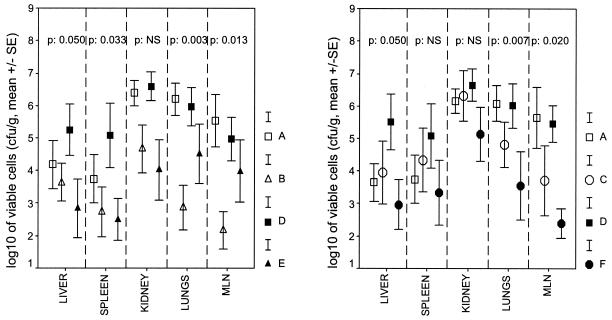
All blood samples drawn at each sampling time yielded the inoculated pathogen on culture. Comparisons of the viable cell counts for the infected kidneys, livers, spleens, lower lobes of the right lung, and mesenteral lymph nodes at the time of death or euthanasia for groups A, B, D, and E and groups A, C, D, and F are shown in Fig. 4. No statistically significant differences in the numbers of viable cells in the infected kidneys were found between the treatment groups. The numbers of isolates in the lungs and mesenteral lymph nodes of group F were statistically significantly lower than those in the lungs and mesenteral lymph nodes of group C (P = 0.019 and 0.026, respectively). The number of isolates in the mesenteral lymph nodes of group F was statistically lower than that in the mesenteral lymph nodes of group E (P = 0.015). E. coli was detected in the livers, lungs, and spleens of three animals in group B (37.5%), three animals in group C (42.8%), and three animals in group D (30%). E. coli was detected in the lungs and spleen of one animal (10%) in group E and one animal in group F (10%).
FIG. 4.
Comparisons of counts of the inoculated P. aeruginosa pathogen in the infected right kidneys, livers, spleens, lower lobes of the right lung, and mesenteral lymph nodes (MLN) of rabbits in groups A to F after death or euthanasia. NS, not significant.
A negative linear correlation was found between survival time and the bacterial load in the livers (r = −0.540; P < 0.0001), spleens (r = −0.575; P < 0.0001), kidneys (r = −0.593; P < 0.0001), lungs (r = −0.609; P < 0.0001), and mesenteral lymph nodes (r = −0.587; P < 0.0001).
The serum clarithromycin and amikacin levels in groups B, C, D, E, and F are given in Table 1. The mean clarithromycin levels in groups B, C, E, and F achieved 1 h after bacterial challenge were within the desired concentration range. However, the concentrations estimated 3 h after bacterial challenge in groups C and F were greater than 10 μg/ml.
TABLE 1.
Concentrations of clarithromycin and amikacin in sera of rabbits in groups B to F with induced pyelonephritis caused by multidrug-resistant P. aeruginosa
| Time (h) of follow-up | Mean ± SE concn (μg/ml) of the indicated drug
|
||||||
|---|---|---|---|---|---|---|---|
| Group B, clarithromycin | Group C, clarithromycin | Group D, amikacin | Group E
|
Group F
|
|||
| Clarithromycin | Amikacin | Clarithromycin | Amikacin | ||||
| 1 | 9.90 ± 1.28 | 13.00 ± 1.43 | 12.99 ± 4.07 | 7.33 ± 1.41 | 13.78 ± 4.71 | 10.50 ± 1.46 | 22.60 ± 4.22 |
| 2 | 7.13 ± 0.48 | 6.46 ± 0.63 | 13.45 ± 1.62 | 5.42 ± 0.78 | 13.90 ± 4.15 | 7.28 ± 0.47 | 17.69 ± 5.87 |
| 3 | 4.90 ± 0.50 | 15.38 ± 0.38 | —a | — | — | 13.50 ± 0.50 | 8.50 ± 1.50 |
| 24 | 1.05 ± 0.05 | NDb | ND | ND | ND | ND | ND |
—, no sampling was performed.
ND, not detectable.
DISCUSSION
In addition to its antibacterial effect, clarithromycin has been found to possess considerable immunomodulatory properties and has become a candidate for the therapy of chronic lung inflammatory states (11, 21, 26). Its effect on human monocytes is expressed at concentrations close to 10 μg/ml (14). The purpose of the present study was to establish a safe dosing regimen for intravenously administered clarithromycin that achieves these levels in serum and to apply it to the management of experimental sepsis. Clarithromycin was administered alone or with amikacin for the treatment of acute pyelonephritis caused by multidrug-resistant P. aeruginosa; P. aeruginosa is a common nosocomial pathogen whose eradication is quite difficult (27). Antimicrobials were administered in parallel with bacterial challenge; clarithromycin was given as single dose or as two doses, with the second dose given after a short time interval, in an attempt to achieve steady-state levels in serum.
The applied model of sepsis was lethal, as shown by the 100% mortality rates for animals in both group A and group D (Fig. 2). Survival analysis revealed a considerable benefit in terms of both the mortality rate and the overall rate of survival after the application of clarithromycin, and this benefit was enhanced in the presence of amikacin. However, no statistically significant differences were encountered between animals administered one dose and animals administered two consecutive doses. Prolongation of survival following treatment with clarithromycin raises questions about the mechanisms of its action. These may involve either an immunomodulatory effect (5) or a direct effect on the bacterial cell (27). Both possibilities were investigated in the present study. All treatment groups had the same degree of endotoxemia (Fig. 3a and b); i.e., the probability that systemic inflammation would be triggered was the same for all treatment groups. Administration of clarithromycin in groups B, C, E, and F restrained the elevation of TNF-α levels in serum, which is contrary to the findings for groups A and D. Serum TNF-α levels were similar between groups B and E as well as between groups C and F (Fig. 3c and d). The delayed increase in TNF-α levels at 48 h in animals in group F might be explained by the absence of clarithromycin from their serum at that time interval (Table 1), when endotoxin triggering was still present (Fig. 3a and b). MDA is a marker of lipid peroxidation, and MDA levels are significantly elevated in sepsis (10). Serum MDA levels decreased over the follow-up period in all groups of animals that received clarithromycin (Fig. 3e and f). The anti-TNF-α and antioxidant properties of clarithromycin are indicative of its anti-inflammatory mode of action. The latter is also supported by previous results that showed the inhibition of proinflammatory cytokines (11, 23, 24) and oxidative burst of monocytes and neutrophils (5, 20) as a result of clarithromycin treatment.
Clarithromycin is reported to be bactericidal for mucoid P. aeruginosa isolates; these isolates are often colonizers and pathogens of the respiratory tract and form biofilms that limit the antibacterial activities of numerous antimicrobials (3). Clarithromycin may alter the rate of protein synthesis and the subsequent production of virulence factors by these isolates (12, 22), and it may act in synergy both in vitro and in vivo with antipseudomonal agents to decrease their growth (3, 28). The hypothesis that the synergy of clarithromycin and amikacin against multidrug-resistant P. aeruginosa explains the prolonged survival of the animals in the present study is not likely for three reasons: (i) the MIC of clarithromycin was significantly higher than the levels achieved in serum (Table 1); (ii) time-kill assays performed with concentrations of clarithromycin and amikacin equal to those in serum failed to disclose any additive or synergistic interaction (Fig. 1); and (iii) on necropsy, the numbers of viable cells in the infected kidneys were similar in all treatment groups. Nevertheless, clarithromycin was administered for a short time period; for clarithromycin to bring about a significant reduction in bacterial colony counts, 7 days of treatment of mice with pneumonia caused by P. aeruginosa was required (3, 27, 28).
The finding that after clarithromycin administration the numbers of colonies in the liver, spleen, lungs, and mesenteral lymph nodes were significantly decreased (Fig. 4) should not be ignored, however. Although it might be hypothesized that comparisons of the bacterial loads between animals with different survival times should not be performed, the existence of a negative correlation between survival and organ load allowed it. The decrease in viable cell counts in different organs might be an index of antibacterial synergy between clarithromycin and amikacin; however, the possibility of the beneficial phagocytosis of the pathogen by the host, which has been proposed to occur after treatment with clarithromycin, should be kept in mind (25). The immunomodulatory mechanism of action of clarithromycin might be further enhanced by recent evidence revealing that pretreatment of the bacterial pathogen with the macrolide antimicrobial before inoculation did not influence the course to death in a model of murine pneumonia (15).
Intravenous administration of clarithromycin and amikacin resulted in concentrations below the MICs for the test pathogen (Table 1). Although clarithromycin was well tolerated by all animals, administration of a second dose to animals in groups C and F resulted in an increase in serum drug levels to concentrations close to those considered to produce prolongations of the Q-T interval in rats (17). This observation and the similar effect of either one dose or two consecutive doses on survival indicate that consecutive dosing might not be needed for the management of experimental sepsis.
The present study revealed that the intravenous administration of clarithromycin that achieves concentrations close to 10 μg/ml might constitute a promising immunomodulatory therapy for the management of sepsis caused by multidrug-resistant P. aeruginosa; when administered singly or with amikacin, a considerable prolongation of survival in an experimental model was achieved. The presented findings intensify the need for further animal studies to fully document the exact role of clarithromycin in the management of sepsis caused by susceptible and multidrug-resistant gram-negative bacteria.
REFERENCES
- 1.Arndt, P., and E. Abraham. 2001. Immunological therapies for sepsis. Intensive Care Med. 27(Suppl. 1):S104-S115. [DOI] [PubMed] [Google Scholar]
- 2.Berry, V., R. Page, J. Satterfield, C. Singley, R. Straub, and G. Woodnut. 2000. Comparative efficacy of gemifloxacin in experimental models of pyelonephritis and wound infection. J. Antimicrob. Chemother. 45(Suppl. 1):87-93. [DOI] [PubMed] [Google Scholar]
- 3.But, K. Q., M. A. Banevicius, C. H. Nightingale, R. Quintiliani, and D. P. Nicolau. 2000. In vitro and in vivo influence of adjunct clarithromycin on the treatment of mucoid Pseudomonas aeruginosa. J. Antimicrob. Chemother. 45:57-62. [DOI] [PubMed] [Google Scholar]
- 4.Chapin-Robinson, K., and S. C. Edberg. 1991. Measurement of antibiotics in human body fluids: techniques and significance, p. 295-366. In V. Lorian (ed.), Antibiotics in laboratory medicine, 2nd ed. The Williams & Wilkins Co., Baltimore, Md.
- 5.Culic, O., V. Erakovic, and M. J. Parnham. 2001. Anti-inflammatory effects of macrolide antibiotics. Eur. J. Pharmacol. 429:209-229. [DOI] [PubMed] [Google Scholar]
- 6.Engelhard, D., S. Pomernz, S. Gallily, N. Strauss, and E. Tuomanen. 1997. Serotype-related differences in inflammatory response to Streptococcus pneumoniae in experimental meningitis. J. Infect. Dis. 175:979-982. [DOI] [PubMed] [Google Scholar]
- 7.Frendéus, B., G. Godaly, L. Hang, D. Karpman, A. C. Lundstedt, and C. Svanborg. 2000. Interleukin 8 receptor deficiency confers susceptibility to acute experimental pyelonephritis and may have a human counterpart. J. Exp. Med. 192:881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giamarellos-Bourboulis, E. J., P. Grecka, A. Dionyssiou-Asteriou, and H. Giamarellou. 1998. In vitro activity of polyunsaturated fatty acids on Pseudomonas aeruginosa: relationship to lipid peroxidation. Prostaglandins Leukot. Essent. Fatty Acids 58:283-287. [DOI] [PubMed] [Google Scholar]
- 9.Giamarellos-Bourboulis, E. J., H. Poulaki, N. Kostomitsopoulos, I. Dontas, D. Perrea, P. E. Karayannacos, and H. Giamarellou. 2003. Effective immunomodulatory treatment of Escherichia coli experimental sepsis with thalidomide. Antimicrob. Agents Chemother. 47:2445-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goode, H. F., H. C. Cowley, B. E. Walker, P. D. Howdle, and N. R. Webster. 1995. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Crit. Care Med. 23:646-651. [DOI] [PubMed] [Google Scholar]
- 11.Ianaro, A., A. Ialenti, P. Maffia P, L. Suatebin, L. Rompola, R. Carnuccio, T. Iuvone, F. D'Acquisto, and M. Di Rosa. 2000. Anti-inflammatory activity of macrolide antibiotics. J. Pharmacol. Exp. Toxicol. 292:156-163. [PubMed] [Google Scholar]
- 12.Kawamura-Sato, K., Y. Iinuma, T. Hasegawa, T. Horii, T. Yamashino, and M. Ohta. 2000. Effect of subinhibitory concentrations of macrolides on expression of flagellin in Pseudomonas aeruginosa and Proteus mirabilis. Antimicrob. Agents Chemother. 44:2869-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalil, A., K. Tullus, T. Bartfai, M. Bakhiet, G. Jaremko, and A. Brauner. 2000. Renal cytokine responses in acute Escherichia coli pyelonephritis in IL-6-deficient mice. Clin. Exp. Immunol. 122:200-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kikuchi, T., K. Hagiwara, Y. Honda, Y., K. Gomi, T. Kobayashi, H. Takahashi, Y. Tokue, A. Watanabe, and T. Nukiwa. 2002. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-kappa B transcription factors. J. Antimicrob. Chemother. 49:745-755. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi, T., K. Tatade, T. Matsumoto, S. Miyazaki, A. Watanabe, T. Nukiwa, and K. Yamaguchi. 2002. Macrolide-treated Pseudomonas aeruginosa induces paradoxical host responses in the lungs of mice and a high mortality rate. J. Antimicrob. Chemother. 50:59-66. [DOI] [PubMed] [Google Scholar]
- 16.Muzio, G., R. A. Salvo, A. Trombetta, R. Autelli, M. Maggiora, M. Terreno, M. U. Dianzani, and R. A. Canuto. 1999. Dose-dependent inhibition of cell proliferation induced by lipid peroxidation products in rat hepatoma cells after enrichment with arachidonic acid. Lipids 34:705-711. [DOI] [PubMed] [Google Scholar]
- 17.Ohtani, H., C. Taninaka, E. Hanada, H. Kotaki, H. Sato, Y. Sawada, and T. Iga. 2000. Comparative pharmacodynamic analysis of Q-T interval prolongation induced by the macrolides clarithromycin, roxithromycin, and azithromycin in rats. Antimicrob. Agents Chemother. 44:2630-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rangel Frausto, M. S. 1999. The epidemiology of bacterial sepsis. Infect. Dis. Clin. N. Am. 13:299-311. [DOI] [PubMed] [Google Scholar]
- 19.Robaux, M. A., L. Dube, J. Caillon, D. Bugnon, M. F. Kergueris, D. Navas, P. Le Conte, D. Barron, and G. Patel. 2001. In vivo efficacy of continuous infusion versus intermittent dosing of ceftazidime alone or in combination with amikacin relative to human kinetic profiles in a Pseudomonas aeruginosa rabbit endocarditis model. J. Antimicrob. Chemother. 47:617-622. [DOI] [PubMed] [Google Scholar]
- 20.Scaglione, F., and G. Rossoni. 1998. Comparative anti-inflammatory effects of roxithromycin, azithromycin and clarithromycin. J. Antimicrob. Chemother. 41(Suppl. B):47-50. [DOI] [PubMed] [Google Scholar]
- 21.Tagaya, E., J. Tamaoki, M. Kondo, and A. Nagai. 2002. Effect of short course of clarithromycin therapy on sputum production in patients with chronic airway obstruction. Chest 122:213-218. [DOI] [PubMed] [Google Scholar]
- 22.Tateda, K., Y. Ishii, T. Matsumoto, T. Kobayashi, S. Miyazaki, and K. Yamagushi. 2000. Potential of macrolide antibiotics to inhibit protein synthesis of Pseudomonas aeruginosa: suppression of virulence factors and stress response. J. Infect. Chemother. 6:1-7. [DOI] [PubMed] [Google Scholar]
- 23.Tessier, P. R., M. K. Kim, W. Zhou, D. Xuan, M. Ye, C. H. Nightingale, and D. P. Nicolau. 2002. Pharmacodynamic assessment of clarithromycin in a murine model of pneumococcal pneumonia. Antimicrob. Agents Chemother. 46:1425-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo, P. C. Y., L. W. C. Chow, E. S. K. Ma, and K. Y. Yuen. 1999. Clarithromycin attenuates the inflammatory response induced by surgical trauma in a guinea pig model. Pharmacol. Res. 39:49-54. [DOI] [PubMed] [Google Scholar]
- 25.Yamaryo, T., K. Oishi, H. Yoshimine, Y. Tsuchihashi, K. Matsushima, and T. Nagatake. 2003. Fourteen-member macrolide promote the phosphatidylserine receptor-dependent phagocytosis of apoptotic neutrophils by alveolar macrophages. Antimicrob. Agents Chemother. 47:48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanagihara, K., K. Tomono, M. Kuroki, Y. Kaneko, T. Sawai, H. Ohno, Y. Miyazaki, Y. Higashiyama, S. Maesaki, and J. Kadota. 2000. Intrapulmonary concentrations of inflammatory cytokines in a mouse model of chronic respiratory infection caused by Pseudomonas aeruginosa. Clin. Exp. Immunol. 122:67-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yanagihara, K., K. Tomono, Y. Imamura, Y. Kaneko, T. Sawai, Y. Miyasaki, Y. Hirakata, H. Mukae, J. Kadota, and S. Kohno. 2002. Effect of clarithromycin on chronic respiratory infection caused by Pseudomonas aeruginosa with biofilm formation in an experimental murine model. J. Antimicrob. Chemother. 49:867-870. [DOI] [PubMed] [Google Scholar]
- 28.Yanagihara, K., K. Tomono, T. Sawai, M. Kuroki, Y. Kaneko, H. Ohno, Y. Higashiyama, Y. Miyazaki, Y. Hirakata, S. Maesaki, J. Kadota, T. Tashiro, and S. Kohno. 2000. Combination therapy for chronic Pseudomonas aeruginosa respiratory infection associated with biofilm formation. J. Antimicrob. Chemother. 46:69-72. [DOI] [PubMed] [Google Scholar]